Abstract
This study seeks to ascertain whether a culturally tailored art calendar could improve participation in cancer screening activities. We conducted a randomized, controlled calendar mail-out in which a Native art calendar was sent by first class mail to 5,633 patients seen at an urban American Indian clinic during the prior 2 years. Using random assignment, half of the patients were mailed a “message” calendar with screening information and reminders on breast, colorectal, lung, and prostate cancer; the other half received a calendar without messages. The receipt of cancer screening services was ascertained through chart abstraction in the following 15 months. In total, 5,363 observations (health messages n=2,695; no messages n=2,668) were analyzed. The calendar with health messages did not result in increased receipt of any cancer-related prevention outcome compared to the calendar without health messages. We solicited clinic input to create a culturally appropriate visual intervention to increase cancer screening in a vulnerable, underserved urban population. Our results suggest that printed materials with health messages are likely too weak an intervention to produce the desired behavioral outcomes in cancer screening.
Keywords: Cancer, Prevention, Screening, American Indians, Health disparities, Randomized, Controlled trial
Introduction
Despite their benefit to individuals and to our cost-conscious society, preventive services are still underused, especially among older, poorer, less-educated, and non-White populations [1]. For American Indians and Alaska Natives, access to optimal preventive services may be limited by their insurance status, the type of settings in which they receive care, the high turnover of providers in the Indian Health Service, and overburdened physicians’ lack of time [2]. Further, more than half of Native people live in urban or non-reservation areas [3], removed from Indian Health Service facilities. As a result, American Indian peoples experience the worst cancer-related health disparities of any minority, after controlling for socioeconomic status, lack of health-encouraging behaviors such as cancer screening, and lack of high-quality facilities and consistent care [4]. Cancer mortality, specifically breast, colorectal, lung, and prostate mortality, is increasing among American Indian and Alaska Native peoples, and has become the second leading cause of death in this population despite the decline in overall rates for the USA [5].
Data from the Washington State Cancer Registry indicate that the highest-incidence cancers in American Indians and Alaska Natives in Washington State are breast (female), prostate (male), lung and bronchus, and colorectal [6]. With the exception of lung cancer, screening procedures are recommended for these cancers: mammography for breast cancer, prostate specific antigen (although controversial) for prostate cancer, and fecal occult blood testing or colonoscopy for colorectal cancer. Risk factors for these cancers can also be modified through education and behavior change, such as smoking cessation. Unfortunately, little information is available about the use of most cancer screening services among American Indian and Alaska Native people [7]. Moreover, few randomized, controlled trials have been conducted to increase preventive cancer care in this vulnerable group [8, 9].
We collaborated with an urban American Indian health facility to design and implement a randomized, controlled calendar mail-out to increase cancer screening services in an urban population of American Indians and Alaska Natives [10]. We tested the hypothesis that brief messages communicating specific cancer facts and action items pertinent to American Indian peoples, presented in the format of a Native art calendar, could increase receipt of cancer screening services for breast, cervical, colorectal, lung, and prostate cancer.
Methods
Site
The Seattle Indian Health Board (SIHB) is a multidisciplinary, community-based organization that provides health and social services to individuals from more than 200 tribes and other low-income residents living in the Pacific Northwest [11]. As the major source of healthcare for the urban King County Native population, the SIHB employs seven clinicians and serves more than 6,000 persons who make more than 40,000 visits per year. Over half the patients are at least 45 years old, approximately 50% are unemployed, 58% lack health insurance, and 80% have incomes that fall under the federal poverty line [11].
Intervention Development
The Partnerships for Native Health at the University of Washington and the SIHB have worked together for more than 15 years. The university-based Principal Investigator obtained funding for a study to improve preventive care at the SIHB. To modify and refine the study, we convened a group consisting of SIHB staff members and the Executive Director, along with University of Washington faculty, students, and staff. The group helped design the study, reviewed the health topics, chose appropriate Native artwork, and edited and refined the messages. Because visual means of communication are important in Native culture [12] and because a prior Native art calendar had been popular at the SIHB, the group felt that a Native art calendar would be a culturally sensitive and well-accepted intervention.
We prepared two versions of the calendar, one with messages and the other without. In addition to the prominent display of a unique piece of Native art each month, the message version of the calendar featured a different cancer health-related message each month. We worked with a research librarian at the University of Washington to find appropriate health and cancer statistics. The topics were chosen based on recommendations for Native populations from several national groups, such as the U.S. Preventive Task Force [13]. We then simplified these facts into short statements or bullet points for the calendar. An epidemiologist reviewed the simplified messages and corrections were made. The SIHB was presented with a compendium of these messages. Leadership and staff made further changes, which improved the readability of the material without altering its meaning. The final messages consisted of a brief statement of the problem (e.g., “Breast cancer is the second leading cause of cancer death among American Indian women”) followed by a recommendation (e.g., “Mammograms can detect early breast cancer. Ask your doctor if you need a mammogram”).
To the extent possible, topics and art were matched. The SIHB owns a large portfolio of Native art that it has collected over many decades. The images we used were drawn primarily from this collection and then matched to topic and month. For example, in the month when the calendar promoted breast cancer screening, it displayed the image of healing mother earth. Both calendars noted events at the SIHB, such as the annual health fair, and included contact information for the SIHB.
Sample
The SIHB maintains a database of medical records of patients, including name, age, sex, race, address, telephone numbers, and emergency contact information, which is updated on each patient visit. All patients over the age of 18 who accessed primary care, pharmacy, nutrition, dental, or behavioral health services at the SIHB within the previous 2 years were eligible for the study (n=5,633). Potential participants were mailed an “opt-out” letter and asked to return the letter if they did not want to participate in the study. Twenty-eight patients returned the opt-out letter. The remaining participants were randomly assigned by a computer-generated algorithm either to a group that received a “message” calendar (n=2,805) or to a group that received a calendar without messages (n=2,800). The human subjects review bodies of the University of Washington and the SIHB approved all study procedures.
Outcomes
We attempted to abstract 5,605 charts. Chart abstraction covered the calendar year plus 3 months into the next year, to allow time after the final December prevention message for recipients to request and receive cancer screening services. We were unable to review the charts of 221 patients, who were therefore lost to follow-up. Chart review included abstraction of basic information (e.g., demographics) and limited clinical information pertinent to the cancer screening procedures. For all cancer types, each outcome was coded as present or absent at baseline and at follow-up. The outcomes for this analysis were receipt of individual cancer screening procedures. Because some types of cancer have multiple screening procedures, we also calculated binary variables indicating receipt of any relevant screening procedure for each cancer type (e.g., colonoscopy for colorectal cancer).
The lung cancer outcomes that we assessed were related to smoking cessation. They included prescription for a nicotine patch or any other smoking cessation product, and either receipt of, or referral to, smoking cessation counseling. We evaluated smoking cessation outcomes for both sexes and all age categories. The breast cancer outcome was receipt of a mammogram. We evaluated breast cancer screening outcomes for women aged 40 years and older. Although no calendar messages explicitly referred to detection or prevention of colorectal or prostate cancer, we evaluated outcomes related to these cancers to determine whether participants might generalize the health messages about other cancers. The colorectal cancer outcomes assessed included fecal occult blood test or colonoscopy; an insufficient number of sigmoidoscopies were performed to include that procedure in the analyses. We evaluated colorectal cancer outcomes for men and women aged 50 years and older. The prostate cancer outcomes included receipt of a prostate specific antigen test or a digital prostate exam. We evaluated prostate cancer screening outcomes for men aged 50 years and older.
Other Variables
The predictor of interest in this analysis was randomized calendar type (with or without health messages). In addition to the cancer-related outcomes, we assessed age (18–39, 40–49, 50–64, and 65–93 years), sex, race (American Indian/Alaska Native vs. other), marital status (single, married, divorced/widowed/separated), and smoking status (current, former/never).
Statistical Analysis
We conducted final analyses for 5,363 patients. One control group participant was excluded for being younger than 18 years old; 20 additional patients (n=11 intervention and n=9 control) were excluded because of missing information for date of birth or sex. The final sample comprised 2,668 patients assigned to the control group and 2,695 patients assigned to the intervention group (see Fig. 1). We calculated percentages to describe age categories, sex, and all baseline cancer outcomes separately for each group, with chi-square p values to assess the success of our randomization process. To evaluate effects of the intervention on cancer outcomes, we calculated the percentage of patients in each group with each outcome during the 15-month follow-up period and used chi-square tests to evaluate the statistical significance of any differences between groups. For each type of cancer, we restricted analyses to the subset of patients who met age and sex guidelines for the procedure. Thus, we evaluated smoking outcomes for men and women of all ages, colorectal cancer outcomes for both sexes aged 50 years and older, breast cancer outcomes for women aged 40 years and older, and prostate cancer outcomes for men aged 50 years and older. We did not restrict smoking outcomes to current smokers because some people who were identified as former or non-smokers received smoking cessation interventions during the follow-up period.
Fig. 1.
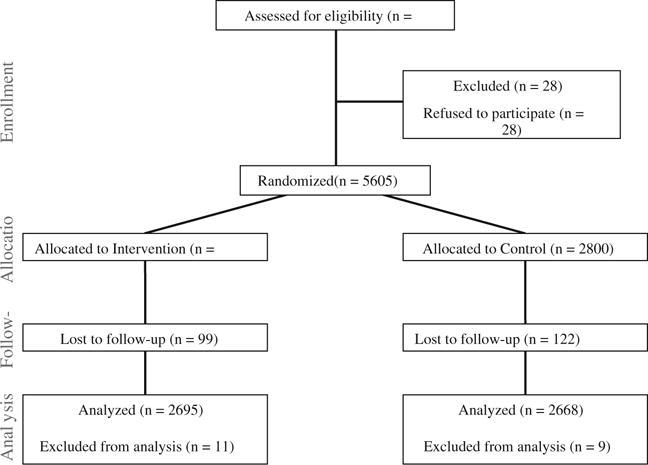
Study diagram
We did not adjust for any covariates in the inferential analysis. In addition to the overall regression models, we ran exploratory models stratifying by age category and sex, restricting the analysis to people who did not receive the relevant intervention or procedure during the baseline period, and restricting the analysis of smoking outcomes to people who were identified as current smokers at baseline. None of the exploratory analyses changed any conclusions from the primary analysis; therefore, we present only the latter in this manuscript. All analyses were conducted using Stata version 10 (StataCorp, TX). We considered an alpha error rate of 0.05 as the threshold for statistical significance.
Results
Table 1 shows baseline distributions for age, sex, and all cancer outcomes by study group. The randomization process was successful, as demonstrated by the similar distributions for all study variables between the two randomized groups. Although the age distribution was statistically significant (p=0.04), there was no indication that this was due to anything other than random chance.
Table 1.
Baseline demographic and cancer-related outcomes in the control group and the intervention group receiving calendars with health messages
| Control (%) | Health messages (%) | Chi-square p value | |
|---|---|---|---|
| Demographicsa | |||
| Age | |||
| 18–39 years | 51 | 47 | 0.04 |
| 40–49 years | 24 | 25 | |
| 50–64 years | 19 | 21 | |
| 65–93 years | 7 | 7 | |
| Sex | |||
| Female | 56 | 55 | 0.56 |
| Male | 44 | 45 | |
| Smoking status | |||
| Not currently smoking | 70 | 71 | 0.65 |
| Current smoker | 30 | 29 | |
| Outcomes | |||
| Smokingb | |||
| Nicotine patch | 1.0 | 1.0 | 0.92 |
| Other smoking treatment | 0.6 | 0.6 | 0.84 |
| Cessation counseling | 6.1 | 5.5 | 0.36 |
| Cessation counseling referral | 0.9 | 0.9 | 0.91 |
| Any smoking outcome | 7.0 | 6.3 | 0.33 |
| Colorectal cancerc | |||
| Stool occult blood | 3.1 | 2.9 | 0.81 |
| Colonoscopy | 0.7 | 0.3 | 0.20 |
| Any colorectal cancer outcome | 3.7 | 3.2 | 0.57 |
| Breast cancerd | |||
| Mammogram | 14.8 | 13.6 | 0.50 |
| Manual breast examination | 10.3 | 13.2 | 0.08 |
| Any breast cancer outcome | 19.4 | 19.4 | 0.97 |
| Prostate cancere | |||
| PSA test | 3.6 | 2.5 | 0.41 |
| Digital prostate examination | 1.9 | 2.1 | 0.86 |
| Any PSA outcome | 4.5 | 4.3 | 0.89 |
Demographics, control (%)—n=2,668 and health messages (%)—n= 2,695
Evaluated for both sexes, all age categories, and all smoking status categories due to smoking intervention outcomes in some people who did not identify as current smokers; control (%)—n=2,668 and health messages (%)—n=2,695
Evaluated for both sexes ≥50 years old; control (%)—n=677 and health messages (%)—n=763
Evaluated for women ≥40 years old; control (%)—n=708 and health messages (%)—n=772
Evaluated for men ≥50 years old; control (%)—n=310 and health messages (%)—n=327
The calendar with health messages did not result in increased receipt of any cancer-related outcome compared to the calendar without health messages (Table 2). None of the exploratory analyses changed our conclusions for any cancer outcomes.
Table 2.
Follow-up prevalence and odds ratios of cancer-related outcomes in the control group and intervention group receiving calendars with health messages
| Outcome: | Control (%) | Health messages (%) | Chi-square p value |
|---|---|---|---|
| Smokinga | |||
| Nicotine patch | 0.8 | 0.6 | 0.48 |
| Other smoking treatment | 0.3 | 0.3 | 0.99 |
| Cessation counseling | 4.5 | 4.5 | 0.99 |
| Cessation counseling referral | 0.9 | 0.7 | 0.51 |
| Any smoking outcome | 5.4 | 5.2 | 0.65 |
| Colorectal cancerb | |||
| Stool occult blood | 3.8 | 3.4 | 0.66 |
| Colonoscopy | 0.9 | 0.9 | 0.95 |
| Any colorectal cancer outcome | 4.4 | 4.1 | 0.73 |
| Breast cancerc | |||
| Mammogram | 13.6 | 14.0 | 0.81 |
| Manual breast examination | 9.2 | 11.3 | 0.19 |
| Any breast cancer outcome | 17.0 | 18.9 | 0.33 |
| Prostate cancerd | |||
| PSA test | 2.6 | 2.5 | 0.91 |
| Digital prostate examination | 1.9 | 1.5 | 0.69 |
| Any PSA outcome | 4.5 | 3.4 | 0.45 |
Evaluated for both sexes, all age categories, and all smoking status categories due to smoking intervention outcomes in some people who did not identify as current smokers; control (%)—n=2,668 and health messages (%)—n=2,695
Evaluated for both sexes ≥50 years old; control (%)—n=677 and health messages (%)—n=763
Evaluated for women ≥40 years old; control (%)—n=708 and health messages (%)—n=772
Evaluated for men ≥50 years old; control (%)—n=310 and health messages (%)—n=327
Discussion
To our knowledge, this is the largest randomized, controlled cancer screening trial conducted among American Indians and Alaska Natives. Furthermore, it assessed screening outcomes for multiple cancer types. Most previous randomized, controlled trials to encourage cancer screening among Native peoples have attempted to enhance only one type of screening. Our study relied solely on mailed materials, focused on an urban population, and included men as well as women. We found that the group that received calendars with health messages did not differ in receipt of cancer screening interventions from the group that received calendars without messages. These results differ from those found in studies of Medicare beneficiaries. In one such study, mass mailings had an effect—although small—on receipt of influenza vaccine [14]. In another, mailed reminders with tailored messages likewise had a small effect on mammography screening [15].
Studies using in-person interventions aimed at increasing cancer prevention in American Indian communities have sometimes [8] produced better results. For example, a randomized, controlled trial of personalized health education delivered by female lay health educators demonstrated that the educational program was associated with greater knowledge about cervical cancer prevention and higher proportions of Lumbee women obtaining Pap smears in the prior year [9]. Another intervention involved 400 American Indian women at urban and rural clinics in California who participated in a cervical cancer screening educational program delivered through the culturally consonant format of “talking circles.” This study did not report data on subsequent receipt of screening but did report participants’ favorable response [16]. The fact that our intervention did not produce the desired outcomes for any type of cancer suggests that printed materials with health messages are unlikely to enhance rates of cancer screening, at least in American Indian and Alaska Native populations.
We used a collaborative approach to decide on the art images, intervention vehicle (calendar), and delivery method (U.S. Postal Service). Our community partner, the SIHB, played a crucial role in selecting the health topics and Native artwork featured in the calendar. Without the participation of SIHB leadership and staff, this study might have lacked the requisite cultural sensitivity and awareness. Our partners decided to use postal mail because it was a relatively inexpensive, fast, and private [17] way to distribute the intervention. We recognize that the SIHB operates under considerable financial constraints, making other, more costly forms of communication (e.g., television and radio) impossible [18]. Our previous research suggests that it is feasible, although challenging, to use the U.S. Postal Service to reach patients seen at an urban American Indian health facility [19].
This study has several limitations. First, although we confirmed addresses, we could not verify that our intended addressees actually received calendars. Second, receipt of the calendar does not assume that it was used. Per CONSORT guidelines, [20] however, we used intention-to-treat analyses, assuming that non-receipt of mailed materials or non-use of calendars was similar across groups. Third, our results are not generalizable to other Native populations, especially in rural, reservation-based settings.
In summary, this collaboration between the University of Washington and the SIHB highlights the successful use of partnership approaches in health services research, even in randomized, controlled studies. We relied on the SIHB for culturally appropriate guidance for choosing the health topics, providing Native artwork for the calendar, obtaining patient contact information, and conducting the mailings. Future studies using mail-outs should consider attaching a pre-paid postage card asking about the health messages in the calendar. This would allow researchers verify receipt and draw attention to the screening messages. Researchers should also identify and test more powerful, yet feasible, strategies for delivering cancer prevention and other health messages to large numbers of Native people.
Acknowledgments
This effort was supported by the National Institute of Aging (P30 AG15297, S. Manson), Agency for Healthcare Research and Quality (P01 HS10854, S. Manson), National Center for Minority Health and Health Disparities (P60 MD000507, S. Manson), and by Native People for Cancer Control, a Community Networks Program of the National Cancer Institute (U01 CA114642, D. Buchwald). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest of any kind.
Contributor Information
Ardith Z. Doorenbos, School of Nursing, University of Washington, Seattle, WA 98177, USA
Clemma Jacobsen, Partnerships for Native Health, University of Washington, Seattle, WA, USA.
Rebecca Corpuz, Seattle Indian Health Board, Seattle, WA, USA.
Ralph Forquera, Seattle Indian Health Board, Seattle, WA, USA.
Dedra Buchwald, School of Medicine, University of Washington, Seattle, WA, USA.
References
- 1.Aspy CB, Mold JW, Thompson DM, Blondell RD, Landers PS, Reilly KE, Wright-Eakers L. Integrating screening and interventions for unhealthy behaviors into primary care practices. Am J Prev Med. 2008;35(5):S373. doi: 10.1016/j.amepre.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM. Surveillance for health behaviors of American Indians and Alaska Natives—findings from the behavioral risk factor surveillance system, 2000–2006. Cancer. 2008;113(5):1131–1141. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Census Bureau. The American Indian and Alaska Native population 2000: Census 2000 Brief. Washington D.C: U.S. Bureau of the Census; 2002. [Google Scholar]
- 4.Kolb B, Wallace AM, Hill D, Royce M. Disparities in cancer care among racial and ethnic minorities. Oncology. 2006;20(10):1256–1270. [PubMed] [Google Scholar]
- 5.Indian Health Service. Trends in Indian health. Rockville: U.S. Department of Health and Human Services; 2001. [Google Scholar]
- 6.Portland Area Indian Health Board. American Indian and Alaska Native cancer incidence and screening: Washington, 2001–2005. 2010 http://www.npaihb.org/images/projects_docs/NWTCCP/WashingtonAI-ANfactsheet2009.pdf Accessed 26 November 2010.
- 7.Schumacher M, Slattery M, Lanier A, Ma Khe-Ni, Edwards S, Ferucci E, Tom-Orme L. Prevalence and predictors of cancer screening among American Indian and Alaska native people: the EARTH study. Cancer Causes Control. 2008;19(7):725–737. doi: 10.1007/s10552-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz M, Kauffman R, Tatum C, Paskett E. Influence of church attendance and spirituality in a randomized controlled trial to increase mammography use among a low-income, tri-racial, rural community. J Relig Health. 2008;47(2):227–236. doi: 10.1007/s10943-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dignan MB, Robert Michielutte H, Wells B, Sharp P, Karen Blinson L, Case D, Bell R, Konen J, Davis S, McQuellon RP. Health education to increase screening for cervical cancer among Lumbee Indian Women in North Carolina. Health Educ Res. 1998;13(4):545–556. doi: 10.1093/her/13.4.545. [DOI] [PubMed] [Google Scholar]
- 10.Forquera, Ralph. The Seattle Indian Health Board. Urban Indian Health. 2010 http://www.kff.org/minorityhealth/6006-index.cfm. Accessed 8 September 2010.
- 11.Seattle Indian Health Board. Mission statement. 2010 http://www.sihb.org/. Accessed 8 September 2010.
- 12.Roppolo K. Vision, voice, and intertribal metanarrative: the American Indian visual-rhetorical tradition and Leslie Marmon Silko’s Almanac of the Dead. Am Indian Q. 2007;31(4):534–558. [Google Scholar]
- 13.U.S. Preventative Task Force. Recommendations. 2010 http://www.ahrq.gov/CLINIC/uspstfix.htm#Recommendations. Accessed 30 September 2010.
- 14.Maglione MA, Stone EG, Shekelle PG. Mass mailings have little effect on utilization of influenza vaccine among Medicare beneficiaries. Am J Prev Med. 2002;23(1):43–46. doi: 10.1016/s0749-3797(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 15.McCaul KD, Wold KS. The effects of mailed reminders and tailored messages on mammography screening. J Community Health. 2002;27(3):181–190. doi: 10.1023/a:1015249906674. [DOI] [PubMed] [Google Scholar]
- 16.Hodge FS, Fredericks L, Rodriguez B. American Indian women’s talking circle: a cervical cancer screening and prevention project. Cancer. 1996;78(7):1592–1597. [PubMed] [Google Scholar]
- 17.Dillman DA, Smyth JD, Christian LM. Internet, mail and mixed-mode surveys: the tailored design method. Wiley; New York: 2009. [Google Scholar]
- 18.Garrett SK, Thomas AP, Cicuttini F, Silagy C, Taylor HR, McNeil JJ. Community-based recruitment strategies for a longitudinal interventional study. J Clin Epidemiol. 2000;53(5):541–548. doi: 10.1016/s0895-4356(99)00153-5. [DOI] [PubMed] [Google Scholar]
- 19.Duffy D, Goldberg J, Buchwald D. Using mail to reach patients seen at an urban health care facility. J Health Care Poor Underserved. 2006;17(3):522–531. doi: 10.1353/hpu.2006.0104. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134(8):657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]


