Abstract
Colocalization of small-molecule and neuropeptide transmitters is common throughout the nervous system of all animals. The resulting co-transmission, which provides conjoint ionotropic (‘classical’) and metabotropic (‘modulatory’) actions, includes neuropeptide-specific aspects that are qualitatively different from those that result from metabotropic actions of small-molecule transmitter release. Here, we focus on the flexibility afforded to microcircuits by such co-transmission, using examples from various nervous systems. Insights from such studies indicate that co-transmission mediated even by a single neuron can configure microcircuit activity via an array of contributing mechanisms, operating on multiple timescales, to enhance both behavioural flexibility and robustness.
Neural circuit flexibility
Today, much attention is focused on understanding the circuit mechanisms that underlie complex behaviours in animals with large numbers of neurons, associated with a tendency to assume that the details of individual synaptic events are relatively insignificant. This comes at a substantial price because many aminergic and peptidergic synaptic actions provide a rich library of modulatory mechanisms that can operate on different timescales and can be crucial for setting circuit dynamics. Here, we discuss some of that richness and show how circuit reconfiguration can be achieved by interesting features of peptide co-transmitter action.
Neurons release a wide range of signalling molecules including the classical small-molecule transmitters (acetylcholine (ACh), glutamate, GABA, glycine), biogenic amines (histamine, 5-hydroxytryptamine (5-HT; also known as serotonin), dopamine, noradrenaline, octopamine), neuropeptides, a gas (nitric oxide), purines (ATP, adenosine) and lipid-derived molecules. These molecules may act ionotropically, by binding to ligand-gated ion channels to rapidly influence the conductance of postsynaptic neurons or by binding to metabotropic receptors to evoke modulatory actions, commonly through one or more G protein-coupled receptors (GPCRs). Neuropeptides, although commonly having only modulatory actions, do sometimes have ionotropic actions1–3.
Neurons containing more than one neurotransmitter are a common occurrence in all species. A growing literature describes neurons with multiple small-molecule transmitters4–10. Consequently, we focus here on co-transmission featuring small molecules colocalized with peptide transmitters, including the description of some of the additional degrees of freedom provided by neuropeptide signalling for neuronal circuit function.
Fundamentals of co-transmission
Co-transmission provides opportunities for circuit flexibility5,6,11–14, as co-transmitters can act on different targets using either the same, different or all co-transmitters15–17 (FIG. 1a,b). The same transmitter can also act on different targets by activating different receptors. The co-transmitting neuron can also produce distinct firing rate-dependent actions (FIG. 1c), temporally distinct effects during a single episode of an unchanging firing rate and/or state-dependent effects when the release of, or the response to, different co-transmitters is separately regulated (FIG. 1d).
Figure 1. Co-transmission of small molecules and neuropeptides provides many degrees of freedom to microcircuit output.
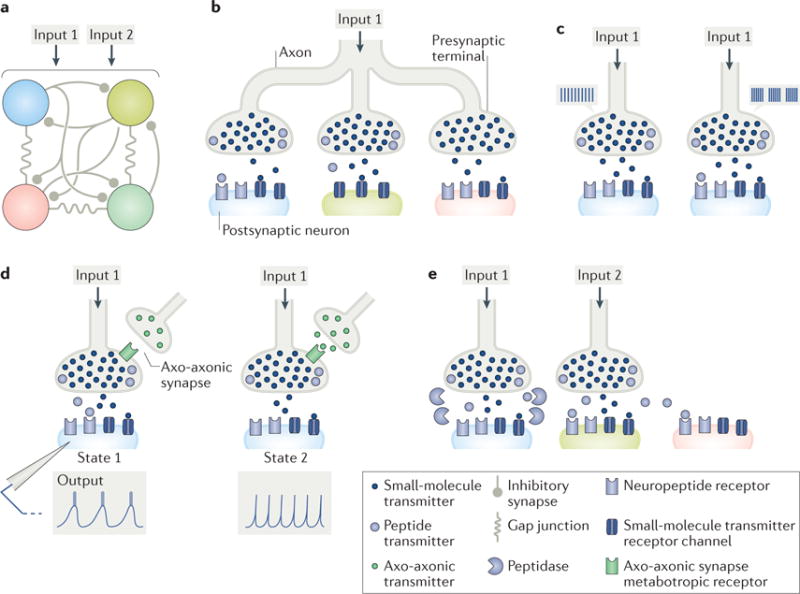
a| A schematic four-neuron microcircuit, interconnected by inhibitory synapses and electrical coupling, receiving input from two small-molecule–neuropeptide co-transmitting projection neurons (Input 1, Input 2) is shown. b | Co-transmission enables a single presynaptic neuron to have distinct actions on different postsynaptic neurons. Input 1 influences the blue circuit neuron via both co-transmitters, and the green and pink circuit neurons via only its small-molecule co-transmitter, albeit through different mechanisms (green neuron: no peptide receptors (postsynaptic mechanism); pink neuron: no peptide released nearby (presynaptic mechanism)). c | Co-transmitters can have distinct activity thresholds for their release. For example, Input 1 influences the blue circuit neuron by releasing only its small-molecule transmitter when firing at a low, tonic frequency (left), but it influences this circuit neuron by releasing both co-transmitters when firing in a rhythmic bursting pattern (right). d | Co-transmission is state dependent. Here, the state change is presynaptic, resulting from an axo-axonic synapse (green transmitter). When the axo-axonic synapse is not active (state 1), Input 1 co-releases both transmitters, and the blue circuit neuron responds with a rhythmic bursting pattern. When the axo-axonic synapse is active (state 2), peptide release is inhibited, and the blue circuit neuron responds with a tonic firing pattern. e | The diffusion distance of a neuronally released peptide can be regulated by the location of extracellular peptidases. Here, extracellular peptidases limit receptor access of Input 1-released peptide to the blue circuit neuron but do not limit Input 2-released peptide. Consequently, the green and pink circuit neurons are unlikely to be influenced by Input 1-released peptide, whereas both would be responsive to Input 2-released peptide, in addition to the green circuit neuron responding to the co-released small-molecule transmitter from Input 2. The peptidase activity near the blue circuit neuron would limit or possibly prevent its response to Input 2-released peptide.
A firing rate-dependent response can also result from small-molecule transmission, when the target neuron has ionotropic and metabotropic receptors for the same small-molecule transmitter. This distinction can occur because low firing rates tend to release insufficient transmitter to activate perisynaptically located GPCRs. By contrast, the firing rate-dependent consequences of small-molecule–neuropeptide co-transmission result from the fact that, at low firing rates, small-molecule release may occur without substantial neuropeptide release (FIG. 1c). However, it is noteworthy that, despite the apparently commonly held belief that neuropeptide release requires prolonged high-frequency firing, neuropeptide release can occur at low firing rates and/or at firing rates that are within the natural activity range of the studied neuron18–26. A more accurate generalization is that neuropeptide release tends to require higher firing rates than those required for the release of small-molecule transmitters.
Additional flexibility results from the existence of neuropeptide families, post-release regulation by peptidase activity (FIG. 1e) and the ability of released neuropeptide to diffuse relatively long distances, thereby binding to membrane receptors that are not accessible to their co-released small-molecule transmitters27–31 (FIG. 1e).
Many neuropeptides are members of large ‘neuropeptide families’ that share an amino acid sequence, commonly including the receptor-binding domain (for reviews, see REFS27,29). These family members can differ by as little as one amino acid, and these families can be large. For example, in the crab Cancer borealis, there are at least 35 A type allatostatin (AST) peptides and 40 FMRFamide-related peptides, as identified by mass spectrometric analyses32,33. In some cases, multiple family members are colocalized within the same neuron32–36.
One might be tempted to conclude that different peptide family members simply represent conservative mutations that are functionally degenerate. However, as one example, although peptide family members can have comparable influences on the well-defined microcircuits in the C. borealis stomatogastric ganglion (STG)37–40 (FIG. 2), members of the same peptide family can have distinctly different potencies41–43. Interestingly, although neuropeptide family members commonly share GPCRs, in some cases, distinct intracellular signalling systems are activated when different family members bind to the same GPCR44,45. Different peptide family members can also bind to distinct receptors46, and unrelated peptides in the same species can bind to the same receptor with comparable affinity47–50.
Figure 2. The crab Cancer borealis stomatogastric nervous system.
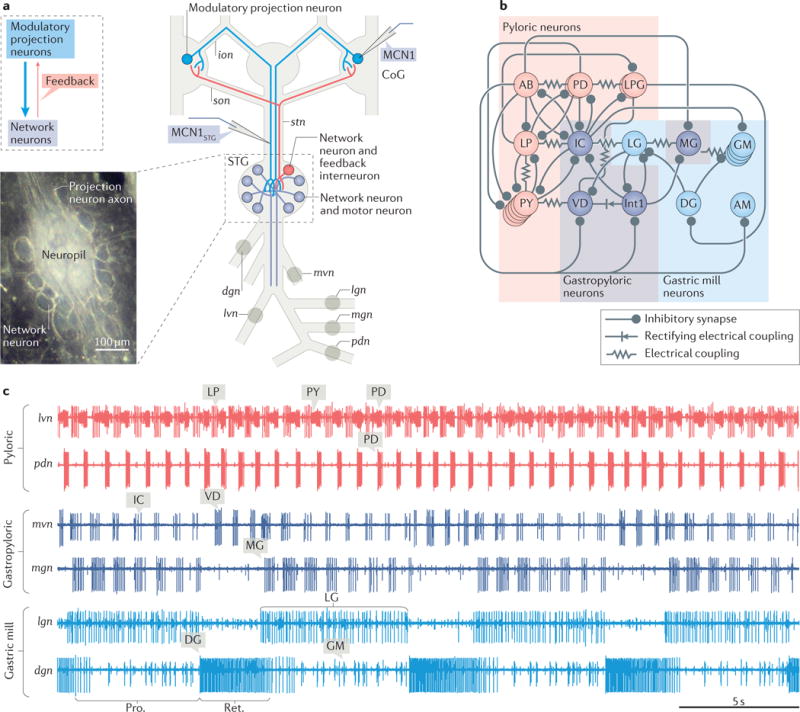
a| A schematic of the isolated stomatogastric nervous system of the crab Cancer borealis is shown. The inset shows a whole-mount image of the desheathed stomatogastric ganglion (STG) under dark-field illumination (anterior, top; posterior, bottom). As it is evident in both the schematic and the inset, the 26 neuronal somata form a single layer surrounding the neuropil. Circles on nerves indicate recording sites for traces shown in part c. b | A schematic of the gastric mill and pyloric circuit is shown. The arrangement of neurons in the schematic represents the relative timing of activity for each neuron during the gastric mill and pyloric rhythms. Specifically, the neurons that exhibit pyloric rhythm-timed activity (‘pyloric neurons’ and ‘gastropyloric neurons’) are displayed such that the top-row neurons are co-active, followed by the middle-row neurons and then the bottom-row neurons, after which the top-row neurons are again active. The neurons that exhibit gastric mill rhythm-timed activity (‘gastric mill neurons’ and ‘gastropyloric neurons’) are displayed such that the top-row neurons are co-active and burst in alternation with the bottom-row neurons. As shown, there are eight gastric mill circuit neuron types, one of which is present as four apparently equivalent copies (GM neurons). All eight neuron types contribute to gastric mill pattern generation, whereas only two (LG and Int1) are also rhythm generator neurons119,134,157. There are seven pyloric circuit neuron types, including the rhythm generator (‘pacemaker’) group AB/PD/LPG110. Three of these neuron types are present as multiple, apparently equivalent copies (PD: 2; LPG: 2; PY: 5). c | Simultaneous extracellular nerve recordings of the gastric mill and pyloric rhythms during tonic stimulation of the modulatory projection neuron modulatory commissural neuron 1 (MCN1) are shown. The pyloric rhythm exhibits a rhythmically repeating triphasic pattern (for example, lateral ventricular nerve (lvn): PD, LP, PY) that is continuously active, in vivo and in vitro, with a cycle period of ~1 s. The gastric mill rhythm (cycle period ~10–20 s) is silent except when driven by modulatory neurons (for example, MCN1), which themselves require activation in vivo and in vitro. It is a rhythmically repeating biphasic pattern, consisting of teeth protraction (Pro.) and teeth retraction (Ret.), which drives the motor response (chewing). Note that some neurons exhibit activity patterns time-locked to both rhythms (gastropyloric neurons). CoG, commissural ganglion; dgn, dorsal gastric nerve; ion, inferior oesophageal nerve; lgn, lateral gastric nerve; mgn, medial gastric nerve; mvn, medial ventricular nerve; pdn, pyloric dilator nerve; son, superior oesophageal nerve; stn, stomatogastric nerve. Part b is adapted with permission from REF.158, Macmillan Publishers Limited. STG photo courtesy of Marie Suver, New York University, USA, and Wolfgang Stein, Illinois State University, USA.
Neuropeptides are inactivated by extracellular peptidase activity, and this provides additional mechanisms for flexible neuromodulation51,52. Whereas the actions of most small-molecule transmitters are brief and limited by membrane-bound transporters or degradative enzymes located close to the release site, the peptidases regulating released peptides are not necessarily comparably constrained. Consequently, released peptides can diffuse longer distances and bind to receptors on neurons not directly influenced by a co-released small-molecule transmitter28,30 (FIG. 1e). In some cases, extracellular peptidase activity does not inactivate the peptide but activates and enhances peptide actions or alters its biological activity by generating cleavage products that either bind to different receptors or act as peptidase inhibitors to enhance the actions of other peptides16,53.
Many neurons contain more than one small-molecule transmitter and more than one neuropeptide. In such cases, the consequences of co-transmission for each target neuron, and thus for the associated circuit, are likely to be considerably more complex.
Co-transmission at identified synapses
Small-molecule–neuropeptide co-transmission has been studied in detail at different synapses. Both convergent (that is, co-transmitters influencing the same target) and divergent (that is, co-transmitters influencing different targets) actions occur, including both actions onto different targets of the same co-transmitting neuron11,17 (FIG. 1b,e). Such co-transmission has received extensive attention in nematode worms (Caenorhabditis elegans), fruitflies (Drosophila melanogaster), sea hares (Aplysia californica), mice (Mus musculus) and rats (Rattus norvegicus). Despite the opportunities for flexibility afforded by co-transmission, as described below, there is a conservation of mechanisms across these animal models.
Co-transmission in C. elegans
Co-transmission studies in C. elegans have focused largely on their impact on behaviour, based on manipulations that selectively reduce or eliminate, or strengthen, the impact of the co-transmitters. These studies have highlighted divergent co-transmitter actions of identified sensory neurons54,55. For example, the olfactory sensory neuron AWC uses glutamate and the buccalin-related peptide NLP1 to influence different postsynaptic target neurons and regulate food searching-related behaviours54. Specifically, glutamate is used to promote food-searching behaviours, whereas NLP1 release initiates a negative feedback loop that limits the duration of these behaviours. Interestingly, the AWC neuron is also conscripted into a high-salt (NaCl) sensing circuit by the salt-sensory neuron ASEL, another small-molecule–neuropeptide co-transmitting neuron with divergent co-transmitter actions56. In low-salt environments, ASEL seems to act only via its small-molecule transmitter to activate a low-salt sensing circuit, whereas, in high-salt environments, ASEL also releases insulin-like peptides and recruits AWC into the high-salt sensing circuit. ASEL also contains additional neuropeptides that are likely to have separate postsynaptic targets56.
Several additional wrinkles resulting from small-molecule–neuropeptide co-transmission are revealed by studies of aversive behaviours in C. elegans55,57,58. One pivotal insight is that different subsets of co-transmitters are released from a single neuron in response to different inputs, enabling the selective generation or regulation of different aversive behaviours. Selective regulation of peptide co-transmitter release also occurs in other systems (see below and REFS59,60).
Co-transmission in Drosophila
Small-molecule–neuropeptide or multiple neuropeptide co-transmission in Drosophila is implicated in microcircuits underlying aspects of olfaction61–64, stress-related responses65,66 and circadian rhythms67. Interestingly, the neuropeptide co-transmitter for many of these studies is sNPF62,63,66,68, reinforcing the notion that neuropeptides, like other transmitters, have many unrelated roles in microcircuit operations. In fact, selective knockdown (RNA interference) of sNPF in one set of Drosophila brain neurons decreased survival under starvation conditions, whereas it increased survival duration when the knockdown occurred in a distinct set of brain neurons65,66. This sampling of co-transmission studies from Drosophila includes examples of apparent postsynaptic convergence66, presynaptic convergence61, neuropeptide autoreceptor-mediated increase in small-molecule co-transmitter release67 and divergence across a single synapse (sNPF acting presynaptically; small-molecule transmitter acting postsynaptically)63.
Co-transmission in Aplysia
Studies in Aplysia have elucidated functional consequences of convergent and divergent co-transmission, including some pivotal roles for peptidase regulation of peptide co-transmitters. For example, a population of neuroendocrine neurons (‘bag cells’) release several neuropeptides that have convergent and divergent influences on neurons associated with various egg laying-related behaviours16,17. The duration of action of these peptides differs, owing to their differential regulation by extracellular peptidases16. A separate, carboxypeptidase-mediated cleavage that alters α-BCP (1–9) to α-BCP (1–8) increases the potency of this peptide16,69.
Convergent co-transmission is also established from a cholinergic projection neuron (CBI-2, peptide co-transmitters FCAP and CP2), which drives the Aplysia feeding microcircuit, and from two feeding-related cholinergic motor neurons (B-15, peptide co-transmitters BUC and SCP; B-16, peptides BUC and MYO). CBI-2 activates the feeding motor programme in part via its convergent presynaptic facilitation of its cholinergic synaptic actions (FCAP and CP2 act on autoreceptors on CBI-2) and alters the electrophysiological properties of a feeding-related motor neuron70–72. In motor neurons B-15 and B-16, the co-released peptides elicit functionally antagonistic actions21–23, as one peptide co-transmitter increases muscle contraction amplitude, and the other increases its relaxation rate. Despite being apparently antagonistic, these are complementary actions that facilitate rhythmic feeding movements by enabling each muscle to maintain its rhythmic contraction pattern. If only contraction amplitude increased, the muscle would not fully relax between contractions, resulting instead in a sustained contraction. If only relaxation rate increased, then contraction amplitude would be compromised, and feeding would be unsuccessful. For these three feeding-related co-transmitter neurons, their peptidergic actions routinely occurred within their behaviourally relevant firing rate.
Co-transmission in rodents
Studies in the rodent thalamus, hypothalamus and brainstem have provided numerous insights regarding the presence and flexibility afforded by co-transmission, particularly regarding the consequences of convergent and divergent co-transmitter actions, and the role of co-transmission in bidirectional communication across individual synapses73–78. For example, in the thalamus, reticular thalamic (RT) neurons use neuropeptide Y (NPY) and GABA as divergent inhibitory co-transmitters, acting via NPY modulation onto their RT neuron targets while eliciting only GABA type A receptor (GABAAR) and GABABR responses in their thalamocortical relay cell targets73. By contrast, convergent co-transmission by 5-HT and substance P underlies excitatory modulation by nucleus raphé obscurus neurons of respiratory interneurons and motor neurons74.
In the hypothalamus, the magnocellular neurosecretory neurons of the paraventricular and supraoptic nuclei, which contain oxytocin (OXT) or vasopressin (VP), release their peptide content from axon terminals in the posterior pituitary and also display somatodendritic transmitter release25,79. An early study established that somatodendritically released OXT and VP inhibit their glutamatergic excitatory input by binding to OXT receptors, putatively on the presynaptic membrane50. Subsequently, OXT neurons were shown to also have a concentration-dependent, retrograde influence on their GABAergic input. At concentrations lower than 100 nM, OXT facilitates presynaptic GABA release80,81. By contrast, at higher concentrations, OXT binds to autoreceptors, eliciting retrograde endocannabinoid (eCB) co-transmitter release, which inhibits presynaptic GABAergic transmission81. Interestingly, application of the peptide α-MSH selectively elicits somatodendritic OXT release by an action potential-independent mechanism, while simultaneously inhibiting OXT release from the axon terminals by suppressing action potential generation82,83.
VP neurons use the opioid peptide dynorphin (DYN) as a co-transmitter79. VP and DYN colocalize to the same large dense-core vesicles, despite the fact that VP is commonly excitatory and DYN is inhibitory84. VP and DYN receptors are also present on these vesicles, so the vesicle fusion that produces peptide release also introduces their receptors onto the plasma membrane85,86. This provides a novel level of flexibility to neuronal signalling that remains under-studied. Similar to OXT actions on autoreceptors81, somatodendritically released VP binding to autoreceptors triggers release of eCB, a third co-transmitter, which acts presynaptically to inhibit GABA release. VP also inhibits presynaptic GABA release via an eCB-independent pathway87.
There are also retrograde VP, DYN and eCB actions on their glutamatergic inputs. Somatodendritic DYN release produces long-term depression (LTD) of presynaptic glutamate release, reducing excitatory drive to the VP–DYN–eCB neurons88. A dynamic interplay exists between the retrograde actions of DYN and VP-triggered eCBs on the glutamatergic inputs60. Depending on the relative intensity of presynaptic and postsynaptic activity, eCB or DYN retrograde release dominates. When the eCB action dominates, it acutely inhibits presynaptic glutamate release, limiting glutamate access to postsynaptic metabotropic glutamate receptors (mGluRs), the activation of which is necessary for enhanced VP and DYN release and subsequent LTD generation60. Consequently, eCB release indirectly inhibits release of its co-transmitters VP and DYN.
Complexity in signalling due to temporal aspects of co-transmission is illustrated in orexinergic (also known as hypocretinergic) neurons in the lateral hypothalamus, which also use DYN and glutamate as co-transmitters89,90. Similar to the VP–DYN neurons, orexin and DYN colocalize to the same large dense-core vesicles despite orexin being excitatory and DYN inhibitory91,92. Their co-release leads to convergent and divergent actions, with some divergent actions being complementary on their respective targets, despite the opposing actions of the two peptides13,89–93. The distinct time course of ionotropic and metabotropic co-transmission is displayed to the extreme in the influence of orexinergic–glutamatergic neurons on their histamine neuron targets in the tuberomammillary nucleus93. Specifically, the convergent glutamate (ionotropic) and orexin (metabotropic) actions on these histamine neurons are excitatory but temporally non-overlapping, and the selective suppression of the action of either co-transmitter does not alter the histamine neuron response to the still effective co-transmitter.
Co-transmission during development
Small-molecule–neuropeptide co-transmission provides functional flexibility during nervous system development, as different co-transmitters and/or their receptor (or receptors) appear at different developmental stages94–97. Co-transmitters and/or receptors expressed by a neuron can change as development progresses. Another interesting twist is that co-transmitter regulation can be sensitive to environmental conditions and physiological state, even in adults98,99. A state-dependent switch in co-transmitter expression, whether during development or in response to physiological state, can have dramatic consequences for circuits and behaviour.
This section on co-transmission at identified synapses has elucidated many degrees of freedom, including insights into the logic of co-transmitters having opposite modes of action (excitation and inhibition), made available by small-molecule–neuropeptide co-transmission and their impact on circuits and behaviour. There are conserved mechanisms across behaviours in a single species, as well as across animal models (for example, convergent and divergent co-transmitter actions; and intrinsically or extrinsically regulated release of different co-transmitters). In the next section, we synthesize the results of numerous studies to understand the roles of co-transmission within the context of a set of small, well-defined microcircuits that underlie aspects of feeding behaviour in decapod crustaceans (FIG. 2).
Co-transmission: stomatogastric system
Applied versus released neuropeptide
Direct application of a neuropeptide is commonly used to evaluate its influence on neurons and networks, particularly because it is often challenging to directly manipulate neuronally released neuropeptides. Direct peptide application is a reasonable first approach, as neuronally released peptides commonly have relatively long-lasting, paracrine-like actions resulting from diffusion throughout a region of neuropil28,30,52. However, there are features of peptidergic neurons that limit the likelihood that an applied neuropeptide mimics the actions resulting from its neuronal release, sometimes leading to inaccurate interpretation of the results from exogenous peptide application. These features include the presence of co-transmitters, the possibility that released neuropeptides do not have access to all available receptors or have access to receptors on different membranes at different concentrations, and the fact that neuropeptide release can be regulated. The microcircuits in the crab C. borealis STG and the modulatory projection neurons that influence them (FIG. 2) represent one of the few sufficiently well-defined systems enabling a detailed determination of the extent to which the microcircuit response to neuronally released peptides is effectively mimicked by bath application of the same peptide.
The crab STG neuropil contains the neuropeptide proctolin exclusively within the axon terminals of three pairs of projection neurons, all of which influence the feeding-related gastric mill (chewing) and pyloric (pumping and filtering of chewed food) microcircuits33,100,101 (FIGS 2,3). These three proctolin-containing projection neurons include the modulatory proctolin neuron (MPN), modulatory commissural neuron 1 (MCN1) and MCN7, all of which occur as pairs of apparently identical neurons102–105. Selectively stimulating each proctolinergic neuron elicits different outputs from the gastric mill and/or pyloric microcircuits105 (FIG. 3a). The distinct actions of these proctolinergic neurons do not seem to result from their axon terminals being restricted to different regions of the STG neuropil. In C. borealis, this neuropil is relatively compact (~200 μm wide, ~400 μm long, and ~65 μm in the z axis106), and there is no evident compartmentalization within it either for proctolin immunolabelling or the branching structure of MCN1 (REFS106,107). The co-transmitter complement is, at least partly, identified for MPN (proctolin and GABA) and MCN1 (proctolin, C. borealis tachykinin-related peptide Ia (CabTRP Ia) and GABA)102,105,108. MCN7 is immunonegative for CabTRP Ia and GABA105.
Figure 3. The microcircuit response to peptidergic neuron activity is not necessarily mimicked by bath application of that neuropeptide.
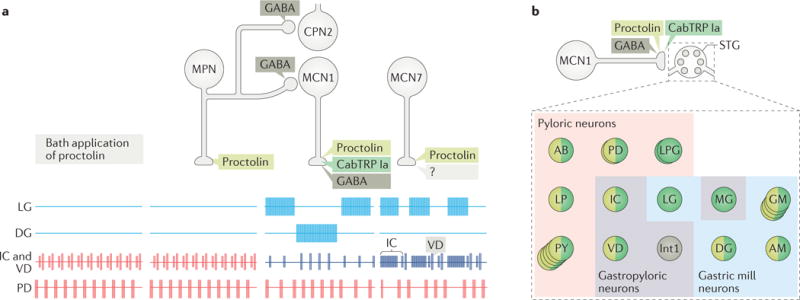
a| This part shows schematic extracellular recordings of identified neurons in the crab Cancer borealis stomatogastric ganglion (STG), which are active during the gastric mill rhythm (LG and DG neurons), pyloric rhythm (PD neuron) or both rhythms (IC and VD neurons). In the isolated crab STG, bath-applied proctolin (far left set of responses) selectively excites the pyloric rhythm100,102. This action mimics the response to activation of only one (modulatory proctolin neuron (MPN)) of the three proctolinergic projection neurons that innervate the STG (MPN, modulatory commissural neuron 1 (MCN1) and MCN7), even though MPN also contains a small-molecule co-transmitter (GABA)102,105. As indicated, MPN also inhibits two projection neurons (MCN1 and commissural projection neuron 2 (CPN2)) by releasing GABA from a separate axon projecting to a separate location (commissural ganglion (CoG))130,140. The other two proctolinergic projection neurons (MCN1 and MCN7) also influence STG microcircuit activity but elicit activity patterns from the circuit neurons that are distinct from proctolin bath application104,105. MCN1-released C. borealis tachykinin-related peptide Ia (CabTRP Ia) and GABA are pivotal for MCN1 activation of the gastric mill rhythm, whereas its release of CabTRP Ia and proctolin dominates its excitation of the pyloric rhythm (see part b). The MCN7 actions on these rhythms result partly from proctolin and probably also from one or more yet-to-be-identified co-transmitters (indicated by ‘?’). In the figure, pyloric rhythm activity is shown in red; gastric mill rhythm activity is shown in blue; gastropyloric activity is shown in purple. b | In the crab STG, MCN1 innervates all pyloric, gastropyloric and gastric mill neurons. The figure shows a representation of responsiveness of each STG circuit neuron to the MCN1-released co-transmitters proctolin (light green), CabTRP Ia (dark green) and GABA (dark grey)116,117. Examples of convergent peptide co-transmitter action (proctolin and CabTRP Ia), selective peptide co-transmitter action (CabTRP Ia) and selective GABA action are shown. In some cases, the STG neuron only responds to the indicated co-transmitter (or co-transmitters) (for example, Int1). In other cases, the STG neuron does respond to an additional co-transmitter but not when it is released from MCN1 (for example, LG responds to applied GABA but not GABA released from MCN1). No information is available regarding whether these co-transmitters are colocalized to all MCN1 terminals or are localized to separate terminals for their release. Part a is adapted with permission from REF.11, Elsevier.
Bath application of proctolin to the isolated STG reproducibly elicits a dose-dependent (threshold: ~10−9 M) excitation of the pyloric rhythm but does not activate the gastric mill rhythm100,103,109. This pyloric rhythm response is distinct from that elicited by application of other neuropeptides, amines or muscarinic agonists present in the STG110,111. Despite the fact that all three proctolin projection neurons influence the pyloric rhythm, selective stimulation of only one of them (MPN) modulates the pyloric rhythm comparably to bath-applied proctolin102,103,105 (FIG. 3a).
Given the presence of a co-transmitter in MPN, it was surprising that MPN stimulation and proctolin application elicited comparable pyloric rhythms. This distinction and the different actions of MCN1 and MCN7 highlight the point that, whereas direct neuropeptide application is a valuable tool for determining individual neuronal responses or modelling the circuit response to a circulating peptide hormone, one cannot always predict the microcircuit response to a co-transmitting peptidergic neuron on the basis of the circuit response to direct application of the associated peptide.
Complementary co-transmitter actions
As discussed above, co-released neuropeptides can produce diverse responses in target neurons and circuits. In the lobster Homarus americanus, co-released peptides have divergent but complementary actions that enable complete microcircuit activation. In this case, a pair of projection neurons innervating the lobster STG is immunoreactive (IR) for the neuropeptides red pigment-concentrating hormone (RPCH) and CabTRP Ia112. This represents one of two pair of RPCH-IR projection neurons innervating the H. americanus STG and is the sole source of CabTRP Ia in this STG. This dual peptide projection neuron is yet to be identified physiologically, but studies in the isolated lobster STG using separately applied and co-applied RPCH and CabTRP Ia show that they have complementary excitatory actions on different pyloric circuit neurons113 (FIG. 4). With descending inputs removed, the normally triphasic pyloric rhythm is monophasic (only the pacemaker neurons are active). Surprisingly, separate RPCH and CabTRP Ia application each activated one of the two inactive motor neuron types and excited the pacemaker neurons, whereas their co-application revived the complete triphasic rhythm113 (FIG. 4). It is not yet clear why this system is designed with one neuron using separate signalling molecules to promote activity in different circuit neurons, but one reasonable explanation involves expanding the flexibility of the actions of this peptidergic neuron by enabling separate regulation of each co-transmitter, either before or after release55,57–60.
Figure 4. Peptide co-transmitters can have complementary actions on microcircuit output.
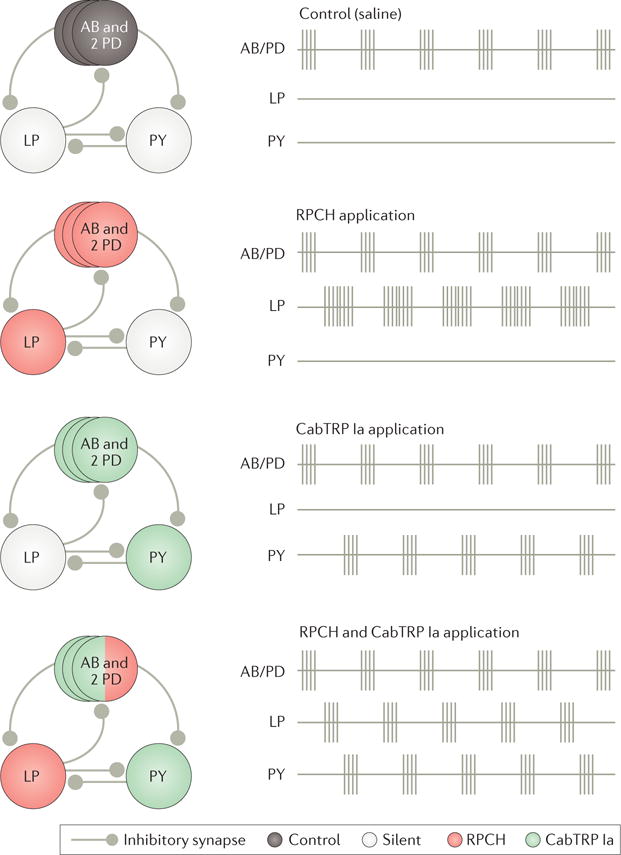
This figure shows the schematic recordings of the core pyloric circuit neurons in the isolated stomatogastric ganglion (STG) of the lobster Homarus americanus under control conditions (saline) and during bath application of the peptide co-transmitters red pigment-concentrating hormone (RPCH) or Cancer borealis tachykinin-related peptide Ia (CabTRP Ia), or both together113. Before STG isolation, the complete pyloric rhythm is expressed, including rhythmic sequential bursting of circuit neurons AB/PD, LP and PY (not shown). Under control conditions in the isolated STG, only the pyloric pacemaker ensemble (AB and both PDs) remains rhythmically active. Applying RPCH alone recruits the LP neuron to resume pyloric-timed bursting, whereas applying CabTRP Ia alone recruits pyloric-timed bursting in the PY neuron. Co-applying both peptides reactivates the complete pyloric rhythm. Adapted with permission of Society for Neuroscience from: Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets, Thirumalai V. & Marder E., J. Neurosci. 22, 1874–1882, 2002; permission conveyed through Copyright Clearance Center, Inc.
Convergent co-transmission: microcircuits
One well-established example of neuropeptide co-transmitter convergence in a microcircuit context is the influence of the projection neuron MCN1 on the pyloric circuit in the crab C. borealis STG (FIGS 2,3). This is not only convergent co-transmitter action onto the same circuit neurons but also a convergent activation of the same ionic current. MCN1 activity excites six of the seven types of pyloric circuit neurons, as well as five of the eight types of gastric mill circuit neurons, via both peptide co-transmitters114–117 (FIG. 3b). This convergent action alters the pattern of the pyloric rhythm and increases its cycle frequency, relative to times when MCN1 is silent118. This same convergent modulation onto pyloric circuit neurons also regulates the speed of the gastric mill rhythm, owing to inter-circuit interactions118–120 (FIGS 2b,5a).
Figure 5. The response of a microcircuit to co-transmission can be sculpted by feedback to the co-transmitting neuron.
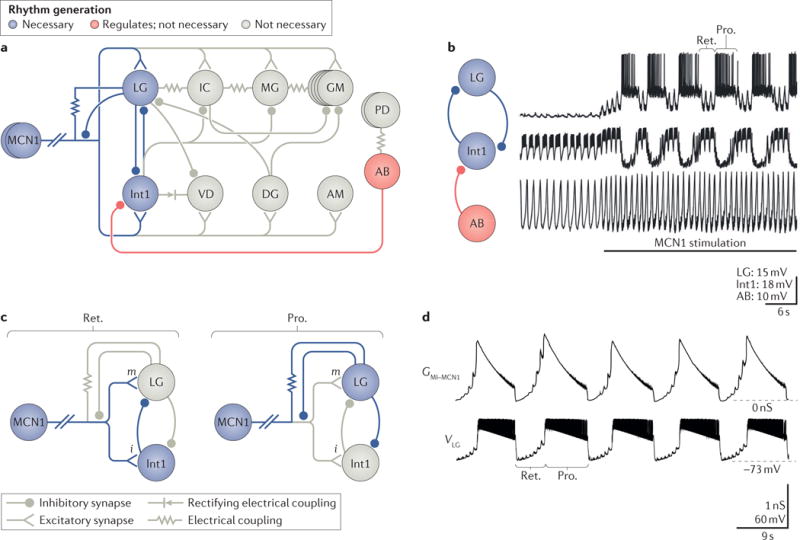
a| Modulatory commissural neuron 1 (MCN1) synaptic interactions with the gastric mill microcircuit are shown. The core rhythm generator includes the synaptic interactions between and intrinsic properties of MCN1, LG and Int1. These three neurons are necessary and sufficient to generate the gastric mill rhythm. The pyloric pacemaker interneuron AB regulates the gastric mill rhythm generator via its inhibitory synapse onto Int1. However, AB is not necessary for rhythm generation; the rhythm slows but continues when AB activity is eliminated119,134. The motor neurons shown in grey are not necessary for rhythm generation159, but some contribute to pattern generation via their intra-circuit synapses, and all contribute to movement via their synapses onto specific gastric mill muscles. The top row shows gastric mill protractor neurons (that is, motor neurons where their activation causes the teeth to move towards each other); the bottom row shows retractor neurons (that is, motor neurons, the activation of which causes the teeth to move away from one another and that are co-active with Int1 neuron); AB and PD are pyloric pacemaker neurons. All neurons are motor neurons except Int1 and AB, which are interneurons that project to the commissural ganglion (CoG). MCN1 excitation of Int1 is ionotropic; all other MCN1 co-transmitter actions are metabotropic. b | Tonic MCN1 stimulation drives the gastric mill rhythm (LG and Int1) and speeds up the pyloric rhythm (AB)118. MCN1 activation of the rhythm generator neurons LG and Int1 establishes the rhythmic alternating bursting pattern (LG bursting during the teeth protraction (Pro.) phase; Int1 bursting during the teeth retraction (Ret.) phase) that is then imposed on all gastric mill neurons. c | The gastric mill rhythm generator circuit during Ret. and Pro. phases of the MCN1-gastric mill rhythm118,127 is shown. During Ret. (left), MCN1 activity causes co-transmitter release, which drives Int1 activity, via ionotropic (i) excitation and provides a slow build-up of metabotropic (m) excitation that eventually enables LG to escape from Int1 inhibition and fire a burst of action potentials. The onset of a LG burst triggers the switch to the Pro. phase (right), during which LG is active and inhibits Int1. During Pro., LG activity also provides ionotropic inhibition to the stomatogastric ganglion (STG) terminals of MCN1 that prevents further co-transmitter release from MCN1 but enables MCN1 activity to sustain LG activity via an electrical synapse until the slowly decaying metabotropic excitation in LG falls below a critical level (see part d). In the figure, active neurons and synapses are shown in blue; silent neurons and synapses are shown in grey; the slanted double hashmarks indicate the abbreviated MCN1 axon. d | This part shows the output of a computational model showing the MCN1 Cancer borealis tachykinin-related peptide Ia (CabTRP Ia)-activated conductance GMI–MCN1 in the LG neuron waxing and waning during the gastric mill Ret. and Pro. phases, respectively (lower VLG trace)138. GMI–MCN1 increases during Ret. owing to continual CabTRP Ia release from MCN1, whereas it decays during Pro. owing to LG inhibition of the MCN1 terminals in the STG (see part c). The peak conductance occurs at the LG burst onset threshold, whereas the steep drop in conductance at the end of the LG burst occurs at the LG burst offset threshold. Part a is adapted with permission of Society for Neuroscience from: Convergent rhythm generation from divergent cellular mechanisms, Rodriguez J. C., Blitz D. M. & Nusbaum M. P., J. Neurosci. 33, 18047–18064, 2013; permission conveyed through Copyright Clearance Center, Inc. Part b is adapted with permission of Society for Neuroscience from: Coordination of fast and slow rhythmic neuronal circuits, Bartos M., Manor Y., Nadim F., Marder E. & Nusbaum M. P., J. Neurosci. 19, 6650–6660, 1999; permission conveyed through Copyright Clearance Center, Inc. Part c is adapted with permission from REF.134, Macmillan Publishers Limited. Part d is adapted with permission of Society for Neuroscience from: Parallel regulation of a modulator-activated current via distinct dynamics underlies comodulation of motor circuit output, DeLong N. D., Kirby M. S., Blitz D. M. & Nusbaum M. P., J. Neurosci. 29, 12355–12367, 2009; permission conveyed through Copyright Clearance Center, Inc.
The MCN1-released proctolin and CabTRP Ia actions are additive. Each peptide depolarizes and increases the firing rate of its STG targets, but not to the level that occurs when both peptides are influencing these neurons117. The similarity of their action results from these two peptides, along with several others, converging to activate the same voltage-dependent ionic current, the modulator-activated inward current (IMI), in pyloric circuit neurons116,121,122.
Given their convergent activation of IMI, one might wonder why MCN1 co-releases proctolin and CabTRP Ia. One possible explanation is that this co-release helps to separate the actions of MPN and MCN1, as neither proctolin application nor MPN stimulation activates the gastric mill rhythm, which MCN1 elicits primarily through CabTRP Ia release (see below). Another possibility, suggested by their additive actions, is that neither proctolin nor CabTRP Ia released alone from MCN1 can activate sufficient IMI to fully drive the pyloric rhythm. The ability of a neuropeptide to activate only a limited amount of the available IMI in some pyloric neurons is established for the peptide hormone crustacean cardioactive peptide (CCAP)123. Another interesting possibility is suggested by a recently identified component of scorpion venom that has high specificity for, and strong inhibitory activity on, neprilysin, a neutral endopeptidase, in humans and arthropods53. Neprilysin cleaves and inactivates tachykinins124,125, and neprilysin inhibitors strengthen and prolong the actions of applied and MCN1-released CabTRP Ia114,117,126. The identified neprilysin inhibitor in the scorpion venom is the short peptide YLPT, designated as [des-Arg1]-proctolin53. YLPT is also the first proctolin cleavage product produced by extracellular aminopeptidase activity in the STG127. Consequently, MCN1 co-release of proctolin and CabTRP Ia may enable proctolin cleavage to enhance and prolong CabTRP Ia actions, by limiting or delaying CabTRP Ia degradation. If so, then MCN1-released proctolin, which, unlike CabTRP Ia, has no direct role in gastric mill rhythm generation (see below), may indirectly enable or tune rhythm generation.
Divergent co-transmission: microcircuits
Elucidating the influence of co-transmission on microcircuits is challenging because of the added complexity that results from different target neurons responding to disparate sets of co-released transmitters (FIGS 1,3b). The circuit neurons unresponsive to one or more co-transmitters often have receptors for those signalling molecules, so, presumably, they can respond to those transmitters when released from a different neuron or as circulating hormones. This situation precludes a first-order determination of the circuit response by directly applying the co-transmitters to the system. There are several examples of divergent co-transmission influence among the identified neurons modulating the feeding-related microcircuits in the decapod crustacean STG59,102,103,117,128–132 (FIG. 2).
Divergent co-transmission underlies the ability of tonic MCN1 firing to drive gastric mill rhythm generation in the crab STG (FIGS 3b,5). Specifically, divergent MCN1 co-transmission influences the gastric mill rhythm generator neurons LG and Int1, whereas peptide convergence occurs on most of the remaining gastric mill neurons114,117. MCN1 influences the LG neuron only by metabotropic CabTRP Ia excitation (plus an electrical synapse) and the Int1 neuron only by ionotropic GABAergic excitatory postsynaptic potentials (EPSPs) (FIG. 5c,d). Neither neuron responds to proctolin, although LG does respond to GABA application108,117,122. Insofar as GABA is best known as an inhibitory neurotransmitter, it is noteworthy that GABA has excitatory, as well as inhibitory, actions in the STG108,117, as is also true in other systems133.
As it is likely to be true for most circuits, knowledge of the synaptic and intrinsic properties of the gastric mill circuit, including the details of the co-transmitters released from the modulatory neurons, is not sufficient to explain how MCN1 activity drives gastric mill rhythm generation. It is equally important to understand the dynamics of these events. For instance, rhythmic pre-synaptic inhibition of MCN1 by the LG neuron limits MCN1 co-transmitter release to the gastric mill retraction phase (LG silent; Int1 active)134 (FIG. 5c,d). This information is available from intra-axonal recordings of MCN1 near the STG107,127. Limited access to events occurring at distant axon terminals in most projection neurons, and the resulting reliance on intra-somatic recordings, has maintained the long-held belief that, because tonic firing by modulatory neurons can drive rhythmic neural activity patterns, such neurons provide no timing cues to the affected circuit. Although this is likely to be accurate in some instances, it is not the only possible outcome. It also illustrates another limitation of bath application studies, in which the dynamics of co-transmitter actions are not present.
The LG-mediated presynaptic inhibition of MCN1 results in LG and Int1 being co-excited by MCN1 during the gastric mill rhythm, despite their exhibiting an alternating bursting pattern at such times134 (FIG. 5b). This co-excitation works to enable sequential bursting by these neurons because the ionotropic excitation of Int1 is fast, and the metabotropic excitation of LG is slow to build up (and its impact is slowed further by Int1 inhibition of LG) and slow to decay (FIG. 5c,d). The MCN1-driven gastric mill activity pattern is also sculpted by extracellular peptidase activity, insofar as the LG burst duration is prolonged considerably in the presence of a neprilysin inhibitor that prevents CabTRP Ia degradation114.
These MCN1-related events continue to influence gastric mill rhythm generation in the more intact system, when the chewing microcircuit is triggered by a mechanosensory pathway both in vitro and in vivo135,136. Under these latter conditions, the chewing pattern is driven by only two projection neurons, MCN1 and commissural projection neuron 2 (CPN2) (REF.137). Making functional sense of this rich tapestry of data, even in such a numerically small circuit, is challenging and has been facilitated by computational modelling studies120,138,139.
Divergent co-transmitter actions can also enable separate, parallel regulation of different microcircuits. The projection neuron MPN drives a pyloric rhythm in the STG that is mimicked by exogenously applied proctolin, its peptide transmitter, despite the presence of GABA as a co-transmitter103,105,122. However, in the commissural ganglion, MPN uses exclusively GABAergic transmission to inhibit the projection neurons MCN1 and CPN2, thereby suppressing gastric mill rhythm generation130,140 (FIG. 3a). MCN1 and CPN2 are both proctolin responsive, but not as a result of MPN stimulation130. There is a similar, functionally and spatially separate action of histamine and its peptide co-transmitter in the inferior ventricular neuron (IVN)/pyloric suppressor (PS) projection neuron in the crab (IVN) and lobster (PS) stomatogastric system (see below)131,132.
Separate regulation of co-transmitters
Co-transmission also allows different co-transmitters to be regulated separately58,60. Such regulation provides added flexibility (and uncertainty, for the experimentalist) to the microcircuit response to a co-transmitting neuronal input. Examples of this selective regulation include the impact of hormonal modulation and proprioceptor feedback to the MCN1-driven gastric mill rhythm59,138,141,142 (FIG. 6). Compared with controls (FIG. 6Aa), bath application of the peptide hormone CCAP to the C. borealis STG modifies the MCN1-driven gastric mill rhythm by selectively prolonging the protractor phase, although it does also influence the retractor phase by preventing a change in its duration24,138 (FIG. 6Aa,Ba). These events result from continuous CCAP activation of IMI in the LG neuron, which reduces the LG neuron reliance on periodic IMI activation by MCN1-released CabTRP Ia138 (FIG. 6Bb,c).
Figure 6. The muscle stretch-sensitive GPR neuron causes a state-dependent prolongation of the gastric mill retractor phase by selectively inhibiting CabTRP Ia release from MCN1.
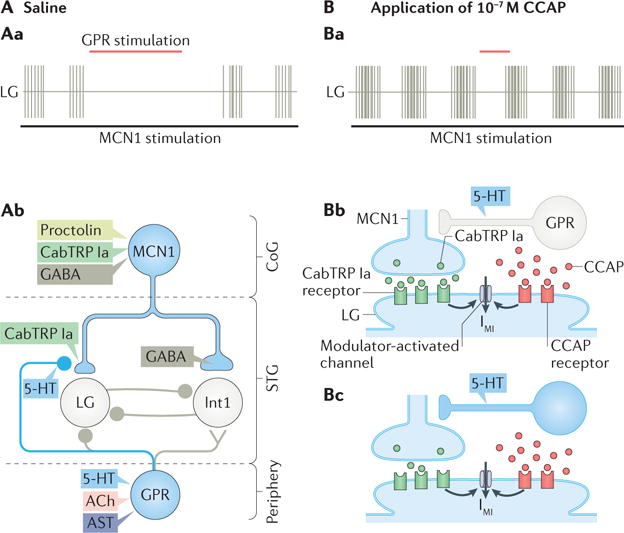
Aa| The schematic recordings show that stimulating the gastropyloric receptor neurons (GPR) during an ongoing modulatory commissural neuron 1 (MCN1)-stimulated gastric mill rhythm delays LG firing and selectively prolongs the gastric mill retractor phase59,138,141,142. Ab | The schematic circuit diagram depicts that, during the MCN1-gastric mill rhythm, GPR stimulation selectively inhibits Cancer borealis tachykinin-related peptide Ia (CabTRP Ia) release from MCN1 (represented by the smaller MCN1 terminal onto LG, as well as the placement of the GPR synapse) using only one of its co-transmitters (5-hydroxytryptamine (5-HT))59,138,141,142. By reducing CabTRP Ia release from MCN1, GPR slows the build-up of modulator-activated inward current (IMI) in LG, thereby delaying the next LG burst onset59. The grey-coloured GPR synapses are too weak to influence the rhythm relative to the other synaptic events occurring during the MCN1-gastric mill rhythm59,138,141,142. In the figure, the MCN1 co-transmitter separation is only for schematic presentation, as it is not known whether there is a spatial separation of the MCN1 co-transmitters to different terminals. Ba | The schematic recordings show that the prolongation of the gastric mill retractor phase caused by GPR stimulation during the MCN1-stimulated gastric mill rhythm in the presence of normal saline (part Aa) is suppressed by the peptide hormone crustacean cardioactive peptide (CCAP)142. CCAP also strengthens and slightly prolongs the protractor phase (LG burst) without altering retractor phase duration24,142. Bb | CCAP and MCN1-released CabTRP Ia bind to separate G protein-coupled receptors (GPCRs) on the LG neuron, but nevertheless each activates IMI current in LG59,138,141,142. GPR is silent (grey). Bc | The amount of IMI activation is not reduced when GPR is active (blue) and inhibiting CabTRP Ia release from MCN1, owing to the parallel IMI activation by CCAP59,138,141,142. ACh, acetylcholine; AST, A type allatostatin; CoG, commissural ganglion; STG, stomatogastric ganglion. Part Ab is adapted with permission from REF.59, APS. Part Bb and part c are adapted with permission of Society for Neuroscience from: Hormonal modulation of sensorimotor integration, DeLong N. D. & Nusbaum M. P., J. Neurosci. 30, 2418–2427, 2010; permission conveyed through Copyright Clearance Center, Inc.
The proprioceptive gastropyloric receptor neurons (GPRs) provide an example of peptide co-transmission being selectively inhibited. The GPRs fire action potentials in response to stretch of gastric mill protractor muscles during the retraction phase143,144, during which MCN1 co-transmitter release occurs (FIG. 5c). The GPRs are multi-transmitter sensory neurons containing ACh, 5-HT and AST38,41,143,145,146 (FIG. 6Ab). The GPR actions on the pyloric and gastric mill neurons include examples of both convergent (ionotropic ACh; metabotropic 5-HT) and divergent (metabotropic 5-HT only) co-transmission. No roles are yet attributed to GPR-released AST, despite extensive AST immunolabelling in the STG neuropil originating from the GPRs41.
In the crab STG, GPR stimulation during the MCN1-gastric mill rhythm retraction phase selectively prolongs that phase141,147 (FIG. 6Aa). By contrast, stimulating GPRs during protraction does not alter either protraction or retraction duration59. This phase-specific action results from the GPR site of action, which is its presynaptic inhibition of the MCN1 axon terminals in the STG141 (FIG. 6Ab). This also explains the lack of GPR influence when stimulated during protraction, because MCN1 is already being inhibited by the LG neuron.
This GPR inhibition changes the balance of the MCN1 co-transmitter influence on the gastric mill rhythm generator, because it selectively weakens the MCN1 peptidergic influence on LG without a parallel change in the MCN1 GABAergic synapse to Int1 (REFS59,141) (FIG. 6Ab). Consequently, the build-up of CabTRP Ia-activated IMI in LG during the retraction phase is slowed, prolonging this phase. This interaction also represents another example of divergent co-transmission, because this GPR synapse onto MCN1 is exclusively serotonergic59 (FIG. 6Ab). Interestingly, this GPR action is state dependent; it is eliminated in the presence of CCAP, as CCAP-mediated activation of IMI in the LG neuron compensates for the GPR-mediated reduction in IMI activation by MCN1 in LG142 (FIG. 6Ba–c).
Computational modelling and subsequent physiological manipulations also argue that selectively regulating peptide co-transmission is necessary for this phase-specific GPR action59. The subcellular mechanism underlying the selective regulation of peptidergic transmission from MCN1 remains to be determined, but it is likely to result from reduced neuropeptide release due to a metabotropic 5-HT action on an aspect of release specific to neuropeptides. These studies highlight the fact that, to understand the impact of co-transmitting neurons on microcircuit output, it is necessary to understand both how such neurons influence each circuit neuron and the extent to which the particular state in which the circuit is being studied regulates the release of each co-transmitter.
Neurons with shared co-transmitters
Insofar as neuronally released peptides act in a paracrine-like manner, it is often expected that different neurons that arborize in the same neuropil, influence the same microcircuit and release the same peptide transmitter have comparable actions on that microcircuit. However, this expectation was not fulfilled in the isolated crab STG with respect to the pyloric circuit response to the proctolin-containing projection neurons MCN1 (CabTRP Ia, proctolin and GABA) and MPN (proctolin and GABA) in experiments in which the CabTRP Ia actions were suppressed with a tachykinin receptor antagonist114,115.
In normal saline, during the MCN1-driven gastric mill rhythm, LG neuron inhibition of MCN1 results in the pyloric rhythm being slower and weaker during protraction than during retraction105,118 (FIGS 2c,5). Both of these pyloric rhythm epochs are distinct from the MPN-driven pyloric rhythm with respect to pyloric cycle frequency, pyloric neuron phase relationships and spike number per burst105,114.
Suppressing CabTRP Ia actions eliminates MCN1 activation of the gastric mill rhythm and thus removes the inhibitory feedback from the LG neuron onto the MCN1 axon terminals114. Under this condition, like MPN, tonic MCN1 activity drives steady excitation of the pyloric rhythm and the MCN1-driven and MPN-driven pyloric rhythms become more similar, but still not equivalent114. The persisting differences could result from differences in proctolinergic transmission, perhaps owing to differential regulation by the extra cellular aminopeptidase activity that cleaves and inactivates proctolin in the crab STG127. This might differentially limit proctolin access to its receptors when released by MCN1 or MPN, resulting in different proctolin concentrations. There is a concentration-dependent action of proctolin on the pyloric rhythm100. This possibility is supported by the finding that the MCN1-driven and MPN-driven pyloric rhythms do become more similar when using aminopeptidase inhibitors to prevent proctolin inactivation and suppressing CabTRP Ia actions115.
The remaining differences might result from yet unexplored aspects of GABAergic signalling by MCN1 or perhaps from another not yet identified co-transmitter present in one or both projection neurons. Alternatively, the remaining differences might result from release of different amounts of proctolin per action potential, as occurs for a different neuropeptide shared by two different neurons in Aplysia22. This latter possibility is supported by the fact that, with aminopeptidase activity suppressed, the proctolinergic actions of MCN1 on the pyloric rhythm were not only prolonged but outlasted the period of its stimulation ~4-fold longer than occurred after MPN stimulation, despite using the same stimulation frequency and duration for both neurons115. By contrast, under control conditions, their excitation of the pyloric rhythm outlasted the stimulation period for similar durations. These distinctions between the proctolinergic actions of MCN1 and MPN on the pyloric microcircuit bring into sharp focus the fact that bath application of neuropeptides is not ideal for elucidating peptidergic modulation of microcircuit activity, even when patterns of peptide release and local regulation of that release are not in play.
Species-specific co-transmission
The stomatogastric system has also provided insight into the detailed similarities and differences that accompany the same microcircuit operation in even closely related species110. Recordings of the pyloric and gastric mill rhythms are readily recognizable whether the recordings come from different species of crab, lobster, crayfish or shrimp, however, the microcircuit details differ across these species110,148,149. With respect to co-transmission, two identified projection neuron pairs are well studied across species. These include MPN in C. borealis and GABA neuron 1/2 (GN1/2) in the lobster Homarus gammarus, and IVN in C. borealis and PS in H. americanus and H. gammarus102,103,131,132,148.
In C. borealis, MPN (proctolin and GABA) stimulation directly elicits a proctolin-equivalent pyloric rhythm and indirectly suppresses gastric mill rhythm generation by GABAergic inhibition of identified projection neurons103,130 (FIG. 3a). The H. gammarus GN1/2 neurons share morphology and GABA with MPN, but GN1/2 contains a CCK-like peptide and FLRFamide-like peptide and not proctolin148,150. Despite not containing proctolin, GN1/2 stimulation shares with MPN stimulation the ability to activate and excite the pyloric rhythm. This GN1/2 action is likely to result from both GABAergic and peptidergic transmission, because GN1/2 stimulation elicits ionotropic EPSPs in several pyloric neurons, as well as a slow, persisting excitation that outlasts the stimulation148. In contrast to MPN stimulation, GN1/2 stimulation excites and activates the gastric mill rhythm. Thus, the co-transmitter similarity (neuropeptide (or neuropeptides) and GABA) does not lead to fully comparable physiological actions (shared pyloric excitation, but distinct effect on the gastric mill rhythm).
IVN neurons and PS neurons also share some characteristics, including morphology and a peptide–histamine co-transmitter phenotype131,132. The neuropeptide in the PS neurons is called crustacean myosuppressin (Crust-MS), a FMRFamide-related peptide132. The IVNs also contain a FLRFamide-related peptide131. Despite their similar co-transmitter phenotype, the IVNs have a predominantly histamine action in the STG, inhibiting the pyloric and gastric mill rhythms, whereas the PS neurons act in the STG primarily via Crust-MS to slow these rhythms. In the commissural ganglia, to which these neurons also project, the IVNs and PS neurons both excite the oesophageal rhythm131,132. In H. americanus, this latter action is entirely histaminergic, providing another example (like MPN) of divergent co-transmitter actions onto different microcircuits in different ganglia. The effective co-transmitter (or co-transmitters) are not known in C. borealis. In the spiny lobster Panulirus interruptus, the IVN neurons elicit in some STG neurons a multicomponent post-synaptic potential (PSP)151–153 with a rapid EPSP (which is thought to be cholinergic152), followed by a slower inhibitory postsynaptic potential (IPSP; which is thought to be histaminergic154), followed then by a slow burst enhancement (which is thought to be cholinergic)152. Clearly, caution is appropriate when extending the results obtained in one species to those in even closely related groups.
Lessons learned
This Review of small-molecule–neuropeptide co-transmission highlights the flexibility that such co-transmission provides to synapses and circuits, including the surprising range of degrees of freedom afforded by peptidergic co-transmission (for example, co-transmitters can have opposing yet complementary actions; divergent co-transmission leads to many possible outcomes; and release of different co-transmitters can be separately regulated). It also accentuates our understanding that circuit output depends not only on the temporal dynamics of all the synaptic and intrinsic currents within the circuit but also on the temporal dynamics of the release of co-transmitters from modulatory neurons and their subsequent actions. The consequence of activating peptidergic neurons also depends on the state of the target circuit, and in turn the target circuit can alter the activity of the modulatory inputs through feedback connections.
Studies of co-transmission underscore that establishing the connectome of a circuit important for a specific behaviour is only the beginning155,156. Insofar as microcircuits often, and perhaps always, receive parallel co-transmitter inputs from different pathways, understanding their collective impact on circuit output will require the collaborative efforts of experimentalists and theorists. We look forward to understanding how a nervous system accomplishes all its tasks, in part by taking advantage of the complex dynamics that arise from small molecule–peptide co-transmitters.
Acknowledgments
Research in the authors’ laboratories is funded by the US National Institutes of Health (NIH) grant NS-29436 (MPN), NSF grant IOS-1153417 (D.M.B.) and NIH grant NS17813 (E.M.).
Glossary
- Biogenic amines
Amine-containing neurotransmitters (dopamine, histamine, 5-hydroxytryptamine (vertebrates and invertebrates), noradrenaline (vertebrates) and octopamine (invertebrates)) that commonly, but not exclusively, act via G protein-coupled receptors to evoke metabotropic responses
- Stomatogastric ganglion
(STG). A small well-defined ganglion in the decapod crustacean (for example, crabs and lobsters) stomatogastric nervous system containing 25–30 neurons (depending on species), nearly all of which contribute to one or both microcircuits (gastric mill circuit (chewing), pyloric circuit (pumping and filtering of chewed food)) located therein
- Postsynaptic convergence (of co-transmitters)
Multiple neurotransmitters released from the same neuron that bind to their respective receptors on the same postsynaptic neuron to regulate neuronal activity
- Presynaptic convergence (of co-transmitters)
Multiple neurotransmitters released from the same neuron that bind to their respective receptors on the same presynaptic terminal (or terminals) to regulate neurotransmitter release from said terminal (or terminals)
- Retraction
Defines the phase of chewing when the teeth move apart; during the crab or lobster gastric mill rhythm, retraction defines the phase of neuronal activity in the sole interneuron (Int1) and the motor neurons (for example, DG neuron) that drive contraction of the ‘retractor’ muscles, which cause the teeth to move away from midline in the intact animal
- Protraction
Defines the phase of chewing when the teeth come together; during the crab or lobster gastric mill rhythm, protraction defines the phase of neuronal activity in the motor neurons (for example, LG neuron) that drive contraction of the ‘protractor’ muscles, which cause the teeth to come together at the midline in the intact animal
Footnotes
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- 2.Durrnagel S, et al. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. J Biol Chem. 2010;285:11958–11965. doi: 10.1074/jbc.M109.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunder S, Assmann M. Peptide-gated ion channels and the simple nervous system of Hydra. J Exp Biol. 2015;218:551–561. doi: 10.1242/jeb.111666. [DOI] [PubMed] [Google Scholar]
- 4.Trudeau LE, et al. The multilingual nature of dopamine neurons. Prog Brain Res. 2014;211:141–164. doi: 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tritsch NX, Granger AJ, Sabatini BL. Mechanisms and functions of GABA co-release. Nat Rev Neurosci. 2016;17:139–145. doi: 10.1038/nrn.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaaga CE, Borisovska M, Westbrook GL. Dual-transmitter neurons: functional implications of co-release and co-transmission. Curr Opin Neurobiol. 2014;29:25–32. doi: 10.1016/j.conb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy C. ATP as a cotransmitter in the autonomic nervous system. Auton Neurosci. 2015;191:2–15. doi: 10.1016/j.autneu.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ, Root DH, Zhang S, Morales M. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J Chem Neuroanat. 2016;73:33–42. doi: 10.1016/j.jchemneu.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granger AJ, Mulder N, Saunders A, Sabatini BL. Cotransmission of acetylcholine and GABA. Neuropharmacology. 2016;100:40–46. doi: 10.1016/j.neuropharm.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 12.Sámano C, Cifuentes F, Morales MA. Neurotransmitter segregation: functional and plastic implications. Prog Neurobiol. 2012;97:277–287. doi: 10.1016/j.pneurobio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Schöne C, Burdakov D. Glutamate and GABA as rapid effectors of hypothalamic “peptidergic” neurons. Front Behav Neurosci. 2012;6:81. doi: 10.3389/fnbeh.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015;18:386–392. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. This seminal paper was among the first to determine that neuronally released peptides are transmitters, are co-released with a small-molecule transmitter (thereby establishing the concept of co-transmission) and can influence target neurons at a distance (that is, with no direct synaptic contacts) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigvardt KA, Rothman BS, Brown RO, Mayeri E. The bag cells of Aplysia as a multitransmitter system: identification of alpha bag cell peptide as a second neurotransmitter. J Neurosci. 1986;6:803–813. doi: 10.1523/JNEUROSCI.06-03-00803.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupfermann I. Functional studies of cotransmission. Physiol Rev. 1991;71:683–732. doi: 10.1152/physrev.1991.71.3.683. [DOI] [PubMed] [Google Scholar]
- 18.Whim MD, Lloyd PE. Frequency-dependent release of peptide cotransmitters from identified cholinergic motor neurons in Aplysia. Proc Natl Acad Sci USA. 1989;86:9034–9038. doi: 10.1073/pnas.86.22.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whim MD, Lloyd PE. Neuropeptide cotransmitters released from identified cholinergic motor neurons in Aplysia. J Neurosci. 1990;10:3313–3322. doi: 10.1523/JNEUROSCI.10-10-03313.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng YY, Horn JP. Continuous repetitive stimuli are more effective than bursts for evoking LHRH release in bullfrog sympathetic ganglia. J Neurosci. 1991;11:85–95. doi: 10.1523/JNEUROSCI.11-01-00085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Release of peptide cotransmitters in Aplysia: regulation and functional implications. J Neurosci. 1996;16:8105–8014. doi: 10.1523/JNEUROSCI.16-24-08105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Peptide cotransmitter release from motorneuron B16 in Aplysia californica: costorage, corelease, and functional implications. J Neurosci. 2000;20:2036–2042. doi: 10.1523/JNEUROSCI.20-05-02036.2000. This work reported that neuropeptide release can occur within the natural range of a neuron firing frequency, that co-released peptides with antagonist actions can be co-stored in the same dense-core vesicles (see also references84 and 91) and that co-released peptides can have complementary actions on their shared target cells, even when their individual actions seem to be antagonistic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilim FS, Price DA, Lesser W, Kupfermann I, Weiss KR. Costorage and corelease of modulatory peptide cotransmitters with partially antagonistic actions on the accessory radula closer muscle of Aplysia californica. J Neurosci. 1996;16:8092–8104. doi: 10.1523/JNEUROSCI.16-24-08092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirby MS, Nusbaum MP. Peptide hormone modulation of a neuronally modulated motor circuit. J Neurophysiol. 2007;98:3206–3220. doi: 10.1152/jn.00795.2006. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 26.Whim MD. Near simultaneous release of classical and peptide cotransmitters from chromaffin cells. J Neurosci. 2006;26:6637–6642. doi: 10.1523/JNEUROSCI.5100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nusbaum MP, Blitz DM. Neuropeptide modulation of microcircuits. Curr Opin Neurobiol. 2012;22:592–601. doi: 10.1016/j.conb.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nässel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Nässel DR. Neuropeptide signaling near and far: how localized and timed is the action of neuropeptides in brain circuits? Invert Neurosci. 2009;9:57–75. doi: 10.1007/s10158-009-0090-1. [DOI] [PubMed] [Google Scholar]
- 31.Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma M, Szabo TM, Jia C, Marder E, Li L. Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides. 2009;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma M, Wang J, Chen R, Li L. Expanding the crustacean neuropeptidome using a multifaceted mass spectrometric approach. J Proteome Res. 2009;8:2426–2437. doi: 10.1021/pr801047v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winther AM, Siviter RJ, Isaac RE, Predel R, Nässel DR. Neuronal expression of tachykinin-related peptides and gene transcript during postembryonic development of Drosophila. J Comp Neurol. 2003;464:180–196. doi: 10.1002/cne.10790. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, Hui L, Sturm RM, Li L. Three dimensional mapping of neuropeptides and lipids in crustacean brain by mass spectral imaging. J Am Soc Mass Spectrom. 2009;20:1068–1077. doi: 10.1016/j.jasms.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickinson PS, et al. Molecular, mass spectral, and physiological analyses of orcokinins and orcokinin precursor-related peptides in the lobster Homarus americanus and the crayfish Procambarus clarkii. Peptides. 2009;30:297–317. doi: 10.1016/j.peptides.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE. Identification, physiological actions, and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J Neurochem. 2007;101:1351–1366. doi: 10.1111/j.1471-4159.2007.04520.x. [DOI] [PubMed] [Google Scholar]
- 38.Szabo TM, et al. Distribution and physiological effects of B-type allatostatins (myoinhibitory peptides, MIPs) in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol. 2011;519:2658–2676. doi: 10.1002/cne.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui L, et al. Discovery and functional study of a novel crustacean tachykinin neuropeptide. ACS Chem Neurosci. 2011;2:711–722. doi: 10.1021/cn200042p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickinson PS, et al. Distinct or shared actions of peptide family isoforms: II. Multiple pyrokinins exert similar effects in the lobster stomatogastric nervous system. J Exp Biol. 2015;218:2905–2917. doi: 10.1242/jeb.124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skiebe P, Schneider H. Allatostatin peptides in the crab stomatogastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. J Exp Biol. 1994;194:195–208. doi: 10.1242/jeb.194.1.195. [DOI] [PubMed] [Google Scholar]
- 42.Nusbaum MP, Marder E. A neuronal role for a crustacean red pigment concentrating hormone-like peptide: neuromodulation of the pyloric rhythm in the crab, Cancer borealis. J Exp Biol. 1988;135:165–181. [Google Scholar]
- 43.Dickinson PS, Sreekrishnan A, Kwiatkowski MA, Christie AE. Distinct or shared actions of peptide family isoforms: I. Peptide-specific actions of pyrokinins in the lobster cardiac neuromuscular system. J Exp Biol. 2015;218:2892–2904. doi: 10.1242/jeb.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poels J, et al. Pharmacology of stomoxytachykinin receptor depends on second messenger system. Peptides. 2005;26:109–114. doi: 10.1016/j.peptides.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Poels J, et al. Functional comparison of two evolutionary conserved insect neurokinin-like receptors. Peptides. 2007;28:103–108. doi: 10.1016/j.peptides.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Poels J, et al. Characterization and distribution of NKD, a receptor for Drosophila tachykinin-related peptide 6. Peptides. 2009;30:545–556. doi: 10.1016/j.peptides.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Poels J, et al. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell Mol Life Sci. 2010;67:3511–3522. doi: 10.1007/s00018-010-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 49.Ollmann MM, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 50.Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- 51.Isaac RE, Bland ND, Shirras AD. Neuropeptidases and the metabolic inactivation of insect neuropeptides. Gen Comp Endocrinol. 2009;162:8–17. doi: 10.1016/j.ygcen.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol. 2002;60:378–387. doi: 10.1159/000067791. [DOI] [PubMed] [Google Scholar]
- 53.Duzzi B, et al. [des-Arg1]-proctolin: a novel NEP-like enzyme inhibitor identified in Tityus serrulatus venom. Peptides. 2016;80:18–24. doi: 10.1016/j.peptides.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 54.Chalasani SH, et al. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. This work established that divergent co-transmission can both elicit (via fast synaptic transmission) a complex behaviour and regulate its duration (via slow, peptidergic transmission), the latter via a peptidergic negative feedback loop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mills H, et al. Opiates modulate noxious chemical nociception through a complex monoaminergic/peptidergic cascade. J Neurosci. 2016;36:5498–5508. doi: 10.1523/JNEUROSCI.4520-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leinwand SG, Chalasani SH. Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat Neurosci. 2013;16:1461–1467. doi: 10.1038/nn.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris G, et al. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci. 2010;30:7889–7899. doi: 10.1523/JNEUROSCI.0497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hapiak V, et al. Neuropeptides amplify and focus the monoaminergic inhibition of nociception in Caenorhabditis elegans. J Neurosci. 2013;33:14107–14116. doi: 10.1523/JNEUROSCI.1324-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeLong ND, Beenhakker MP, Nusbaum MP. Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J Neurophysiol. 2009;102:3492–3504. doi: 10.1152/jn.00833.2009. This paper demonstrated that neuropeptide co-release (with GABA) from an identified modulatory projection neuron is selectively inhibited (by an identified sensory feedback pathway, acting via its own divergent co-transmitter action), altering the motor pattern generated by the target microcircuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iremonger KJ, Kuzmiski JB, Baimoukhametova DV, Bains JS. Dual regulation of anterograde and retrograde transmission by endocannabinoids. J Neurosci. 2011;31:12011–12020. doi: 10.1523/JNEUROSCI.2925-11.2011. This study revealed the complexity of co-transmission by showing that, in the hypothalamus, dendritic release of eCB can regulate, via its actions on the presynaptic neuron, release of its retrograde peptide co-transmitters (VP and DYN) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ignell R, et al. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci USA. 2009;106:13070–13075. doi: 10.1073/pnas.0813004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnstedt O, et al. Memory-relevant mushroom body output synapses are cholinergic. Neuron. 2016;89:1237–1247. doi: 10.1016/j.neuron.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. This study showed that, during a particular behavioural state (starvation), a neuropeptide is released, binds to autoreceptors on identified olfactory neuron terminals and strengthens the co-release of that peptide and its small-molecule transmitter, thus driving increased food search behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang JW. Presynaptic modulation of early olfactory processing in Drosophila. Dev Neurobiol. 2012;72:87–99. doi: 10.1002/dneu.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kahsai L, Kapan N, Dircksen H, Winther AM, Nässel DR. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. PLoS ONE. 2010;5:e11480. doi: 10.1371/journal.pone.0011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kapan N, Lushchak OV, Luo J, Nässel DR. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol Life Sci. 2012;69:4051–4066. doi: 10.1007/s00018-012-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi C, et al. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. This study demonstrated that a neuropeptide that is released and binds to autoreceptors on circadian clock neurons facilitates its own release and that of its small-molecule co-transmitter, in this case shifting more of the fly’s circadian-regulated activity from evening to morning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kahsai L, Kapan N, Dircksen H, Winther AM, Nassel DR. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. PLoS ONE. 2010;5:e11480. doi: 10.1371/journal.pone.0011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pulst SM, Rothman BS, Mayeri E. Presence of immunoreactive alpha-bag cell peptide[1–8] in bag cell neurons of Aplysia suggests novel carboxypeptidase processing of neuropeptides. Neuropeptides. 1987;10:249–259. doi: 10.1016/0143-4179(87)90075-8. [DOI] [PubMed] [Google Scholar]
- 70.Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J Neurophysiol. 2003;90:2074–2079. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- 71.Koh HY, Weiss KR. Activity-dependent peptidergic modulation of the plateau-generating neuron B64 in the feeding network of Aplysia. J Neurophysiol. 2007;97:1862–1867. doi: 10.1152/jn.01230.2006. [DOI] [PubMed] [Google Scholar]
- 72.Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J Neurosci. 2010;30:8906–8919. doi: 10.1523/JNEUROSCI.1287-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun QQ, Baraban SC, Prince DA, Huguenard JR. Target-specific neuropeptide Y-ergic synaptic inhibition and its network consequences within the mammalian thalamus. J Neurosci. 2003;23:9639–9649. doi: 10.1523/JNEUROSCI.23-29-09639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ptak K, et al. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jego S, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chee MJ, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu J, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. doi: 10.7554/eLife.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans. 2007;35:1236–1242. doi: 10.1042/BST0351236. [DOI] [PubMed] [Google Scholar]
- 80.Israel JM, Poulain DA, Oliet SH. Oxytocin-induced postinhibitory rebound firing facilitates bursting activity in oxytocin neurons. J Neurosci. 2008;28:385–394. doi: 10.1523/JNEUROSCI.5198-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sabatier N, et al. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabatier N. α-Melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol. 2006;18:703–710. doi: 10.1111/j.1365-2826.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- 84.Whitnall MH, Gainer H, Cox BM, Molineaux CJ. Dynorphin-A-(1–8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983;222:1137–1139. doi: 10.1126/science.6648526. [DOI] [PubMed] [Google Scholar]
- 85.Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of κ opioid receptors to the plasma membrane. J Neurosci. 1999;19:2658–2664. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–466. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- 87.Wang L, Armstrong WE. Tonic regulation of GABAergic synaptic activity on vasopressin neurones by cannabinoids. J Neuroendocrinol. 2012;24:664–673. doi: 10.1111/j.1365-2826.2011.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Apergis-Schoute J, et al. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci. 2015;35:5435–5441. doi: 10.1523/JNEUROSCI.5269-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muschamp JW, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X, Marchant NJ, Shaham Y. Opposing roles of cotransmission of dynorphin and hypocretin on reward and motivation. Proc Natl Acad Sci USA. 2014;111:5765–5766. doi: 10.1073/pnas.1403603111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schöne C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7:697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cape SS, Rehm KJ, Ma M, Marder E, Li L. Mass spectral comparison of the neuropeptide complement of the stomatogastric ganglion and brain in the adult and embryonic lobster, Homarus americanus. J Neurochem. 2008;105:690–702. doi: 10.1111/j.1471-4159.2007.05154.x. [DOI] [PubMed] [Google Scholar]
- 95.Rehm KJ, Deeg KE, Marder E. Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus. J Neurosci. 2008;28:9828–9839. doi: 10.1523/JNEUROSCI.2328-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandvik GK, Hodne K, Haug TM, Okubo K, Weltzien FA. RFamide peptides in early vertebrate development. Front Endocrinol (Lausanne) 2014;5:203. doi: 10.3389/fendo.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sillar KT, Combes D, Simmers J. Neuromodulation in developing motor microcircuits. Curr Opin Neurobiol. 2014;29:73–81. doi: 10.1016/j.conb.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–453. doi: 10.1126/science.1234152. This paper established that environmental conditions (short versus long photoperiods) can trigger a switch in the balance of co-transmitters (dopamine and somatostatin) and their receptors in rodent hypothalamic neurons, altering behaviours associated with these neurons. [DOI] [PubMed] [Google Scholar]
- 99.Spitzer NC. Neurotransmitter switching? No surprise. Neuron. 2015;86:1131–1144. doi: 10.1016/j.neuron.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marder E, Hooper SL, Siwicki KK. Modulatory action and distribution of the neuropeptide proctolin in the crustacean stomatogastric nervous system. J Comp Neurol. 1986;243:454–467. doi: 10.1002/cne.902430403. [DOI] [PubMed] [Google Scholar]
- 101.Coleman MJ, Nusbaum MP, Cournil I, Claiborne BJ. Distribution of modulatory inputs to the stomatogastric ganglion of the crab, Cancer borealis. J Comp Neurol. 1992;325:581–594. doi: 10.1002/cne.903250410. [DOI] [PubMed] [Google Scholar]
- 102.Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). I. Identification and characterization. J Neurosci. 1989;9:1591–1599. doi: 10.1523/JNEUROSCI.09-05-01591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). II. State-dependent modulation of rhythmic motor activity. J Neurosci. 1989;9:1600–1607. doi: 10.1523/JNEUROSCI.09-05-01600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coleman MJ, Nusbaum MP. Functional consequences of compartmentalization of synaptic input. J Neurosci. 1994;14:6544–6552. doi: 10.1523/JNEUROSCI.14-11-06544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blitz DM, et al. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. This study established that different identified neurons containing the same peptide co-transmitter and influencing the same microcircuits can elicit different motor patterns from these circuits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christie AE, Baldwin DH, Marder E, Graubard K. Organization of the stomatogastric neuropil of the crab, Cancer borealis, as revealed by modulator immunocytochemistry. Cell Tissue Res. 1997;288:135–148. doi: 10.1007/s004410050801. [DOI] [PubMed] [Google Scholar]
- 107.Nusbaum MP, Weimann JM, Golowasch J, Marder E. Presynaptic control of modulatory fibers by their neural network targets. J Neurosci. 1992;12:2706–2714. doi: 10.1523/JNEUROSCI.12-07-02706.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swensen AM, et al. GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol. 2000;203:2075–2092. doi: 10.1242/jeb.203.14.2075. [DOI] [PubMed] [Google Scholar]
- 109.Hooper SL, Marder E. Modulation of the lobster pyloric rhythm by the peptide proctolin. J Neurosci. 1987;7:2097–2112. doi: 10.1523/JNEUROSCI.07-07-02097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 111.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fénelon VS, Kilman V, Meyrand P, Marder E. Sequential developmental acquisition of neuromodulatory inputs to a central pattern-generating network. J Comp Neurol. 1999;408:335–351. doi: 10.1002/(sici)1096-9861(19990607)408:3<335::aid-cne3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 113.Thirumalai V, Marder E. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J Neurosci. 2002;22:1874–1882. doi: 10.1523/JNEUROSCI.22-05-01874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor patterns from the same neuronal circuit. J Neurosci. 2000;20:8943–8953. doi: 10.1523/JNEUROSCI.20-23-08943.2000. This paper showed that two different identified projection neurons use the same two co-transmitters (GABA and proctolin) to elicit different motor patterns from the same microcircuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wood DE, Nusbaum MP. Extracellular peptidase activity tunes motor pattern modulation. J Neurosci. 2002;22:4185–4195. doi: 10.1523/JNEUROSCI.22-10-04185.2002. This paper showed that the same extracellular peptidase activity differentially influences how different identified projection neurons using the same neuropeptide co-transmitter modulate the same microcircuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci. 2001;21:4050–4058. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci. 2007;26:1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x. [DOI] [PubMed] [Google Scholar]
- 118.Bartos M, Nusbaum MP. Intercircuit control of motor pattern modulation by presynaptic inhibition. J Neurosci. 1997;17:2247–2256. doi: 10.1523/JNEUROSCI.17-07-02247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci. 1999;19:6650–6660. doi: 10.1523/JNEUROSCI.19-15-06650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nadim F, Manor Y, Nusbaum MP, Marder E. Frequency regulation of a slow rhythm by a fast periodic input. J Neurosci. 1998;18:5053–5067. doi: 10.1523/JNEUROSCI.18-13-05053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Golowasch J, Marder E. Proctolin activates an inward current whose voltage dependence is modified by extracellular Ca2+ J Neurosci. 1992;12:810–817. doi: 10.1523/JNEUROSCI.12-03-00810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20:6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia VJ, Daur N, Temporal S, Schulz DJ, Bucher D. Neuropeptide receptor transcript expression levels and magnitude of ionic current responses show cell type-specific differences in a small motor circuit. J Neurosci. 2015;35:6786–6800. doi: 10.1523/JNEUROSCI.0171-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saleh TM, Kombian SB, Zidichouski JA, Pittman QJ. Peptidergic modulation of synaptic transmission in the parabrachial nucleus in vitro: importance of degradative enzymes in regulating synaptic efficacy. J Neurosci. 1996;16:6046–6055. doi: 10.1523/JNEUROSCI.16-19-06046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 126.Blitz DM, et al. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol. 2008;211:1000–1011. doi: 10.1242/jeb.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coleman MJ, Konstant PH, Rothman BS, Nusbaum MP. Neuropeptide degradation produces functional inactivation in the crustacean nervous system. J Neurosci. 1994;14:6205–6216. doi: 10.1523/JNEUROSCI.14-10-06205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katz PS, Harris-Warrick RM. Neuromodulation of the crab pyloric central pattern generator by serotonergic/cholinergic proprioceptive afferents. J Neurosci. 1990;10:1495–1512. doi: 10.1523/JNEUROSCI.10-05-01495.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Katz PS, Harris-Warrick RM. Recruitment of crab gastric mill neurons into the pyloric motor pattern by mechanosensory afferent stimulation. J Neurophysiol. 1991;65:1442–1451. doi: 10.1152/jn.1991.65.6.1442. [DOI] [PubMed] [Google Scholar]
- 130.Blitz DM, Nusbaum MP. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci. 1999;19:6774–6783. doi: 10.1523/JNEUROSCI.19-16-06774.1999. This study demonstrated that a single projection neuron uses spatially segregated actions of different co-transmitters to regulate separate microcircuits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Christie AE, et al. Actions of a histaminergic/peptidergic projection neuron on rhythmic motor patterns in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol. 2004;469:153–169. doi: 10.1002/cne.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kwiatkowski MA, et al. Coordination of distinct but interacting rhythmic motor programs by a modulatory projection neuron using different co-transmitters in different ganglia. J Exp Biol. 2013;216:1827–1836. doi: 10.1242/jeb.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ostroumov A, et al. Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron. 2016;92:493–504. doi: 10.1016/j.neuron.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coleman MJ, Meyrand P, Nusbaum MP. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature. 1995;378:502–505. doi: 10.1038/378502a0. [DOI] [PubMed] [Google Scholar]
- 135.Beenhakker MP, Blitz DM, Nusbaum MP. Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system. J Neurophysiol. 2004;91:78–91. doi: 10.1152/jn.00741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Diehl F, White RS, Stein W, Nusbaum MP. Motor circuit-specific burst patterns drive different muscle and behavior patterns. J Neurosci. 2013;33:12013–12029. doi: 10.1523/JNEUROSCI.1060-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beenhakker MP, Nusbaum MP. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci. 2004;24:6741–6750. doi: 10.1523/JNEUROSCI.1682-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeLong ND, Kirby MS, Blitz DM, Nusbaum MP. Parallel regulation of a modulator-activated current via distinct dynamics underlies comodulation of motor circuit output. J Neurosci. 2009;29:12355–12367. doi: 10.1523/JNEUROSCI.3079-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marder E, Manor Y, Nadim F, Bartos M, Nusbaum MP. Neuronal Mechanisms for Generating Locomotory Activity. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Ann NY Acad Sci. 1998. pp. 226–237. [Google Scholar]
- 140.Blitz DM, Nusbaum MP. Motor pattern selection via inhibition of parallel pathways. J Neurosci. 1997;17:4965–4975. doi: 10.1523/JNEUROSCI.17-13-04965.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beenhakker MP, DeLong ND, Saideman SR, Nadim F, Nusbaum MP. Proprioceptor regulation of motor circuit activity by presynaptic inhibition of a modulatory projection neuron. J Neurosci. 2005;25:8794–8806. doi: 10.1523/JNEUROSCI.2663-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DeLong ND, Nusbaum MP. Hormonal modulation of sensorimotor integration. J Neurosci. 2010;30:2418–2427. doi: 10.1523/JNEUROSCI.5533-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Katz PS, Eigg MH, Harris-Warrick RM. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol. 1989;62:558–570. doi: 10.1152/jn.1989.62.2.558. [DOI] [PubMed] [Google Scholar]
- 144.Birmingham JT, Szuts Z, Abbott LF, Marder E. Encoding of muscle movement on two time scales by a sensory neuron that switches between spiking and burst modes. J Neurophysiol. 1999;82:2786–2797. doi: 10.1152/jn.1999.82.5.2786. [DOI] [PubMed] [Google Scholar]
- 145.Beltz B, et al. Serotonergic innervation and modulation of the stomatogastric ganglion of three decapod crustaceans (Panulirus interruptus, Homarus americanus and Cancer irroratus) J Exp Biol. 1984;109:35–54. doi: 10.1242/jeb.109.1.35. [DOI] [PubMed] [Google Scholar]
- 146.Katz PS, Harris-Warrick RM. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. II. Rapid nicotinic and prolonged modulatory effects on neurons in the stomatogastric ganglion. J Neurophysiol. 1989;62:571–581. doi: 10.1152/jn.1989.62.2.571. [DOI] [PubMed] [Google Scholar]
- 147.Beenhakker MP, Kirby MS, Nusbaum MP. Mechanosensory gating of proprioceptor input to modulatory projection neurons. J Neurosci. 2007;27:14308–14316. doi: 10.1523/JNEUROSCI.4404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyrand P, Faumont S, Simmers J, Christie AE, Nusbaum MP. Species-specific modulation of pattern-generating circuits. Eur J Neurosci. 2000;12:2585–2596. doi: 10.1046/j.1460-9568.2000.00121.x. This study showed that likely-equivalent projection neurons in different species share a co-transmitter phenotype (small-molecule plus peptide co-transmitters) but exhibit some distinct actions on their shared target microcircuits. [DOI] [PubMed] [Google Scholar]
- 149.Bucher D, Taylor AL, Marder E. Central pattern generating neurons simultaneously express fast and slow rhythmic activities in the stomatogastric ganglion. J Neurophysiol. 2006;95:3617–3632. doi: 10.1152/jn.00004.2006. [DOI] [PubMed] [Google Scholar]
- 150.Cournil I, Meyrand P, Moulins M. Identification of all GABA-immunoreactive neurons projecting to the lobster stomatogastric ganglion. J Neurocytol. 1990;19:478–493. doi: 10.1007/BF01257238. [DOI] [PubMed] [Google Scholar]
- 151.Marder E, Eisen JS. Electrically coupled pacemaker neurons respond differently to the same physiological inputs and neurotransmitters. J Neurophysiol. 1984;51:1362–1374. doi: 10.1152/jn.1984.51.6.1362. [DOI] [PubMed] [Google Scholar]
- 152.Russell DF, Hartline DK. A multiaction synapse evoking both EPSPs and enhancement of endogenous bursting. Brain Res. 1981;223:19–38. doi: 10.1016/0006-8993(81)90803-9. [DOI] [PubMed] [Google Scholar]
- 153.Sigvardt KA, Mulloney B. Properties of synapses made by IVN command-interneurones in the stomatogastric ganglion of the spiny lobster Panulirus interruptus. J Exp Biol. 1982;97:153–168. doi: 10.1242/jeb.97.1.153. [DOI] [PubMed] [Google Scholar]
- 154.Claiborne BJ, Selverston AI. Histamine as a neurotransmitter in the stomatogastric nervous system of the spiny lobster. J Neurosci. 1984;4:708–721. doi: 10.1523/JNEUROSCI.04-03-00708.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 156.Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 157.Saideman SR, et al. Modulation of rhythmic motor activity by pyrokinin peptides. J Neurophysiol. 2007;97:579–595. doi: 10.1152/jn.00772.2006. [DOI] [PubMed] [Google Scholar]
- 158.Nusbaum MP, Beenhakker MP. A small systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.White RS, Nusbaum MP. The same core rhythm generator underlies different rhythmic motor patterns. J Neurosci. 2011;31:11484–11494. doi: 10.1523/JNEUROSCI.1885-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


