Summary
There are many similar risk factors for both cancer and cardiovascular disease. There are substantial biological data to support the hypotheses that the pathogenesis of these 2 diseases overlaps. Exercise should be strongly encouraged and should continue to be a focus of ongoing research. The management of risk factors for cardiovascular disease and cancer, including tobacco use, a healthy diet, and normal weight, should be further addressed. Treatment of cardiac risk factors, including hypertension, hyperlipidemia, and diabetes, in cancer patients may actually improve overall outcomes not only in cancer patients on active therapy but also in cancer survivors. As a result, continued collaboration between oncology and cardiology is essential.
Keywords: Cardiooncology, Risk factors, Cancer, Heart disease, Risk
Cardiovascular disease and cancer are 2 of the largest contributors to death in the United States. Currently, there are more than 15 million individuals with a history of cardiovascular disease and 14 million with a history of cancer.1 This number is only expected to rise as the population in the United States ages and advances in the management of both cardiovascular disease and cancer are made. There is a growing body of evidence that suggests cancer and cardiovascular disease share a biological mechanism. In the complex process of carcinogenesis, cells go through several genetic “hits” before the full neoplastic phenotype of growth, invasion, and metastasis occurs. This process of malignant transformation can be divided into the following: initiation (irreversible step), promotion (stimulation of the growth of initiated cells), and progression (development of a more aggressive phenotype of promoted cells).2 Similarly, the pathogenesis of cardiovascular disease is a multistep process. Although inflammation seems to play a major role in both diseases, other mechanisms have also been described, including the role of hyperglycemia, hyperinsulinemia, and IGF-1.3 Gaining a better understanding of these shared mechanisms is vital. This review explores the risk factors common to cardiovascular disease and cancer, focusing on the epidemiologic studies that have been completed as well as how underlying cardiovascular disease affects cancer and cancer treatments.
Mechanisms Common to Cancer and Cardiovascular Disease
Inflammation and Cancer
The connection between inflammation and cancer has long been established. Initially in the nineteenth century, Virchow appreciated the presence of leukocytes within neoplastic tissue. From his work, speculations arose as to whether cancer started in inflammatory sites. Further work demonstrated the associations between some chronic infections and cancers, that is, human papilloma-virus and cervical cancer,4 Helicobacter pylori, and stomach cancer,5 Epstein-Barr virus and cancers.6,7 Nonhealing wounds of the skin can develop into squamous cell carcinomas. There is also an association between chronic inflammatory diseases and cancer, that is, systemic sclerosis and cancer,8 celiac disease, and small bowel lymphomas.9 Approximately 10% to 20% of cancers arise at the site of chronic inflammation.10 Through time, it has been appreciated that inflammation in the tumor microenvironment promotes malignant transformation and carcinogenesis. The presence of signaling between immune cells is required for tumor growth. There have been suggestions that stopping this inflammation with the use of anti-inflammatory medication actually delays cancer growth. For example, the use of aspirin, a cyclooxygenase 2 inhibitor, prevents the growth of primary and secondary polyps.11 Similarly, aspirin use may be preventative for lung, esophagus, and stomach cancer.12 There is ongoing research that perhaps statins, a cholesterol-lowering agent, may decrease inflammation and result in improvements in cancer outcomes.3
Inflammation and Cardiovascular Disease
Atherosclerotic disease, initially thought a lipid storage disease, is now known to be well-characterized by inflammation. Many risk factors for cardiovascular disease (hypertension, tobacco use, hyperlipidemia, and insulin resistance) trigger atherosclerosis through the promotion of adhesion of endothelial cells and the stimulation of leukocyte attachment to blood vessel walls that normally resist attachment.3,13 With hyperlipidemia, excess lipoproteins accumulate in the subendothelial space, leading to oxidation and uptake by monocytes and macrophages.14 Similarly with hypertension, there is oxidative stress on blood vessel walls. Elevations in inflammatory biomarkers, such as the C-reactive protein (CRP), have been associated with both primary and secondary cardiovascular events.15–17 There are ongoing investigations looking at immunotherapy for the reduction in cardiovascular events. For example, an inhibitor of interleukin (IL)-1B, a proinflammatory cytokine involved in atherosclerosis and the regulation of CRP, is currently being studied for its effects on reduction in cardiovascular disease events in the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial (NCT01327846) as well as its effects on vascular endothelial function (NCT02272946). Additional trials are also ongoing looking at the effects of statins and its effects on a reduction in inflammation.
Although diabetes, smoking, and obesity contribute to inflammation, there are a variety of other mechanisms that may also result in the shared risk factors between cancer and cardiovascular disease. These mechanisms are discussed.
Shared Risk Factors for Cancer and Cardiovascular Disease
There are many potential risk factors (modifiable and nonmodifiable) common to both cancer and cardiovascular disease, include aging, gender, obesity, diabetes mellitus, physical activity, diet, and smoking.
Age
The incidence of most cancers increases with advancing age. In developed countries, 78% of all newly diagnosed cancers occurs in individuals over the age of 55 years.18 Similarly, cardiovascular risk increases with advancing age. Although 4.3% of individuals ages 18 to 44 years have heart disease, this prevalence increases to 12% for those ages 45 to 64 years, 24.6% for those ages 65 to 74 years, and 35% in those over the age of 75 years.19
Gender
Although certain cancers are gender-specific (cervix, testicular, uterine, and prostate), overall, cancer occurs more frequently in men. Similarly, men have a higher risk of developing cardiovascular disease.19,20
Obesity
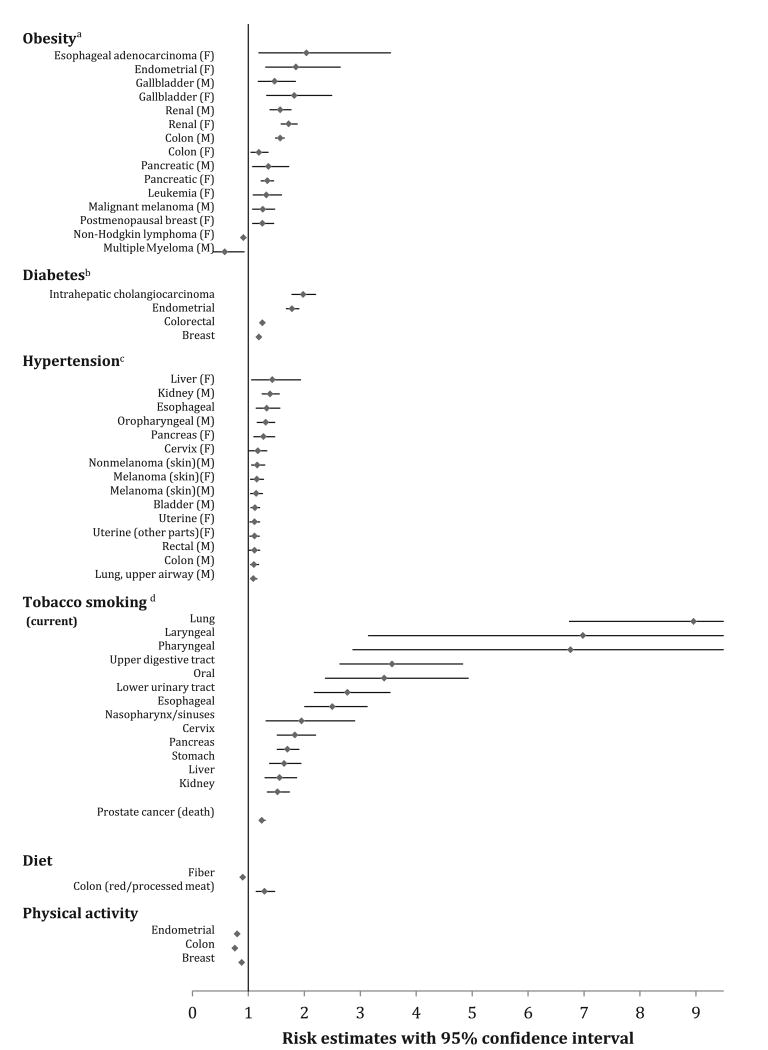
According to epidemiologic data, 20% of all cancers are considered weight related.21,22 According to the American Institute of Cancer Research and the World Cancer Research Fund, there is a link between obesity and many cancers, including esophageal adenocarcinoma, pancreatic, liver, colorectal, postmenopausal breast cancer, endometrial, and kidney cancers (Fig. 1).23,24 It also seems that among those with a higher body mass index (BMI), cancer risk is higher. For those with a BMI between 27.5 kg/m2 and 29.9 kg/m2, the risk of cancer increases by 12% whereas those with a BMI greater than 40 kg/m2 have a 70% increased risk25 of cancer compared with those with a normal BMI. Similarly, in a large meta-analysis by Renehan and colleagues,26 a 5-kg/m2 increase in BMI was strongly associated with the risk of esophageal adenocarcinoma (relative risk [RR] 1.52, P<.001), thyroid cancer (RR 1.33, P = .02), colon cancer (RR 1.24, P<.001), and kidney cancer (RR 1.24, P<.0001) in men and endometrial cancer (RR 1.59, P<.0001), gallbladder cancer (RR 1.59, P = .04), esophageal adenocarcinoma (RR 1.51, P<.0001), and kidney cancer (RR 1.34, P<.0001) in women. On the contrary, dramatic weight loss from gastric bypass surgery is associated with reduced cancer mortality by approximately 60%.27,28 These data suggest that promoting healthy weight change in adults can have important health benefits and outcomes from a cancer perspective.
Fig. 1.
Modifiable cardiac risk factors with their estimated cancer risk. Figure limited to only the positive and/or negative associations using the most recently published meta-analysis or prospective cohort study investigating the associations between a cardiac risk factor and various cancer sites; hyperlipidemia was excluded from the figure due to inconclusive evidence that it is associated with cancer risk. All cancer estimates were reported as a RR except where noted. aRisk for BMI greater than 30 kg/m2. bNote, there were several additional positive (bladder, esophageal, extrahepatic cholangiocarcinoma, gallbladder, gastric, hepatocellular carcinoma, kidney, leukemia, multiple myeloma, non-Hodgkin lymphoma, ovarian cancer, and pancreatic cancer) and negative (lung and prostate) associations by fixed effects models, but the authors did not deem these positive associations because their 95% prediction intervals included the null value. cProspective observational cohort; Cox regression was used to calculate HRs of cancer per 10–mm Hg increments of mid–blood pressure. dOnly cancer sites with sufficient evidence of carcinogenicity related to tobacco exposure in humans were considered in the meta-analysis. (From Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133(11):1106; with permission.)
Obesity has been associated with an increased risk of cardiovascular disease as outlined by the Framingham data.29 The age-adjusted RR for cardiovascular disease is higher for those who are overweight (men RR 1.21 [1.05–1.40]; women RR 1.20 [1.03–1.41]) or obese (men RR 1.46 [1.20–1.77]; women RR 1.64 [1.37–1.98]). Obesity has been associated with a proinflammatory state, a prothrombotic state, insulin resistance, and atherogenic dyslipidemia.30,31 Obesity causes an increase in cardiac output and hence overall cardiac workload to meet the metabolic demands of the expanded adipose tissue. Obesity has been linked to several cardiovascular changes, including hyperdynamic circulation, structural changes, sleep apnea, and overt heart failure.32 This combination of factors leads to a concentric left ventricular hypertrophy and cardiovascular effects, even without associated hypertension.33
Biologically, there are plausible overlapping mechanisms associating the inflammatory state of obesity with both cardiovascular disease and cancer (Box 1). Elevated levels of IGF-1, found in obesity and the metabolic syndrome, are associated in preclinical models with tumor growth.34,35 IGF-1 and its effects are discussed later.36–41 Elevated estrogen levels are commonly seen in obesity; elevated estrogen levels are associated with breast and endometrial cancers. Biologically, estrogen is associated with tumor proliferation. Higher levels of IL-6, produced by adipose tissue, have been associated with hyper-tension and hepatic production of CRP, known to be associated with cardiovascular disease. From a cancer perspective, IL-6 has been shown to inhibit cancer cell apoptosis, stimulate angiogenesis, and play a role in drug resistance and tumor progression.42,43 Leptin, thought to be related to cardiovascular disease,44 has been associated with obesity and tumor growth and invasion. It has been associated with hepatocellular carcinoma, prostate cancer, and ovarian cancer. Leptin seems to up-regulate the expression of cyclin D1 and Mcl-1 to stimulate cell growth by activating the PI3K/Akt and MEK/ERK1/2 pathways in ovarian cancer.45,46
Box 1. Biological mediators linking obesity, cancer, and cardiovascular disease.
IGF-1
Estrogen
IL-6
Leptin
Adiponectin
Diabetes
In 2010, the American Diabetes Association issued a statement linking diabetes to colon, liver, endometrial, pancreas, and bladder cancers (see Fig. 1).20 There are also concerns about its association with leukemia, kidney cancer, and esophageal cancer. Although some inclusion criteria in this analysis were debated,47 a recent review of meta-analyses of observational studies provided a significant amount of evidence to support this association of type 2 diabetes mellitus with cancer.48
Diabetes mellitus affects large and small vessels in the vasculature system and has long been associated with cardiovascular disease. The pathophysiology is likely multifaceted. Insulin resistance leads to dyslipidemia and abnormalities in lipoproteins through oxidative stress, glycolysis, and triglyceride enhancement. Endothelial function, an early marker for atherosclerosis, is stimulated by hyperglycemia induced free radical damage.13 With higher levels of glucose, IGF-1 stimulates the migration and proliferation of smooth muscle cells, a part of early cardiovascular disease development.20
From a biological perspective, hyperglycemia, hyperinsulinemia, and the role of IGF-1 all likely contribute to the underlying biological link between cancer, cardiovascular disease, and diabetes mellitus. Many cancers, such as breast cancer, overexpress the insulin receptor.49 IGF-1 promotes cell proliferation by stimulating cancer cell proliferation and metastasis; this seems evident even in cells in which the IGF receptors are deficient.49,50 Multiple signaling pathways are activated after insulin receptors or IGF-1 receptors interact with their ligands. This complex pathway leads to proliferation, protection from apoptotic stimuli, invasion, and metastasis. Additionally, hyperglycemia allows IGF-1 to stimulate vascular smooth muscle proliferation and migration. This mechanism furthers cancer growth and metastasis51; the smooth muscle proliferation and migration is also a hallmark of atherosclerosis pathophysiology.52
Separate from the direct effects of insulin on cancer cells, it has been theorized that hyperinsulinemia may promote carcinogenesis indirectly through IGF-1.53 Insulin reduces the hepatic production of IGF-binding protein, resulting in higher levels of IGF-1.54 IGF-1 has more potent antiapoptotic activities than insulin. It may act as a growth stimulus. Current cancer clinical trials are examining inhibitors of IGF-1.55 Although insulin reduces the hepatic production of IGF-binding protein, it also reduces blood levels of sex hormone–binding globulin, leading to increases in bioavailable estrogen in both men and women. Although this increase in sex hormones may explain the increased risk of premenopausal breast cancer and endometrial cancer seen among those with hyperinsulinemia,21 it does not explain a positive association between hyperinsulinemia and the cardiovascular system, because estrogen has a beneficial effect in cardiovascular disease. Higher levels of IGF have also been associated with colon and prostate cancer.
Tobacco Use
Tobacco usage, in particular cigarette smoking, is a heavily weighted risk factor for multiple types of cancers (see Fig. 1), including lung cancer, esophageal cancer, and pancreas cancer.56 The American Cancer Society estimates that smoking is responsible for 30% of all cancer-related deaths in the United States. Although rates of tobacco use are decreasing in the United States and Canada, trends continue to increase globally, according to the World Health Organization's Global Report on Trends in Prevalence of Tobacco Use 2015.57
Cigarette smoking contributes to all stages of atherosclerosis, including stroke, myocardial infarction, hypertension, and aortic aneurysms; it has also been associated with approximately one-third of all first myocardial infarctions worldwide.56 By decreasing nitric oxide, causing vasomotor dysfunction, increasing oxidative stress, and contributing to endothelial damage, tobacco use contributes to atherosclerosis.58
Biologically, tobacco smoke produces several irritants, carcinogens, proinflammatory stimuli, and oxidizing agents. These processes affect signaling found in both tobacco-related and cardiovascular disease. Nicotine has also been found directly related to the pathogenesis of both cardiovascular disease and cancer by inhibiting apoptosis and increasing angiogenesis.58
Hypertension
Hypertension has a particularly strong association with kidney cancers.59,60 Also, in the Metabolic Syndrome and Cancer Project,34 hypertension was positively correlated to total incident cancer (hazard ratio [HR] per 10–mm Hg increment: 1.07) and to cancers of the oropharynx, colon, rectum, lung, bladder, kidney, and melanoma in men and to cancers of the liver, pancreas, cervix, uterus, and melanoma in women.
Hypertension contributes to cardiovascular disease through various mechanisms, including its direct hemodynamic consequences, neurohormonal activation, autonomic influences, oxidative stress, and inflammation. These mechanisms may also be related to cancer and cancerous growth. In hypertension, vascular endothelial growth factor (VEGF) increases. VEGF is needed for tumors to induce new blood vessel formation. Angiotensin II, increased in hypertension, stimulates VEGF production.61 Additionally further oxidative stress on blood vessel walls in the tumor microenvironment may further carcinogenesis.
Hyperlipidemia
Animal models have predicted that a high cholesterol diet may lead to colon polyps. It has been hypothesized that chronic saturated fat with increased cholesterol intake leads to an increase in hepatic bile acids and promotes carcinogenesis.62–64 Other studies suggest there may also be an association between hyperlipidemia and breast cancer.65,66 The cholesterol metabolite 27-hydroxycholesterol is similar to estradiol. Studies suggest that 27-hydroxycholesterol behaves as an endogenous selective estrogen receptor modulator. Similar to estradiol, 27-hydroxycholesterol, behaving as a partial agonist, positively regulates gene transcription and cell proliferation in cellular models of breast cancer and, as a result, may influence the pathology of breast cancer.67 Several recent studies suggest there are improved cancer outcomes in those on cholesterol-lowering agents; these are discussed later.
In cardiovascular disease, the link between hyperlipidemia and cardiovascular disease has long been established. Excess lipoproteins in hyperlipidemia accumulate in the subendothelial space. This accumulation leads to oxidation, and further uptake by monocytes and macrophages causing further inflammation, thus promoting carcinogenesis.
Diet
Diet has been linked to both cancer and cardiovascular disease in both direct and indirect ways. There are direct carcinogens in foods, such aflatoxins and nitrosamines. Aflatoxins can cause liver cancer. Nitrosamines, produced from nitrates and added to processed meat for conservation, are genotoxic substances that can act directly on DNA, causing point mutations, deletions, and insertions. Additionally, dietary fats found primarily in beef and dairy products can lead to inflammation and increases in carcinogenic bile acids and nitrosamines. In animal species as well as in human case-control studies, nitrosamines have been associated with gastric cancer and esophageal cancer.68 In contrast, the Mediterranean-style diet, focused on high amounts of fruits, vegetables, whole grains, and unsaturated fats, has been associated with an approximately 10% decrease in cancer mortality.69 It has been linked to a decrease in the incidence of many cancers, in particular gastric cancer and colon cancer.70–73 It is hypothesized that the Mediterranean diet results in a decrease in inflammatory cytokines and, as result, a decrease in cancer incidence. The beneficial role of fruits and vegetables on cancer incidence is thought to be a result of the high levels of polyphenols. Polyphenols seem to affect several metabolic pathways, including mitogen-activated protein kinases, phosphatidylinositol 3-kinases, IGF-1, nuclear factor κB, and reactive oxygen species, leading to reductions in both cardiovascular disease and cancer.74
Finally, a Mediterranean-style diet indirectly reduces cancer risk by improving intermediaries associated with cancer, such as obesity, hyperlipidemia, hyperinsulinemia, and inflammation (discussed previously).
Diet and nutrition have long been associated with cardiovascular disease. Based on several previously published articles, there is an increased risk of cardiovascular disease associated with high content of saturated fats, red meat, sugar, and processed food.71,75–78 In contrast, the Dietary Approaches to Stop Hypertension (DASH) diet and a Mediterranean-style diet focused on high amounts of fruits, vegetables, whole grains, and unsaturated fats are associated with better cardiovascular outcomes. The Mediterranean diet has been linked to approximately one-third fewer cardiovascular events.79–81 Although the biological mechanisms linking diet and cardiovascular disease are not completely understood, there are several intermediaries associated with diet and cardiovascular disease, including high body weight, high blood pressure, and uncontrolled lipids. With the Mediterranean diet, there is typically less obesity (and its other intermediaries, discussed previously), a decrease in adipokines, and a decrease in inflammatory cytokines, all contributing to fewer cardiovascular events.
Sedentary Behavior
Exercise can also have a favorable effect on improving cancer risk. Modest exercise has been associated with reduced incidence of prostate, breast, bladder, esophageal, kidney, and endometrial cancers.82–85 Exercise seems to decrease not only primary incidence of cancer but also recurrence in cancer survivors. Sedentary women who begin exercising 150 minutes per week can decrease their risk of breast cancer recurrence by 6%.86 Exercise has also been associated with a decrease in cancer mortality.87,88 For each 15 minutes of exercise per day, cancer mortality decreases by 1%.89 The cancer preventative effects also seem independent of weight. An individual with a healthy body weight who is inactive still has a higher risk for cancer compared with those who exercise.90
Exercise can have a favorable effect on cardiovascular profiles and heart failure.91 Exercise can result in improvements in obesity, diabetes, and lipid profiles; a decrease in cardiovascular and cancer events with exercise can occur through these mechanisms (discussed previously). Through improvements in aerobic capacity, blood vessel capacitance, and vascular wall function, however, exercise can also improve cardiovascular disease.92 Additionally, not all the benefits of exercise are related to weight management: as outlined in the INTERHEART Study, sedentary behavior, even in individuals with a healthy weight, results in an increase in cardiovascular mortality.93 It seems the effects of exercise are dose related, with exercise of 150 minutes per week reducing the risk of cardiovascular events by 14%, with exercise of 200 minutes per week reducing the risk of cardiovascular events by 20%.90,94,95
Biologically, the effects of exercise on cancer and cardiovascular disease prevention are likely multifactorial. Some of these mechanisms overlap and have been described previously. For instance, exercise is often seen in conjunction with a healthier diet. Exercise decreases diabetes, hypertension, hyperlipidemia, and obesity. With physical activity, there is an overall decrease in adipose tissue. Thus the overall effect of exercise is a reduction in circulating sex and metabolic hormones, a decrease in insulin, decrease in leptin, and a decrease in inflammatory markers (CRP, tumor necrosis factor, and IL-6), several of which are known to be carcinogenic.87 Additionally, the impacts of exercise on decreasing cancer mortality are likely related to better cardiac reserve and less cardiac toxicity of cancer treatment.
Cardiovascular Disease and Oncologic Diseases
Given the shared risk factors for cancer and cardiac disease, it is not surprising that many individuals with cancer also have cardiovascular disease. Similarly, many individuals with cardiovascular disease also have a history of cancer. In a recent study on cancer survivors with a variety of cancers, 62% were overweight or obese, 55% had hypertension, and 21% had diabetes.96 This study is consistent with many other published articles where survivors have higher rates of obesity, hyperglycemia, hyperlipidemia, and elevations in cardiac biomarkers, such as CRP.97 In looking at childhood cancer survivors, compared with siblings without cancer, childhood cancer survivors were more likely to be on medications for hypertension, hyperlipidemia, and diabetes, even if their prior cancer treatment did not include chest radiation.98
Although some of the increased cardiac risk may be related to cancer treatments, such as chemotherapy and radiation, other studies suggest that patients with cancer likely have an elevated cardiac risk at the time of diagnosis, before receiving any chemotherapy or radiation. In a Dutch study, calcium artery scores were elevated in those with a new cancer diagnosis when compared with healthy controls in the Multi-Ethnic Study of Atherosclerosis (MESA) study.99 In studies of prostate cancer patients in Canada, before starting androgen deprivation, individuals with prostate cancer had elevations in hypertension, hyperlipidemia, and glucose intolerance compared with population controls.100 Additionally, the prostate cancer patients were also more likely to have metabolic syndrome compared with controls.101 In summary, the findings of increased cardiac risk factors at the time of cancer diagnoses, compared with matched controls, provide further evidence for the shared biology underlying both cardiovascular disease and cancer.
Controlling cardiac risk factors may also improve cancer incidence and outcomes (Box 2). In the large European Prospective Investigation into Cancer and Nutrition (EPIC) study of 23,153 individuals ages 35 to 65 years, researchers examined whether those who adhered to lifestyle events of a BMI less than 30 kg/m2, no tobacco use, exercise greater than 3.5 h/wk, and a healthy diet had improvements in chronic disease outcomes. After a mean follow-up of 7.8 years, adherence to all 4 lifestyles events compared with 0 lifestyle events resulted in decreases in chronic disease (adjusted HR 0.22), diabetes (adjusted HR 0.07), myocardial infarction (adjusted HR 0.19), stroke (adjusted HR 0.50), and cancer (adjusted HR 0.64).102 Additionally, the Atherosclerosis Risk in Communities Study (ARIC) researchers examined the impact of 7 ideal cardiovascular health metrics (see Box 2) on individuals ages 45 to 64 years between 1987 to 2006. Adhering to 6 of the 7 health metrics resulted in a 51% lower incidence of cancer, after adjusting for age, gender, race, and ARIC study site.103 Although these risk factors were picked to look at the reduction in cardiovascular events, they also resulted in a decrease in cancer risk.
Box 2. Cardiovascular health metrics also associated with decreases in cancer incidence.
BMI less than 25 kg/m2
Glucose (untreated fasting glucose <100 mg/dL)
Tobacco use (never smoking or quitting >12 months previously)
Physical activity (>75 min/wk of vigorous physical activity or 150 min/wk of moderate or moderate 1 vigorous activity)
Hypertension (untreated blood pressure <120 mm Hg systolic and 80 mm Hg diastolic)
Hyperlipidemia (untreated total cholesterol <200 mg/dL)
Healthy dieta
aHealthy diet is defined as having 4 to 5 components of a healthy diet score.
Data from Professional Heart Daily. Guidelines and Statements. 2010. Available at: http://my.americanheart.org/professional/StatementsGuidelines/ByPublicationDate/PreviousYears/2010-Publications_UCM_322319_Article.jsp.
Cardiac Risk Factors and Cancer Outcomes
Cardiac Medications and Cancer Outcomes
Several studies have examined the impact of cardiovascular medications on cancer outcomes and incidence. Antiglycemic agents as a whole have reported mixed results in cancer risk. Although small studies have suggested a possible increase in cancer risk with the use of insulin analogs,104 a significant amount of data suggests metformin may actually reduce cancer risk.105,106 Based on the hyperinsulinemia hypothesis discussed previously, biologically it seems feasible that antiglycemic agents are associated with a reduction in cancer. Mechanistically, metformin activates adenosine monophosphate-activated protein kinase in hepatocytes. This kinase is not only a regulator of lipids and glucose but also a tumor suppressor. Currently, there are ongoing studies looking at metformin and cancer in prostate cancer, colorectal cancer, breast cancer, nonsmall cell lung cancer, bladder cancer, ovarian, and endometrial cancers (NCT02360618, NCT02115464, NCT02285855, NCT01340300, NCT02122185, NCT02065687, and NCT01243385).
Antihypertensives have mixed results in whether they may increase or decrease cancer risk. In animal models, furosemide and hydrochlorothiazide use results in a marginal increase in renal cell carcinoma and adrenal adenomatous growths.107 A larger meta-analysis of studies in humans by Grossman and colleagues60 also found an association between diuretics and renal cell carcinoma (odds ratio [OR] 1.55; 95% CI, 1.42–1.71). This risk has also been observed in individuals on diuretics for nonhypertensive reasons.108 Other groups have examined the impact of β-blockers and angiotensin-converting enzyme (ACE) inhibitors on cancer. A review of 11 observational studies examining 113,048 individuals found a 6% decreased incidence of colon cancer in individuals on ACE inhibitors or angiotensin receptor blockers (ARBs).109 Most other work has examined whether the use of ACE inhibitors, ARBs and β-blockers affect cancer outcomes.110–112 In the Life After Cancer Epidemiology (LACE) cohort of breast cancer survivors, risk of breast cancer recurrence was slightly increased in women on ACE inhibitors (HR 1.56; 95% CI, 1.02–2.39) and decreased in those on β-blockers (HR 0.86; 95% CI, 0.57–1.32).113 Another group examining 1449 patients found no difference in disease-free survival rates or overall survival rates in breast cancer survivors using ACEs or ARBs110 but also found an improvement in responses with the use of β-blockers.114 Given the mixed results from these studies and others, it is clear that further investigation needs to be performed to better understand the connection between ACE inhibitors, ARBs and β-blockers on cancer outcomes.
Inhibitors of hydroxymethylglutaryl coenzyme A reductase (HMGCR), otherwise known as statins, are well known to cause a reduction in cardiovascular mortality. Statins lead to a decrease in mevalonate and downstream regulators of cholesterol. Mevalonate and the downstream regulators of cholesterol can be critical to cancer growth and progression. In addition to their anti-inflammatory effects, statins likely lead to anti-tumor effects through this pathway and mechanism. These biological data have been confirmed in clinical studies.115 Improved disease-free survivals have been demonstrated in breast cancer survivors and prostate cancer survivors taking statins.116,117 Recently, a large study of more than 4000 veterans with multiple myeloma demonstrated a 12-month overall survival advantage in individuals with multiple myeloma on a statin.118 Similarly, in a large study by Nielsen and colleagues119 of the Danish population with a cancer diagnosis between 1995 and 2007, there was a reduced cancer mortality among statin users compared with those who had never used statins in 13 different types of cancers (adjusted HR for death of any cause 0.82; 95% CI, 0.81–0.85).
Finally, the role of inflammatory drugs may also be associated with a decrease in many cancers. Aspirin use affects cyclooxygenase-dependent and cyclooxygenase-independent mechanisms; its use has been associated with a 24% risk reduction of primary colon cancer120; other studies suggest it is useful as a secondary preventative in colon cancer survivors. Additional ongoing clinical trials looking at the benefits of aspirin on breast cancer recurrence are being proposed by the Alliance for Clinical Trials in Oncology cancer clinical trial network.121
Controlling Cardiac Risk Factors May Actually Improve Cancer Outcomes
Risk factors for chemotherapy-related cardiac complications should be assessed in all patients diagnosed with cancer who are considered for cancer therapy, whether the administration of biologics, chemotherapy, or radiation therapy. Given that advancing age, prior cardiac dysfunction, coronary artery disease, hypertension, tobacco use, and obesity have been associated with an increased risk of anthracycline cardiac toxicity, it is recommended that all patients prescribed anthracyclines should be consulted about risk stratification and risk modification.122 Given that a large number of other chemotherapies and targeted therapies can cause cardiac toxicity as well,123 it is important to evaluate cardiac risk factors in all oncology patients. Hypertension has been linked as a risk factor for VEGF-induced hypertension124 as well as trastuzumab cardiac toxicity.125 Other models are being developed to look at risk factors for tyrosine kinase–induced cardiac toxicity.126 Collaborative assessment by oncologists and cardiologists before the start of chemotherapy can lead to early identification of patients at risk as well as discussions about the utility and benefits of cardiac toxic medications as opposed to potential alternative therapies.127 In some situations, as discussed previously, alternative noncardiac toxic chemotherapy regimens may be considered. A lower-intensity chemo-therapy regimen, however, given out of concern for potential cardiac toxicity, may actually result in worsened cancer survival. In a large study of 17,000 individuals, management of these cardiac comorbidities, such as hypertension and diabetes, may actually improve cancer survival.128,129 These data suggest a need for better collaboration between cardiologists and oncologists as part of not only primary prevention but also secondary prevention.
Key points.
There is a growing body of evidence that suggests cancer and cardiovascular disease have shared biological mechanisms.
There are several shared risk factors for both cancer and cardiovascular disease: age, gender, obesity, diabetes mellitus, physical activity, diet, and tobacco use.
Although inflammation seems to play a major role in both diseases, other common mechanisms have been described, including the role of hyperglycemia, hyperinsulinemia, and insulinlike growth factor 1 (IGF-1).
Controlling cardiac risk factors may also improve cancer incidence and outcomes.
Further collaboration between oncology and cardiology needs to occur as part of not only primary prevention but also secondary prevention.
Acknowledgments
A. Prizment was supported by the National Center for Advancing Translational Sciences of the National Institute of Health (NIH)Award Number UL1 TR000114. A. Blaes was supported by the Building Interdisciplinary Research Careers in Women's Health (NIH #K12-HD055887).
Footnotes
Disclosure: No author has any financial or commercial conflicts of interest.
References
- 1.Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1–119. [PubMed] [Google Scholar]
- 2.DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman and Rosenberg's cancer: principles and practice of oncology. 10th. River-woods (IL): Wolters Kluwer; 2014. [Google Scholar]
- 3.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83(2):456S–60S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 4.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 5.Huang JQ, Sridhar S, Chen Y, et al. Meta-analysis of the relationship between helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–79. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 6.Thorley-Lawson DA, Gross A. Persistence of the epstein-barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 7.Prabhu SR, Wilson DF. Evidence of epstein-barr virus association with head and neck cancers: a review. J Can Dent Assoc. 2016;82:g2. [PubMed] [Google Scholar]
- 8.Zeineddine N, Khoury LE, Mosak J. Systemic sclerosis and malignancy: a review of current data. J Clin Med Res. 2016;8(9):625–32. doi: 10.14740/jocmr2606w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodward J. Improving outcomes of refractory celiac disease - current and emerging treatment strategies. Clin Exp Gastroenterol. 2016;9:225–36. doi: 10.2147/CEG.S87200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248(3):171–83. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 11.Tarraga Lopez PJ, Albero JS, Rodriguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33–46. doi: 10.4137/CGast.S14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263(2 Pt 2):H321–6. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- 14.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? findings in 356,222 primary screenees of the multiple risk factor intervention trial (MRFIT) JAMA. 1986;256(20):2823–8. [PubMed] [Google Scholar]
- 15.Ridker PM, Koenig W, Fuster V. C-reactive protein and coronary heart disease. N Engl J Med. 2004;351(3):295–8. [author reply: 295–8] [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 17.Parrinello CM, Lutsey PL, Ballantyne CM, et al. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J. 2015;170(2):380–9. doi: 10.1016/j.ahj.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia M, Jemal A, Ward EM, et al. Global cancer facts and figures. Atlanta (GA): American Cancer Society; 2007. [Google Scholar]
- 19. [Accessed September 1, 2016]; Available at: http://www.cdc.gov/nchs/fastats/heart-disease.htm.
- 20.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 23.Dobbins M, Decorby K, Choi BC. The association between obesity and cancer risk: a meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med. 2013;2013:680536. doi: 10.5402/2013/680536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 27.Tee MC, Cao Y, Warnock GL, et al. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surg Endosc. 2013;27(12):4449–56. doi: 10.1007/s00464-013-3127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 29.Wilson PW, D'Agostino RB, Sullivan L, et al. Over-weight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 30.Luft VC, Schmidt MI, Pankow JS, et al. Chronic inflammation role in the obesity-diabetes association: a case-cohort study. Diabetol Metab Syndr. 2013;5(1):31. doi: 10.1186/1758-5996-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emanuela F, Grazia M, Marco de R, et al. Inflammation as a link between obesity and metabolic syndrome. J Nutr Metab. 2012;2012:476380. doi: 10.1155/2012/476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasan RS. Cardiac function and obesity. Heart. 2003;89(10):1127–9. doi: 10.1136/heart.89.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litwin SE. Cardiac remodeling in obesity: time for a new paradigm. JACC Cardiovasc Imaging. 2010;3(3):275–7. doi: 10.1016/j.jcmg.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Stocks T, Van Hemelrijck M, Manjer J, et al. Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and cancer project. Hypertension. 2012;59(4):802–10. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 35.Mendonca FM, de Sousa FR, Barbosa AL, et al. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64(2):182–9. doi: 10.1016/j.metabol.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care. 2013;36(Suppl 2):S233–9. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjorge T, Stocks T, Lukanova A, et al. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol. 2010;171(8):892–902. doi: 10.1093/aje/kwq006. [DOI] [PubMed] [Google Scholar]
- 39.Borena W, Strohmaier S, Lukanova A, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131(1):193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 40.Haggstrom C, Rapp K, Stocks T, et al. Metabolic factors associated with risk of renal cell carcinoma. PLoS One. 2013;8(2):e57475. doi: 10.1371/journal.pone.0057475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ankarfeldt MZ, Angquist L, Stocks T, et al. Body characteristics, [corrected] dietary protein and body weight regulation. Reconciling conflicting results from intervention and observational studies? PLoS One. 2014;9(7):e101134. doi: 10.1371/journal.pone.0101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38(7):904–10. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141(2):125–39. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–10. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C, Chang YC, Lan MS, et al. Corrigendum] leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/akt signaling pathways. Int J Oncol. 2016;49(2):847. doi: 10.3892/ijo.2016.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu CL, Chang YC, Cheng SP, et al. The roles of serum leptin concentration and polymorphism in leptin receptor gene at codon 109 in breast cancer. Oncology. 2007;72(1–2):75–81. doi: 10.1159/000111097. [DOI] [PubMed] [Google Scholar]
- 47.Satija A, Spiegelman D, Giovannucci E, et al. Type 2 diabetes and risk of cancer. BMJ. 2015;350:g7707. doi: 10.1136/bmj.g7707. [DOI] [PubMed] [Google Scholar]
- 48.Tsilidis KK, Kasimis JC, Lopez DS, et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 49.Denley A, Carroll JM, Brierley GV, et al. Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol Cell Biol. 2007;27(10):3569–77. doi: 10.1128/MCB.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker MA, Ibrahim YH, Oh AS, et al. Insulin receptor substrate adaptor proteins mediate prognostic gene expression profiles in breast cancer. PLoS One. 2016;11(3):e0150564. doi: 10.1371/journal.pone.0150564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6(10):821–33. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 52.MacLeod DC, Strauss BH, de Jong M, et al. Proliferation and extracellular matrix synthesis of smooth muscle cells cultured from human coronary atherosclerotic and restenotic lesions. J Am Coll Cardiol. 1994;23(1):59–65. doi: 10.1016/0735-1097(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 53.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11 Suppl):3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 54.Powell DR, Suwanichkul A, Cubbage ML, et al. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem. 1991;266(28):18868–76. [PubMed] [Google Scholar]
- 55.You L, Liu C, Tang H, et al. Advances in targeting insulin-like growth factor signaling pathway in cancer treatment. Curr Pharm Des. 2014;20(17):2899–911. doi: 10.2174/13816128113199990595. [DOI] [PubMed] [Google Scholar]
- 56.Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality–beyond established causes. N Engl J Med. 2015;372(7):631–40. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 57.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4(4):327–38. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris PB, Ference BA, Jahangir E, et al. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the american college of cardiology. J Am Coll Cardiol. 2015;66(12):1378–91. doi: 10.1016/j.jacc.2015.07.037. http://dx.doi.org/10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 59.Bangalore S, Kumar S, Kjeldsen SE, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12(1):65–82. doi: 10.1016/S1470-2045(10)70260-6. [DOI] [PubMed] [Google Scholar]
- 60.Grossman E, Messerli FH, Goldbourt U. Carcinogenicity of antihypertensive therapy. Curr Hypertens Rep. 2002;4(3):195–201. doi: 10.1007/s11906-002-0007-4. [DOI] [PubMed] [Google Scholar]
- 61.Baik SK, Jo HS, Suk KT, et al. Inhibitory effect of angiotensin II receptor antagonist on the contraction and growth of hepatic stellate cells. Korean J Gastroenterol. 2003;42(2):134–41. [PubMed] [Google Scholar]
- 62.Tseng TH, Hsu JD, Chu CY, et al. Promotion of colon carcinogenesis through increasing lipid peroxidation induced in rats by a high cholesterol diet. Cancer Lett. 1996;100(1–2):81–7. doi: 10.1016/0304-3835(95)04073-0. [DOI] [PubMed] [Google Scholar]
- 63.Swamy MV, Patlolla JM, Steele VE, et al. Chemo-prevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66(14):7370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 64.Reddy BS. Dietary fat and its relationship to large bowel cancer. Cancer Res. 1981;41(9 Pt 2):3700–5. [PubMed] [Google Scholar]
- 65.Nelson ER, Wardell SE, Jasper JS, et al. 27-hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–8. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warner M, Gustafsson JA. On estrogen, cholesterol metabolism, and breast cancer. N Engl J Med. 2014;370(6):572–3. doi: 10.1056/NEJMcibr1315176. [DOI] [PubMed] [Google Scholar]
- 67.DuSell CD, Umetani M, Shaul PW, et al. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22(1):65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez CA, Jakszyn P, Pera G, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the european prospective investigation into cancer and nutrition (EPIC) J Natl Cancer Inst. 2006;98(5):345–54. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- 69.Schwingshackl L, Hoffmann G. Adherence to mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer. 2014;135(8):1884–97. doi: 10.1002/ijc.28824. [DOI] [PubMed] [Google Scholar]
- 70.Slattery ML, Potter JD, Sorenson AW. Age and risk factors for colon cancer (united states and australia): are there implications for understanding differences in case-control and cohort studies? Cancer Causes Control. 1994;5(6):557–63. doi: 10.1007/BF01831384. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buckland G, Agudo A, Lujan L, et al. Adherence to a mediterranean diet and risk of gastric adenocarcinoma within the european prospective investigation into cancer and nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381–90. doi: 10.3945/ajcn.2009.28209. [DOI] [PubMed] [Google Scholar]
- 73.Verberne L, Bach-Faig A, Buckland G, et al. Association between the mediterranean diet and cancer risk: a review of observational studies. Nutr Cancer. 2010;62(7):860–70. doi: 10.1080/01635581.2010.509834. [DOI] [PubMed] [Google Scholar]
- 74.Baena Ruiz R, Salinas Hernandez P. Diet and cancer: risk factors and epidemiological evidence. Maturitas. 2014;77(3):202–8. doi: 10.1016/j.maturitas.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66(14):1538–48. doi: 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu H, Flint AJ, Qi Q, et al. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med. 2015;175(3):373–84. doi: 10.1001/jamainternmed.2014.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130(13):1110–30. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 78.Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American diabetes Association. Circulation. 2015;132(8):691–718. doi: 10.1161/CIR.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368(14):1279–90. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 80.de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343(8911):1454–9. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 81.de Lorgeril M, Salen P. Wine ethanol, platelets, and mediterranean diet. Lancet. 1999;353(9158):1067. doi: 10.1016/S0140-6736(99)00454-7. [DOI] [PubMed] [Google Scholar]
- 82.Johnson CB, Davis MK, Law A, et al. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32(7):900–7. doi: 10.1016/j.cjca.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Schmid D, Behrens G, Keimling M, et al. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30(5):397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 84.Cao Y, Keum NN, Chan AT, et al. Television watching and risk of colorectal adenoma. Br J Cancer. 2015;112(5):934–42. doi: 10.1038/bjc.2014.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Behrens G, Matthews CE, Moore SC, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIHAARP diet and health study. Eur J Epidemiol. 2013;28(1):55–66. doi: 10.1007/s10654-013-9767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 87.Friedenreich CM. Physical activity and cancer: lessons learned from nutritional epidemiology. Nutr Rev. 2001;59(11):349–57. doi: 10.1111/j.1753-4887.2001.tb06962.x. [DOI] [PubMed] [Google Scholar]
- 88.Friedenreich CM, Neilson HK, Woolcott CG, et al. Inflammatory marker changes in a yearlong randomized exercise intervention trial among post-menopausal women. Cancer Prev Res (Phila) 2012;5(1):98–108. doi: 10.1158/1940-6207.CAPR-11-0369. [DOI] [PubMed] [Google Scholar]
- 89.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–53. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 90.Hu FB, Willett WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 91.Pandey A, Garg S, Khunger M, et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132(19):1786–94. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 92.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: a statement from the american heart association committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 93.Held C, Iqbal R, Lear SA, et al. Physical activity levels, ownership of goods promoting sedentary behaviour and risk of myocardial infarction: results of the INTERHEART study. Eur Heart J. 2012;33(4):452–66. doi: 10.1093/eurheartj/ehr432. [DOI] [PubMed] [Google Scholar]
- 94.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298(21):2507–16. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113(4):499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7(2):253–61. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whitlock MC, Yeboah J, Burke GL, et al. Cancer and its association with the development of coronary artery calcification: an assessment from the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002533. [pii:e002533] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer–a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):170–81. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mast ME, Heijenbrok MW, Petoukhova AL, et al. Preradiotherapy calcium scores of the coronary arteries in a cohort of women with early-stage breast cancer: a comparison with a cohort of healthy women. Int J Radiat Oncol Biol Phys. 2012;83(3):853–8. doi: 10.1016/j.ijrobp.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 100.Davis MK, Rajala JL, Tyldesley S, et al. The prevalence of cardiac risk factors in men with localized prostate cancer undergoing androgen deprivation therapy in British Columbia, Canada. J Oncol. 2015;2015:820403. doi: 10.1155/2015/820403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morote J, Gomez-Caamano A, Alvarez-Ossorio JL, et al. The metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapy. J Urol. 2015;193(6):1963–9. doi: 10.1016/j.juro.2014.12.086. [DOI] [PubMed] [Google Scholar]
- 102.Nothlings U, Ford ES, Kroger J, et al. Lifestyle factors and mortality among adults with diabetes: findings from the european prospective investigation into cancer and nutrition-potsdam study*. J Diabetes. 2010;2(2):112–7. doi: 10.1111/j.1753-0407.2010.00069.x. [DOI] [PubMed] [Google Scholar]
- 103.Rasmussen-Torvik LJ, Shay CM, Abramson JG, et al. Ideal cardiovascular health is inversely associated with incident cancer: the atherosclerosis risk in communities study. Circulation. 2013;127(12):1270–5. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grouven U, Hemkens LG, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues. Diabetologia. 2010;53(1):209–11. doi: 10.1007/s00125-009-1582-6. Reply to Nagel JM, Mansmann U, Wegscheider K et al. [letter] and Simon D [letter] [DOI] [PubMed] [Google Scholar]
- 105.Pernicova I, Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 106.Johnson JA, Carstensen B, Witte D, et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55(6):1607–18. doi: 10.1007/s00125-012-2525-1. [DOI] [PubMed] [Google Scholar]
- 107.Lijinsky W, Reuber MD. Chronic carcinogenesis studies of acrolein and related compounds. Toxicol Ind Health. 1987;3(3):337–45. doi: 10.1177/074823378700300306. [DOI] [PubMed] [Google Scholar]
- 108.Chow WH, McLaughlin JK, Mandel JS, et al. Risk of renal cell cancer in relation to diuretics, antihypertensive drugs, and hypertension. Cancer Epidemiol Biomarkers Prev. 1995;4(4):327–31. [PubMed] [Google Scholar]
- 109.Dai YN, Wang JH, Zhu JZ, et al. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers therapy and colorectal cancer: a systematic review and meta-analysis. Cancer Causes Control. 2015;26(9):1245–55. doi: 10.1007/s10552-015-0617-1. [DOI] [PubMed] [Google Scholar]
- 110.Chae YK, Brown EN, Lei X, et al. Use of ACE inhibitors and angiotensin receptor blockers and primary breast cancer outcomes. J Cancer. 2013;4(7):549–56. doi: 10.7150/jca.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H, Liao Z, Zhuang Y, et al. Incidental receipt of cardiac medications and survival outcomes among patients with stage III non-small-cell lung cancer after definitive radiotherapy. Clin Lung Cancer. 2015;16(2):128–36. doi: 10.1016/j.cllc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 112.Song T, Choi CH, Kim MK, et al. The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: a meta-analysis. Eur J Cancer Prev. 2016;26(1):78–85. doi: 10.1097/CEJ.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 113.Ganz PA, Habel LA, Weltzien EK, et al. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat. 2011;129(2):549–56. doi: 10.1007/s10549-011-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29(19):2645–52. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ji Y, Rounds T, Crocker A, et al. The effect of atorvastatin on breast cancer biomarkers in high-risk women. Cancer Prev Res (Phila) 2016;9(5):379–84. doi: 10.1158/1940-6207.CAPR-15-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dale KM, Coleman CI, Henyan NN, et al. Statins and cancer risk: a meta-analysis. JAMA. 2006;295(1):74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 117.Mucci LA, Stampfer MJ. Mounting evidence for prediagnostic use of statins in reducing risk of lethal prostate cancer. J Clin Oncol. 2014;32(1):1–2. doi: 10.1200/JCO.2013.53.2770. [DOI] [PubMed] [Google Scholar]
- 118.Sanfilippo KM, Keller J, Gage BF, et al. Statins are associated with reduced mortality in multiple myeloma. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.3482. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792–802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 120.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–50. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 121.Available at: https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path5/public/news-ABC-trial-Nov2015
- 122.Accordino MK, Neugut AI, Hershman DL. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol. 2014;32(24):2654–61. doi: 10.1200/JCO.2013.55.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 124.Hamnvik OP, Choueiri TK, Turchin A, et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121(2):311–9. doi: 10.1002/cncr.28972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ezaz G, Long JB, Gross CP, et al. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3(1):e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hurley PJ, Konety S, Cao Q, et al. Frequency and risk factors for tyrosine kinase inhibitor-associated cardiotoxicity. J Clin Oncol. 2016;34(Suppl) [abstract: 6596] [Google Scholar]
- 127.Lenihan DJ, Cardinale D, Cipolla CM. The compelling need for a cardiology and oncology partnership and the birth of the international CardiOncology society. Prog Cardiovasc Dis. 2010;53(2):88–93. doi: 10.1016/j.pcad.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 128.Piccirillo J, Tierney R, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 129.Emerging Risk Factors Collaboration. Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]