Abstract
Objectives
The Centers for Disease Control and Prevention considers carbapenem-resistant Enterobacteriaceae (CRE) an urgent public health threat; however, its economic burden is unknown.
Methods
We developed a CRE clinical and economics outcomes model to determine the cost of CRE infection from the hospital, third-party payer, and societal, perspectives and to evaluate the health and economic burden of CRE to the USA.
Results
Depending on the infection type, the median cost of a single CRE infection can range from $22 484 to $66 031 for hospitals, $10 440 to $31 621 for third-party payers, and $37 778 to $83 512 for society. An infection incidence of 2.93 per 100 000 population in the USA (9418 infections) would cost hospitals $275 million (95% CR $217–334 million), third-party payers $147 million (95% CR $129–172 million), and society $553 million (95% CR $303–1593 million) with a 25% attributable mortality, and would result in the loss of 8841 (95% CR 5805–12 420) quality-adjusted life years. An incidence of 15 per 100 000 (48 213 infections) would cost hospitals $1.4 billion (95% CR $1.1–1.7 billion), third-party payers $0.8 billion (95% CR $0.6–0.8 billion), and society $2.8 billion (95% CR $1.6–8.2 billion), and result in the loss of 45 261 quality-adjusted life years.
Conclusions
The cost of CRE is higher than the annual cost of many chronic diseases and of many acute diseases. Costs rise proportionally with the incidence of CRE, increasing by 2.0 times, 3.4 times, and 5.1 times for incidence rates of 6, 10, and 15 per 100 000 persons.
Keywords: Carbapenem-resistant Enterobacteriaceae, Cost, CRE, Economic burden, Model
Introduction
Although the Centers for Disease Control and Prevention (CDC) considers carbapenem-resistant Enterobacteriaceae (CRE) to be an urgent public health threat [1] because of its increasing prevalence [2,3] and resistance to many antibiotics, the potential economic impact is currently unknown. CRE detection increased more than fivefold between 2008 and 2012 [4]. Within the first 6 months of 2012, 3.9% of acute care hospitals and 17.8% of long-term acute care hospitals (LTACs) reported at least one CRE infection [2], and as of January 2015, CRE infections have been confirmed in 48 states and are estimated at 2.93 per 100 000 persons [5,6]. With limited treatment options and high associated mortality, there is a pressing need to better understand the economic burden of CRE infection to help guide investment in CRE prevention and control strategies.
Allocating resources towards a response to CRE necessitates a better understanding of the economic impact of CRE infection. Therefore, we developed a clinical and economic model to determine the cost of CRE infection by infection type and the annual cost of CRE infection in the USA.
Materials and methods
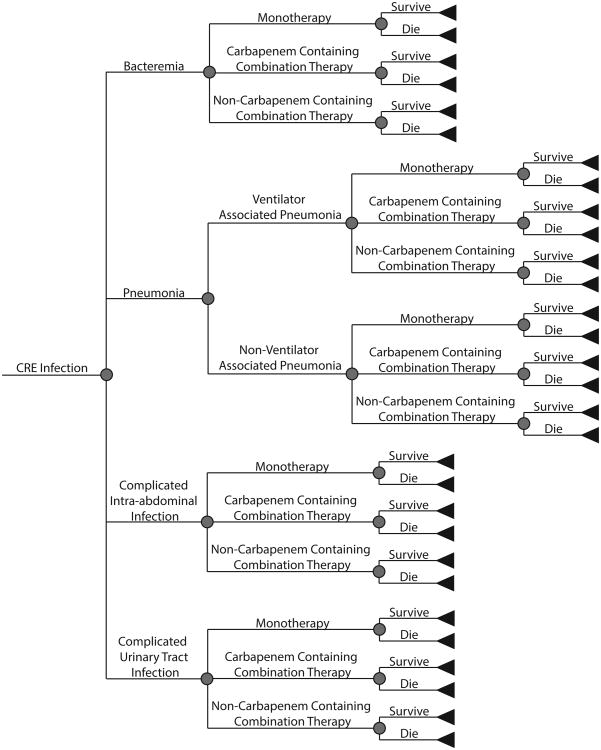
We developed a CRE clinical and economics outcomes model to determine the cost and health effects of CRE infection from the hospital, third-party payer, and societal perspectives for the duration of infection and outcomes. Each patient entered the model with a CRE infection and had a probability of having one of four infection types: bacteraemia, intra-abdominal infection, pneumonia, or complicated urinary tract infection (UTI). Those with pneumonia had a probability of ventilator-associated pneumonia (VAP). All patients, regardless of infection type, had probabilities of receiving different types of treatment: monotherapy (e.g. tigecycline, aminoglycoside), carbapenem-containing combination therapy, or non-carbapenem containing combination therapy. Each patient had a probability of mortality, depending on their infection type and treatment received, attenuated by the attributable mortality of CRE. Additionally, each patient had a probability of being in an intensive care unit (ICU), versus a general ward. Fig. 1 outlines the model structure, and Table 1 shows the model input parameters, values, and sources. Parameter estimates came from review papers published in the literature, systematic reviews, and nationally representative databases when available, other estimates came from an extensive search of the scientific literature. When reviews were not available, data from the USA was preferred, followed by countries with a similar CRE epidemiology. Where multiple studies were used to determine the parameter estimate, raw counts where extracted from studies to calculate the overall number or probability.
Fig. 1.
Model outline.
Table 1. Clinical and economic model input parameters, values, and sources.
| Parameter | Mean or median | Standard deviation or range | Source |
|---|---|---|---|
| Costs (2016 $US) | |||
| ICU bed day | 4893 | 31.66 | [30,31] |
| General ward bed daya,b | 2877 | 25.43 | [8] |
| Hospitalizationa,c | |||
| Bacteraemia | 13 231 | 396 | [8] |
| Intra-abdominal infection | 14 572 | 278 | [8] |
| Pneumonia (non-VAP) | 20 968 | 1844 | [8] |
| Ventilator-associated pneumonia (VAP) | 27 981 | 2351 | [8] |
| Urinary tract infection (UTI) | 7767 | 126 | [8] |
| Drug treatments per day | |||
| Tigecycline | 294.01 | [13] | |
| Meropenem | 132.93 | [13] | |
| Gentamicin | 44.68 | [13] | |
| Amikacin | 59.44 | [13] | |
| Colistin | 87.51 | [13] | |
| PICC line insertion | 98.45 | [32] | |
| Urine analysis | 3.01 | 0.14 | [33] |
| Urine culture | 11.02 | 1.08 | [33] |
| Abdominal CT scan | 260.68 | 76.80 | [32] |
| Bronchoscopy | 138.31 | 25.17 | [32] |
| Wound culture | 11.90 | 0.93 | [33] |
| Chest x-ray | 28.79 | [32] | |
| Sputum cultures | 17.77 | 2.10 | [32] |
| Blood culture | 14.00 | 1.59 | [33] |
| Median hourly wage (all occupations) | 18.13 | 9.36–45.94d | [10] |
| Median annual wage (all occupations) | 37 704.39 | 19 467.52–95 544.65d | [10] |
| Registered nurse hourly wage | 33.99 | 23.40–50.44d | [10] |
| Probabilities (%) | |||
| Bacteraemia | 20.28 | [34–39] | |
| Intra-abdominal infection | 5.92 | [34–39] | |
| Pneumonia (VAP and non-VAP) | 25.70 | [34–39] | |
| Urinary tract infection (UTI) | 48.09 | [34–39] | |
| Ventilator-associated pneumonia (VAP) given CRE pneumonia | 5.76 | [34–39] | |
| Probability of ICU at onset | 44.2 | 4.53 | [36,40] |
| Treatment probabilities | |||
| Monotherapy | 47.03 | [41,42] | |
| Carbapenem containing combination therapy | 38.86 | [41,42] | |
| Non-carbapenem containing combination therapy | 14.11 | [41,42] | |
| Mortality from bacteraemia | |||
| Monotherapy | 46.4 | [42] | |
| Carbapenem containing combination therapy | 40.7 | [42] | |
| Non-carbapenem containing combination therapy | 18.2 | [42] | |
| Mortality from intra-abdominal infection | |||
| Monotherapy | 33.3 | [42] | |
| Carbapenem containing combination therapy | 31.4 | [42] | |
| Non-carbapenem containing combination therapy | 0.0 | [42] | |
| Mortality from pneumonia | |||
| Monotherapy | 46.7 | [42] | |
| Carbapenem containing combination therapy | 30.4 | [42] | |
| Non-carbapenem containing combination therapy | 27.8 | [42] | |
| Mortality from urinary tract infection | |||
| Monotherapy | 38.9 | [42] | |
| Carbapenem containing combination therapy | 28.6 | [42] | |
| Non-carbapenem containing combination therapy | 10.0 | [42] | |
| Mortality attributable to CRE | 26–44 | [12] | |
| Durations (days) and numbers | |||
| Attributable length of stay | |||
| Bacteraemia | 9–10 | [21–23] | |
| Intra-abdominal infection | 14–21 | Expert opinion | |
| Pneumonia (non-VAP) | 4–10 | [43] | |
| Ventilator-associated pneumonia (VAP) | 10–14 | [44–48] | |
| Urinary tract infection (UTI) | 4–8 | [49] | |
| Treatment durations | |||
| Bacteraemia | 14 | [7], Expert opinion | |
| Intra-abdominal infection | 10–14 | [7], Expert opinion | |
| Pneumonia (non-VAP) | 10–14 | [7], Expert opinion | |
| Ventilator-associated pneumonia (VAP) | 14 | [7], Expert opinion | |
| Urinary tract infection (UTI) | 14–21 | [7], Expert opinion | |
| Patient contacts per day | 25–50 | [50] | |
| Weight (kg) adults ≥60 years | 78.35 | 64.8–90.5 | [51] |
| Baseline QALY value | 0.84 | ||
| Utility weights | |||
| Bacteraemia | 0.985 | 0.015 | [52–56] |
| Intra-abdominal infection | 0.518 | 0.179 | [57–62] |
| Pneumonia (non-VAP) | 0.969 | 0.046 | [52,54,63,64] |
| Ventilator-associated pneumonia (VAP) | 0.875 | 0.064 | [65] |
| Urinary tract infection (UTI) | 0.807 | 0.086 | [57,66–68] |
Values are weighted means for those aged 45–64 years and 65–84 years.
Estimated for all non-neonatal, non-material discharges.
Estimated using the following International Classification of Diseases, 9th Revision (ICD-9) codes: 790•7 for bacteraemia; 540•0 for intra-abdominal infection; 482•0 for pneumonia (non-VAP); 997•31 for ventilator-associated pneumonia; and 599•0 for urinary tract infection.
Values are 10–90% range.
Drug treatment therapies and dosing followed Micromedex [7], supplemented with expert opinion. We used meropenem as the carbapenem of choice. For each of the other drug classes, if more than one drug could be used, we modelled the drug with the lowest cost per day to remain conservative. Additionally, to represent the high variability in drug options given to patients for each therapy type, we assumed an equal probability of treatment for each of the various drugs to model the average across all the possible combinations. Those receiving monotherapy had an equal probability of being treated with aminoglycoside, tigecycline, meropenem, or colistin. Those receiving a carbapenem-containing combination therapy had an equal probability of being treated with meropenem plus one of the following: tigecycline, aminoglycoside, or colistin. Patients with a non-carbapenem-containing combination therapy had an equal probability of being treated with tigecycline plus amikacin, tigecycline plus gentamicin, tigecycline plus colistin, amikacin plus colistin, or gentamicin plus colistin. Any patient receiving tigecycline received a loading dose, per clinical standards. Treatment durations are listed in Table 1. Aminoglycoside dosing was based on once-daily dosing.
As the cost per bed day and hospitalization costs were derived from the Healthcare Utilization Project (HCUP) [8], they include service charges (converted to costs) associated with a patient's hospital stay, including room and board, thus capturing additional charges for the use of single-occupancy/private rooms. Additional tests and procedures were infection-specific. All bacteraemic patients had at least two blood cultures, with most (four out of five) having had three. Those with complicated intra-abdominal infections received two wound cultures, one blood culture, and two abdominal CT scans. Non-VAP patients had one sputum culture, one blood culture, and two chest x-rays, while VAP patients received two blood cultures, one bronchoscopy, two sputum cultures, and a daily chest x-ray. All patients with a UTI got at least one urine culture and one urine analysis, while a few (one in three) got two of each.
The hospital perspective measured illness costs in lost bed days (i.e. additional length of stay (LOS) attributable to CRE infection) derived from the infection-specific attributable LOS and cost per bed day, either ICU or general ward (depending on patient location). This represents the opportunity cost of lost bed days because of additional LOS attributable to CRE following a method described by Graves [9]. The third-party payer perspective included direct costs (e.g. hospitalization, drug treatments, and associated tests). The social perspective included direct and indirect (i.e. productivity losses due to absenteeism and mortality) costs. Hourly wages for all occupations in the USA [10] were used as a proxy for productivity losses. Productivity losses for mortality resulted in the net present value of missed lifetime earnings based on the yearly annual wage [10] and years of life lost based on that patient's life expectancy [11]. Given the older age of persons with CRE infection, we assumed patients with CRE infection to be ≥60 years old. All input parameters were age-specific when applicable. All costs were discounted to 2016 $US using a 3% discount rate.
Health effects were measured in quality-adjusted life years (QALYs), and we calculated the QALYs lost due to CRE infection (i.e. accounting only for reductions in health effects because of illness and/or death). Each CRE infection case accrued QALY values based on their age-dependent healthy QALY value attenuated by the infection-specific utility weight for the duration of their infection. Death resulted in the loss of their discounted lifetime QALY values for the remainder of their life expectancy.
Simulations and scenarios
For each scenario, we ran Monte Carlo simulations consisting of 1000 trials varying the distributions throughout their ranges. All results reported are median and 95% credibility ranges (95% CR). Sensitivity analysis varied the attributable mortality of CRE (26-44% [12]) and the carbapenem drug used, that is the cost of treatment with carbapenem (meropenem ($134 per day) versus imipenem/cilastatin ($245 per day) [13]). We also evaluated the impact of drug treatment options with a scenario that uses only colistin for those receiving monotherapy and colistin plus meropenem for those receiving a carbapenem-containing combination therapy. Additional scenarios varied projected CRE incidences in the USA (2.93—15.0 per 100 000), given the rapidly expanding epidemiology.
Results
Cost per CRE infection
The cost of a single CRE infection (regardless of infection type and assuming meropenem was the primary carbapenem used) was $29 157 (95% CR $22 993-$35 503) from the hospital perspective and $15 647 (95% CR $13 701-$18 286) from the third-party payer perspective. Cost from the societal perspective varied with the attributable mortality, ranging from $58 692 (95% CR $32 155-$169 153) to $86 940 (95% CR $43 961-$256 870). Most societal costs were made up of productivity losses due to mortality (approximately 70–79%, depending on the attributable mortality), followed by direct medical costs. CRE infection resulted in the loss of 1 QALY. Increasing the costs of carbapenem therapies to $245 per day (assuming imipenem/cilastatin use) only increased third-party payer costs by a relative 5% ($16 480 vs. $15 647) and societal costs by a relative 0% ($87 059 vs. $86 940) to 2% ($59 671 vs. $58 692), depending on the attributable mortality. Assuming colistin as monotherapy and colistin plus meropenem as the carbapenem-containing combination therapy, did not substantially change the cost of a CRE infection (decreased by a relative 1%). Third-party payer costs decreased to $15 488 (95% CR $13 924-$16,785), while the decrease in the cost to society ranged from $58 026 (95% CR $32 044-$169 890) to $86 484 (95% CR $42 495-$279 174), depending on the attributable mortality.
Table 2 shows the cost of a single CRE infection by infection type from each perspective assuming the use of meropenem. From the hospital perspective, intra-abdominal infections were the most costly, while pneumonia was the most costly from the third-party payer (VAP) and societal (non-VAP) perspectives. Again, most of the productivity losses incurred resulted from mortality.
Table 2. Economic burden (($US, median (95% credibility range)) and QALYs lost because of CRE infection by type of infection assuming meropenem use for carbapenem therapies.
| Bacteraemia | Intra-abdominal infection | Non-VAP | VAP | UTI | |
|---|---|---|---|---|---|
| Attributable mortality 26% | |||||
| Hospital perspective | 35 532 (32 516–39 280) | 65 815 (51 794–81 005) | 26 336 (14 646–38 473) | 45 359 (36 682–54 235) | 22 678 (14 680–31 068) |
| Third-party payer perspective | 16 509 (14 378–18 948) | 18 924 (16 585–22 472) | 23 724 (19 625–28 520) | 31 622 (26 577–36844) | 10 543 (8967–12 564) |
| Productivity losses because of absenteeism | 1354 (619–5526) | 2454 (1059–10 172) | 930 (342–4374) | 1688 (754–6929) | 853 (302–3606) |
| Productivity losses because of death | 48 106 (17 523–168 916) | 33 337 (12 144–117 060) | 42 507 (15 484–149 258) | 2599 (947–9126) | 36 875 (13 432–129 482) |
| Societal perspective | 66 363 (35 558–185 503) | 55 634 (33 184–139 461) | 67 427 (40 422–175 054) | 36 706 (30 357–45 637) | 48 519 (24 823–140 346) |
| QALYs lost | 1 (1–2) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 1 (1–1) |
| Attributable mortality 35% | |||||
| Hospital perspective | 35 980 (32 525–39 089) | 65 663 (51 802–80 719) | 26 190 (14 672–38 622) | 45 694 (36 630–54 382) | 22 569 (14 640–30 822) |
| Third-party payer perspective | 16 365 (14 455–19 023) | 18 844 (16 573–22 515) | 23 669 (19 567–28 294) | 31 565 (26 503–37 222) | 10 485 (8959–12 617) |
| Productivity losses because of absenteeism | 1363 (630–5136) | 2520 (1059–9702) | 973 (321–4396) | 1760 (739–6671) | 843 (308–3120) |
| Productivity losses because of death | 66 269 (25 421–237 397) | 45 925 (17 617–164 518) | 58 557 (22 462–209 769) | 3580 (1373–12 826) | 50 798 (19 486–181 976) |
| Societal perspective | 84 795 (43 018–256 352) | 68 643 (38 972–187 163) | 83 545 (46 510–232 828) | 38 105 (31 030–48 168) | 62 720 (30 714–194 194) |
| QALYs lost | 1 (1–2) | 1 (1–1) | 1 (1–2) | 0 (0–0) | 1 (1–2) |
| Attributable mortality 44% | |||||
| Hospital perspective | 35 321 (32 558–39 236) | 65 896 (51 443–80 403) | 26 336 (14 631–38 511) | 45 782 (36 677–54 405) | 22 622 (14 584–31 150) |
| Third-party payer perspective | 16 594 (14 403–19 009) | 19 053 (16 613–22 564) | 23 722 (19 617–28 234) | 31 813 (26 282–37 351) | 10 628 (8937–12 629) |
| Productivity losses because of absenteeism | 1383 (621–4878) | 2584 (1058–9402) | 1025 (310–4006) | 1771 (756–6424) | 870 (310–3178) |
| Productivity losses because of death | 78 320 (28 780–309 447) | 54 276 (19 945–214 449) | 69 205 (25 431–27 3434) | 4231 (1555–16 719) | 60 036 (22 061–237 205) |
| Societal perspective | 97 288 (47 215–327 373) | 77 281 (42 532–23 6905) | 95 765 (50 771–297 385) | 39 103 (31 328–51 935) | 71 944 (33 921–248 597) |
| QALYs lost | 2 (1–3) | 1 (1–2) | 2 (1–2) | 0 (0–0) | 1 (1–2) |
Cost of CRE infection in the USA
Between 2012 and 2013, the Multi-site Gram-Negative Surveillance Initiative (MuGSI) estimates a CRE infection incidence of 2.93 per 100 000 persons (based on US census estimates of the surveillance area population) [5]. Extrapolating this to the entire US population (an estimated 321 418 820 in 2015 [14]) and assuming CRE incidence has not changed, would equate to 9418 CRE infections in the USA in 2015. Table 3 reports the number of CRE infections, deaths, and economic burden for varying incidence rates in the USA, assuming meropenem use and a 26% attributable mortality. Assuming an incidence of 2.93 per 100 000 persons, these infections would cost hospitals $275 million (95% CR $217-$334 million) and third-party payers $147 million (95% CR $129-$172 million). The number of deaths, cost to society, and QALYs lost vary with CRE's attributable mortality. With a 35% attributable mortality applied to incidence rates of 2.93 to 15.0 per 100 000 population, CRE infection resulted in 1131–5790 deaths and societal costs of $681 million (95% CR $356-$2060 million) to $3489 million (95% CR $1822-$10 547 million), and 11 835 (95% CR 7764 16-648) to 60 590 (95% CR 39 745 to 85 230) QALYs lost. An attributable mortality of 44% would increase the number of CRE infection deaths from 1422 to 7279, and cost $819 million (95% CR $414-$2419 million) to $4.2 billion (95% CR $2.1-$12.4 billion) from the societal perspective, and results in 14 825 (95% CR 9717-20 899) to 75 898 (95% CR 49 746-106 993) QALYs lost (varying with incidence rates 2.93 to 10.0 per 100 000 population).
Table 3. Epidemiologic and economic burden (median (95% credibility range), costs in millions ($US)) of CRE infections in the United States assuming a 26% attributable mortality and meropenem use for carbapenem therapies.
| CRE incidence per 100 000 | ||||
|---|---|---|---|---|
|
|
||||
| 2.93 | 6 | 10 | 15 | |
| Infections | 9418 | 19 285 | 32 142 | 48 213 |
| Deaths | 840 | 1721 | 2868 | 4301 |
| QALYs lost | 8841 (5805–12 420) | 18 105 (11 888–25 433) | 30 174 (19 814–42 388) | 45 261 (29 720–63 581) |
| Hospital perspective | 275 (217–334) | 562 (443–685) | 937 (739–1141) | 1406 (1109–1712) |
| Third-party payer perspective | 147 (129–172) | 302 (264–353) | 503 (440–588) | 754 (661–882) |
| Productivity losses because of absenteeism | 11 (5–46) | 22 (9–94) | 37 (16–157) | 56 (24–236) |
| Productivity losses because of death | 389 (149–1407) | 796 (306–2882) | 1327 (509–4803) | 1990 (764–7205) |
| Societal perspective | 553 (303–1593) | 1132 (620–3262) | 1886 (1034–5437) | 2830 (1550–8155) |
Assuming imipenem/cilastatin use for carbapenem therapies and a 26% attributable mortality, 9418 CRE infections (incidence of 2.93 per 100 000 population) in the USA cost $155 million (95% CR $131-$172 million) to third-party payers and $562 million (95% CR $301-$1554 million) to society. An incidence of 10.0 per 100 000 population (32 142 infections) would cost third-party payers $530 million (95% CR $447-$588 million) and society $1.9 billion (95% CR $1.0-$5.3 billion) assuming imipenem/cil-astatin use. Thus, resulting cost differences compared with meropenem (Table 2) are minimal.
Discussion
Our study demonstrates that a single CRE infection can cost hospitals up to $66 031, third-party payers up to $31 621, and society up to $83 512, depending on infection type. CRE infections in the USA (incidence of 2.93 per 100 000 persons) cost hospitals $275 million, third-party payers $147 million, and society $553 million (26% attributable mortality). However, the rising incidence of CRE suggests a high risk for a rapidly escalating economic burden of CRE infection into billions of dollars ($1.1-$2.8 billion in societal costs for CRE infection incidence 6.0–15.0 per 100 000 persons). Sensitivity analyses showed minimal impact of carbapenem choice (from $134 up to $245 per day) and other drug treatment options (use of colistin for monotherapy and in carbapenem-containing combination therapy), while societal costs varied greatly with attributable CRE mortality. Thus, attributable mortality is a key driver of societal costs and QALYs lost. For every 9% increase in attributable mortality, there is a 20–35% relative increase in CRE deaths, an approximate 21% relative increase in societal costs, and 25% relative increase in QALYs lost. Productivity losses because of mortality make up 70–79% of societal costs for any CRE infection, but this varies by infection type and attributable mortality, ranging from 7.1% (VAP, 26% attributable mortality) to 83.4% (UTI, 44% attributable mortality). Therefore, averting CRE deaths should be a focus of infection control and prevention and the development of potential new drugs or a vaccine.
Quantifying and having a better understanding of the economic burden of CRE infections can assist various decision makers. For example, hospital administrators, infection control practitioners, and policy makers could use this information to determine investment in CRE prevention and control measures. This information can also help policy makers and third-party payers make insurance coverage and reimbursement decisions. Additionally, manufacturers and drug companies can use such information to develop and price CRE tests and treatments.
As the prevalence of CRE has been steadily on the rise [2–4,6], now is an important time to consider investment for infection control and prevention measures for CRE. Reducing the number of CRE carriers not only results in epidemiological benefits of fewer transmission events and subsequent infections, but would also result in substantial reductions in the economic and health burdens. Coordinated approaches to control should be considered, as the gains achieved (i.e. reductions in CRE prevalence) by coordinated approaches are better than those seen when healthcare facilities act alone [15]. Such regional approaches to primary prevention strategies to limit CRE spread could lead to reductions in the cost of CRE infection and may represent a strategy with potential for cost-savings or a return on investment, garnering benefits to all.
It should be noted that our resulting costs to the hospital perspective are consistently higher than costs to third-party payers as they are based on attributable LOS, while the third-party costs are based on hospitalization cost data for the infection outcome and may not reflect only those caused by CRE. Thus, our costs to third-party payers may be underestimates. Additionally, our economic burden estimates of CRE infections in the USA are most likely an underestimate as surveillance for CRE is not routinely in place in the USA. The incidence estimates based on the MuGSI study are limited (based on data from seven metropolitan areas [5]) and thus may not provide an adequate picture of real world incidence across the USA. These estimates may change as more data become available.
Compared with other infection and health conditions, the cost of CRE infection and other healthcare-associated infections (e.g. Acinetobacter baumannii [16,17], norovirus [18], vancomycin-resistant enterococcal (VRE) bloodstream infections [19], methicillin-resistant Staphylococcus aureus (MRSA) [20], and healthcare-acquired Clostridium difficile infection (CDI) [21]) show that infection control and prevention needs more attention. CRE infections are more costly than episodes of other infectious diseases. For example, in 2016 values, the cost of one influenza case is an estimated $2807 to $8889 to society [22]; the societal costs of pertussis in adolescents and adults is an estimated $600 to $1169 [23]; Lyme disease cost societyan estimated $451 per case [24]; and food-borne illness because of salmonella cost society $3899 per case [25]. CRE infections are also more costly compared with the annual costs of some chronic diseases. For example, high blood pressure cost society $672 annually per person [26], asthma cost $4008 per case in direct costs [27], and diagnosed diabetes cost $13 015 annually per person [28] (all in 2016 values). While similar methodologies were used to estimate costs for these conditions, care should be taken when making comparisons. Although our study focuses on the cost of CRE infection in the USA, our results can be used in the context of other countries; and while the estimated value of cost per infection may be different, the magnitude would be similar. Cost estimates would be more comparable for countries that have similar standards of care and economic indicators (e.g. GNI) and less comparable for countries that are less similar.
Our model attempted to be conservative about the costs and health effects of CRE infections. While our attributable LOS estimates are based at the higher end of those reported in the literature for each outcome, we did not directly adjust for carbapenem resistance. Therefore, these estimates may underestimate the true attributable LOS of CRE infection, making our results from the hospitals perspective conservative. Additionally, our antibiotic treatment selection does not include estimates for uptake of novel anti-CRE antibiotics like ceftazidime-avibactam or other drugs in development. These novel antibiotics will likely sell for a premium over the values used in our model, thus our estimates for costs may be artificially low. For example, ceftazidime-avibactam costs approximately $300 a day [13], which would increase the estimated costs. Although the standard of care for CRE infections may also require use of single rooms or cohorting, we did not include potential costs of transferring patients in and out of private rooms based on isolation needs. However, this issue may become more obsolete in the near future as hospitals move toward the use of single-occupancy rooms.
All models, by definition, are simplifications of reality [29] and therefore cannot account for every possible event or outcome. We did not consider any costs that may be incurred outside the duration of hospitalization (e.g. continued treatment or home health care). We used utility values associated with each outcome, but they are not CRE specific nor did we adjust for CRE which may lead to more severe outcomes. Our model was designed to calculate the economic costof CRE infection from the hospital perspective, rather than the financial cost to the hospital, thus we did not consider hospital occupancy rates. Additionally, as we modelled only the duration of hospitalization for each infection, we did not consider the potential for resistance to colistin or tigecycline or further resistance to carbapenems. Our model drew from literature of varying quality and across multiple geographic locations because of disparate sources; thus, results may change as better data become available.
Conclusions
The cost of CRE infection is higher than the annual cost of many chronic diseases and of many acute diseases. Costs rise proportionally with the incidence of CRE infection, increasing by 2.0 times, 3.4 times, and 5.1 times for incidence rates of 6, 10, and 15 per 100 000 persons. Given the increasing trends in CRE prevalence in the USA, prevention and control strategies should be considered now, before becoming endemic.
Acknowledgments
This work was supported by the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Office of Behavioral and Social Sciences Research (OBSSR) and the Global Obesity Prevention Center (GOPC) via grant U54HD070725, and NICHD via grant U01 HD086861. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Transparency declaration: The authors declare no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. Atlanta, GA: Centers for Disease Control and Prevention 2013; 2013. [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Vital signs: Carbapenem-resistant enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Guh AY, Limbago B, Kallen AJ. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti Infect Ther. 2014;12:565–80. doi: 10.1586/14787210.2014.902306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thaden JT, Lewis SS, Hazen KC, Huslage K, Fowler VG, Jr, Moehring RW, et al. Rising rates of carbapenem-resistant enterobacteriaceae in community hospitals: a mixed-methods review of epidemology and microbiology practices in a network of community hospitals in the southeastern United States. Infect Control Hosp Epidemiol. 2014;35:978–83. doi: 10.1086/677157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh AY, Bulens SN, Mu Y, Jacob JT, Reno J, Scott J, et al. Epidemiology of carbapenem-resistant enterobacteriaceae in 7 US communities, 2012–2013. JAMA. 2015;314:1479–87. doi: 10.1001/jama.2015.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Healthcare associated infections. Atlanta, GA: Tracking CRE; 2015. http://www.cdc.gov/hai/organisms/cre/TrackingCRE.html. [Google Scholar]
- 7.Truven Health Analytics. Mircromedex 2.0 (electronic version) Greenwood Village, Colorado, USA: Truven Health Analytics; 2015. [Google Scholar]
- 8.United States Department of Health & Human Services. HCUP facts and figures: Statistics on hospital-based care in the United States. Rockville, MD: AHRQ: Agency for Healthcare Research and Quality; 2012. http://hcupnet.ahrq.gov/HCUPnet.jsp. [Google Scholar]
- 9.Graves N. Economics and preventing hospital-acquired infection. Emerg Infect Dis. 2004;10:561–6. doi: 10.3201/eid1004.020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bureau of Labor Statistics. Occupational employment statistics: May 2014 national occupational employment and wage estimates, United States. Washington, DC: U.S.: Bureau of Labor Statistics Division of Occupational Employment Statistics; 2014. http://www.bls.gov/oes/current/oes_nat.htm#29–0000. [Google Scholar]
- 11.Wilmoth J, Shkolnikov V. Human mortality database. University of California, Berkeley (USA), and Max Planck Institute for Demographic Reseach (Germany) 2010 [Google Scholar]
- 12.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant enterobacteriaceae infections. Emerg Infect Dis. 2014;20:1170–5. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truven Health Analytics. Red book online. Greenwood Village, Colorado, USA: Truven Heatlh Analytics; 2015. [Google Scholar]
- 14.U.S. Census Bureau. Annual estimates of the resident population: April 1, 2010 to July 1, 2015. Washington, DC USA: 2015. Population Division. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=PEP_2015_PEPANNRES&src=pt. [Google Scholar]
- 15.Lee BY, Bartsch SM, Wong KF, McKinnell JA, Slayton RB, Miller LG, et al. The potential trajectory of carbapenem-resistant Enterobacteriaceae, an emerging threat to health-care facilities, and the impact of the Centers for Disease Control and Prevention toolkit. Am J Epidemiol. 2016;183:471–9. doi: 10.1093/aje/kwv299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BY, McGlone SM, Doi Y, Bailey RR, Harrison LH. Economic impact of Acinetobacter baumannii infection in the intensive care unit. Infect Control Hosp Epidemiol. 2010;31:1087–9. doi: 10.1086/656378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson RE, Schweizer ML, Perencevich EN, Nelson SD, Khader K, Chiang HY, et al. Costs and mortality associated with multidrug-resistant healthcare-associated Acinetobacter infections. Infect Control Hosp Epidemiol. 2016;37(10):1212–8. doi: 10.1017/ice.2016.145. [DOI] [PubMed] [Google Scholar]
- 18.Lee BY, Wettstein ZS, McGlone SM, Bailey RR, Umscheid CA, Smith KJ, et al. Economic value of norovirus outbreak control measures in healthcare settings. Clin Microbiol Infect. 2011;17:640–6. doi: 10.1111/j.1469-0691.2010.03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler AM, Olsen MA, Guth RM, Woeltje KF, Camins BC, Fraser VJ. Attributable costs of enterococcal bloodstream infections in a nonsurgical hospital cohort. Infect Control Hosp Epidemiol. 2010;31:28–35. doi: 10.1086/649020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodise TP, McKinnon PS. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2005;52:113–22. doi: 10.1016/j.diagmicrobio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.McGlone SM, Bailey RR, Zimmer SM, Popovich MJ, Tian Y, Ufberg P, et al. The economic burden of Clostridium difficile. Clin Microbiol Infect. 2012;18:282–9. doi: 10.1111/j.1469-0691.2011.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molinari NAM, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the us: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Lee GM, Lett S, Schauer S, LeBaron C, Murphy TV, Rusinak D, et al. Massachusetts Pertussis Study Group. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis. 2004;39:1572–80. doi: 10.1086/425006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Meltzer MI, Pena CA, Hopkins AB, Wroth L, Fix AD. Economic impact of Lyme disease. Emerg Infect Dis. 2006;12:653–60. doi: 10.3201/eid1204.050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman S, Maculloch B, Batz MB. Economic burden of major foodborne illnesses aquired in the United States. U.S: Department of Agriculture Economic Research Service; 2015. [Google Scholar]
- 26.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 27.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Dall TM, Zhang Y, Chen YJ, Quick WW, Yang WG, Fogli J. The economic burden of diabetes. Health Affairs. 2010;29:1–7. doi: 10.1377/hlthaff.2009.0155. [DOI] [PubMed] [Google Scholar]
- 29.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clin Infect Dis. 2008;46:1139–41. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 30.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–71. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 31.Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 32.American Medical Association. CPT code/relative value search. Chicago, IL: 2015. [Google Scholar]
- 33.Centers for Medicare & Medicaid Services. 2015 Clinical Diagnostic Laboratory Fee Schedule (CLAB) Baltimore, MD: U.S. Department of Health & Human Services; 2015. http://www.cms.gov/ClinicalLabFeeSched/ [Google Scholar]
- 34.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 35.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–33. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19:E23–30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 37.Marquez P, Terashita D, Dassey D, Mascola L. Population-based incidence of carbapenem-resistant Klebsiella pneumoniae along the continuum of care, Los Angeles county. Infect Control Hosp Epidemiol. 2013;34:144–50. doi: 10.1086/669087. [DOI] [PubMed] [Google Scholar]
- 38.Borer A, Saidel-Odes L, Eskira S, Nativ R, Riesenberg K, Livshiz-Riven I, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012;40(5):421–5. doi: 10.1016/j.ajic.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Falagas ME, Rafailidis PI, Kofteridis D, Virtzili S, Chelvatzoglou FC, Papaioannou V, et al. Risk factors of carbapenem-resistant klebsiella pneumoniae infections: a matched case control study. J Antimicrob Chemother. 2007;60:1124–30. doi: 10.1093/jac/dkm356. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick M, Zembower T, Malczynski M, Qi C, Bolon M. Outcomes of enhanced surveillance program for carbapenem-resistant enterobacteriaceae. Infect Control Hosp Epidemiol. 2014;35:419–22. doi: 10.1086/675595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58:654–63. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing enterobacteriaceae. Clin Microbiol Infect. 2014;20:862–72. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 43.Eber MR, Laxminarayan R, Perencevich EN, Malani A. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med. 2010;170:347–53. doi: 10.1001/archinternmed.2009.509. [DOI] [PubMed] [Google Scholar]
- 44.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–93. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 45.Melsen WG, Rovers MM, Groenwold RH, Bergmans DC, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–71. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 46.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51(Suppl. 1):S120–5. doi: 10.1086/653060. [DOI] [PubMed] [Google Scholar]
- 47.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–21. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 48.Restrepo MI, Anzueto A, Arroliga AC, Afessa B, Atkinson MJ, Ho NJ, et al. Economic burden of ventilator-associated pneumonia based on total resource utilization. Infect Control Hosp Epidemiol. 2010;31:509–15. doi: 10.1086/651669. [DOI] [PubMed] [Google Scholar]
- 49.Yi SH, Baggs J, Gould CV, Scott RD, 2nd, Jernigan JA. Medicare reimbursement attributable to catheter-associated urinary tract infection in the inpatient setting: a retrospective cohort analysis. Med Care. 2014;52:469–78. doi: 10.1097/MLR.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jernigan JA, Clemence MA, Stott GA, Titus MG, Alexander CH, Palumbo CM, et al. Control of methicillin-resistant Staphylococcus aureus at a university hospital: one decade later. Infect Control Hosp Epidemiol. 1995;16:686–96. doi: 10.1086/647042. [DOI] [PubMed] [Google Scholar]
- 51.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 52.Kulpeng W, Leelahavarong P, Rattanavipapong W, Sornsrivichai V, Baggett HC, Meeyai A, et al. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31:2839–47. doi: 10.1016/j.vaccine.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diez-Domingo J, Ridao-Lopez M, Gutierrez-Gimeno MV, Puig-Barbera J, Lluch-Rodrigo JA, Pastor-Villalba E. Pharmacoeconomic assessment of implementing a universal PCV-13 vaccination programme in the Valencian public health system (Spain) Vaccine. 2011;29:9640–8. doi: 10.1016/j.vaccine.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 54.Tyo KR, Rosen MM, Zeng W, Yap M, Pwee KH, Ang LW, et al. Cost-effectiveness of conjugate pneumococcal vaccination in Singapore: comparing estimates for 7-valent, 10-valent, and 13-valent vaccines. Vaccine. 2011;29:6686–94. doi: 10.1016/j.vaccine.2011.06.091. [DOI] [PubMed] [Google Scholar]
- 55.Rozenbaum MH, Sanders EA, van Hoek AJ, Jansen AG, van der Ende A, van den Dobbelsteen G, et al. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ. 2010;340:c2509. doi: 10.1136/bmj.c2509. [DOI] [PubMed] [Google Scholar]
- 56.Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22:4203–14. doi: 10.1016/j.vaccine.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Lee BY, Bailey RR, Smith KJ, Muder RR, Strotmeyer ES, Lewis GJ, et al. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infec Control Hosp Epidemiol. 2010;31:598–606. doi: 10.1086/652524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Y, Tai JH, Bartsch SM, Zimmerman RK, Muder RR, Lee BY. The potential economic value of a Staphylococcus aureus vaccine among hemodialysis patients. Vaccine. 2012;30:3675–82. doi: 10.1016/j.vaccine.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevenson SM, Danzig MR, Ghandour RA, Deibert CM, Decastro GJ, Benson MC, et al. Cost-effectiveness of neoadjuvant chemotherapy before radical cystectomy for muscle-invasive bladder cancer. Urol Oncol. 2014;32:1172–7. doi: 10.1016/j.urolonc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Wong C, Luk IW, Ip M, You JH. Prevention of gram-positive infections in peritoneal dialysis patients in hong kong: a cost-effectiveness analysis. Am J Infect Control. 2014;42:412–6. doi: 10.1016/j.ajic.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu U, Saito S, Lings Y, Iino N, Kazama JJ, Akazawa K. Cost-effectiveness achieved through changing the composition of renal replacement therapy in Japan. J Med Econ. 2012;15:444–53. doi: 10.3111/13696998.2011.653512. [DOI] [PubMed] [Google Scholar]
- 62.Guest JF, Watson HG, Limaye S. Modeling the cost-effectiveness of pro-thrombin complex concentrate compared with fresh frozen plasma in emergency warfarin reversal in the United Kingdom. Clin Ther. 2010;32:2478–93. doi: 10.1016/j.clinthera.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Marti SG, Colantonio L, Bardach A, Galante J, Lopez A, Caporale J, et al. A cost-effectiveness analysis of a 10-valent pneumococcal conjugate vaccine in children in six Latin American countries. Cost Eff Resour Alloc. 2013;11:21. doi: 10.1186/1478-7547-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bos JM, Rumke H, Welte R, Postma MJ. Epidemiologic impact and cost-effectiveness of universal infant vaccination with a 7-valent conjugated pneumococcal vaccine in The Netherlands. Clin Ther. 2003;25:2614–30. doi: 10.1016/s0149-2918(03)80322-3. [DOI] [PubMed] [Google Scholar]
- 65.Shorr AF, Susla GM, Kollef MH. Linezolid for treatment of ventilator-associated pneumonia: a cost-effective alternative to vancomycin. Crit Care Med. 2004;32:137–43. doi: 10.1097/01.CCM.0000104110.74657.25. [DOI] [PubMed] [Google Scholar]
- 66.Smith KJ, Cook RL, Roberts MS. Time from sexually transmitted infection acquisition to pelvic inflammatory disease development: influence on the cost-effectiveness of different screening intervals. Value Health. 2007;10:358–66. doi: 10.1111/j.1524-4733.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 67.Armstrong N, Vale L, Deverill M, Nabi G, McClinton S, N'Dow J, et al. BPE Study Group. Surgical treatments for men with benign prostatic enlargement: cost effectiveness study. BMJ. 2009;338:b1288. doi: 10.1136/bmj.b1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bermingham SL, Hodgkinson S, Wright S, Hayter E, Spinks J, Pellowe C. Intermittent self catheterisation with hydrophilic, gel reservoir, and non-coated catheters: a systematic review and cost effectiveness analysis. BMJ. 2013;346:e8639. doi: 10.1136/bmj.e8639. [DOI] [PMC free article] [PubMed] [Google Scholar]