Abstract
Background:
Hepatitis B is an immune response-mediated disease. The aim of this study was to explore the differences of ratios of T-helper (Th) 2 cells to Th1 cells and cytokine levels in acute hepatitis B (AHB) patients and chronic hepatitis B virus (HBV)-infected patients in immune-tolerance and immune-active phases.
Methods:
Thirty chronic HBV-infected patients in the immune-tolerant phase (IT group) and 50 chronic hepatitis B patients in the immune-active (clearance) phase (IC group), 32 AHB patients (AHB group), and 13 healthy individuals (HI group) were enrolled in the study. The cell proportions in peripheral blood, cytokine levels in plasma, and serum levels of HBV DNA, hepatitis B surface antigen, and hepatitis B e antigen were detected.
Results:
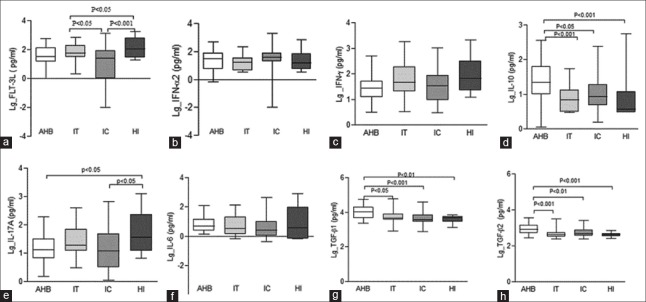
The Th1 cell percentage and Th2/Th1 ratio in the HBV infection group (including IT, IC, and AHB groups) were significantly different from those in HI group (24.10% ± 8.66% and 1.72 ± 0.61 vs. 15.16% ± 4.34% and 2.40 ± 0.74, respectively; all P < 0.001). However, there were no differences in the Th1 cell percentages and Th2/Th1 ratios among the IT, IC, and AHB groups. In HBV infection group, the median levels of Flt3 ligand (Flt3L), interferon (IFN)-γ, and interleukin (IL)-17A were significantly lower than those in HI group (29.26 pg/ml, 33.72 pg/ml, and 12.27 pg/ml vs. 108.54 pg/ml, 66.48 pg/ml, and 35.96 pg/ml, respectively; all P < 0.05). IFN-α2, IL-10, and transforming growth factor (TGF)-β2 median levels in hepatitis group (including patients in AHB and IC groups) were significantly higher than those in IT group (40.14 pg/ml, 13.58 pg/ml, and 557.41 pg/ml vs. 16.74 pg/ml, 6.80 pg/ml, and 419.01 pg/ml, respectively; all P < 0.05), while patients in hepatitis group had significant lower Flt3L level than IT patients (30.77 vs. 59.96 pg/ml, P = 0.021). Compared with IC group, patients in AHB group had significant higher median levels of IL-10, TGF-β1, and TGF-β2 (22.77 pg/ml, 10,447.00 pg/ml, and 782.28 pg/ml vs. 8.66 pg/ml, 3755.50 pg/ml, and 482.87 pg/ml, respectively; all P < 0.05).
Conclusions:
Compared with chronic HBV-infected patients in immune-tolerance phase, chronic HBV-infected patients in immune-active phase and AHB patients had similar Th2/Th1 ratios, significantly higher levels of IFN-α2, IL-10, and TGF-β. AHB patients had significantly higher IL-10 and TGF-β levels than chronic HBV-infected patients in immune-active phase.
Keywords: Acute Hepatitis B, Chronic Hepatitis B, Cytokine, Immune-tolerance, T Lymphocytes
INTRODUCTION
It is estimated that more than 90% of hepatitis B virus (HBV) infection in infants will develop chronic hepatitis,[1] whereas 90–95% of acute HBV infection occurred in adulthood will recover.[2] It is generally agreed in theory that the ineffective immune response may lead to the development of chronic HBV infection, which can be divided into four phases: immune tolerance, hepatitis B e antigen (HBeAg)-positive immune active, inactive carrier, and HBeAg-negative hepatitis. Compared with immune-tolerance patients, patients in immune-active phase or patients with acute hepatitis B (AHB) have obvious liver necroinflammation.[3,4] Numerous studies have confirmed that the severity of liver inflammation and viral clearance were closely related to specific cytotoxic T lymphocytes (CTL).[5] However, the role of CD4+ T-helper (Th) lymphocyte cells and cytokines in the clinical pathogenesis and prognosis of the disease is not very clear. The aim of this study was to explore the differences of ratios of Th2 cells to Th1 cells and cytokine levels in acute and chronic HBV-infected patients.
METHODS
Ethical approval
This study used anonymous data, and all participants agreed to provide their peripheral blood specimens for research. The study protocol followed the ethical guidelines of the Declaration of Helsinki and was approved by the Beijing Ditan Hospital Ethical Review Committee.
Participants
In this prospective study, participants were consecutively enrolled from February 2014 to December 2016 in Liver Diseases Center, Beijing Ditan Hospital. The chronic HBV-infected patients in immune-tolerance and immune-active phases were diagnosed according to the guidelines,[6,7] AHB patients were defined as individuals who had no HBV infection 6 months before, but were positive for hepatitis B surface antigen (HBsAg), abnormal in alanine aminotransferase (ALT) levels and had an anti-HBc-IgM titer >1:1000 or were diagnosed by liver histological examination at enrollment, and healthy individuals, who were the staff in our hospital and had no HBV infection and the other cause for liver diseases, were diagnosed according to the reported criterion (ALT <19 U/L for female and <30 U/L for male).[8] The patients were excluded when they were co-infected with other viruses, such as hepatitis C virus, hepatitis D virus, and human immunodeficiency virus; and had other liver diseases, including alcohol liver disease, fatty liver, autoimmune liver disease, metabolic liver disease, or liver cancer; and had liver fibrosis and cirrhosis diagnosed by Fibroscan.[9] All patients did not receive antiviral or liver function protective drugs at the time of research.
Serological analysis
The parameters of liver function were measured using an automatic biochemical analyzer (7600-020, Hitachi, Japan). HBsAg, anti-HBs, HBeAg, and anti-HBe levels were measured using an Abbott Architect i2000 detection reagent (Abbott Diagnostics, Abbott Park, IL, USA). The dynamic range of HBsAg was 0.05–250 U/ml. Samples with HBsAg levels >250 U/ml were automatically retested at a 1:500 dilution. HBsAg <0.05 U/ml was defined as the disappearance of HBsAg. The serum HBV DNA level was quantitated with a Roche COBAS AmpliPrep/COBAS TaqMan 96 full automatic real-time fluorescence quantitative polymerase chain reaction detection reagent (with a lower limit of 20 U/ml) (Roche, Pleasanton, CA, USA).
Detection of peripheral blood T-helper cells and cytokines
CD4+ T-cells were stained with monoclonal antibodies against CD4-FITC, CD3-PerCP, CD45RO-APC, and CD62L-PE (Becton-Dickinson, San Jose, CA, USA). To measure the expression of CD4+ T-cells, 100 μl of whole peripheral blood samples was incubated with 3 μl mouse anti-human CD4 (FITC), mouse anti-human CD3 (PerCP), mouse anti-human CD45RO (APC), and 5 μl mouse anti-human CD62L (PE) in the appropriate tubes at room temperature in the dark for 20 min. A total of 2 ml of FACS lysing solution (Becton-Dickinson) was added and incubated at room temperature in dark for 5 min and then the samples were centrifuged at 300 ×g for 5 min. The supernatant was aspirated, and 2 ml of phosphate-buffered saline (PBS) was added and vortexed gently and then centrifuged at 300 ×g for 5 min. The supernatant was aspirated, vortexed gently, and resuspended with 200 μl of PBS. The multicolor samples were acquired with a four-color flow cytometry (FACSCalibur Flow Cytometer, Becton-Dickinson). CD4+ T cells were identified as mononuclear cells that were CD3 positive and CD4 positive. CD45RO and CD62L of CD3+ CD4+ cells were measured to assess the subset of CD4+ T-cells. CD3+ CD4+ CD45RO+ CD62L− represented Th1 cells and CD3+ CD4+ CD45RO+ CD62L+ represented Th2 cells.[7]
Peripheral venous blood was collected, and plasma was separated and stored in a refrigerator set at −80°C for cytokine detection. Eight cytokines in the plasma were quantitatively measured by Luminex assays, including Flt3 ligand (Flt3L), interferon (IFN)-α2, IFN-γ, interleukin (IL)-10, IL-17A, IL-6, transforming growth factor (TGF) -β1, and TGF-β2. Each sample was assayed in duplicate, and cytokine standards supplied by the manufacturer were run on each plate. Data were acquired by the Luminex assay and analyzed using the FlexMap 3D analyzer (Austin, TX, USA) according to the manufacturer's instructions.
Statistical analysis
The statistical analysis was performed using SPSS (version 11.5, SPSS In., Chicago, IL, USA). Serum levels of HBV DNA and HBsAg were logarithmically transformed for the analysis. Continuous variable was expressed as the mean ± standard deviation or median (Q1, Q3) and was analyzed using the Mann-Whitney U-test or analysis of variance. Chi-square or Fisher's exact tests were used for the analysis of the categorical variables. A P < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics
A total of 125 participants were included in this study, including 65 males and 60 females aged 32.7 ± 10.3 years (range 20–74 years). There were 30 chronic HBV-infected patients in the immune-tolerant phase (IT group) and 50 chronic HBV-infected patients in the immune-active (clearance) phase (IC group), 32 AHB patients (AHB group), and 13 healthy individuals (HI group). The serum levels of HBV DNA, HBsAg, and HBeAg were significantly lower, and ALT levels were significantly higher in the IC and AHB groups than in the IT group [Table 1].
Table 1.
Clinical characteristics of participants
| Characteristic | IT (n = 30) | IC (n = 50) | AHB (n = 32) | HI (n = 13) | F or χ2 | P |
|---|---|---|---|---|---|---|
| Male/female, n | 14/16 | 28/22 | 21/11 | 2/11 | 10.025* | 0.018 |
| Age (years) | 29.87 ± 7.27 | 32.02 ± 7.65 | 38.72 ± 14.55 | 26.07 ± 1.93 | 7.196 | <0.001 |
| Baseline ALT (U/L) | 34.44 ± 17.66 | 304.10 ± 268.18 | 1187.71 ± 858.28 | 10.48 ± 2.68 | 39.139 | <0.001 |
| HBV DNA (log10 U/ml) | 8.11 ± 0.48 | 7.05 ± 1.23 | 4.61 ± 1.40 | NA | 78.576 | <0.001 |
| HBsAg (log10 U/ml) | 4.66 ± 0.52 | 3.81 ± 0.69 | 2.55 ± 1.40 | NA | 41.405 | <0.001 |
| HBeAg (S/CO ratio) | 1589.40 ± 185.33 | 883.07 ± 495.99 | 144.93 ± 344.07 | NA | 105.444 | <0.001 |
Data are shown as n or mean ± SD. *: χ2. ALT: Alanine aminotransferase; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; S/CO: Sample/cutoff; IT: Chronic HBV-infected patients in immune-tolerance phase; IC: Chronic HBV-infected patients in immune-active phase; AHB: Acute hepatitis B patients; HI: Healthy individuals; NA: Not applicable; SD: Standard deviation.
T-helper 2/T-helper 1 ratio
The Th1 cell percentage and Th2/Th1 ratio in the HBV infection group (including IT, IC, and AHB groups) were significantly different from those in HI group (24.10% ± 8.66% and 1.72 ± 0.61 vs. 15.16% ± 4.34% and 2.40 ± 0.74, respectively; all P < 0.001). However, there were no differences in the Th1 cell percentages and Th2/Th1 ratios among the IT, IC, and AHB groups [Table 2].
Table 2.
The percentages of Th cells and ratio of Th2/Th1 in patients with HBV infection and healthy individuals
| Characteristics | IT (n = 30) | IC (n = 50) | AHB (n = 32) | F | P | HBV-infection group* (n = 112) | HI (n = 13) | t | P |
|---|---|---|---|---|---|---|---|---|---|
| Th1 (%) | 24.60 ± 7.54 | 24.07 ± 9.10 | 23.67 ± 9.14 | 1.466 | 0.235 | 24.10 ± 8.66 | 15.16 ± 4.34 | 6.132 | <0.001 |
| Th2 (%) | 35.19 ± 8.44 | 38.25 ± 8.71 | 38.27 ± 7.86 | 0.088 | 0.916 | 37.44 ± 8.44 | 34.13 ± 7.62 | 1.346 | 0.181 |
| Th2/Th1 ratio | 1.55 ± 0.58 | 1.77 ± 0.66 | 1.77 ± 0.54 | 1.487 | 0.231 | 1.72 ± 0.61 | 2.40 ± 0.74 | 3.743 | <0.001 |
Data are shown as mean ± SD. *IT, IC, and AHB. Th: T-helper; IT: Chronic HBV-infected patients in immune-tolerance phase; IC: Chronic HBV-infected patients in immune-active phase; AHB: Acute hepatitis B patients; HI: Healthy individuals; SD: Standard deviation.
Cytokine levels
In HBV-infected group, the median levels of Flt3L, IFN-γ, and IL-17A were significantly lower than those in HI group (29.26 pg/ml, 33.72 pg/ml, and 12.27 pg/ml vs. 108.54 pg/ml, 66.48 pg/ml, and 35.96 pg/ml, respectively; all P < 0.05). IFN-α2, IL-10, and TGF-β2 median levels in hepatitis group (including AHB and IC groups) were significantly higher than those in IT group (40.14 pg/ml, 13.58 pg/ml, and 557.41 pg/ml vs. 16.74 pg/ml, 6.80 pg/ml, and 419.01 pg/ml, respectively; all P < 0.05), while patients in hepatitis group had significant lower Flt3L level than that in IT group (30.77 vs. 59.96 pg/ml, P = 0.021). Compared with IC group, patients in AHB group had significant higher median levels of IL-10, TGF-β1, and TGF-β2 (22.77 pg/ml, 10,447.00 pg/ml, and 782.28 pg/ml vs. 8.66 pg/ml, 3755.50 pg/ml, and 482.87 pg/ml, respectively; all P < 0.05) [Table 3 and Figure 1].
Table 3.
Cytokine levels in patients with HBV infection and healthy individuals
| Characteristics | IT (n = 30) | IC (n = 50) | AHB (n = 32) | Hepatitis group* (n = 82) | HBV-infection group† (n = 112) | HI (n = 13) | Z (IT vs. hepatitis) | P (IT vs. hepatitis) | Z (IC vs. AHB) | P (IC vs. AHB) | Z (HBV-infection vs. HI) | P (HBV-infection vs. HI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flt3-L (pg/ml) | 59.96 (29.89, 205.49) | 25.45 (0.16, 83.99) | 35.13 (13.35, 161.05) | 30.77 (2.14, 84.13) | 29.26 (0.66, 84.13) | 108.54 (30.67, 643.12) | −2.312 | 0.021 | −1.21 | 0.226 | −3.055 | 0.002 |
| IFN-α2 (pg/ml) | 16.74 (4.36, 33.18) | 39.53 (20.47, 74.19) | 40.14 (5.61, 84.64) | 40.14 (12.94, 76.66) | 23.56 (5.66, 65.42) | 15.35 (5.69, 67.64) | −2.083 | 0.037 | −0.773 | 0.44 | −0.088 | 0.93 |
| IFN-γ (pg/ml) | 45.48 (20.44, 191.01) | 33.59 (9.38, 86.39) | 27.13 (11.26, 50.85) | 31.28 (10.01, 66.48) | 33.72 (12.94, 66.48) | 66.48 (24.31, 342.43) | −1.711 | 0.087 | −0.238 | 0.812 | −2.198 | 0.028 |
| IL-10 (pg/ml) | 6.80 (3.13, 13.31) | 8.66 (4.83, 19.26) | 22.77 (9.28, 67.13) | 13.58 (5.20, 25.34) | 8.60 (3.39, 21.06) | 3.73 (2.98, 11.92) | −2.284 | 0.022 | −2.535 | 0.011 | −1.292 | 0.196 |
| IL-17A (pg/ml) | 19.34 (12.32, 68.76) | 12.30 (3.23, 47.54) | 14.69 (6.29, 33.23) | 12.32 (4.00, 46.01) | 12.27 (3.92, 39.18) | 35.96 (12.22, 272.99) | −1.864 | 0.062 | −0.693 | 0.488 | −2.826 | 0.005 |
| IL-6 (pg/ml) | 3.33 (1.45, 19.99) | 2.57 (1.06, 9.99) | 4.64 (2.38, 12.19) | 3.76 (1.49, 10.21) | 2.84 (1.26, 9.44) | 3.59 (0.69, 132.92) | −0.482 | 0.63 | −1.585 | 0.113 | −0.665 | 0.506 |
| TGF-β1 (pg/ml) | 4713.50 (3638.25, 8294.50) | 3755.50 (2879.00, 7079.00) | 10447.00 (4948.00, 20, 895.00) | 4948.00 (2974.00, 10, 335.00) | 4790 (2911, 8597) | 4842.00 (2787.50, 5635.00) | −0.252 | 0.801 | −3.85 | 0.001 | −1.162 | 0.245 |
| TGF-β2 (pg/ml) | 419.01 (311.80, 562.83) | 482.87 (358.44, 751.19) | 782.28 (499.84, 1424.00) | 557.41 (407.85, 1035.00) | 476.84 (355.05, 772.51) | 440.18 (338.99, 485.87) | −2.302 | 0.021 | −2.813 | 0.005 | −1.138 | 0.255 |
Data are presented as median (Q1, Q3). *IC and AHB; †IT, IC, and AHB. The comparisons between groups were performed with Mann-Whitney U-test. IT: Chronic HBV-infected patients in immune-tolerance phase; IC: Chronic HBV-infected patients in immune-active phase; AHB: Acute hepatitis B patients; HI: Healthy individuals; Flt3-L: Flt3 ligand; IFN: Interferon; IL: Interleukin; TGF: Transforming growth factor; HBV: Hepatitis B virus.
Figure 1.
Plasma cytokine levels were detected by the Luminex assay among patients with acute hepatitis B (n = 32), chronic HBV-infected patients in immune-active (n = 50) and tolerance phases (n = 30), and healthy individuals (n = 13). (a) Flt-3L. (b) IFN-α2. (c) IFN-γ. (d) IL-10. (e) IL-17A. (f) IL-6. (g) TGF-β1.(h) TGF-β2. AHB: Acute hepatitis B patients; IC: Chronic HBV-infected patients in immune-active phase; IT: Chronic HBV-infected patients in immune-tolerance phase; HI: Healthy individuals; Flt-3L: Flt3 ligand; IFN: Interferon; IL: Interleukin; TGF: Transforming growth factor; HBV: Hepatitis B virus.
DISCUSSION
An efficient suppression of virus replication depends on a combination of innate immunity and adaptive cellular and humoral immunities.[10] Hepatitis B is an immune response-mediated disease. The occurrence of hepatitis B is due to the replication of intracellular DNA, expression of viral proteins, and activation of immune cells, including natural killer (NK) cells, specific-T-cells (CD4+, CD8+), dendritic cells (DCs), and mononuclear macrophages. Liver damage is mediated directly by immune cells as while as indirectly by cytokines. Therefore, the occurrence of hepatitis after HBV infection is inseparable from the corresponding cytokine environment in the patients. In this study, the characteristics of Th2/Th1 cell ratios as well as cytokine levels were explored in patients with chronic HBV infection in immune-tolerance and immune-active phases and patients with AHB.
In our study, the levels of HBV DNA, HBsAg, HBeAg, and ALT were significantly different between the HBV-infected groups. HBV DNA, HBsAg, and HBeAg levels in IC and AHB groups were significantly lower than that in the IT group, while ALT levels were significantly higher than that in the IT group. HBsAg and HBeAg are the most important serological markers of HBV infection.[11] The lower levels of HBV DNA, HBsAg, and HBeAg in the IC and AHB groups compared with those in the IT group might be the results of the liver necroinflammation and immune response to HBV.
CD4+ Th cells are divided into two subsets (Th1 and Th2): Th1 cells release IL-2 and IFN-γ, regulate CTL cells to exert their cytotoxic effects, and mediate cell immunity to clear viruses; Th2 cells secrete cytokines such as IL-4, IL-6, and IL-10 and regulate humoral immunity by expressing the costimulatory molecule CD40L and combining it with the B-cell surface CD40 receptor.[12] Studies have shown that the Th1/Th2 imbalance and cellular immune disorders in peripheral blood may be the important reasons that lead to HBV infection that cannot be fully recovered.[13] Th1 cells mainly participate in cell immunity and play an important role in thoroughly clearing viruses in the recovery period of self-resolving HBV infection, but they do not cause elevated aminotransferase.[14] Although there are CD4+ T-cell responses in the immune-tolerant phase, the host immune response appears to be at a lower level.[15] In our study, patients infected with HBV had a significantly higher Th1 level and a lower Th2/Th1 ratio than healthy individuals.
Many cytokines are involved in the development of chronic HBV infection and hepatitis. IFN-α is an important antiviral active cytokine and widely used for the treatment of chronic hepatitis B.[16,17,18,19,20] IFN-γ is the most important cytokine of CTL for controlling HBV replication or eradication in a noncytolytic manner. Flt-3L is also an essential growth factor for DC and NK cell homeostasis in vivo,[21] and as an adjuvant, it can augment humoral and cellular immune responses to the HBV DNA vaccine.[22] IL-10 has inhibitory effects on pro-inflammation cytokines, it is known for sustaining HBV replication and initiating chronic HBV infection and directing fibrogenesis via inhibition of IFN-γ secretion.[23,24,25] The pivotal function of TGF-β in the immune system is to induce tolerance via the regulation of lymphocyte proliferation, differentiation, and survival,[26,27] and IL-6, IL-21, and IL-1β induce the differentiation of TH17.[28,29] IL-17A is a proinflammatory cytokine with dual effects on the immune responses and may play an important role in the development of HBV-related liver diseases.[30] The results of this study showed that Flt3L, IFN-γ, and IL-17A levels in AHB and IC groups were lower than those in the HI group. However, the levels of IL-10 and TGF-β in AHB group were significantly higher than those in IC group. These results suggest that the immune response may be subject to negative regulation in individuals with acute self-limited HBV infections at the stage of severe inflammation. It has been shown that acute HBV infection usually triggers a rapid induction of IFN-α.[31,32,33] In our study, compared with HI group, an elevated IFN-α level was observed in patients with HBV infection, and IFN-α level was significantly enhanced in IC and AHB groups compared with patients in IT group; it might be indicated that the development of hepatitis B was associated with IFN-α level elevation. Compared with healthy individuals, IL-10 level was enhanced in patients with HBV infection, and it was significantly higher in IC and AHB groups compared with IT group. There might be a downregulated immune response to HBV mediated by IL-10 in chronic HBV infection and AHB. In HBV-infected patients, the generation of IFN-γ might be also downregulated by the elevated IL-10 level and HBV replication.[31] Our result also showed that compared with IC group, patients in AHB group had significantly higher IL-10 and TGF-β levels. As an important immune downregulating cytokine, IL-10 is mainly produced by Treg cell and act as an inhibition factor for plasmacytoid DC, NK, and HBV-specific T-cells.[34,35] It should be noted that the immune response to HBV was further downregulated in AHB.
Several limitations should be noted in the present study: as the participants were consecutively enrolled, it was difficult to match baseline characteristics and sample size among four groups during the study, and there was a bias of age and gender among four groups. Besides, the sample size is relatively small, and the result of the present study should be further validated by well-designed studies with large sample size.
Results in our study showed that compared with healthy individual, HBV-infected patients had increased Th1 cell percentages and Th2/Th1 ratios, but no significant differences were found in the Th cell percentages and Th2/Th1 ratios among chronic HBV-infected patients in immune-tolerance phase, chronic HBV-infected patients in immune-active phase, and AHB patients. Chronic HBV-infected patients in immune-active phase or AHB patients had elevated IFN-α2, IL-10, and TGF-β2 levels than chronic HBV-infected patients in immune-tolerance phase. Moreover, AHB patients had significantly higher IL-10, TGF-β1, and TGF-β2 levels than chronic HBV-infected patients in immune-active phase.
Financial support and sponsorship
The work was supported by a grant from the Basic and Clinical Fund of Capital Medical University (No. 17JL88).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Jonas MM. Hepatitis B and pregnancy: An underestimated issue. Liver Int. 2009;29(Suppl 1):133–9. doi: 10.1111/j.1478-3231.2008.01933.x. doi: 10.1111/j.1478-3231.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 2.Ozasa A, Tanaka Y, Orito E, Sugiyama M, Kang JH, Hige S, et al. Influence of genotypes and precore mutations on fulminant or chronic outcome of acute hepatitis B virus infection. Hepatology. 2006;44:326–34. doi: 10.1002/hep.21249. doi: 10.1002/hep.212490. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.03.021. [Epub ahead of print] doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Han Y, Jin K, Wan Y, Wang S, Liu B, et al. Dynamic changes of cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, and natural killer T (NKT) cells in patients with acute hepatitis B infection. Virol J. 2011;8:199. doi: 10.1186/1743-422X-8-199. doi: 10.1186/1743-422X-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of Liver. EASL clinical practical guidelines: Management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 9.European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. doi: 10.1016/j.jhep.2015.04.006. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Martin P, Dubois C, Jacquier E, Evlachev A, Boukhebza H, Dion S, et al. Multivalent adenovirus-based immunotherapeutic for treatment of chronic hepatitis B induces broad robust and polyfunctional T cells in naive and HBV tolerant mice. J Hepatol. 2013;58(Suppl 1):S57–8. doi: 10.1016/S0168-8278(13)60132-4. [Google Scholar]
- 11.Matsuzaki S, Shinozaki K, Kobayashi N, Agematsu K. Polarization of Th1/Th2 in human CD4 T cells separated by CD62L: Analysis by transcription factors. Allergy. 2005;60:780–7. doi: 10.1111/j.1398-9995.2005.00793.x. doi: 10.1111/j.1398-9995.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Wang Y, Chen Y. Cellular immune response in patients with chronic hepatitis B virus infection. Microb Pathog. 2014;74:59–62. doi: 10.1016/j.micpath.2014.07.010. doi: 10.1016/j.micpath.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Aalaei-Andabili SH, Alavian SM. Regulatory T cells are the most important determinant factor of hepatitis B infection prognosis: A systematic review and meta-analysis. Vaccine. 2012;30:5595–602. doi: 10.1016/j.vaccine.2012.06.063. doi: 10.1016/j.vaccine.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 14.Han YP, Li J, Jiang LF, Xu QQ, Liu B, Dong L, et al. Hepatitis B e antigen from chronic hepatitis B patients induces Th1/Th2 cytokine imbalance in vitro. Chin J Hepatol. 2013;21:584–9. doi: 10.3760/cma.j.issn.1007-3418.2013.08.006. doi: 10.3760/cma.j.issn.1007-3418.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: Current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MH, Zhang L, Qu XJ, Lu Y, Shen G, Wu SL, et al. Kinetics of hepatitis B surface antigen level in chronic hepatitis B patients who achieved hepatitis B surface antigen loss during pegylated interferon alpha-2a treatment. Chin Med J. 2017;130:559–65. doi: 10.4103/0366-6999.200554. doi: 10.4103/0366-6999.200554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MH, Zhang L, Qu XJ, Lu Y, Shen G, Li ZZ, et al. The predictive value of baseline HBsAg level and early response for HBsAg loss in patients with HBeAg-positive chronic hepatitis B during pegylated interferon alpha-2a treatment. Biomed Environ Sci. 2017;30:177–84. doi: 10.3967/bes2017.025. doi: 10.3967/bes2017.025. [DOI] [PubMed] [Google Scholar]
- 18.Li MH, Xie Y, Zhang L, Lu Y, Shen G, Wu SL, et al. Hepatitis B surface antigen clearance in inactive hepatitis B surface antigen carriers treated with peginterferon alfa-2a. World J Hepatol. 2016;8:637–43. doi: 10.4254/wjh.v8.i15.637. doi: 10.4254/wjh.v8.i15.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viganò M, Invernizzi F, Lampertico P. Optimal therapy of chronic hepatitis B: How do I treat my HBeAg-negative patients? Liver Int. 2015;35(Suppl 1):107–13. doi: 10.1111/liv.12717. doi: 10.1111/liv.12717. [DOI] [PubMed] [Google Scholar]
- 20.Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study) Hepatology. 2015;61:1512–22. doi: 10.1002/hep.27586. doi: 10.1002/hep.27586. [DOI] [PubMed] [Google Scholar]
- 21.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–97. [PubMed] [Google Scholar]
- 22.Zhou Q, Wang F, Yang F, Wang Y, Zhang X, Sun S. Augmented humoral and cellular immune response of hepatitis B virus DNA vaccine by micro-needle vaccination using Flt3L as an adjuvant. Vaccine. 2010;28:1357–62. doi: 10.1016/j.vaccine.2009.11.006. doi: 10.1016/j.vaccine.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Wu JF, Wu TC, Chen CH, Ni YH, Chen HL, Hsu HY, et al. Serum levels of interleukin-10 and interleukin-12 predict early, spontaneous hepatitis B virus e antigen seroconversion. Gastroenterology. 2010;138:165–72.e1-3. doi: 10.1053/j.gastro.2009.09.018. doi: 10.1053/j.gastro.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–35. doi: 10.4049/jimmunol.1103139. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 28.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 29.Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arababadi MK, Bidaki MZ, Kennedy D. IL-17A in hepatitis B infection: Friend or foe? Arch Virol. 2014;159:1883–8. doi: 10.1007/s00705-014-2002-x. doi: 10.1007/s00705-014-2002-x. [DOI] [PubMed] [Google Scholar]
- 31.Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–300. doi: 10.1053/j.gastro.2009.06.054. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. doi: 10.1016/j.immuni.2005.05.00.5. [DOI] [PubMed] [Google Scholar]
- 33.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. doi: 10.1128/CMR.14.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG, et al. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52:330–9. doi: 10.1016/j.jhep.2009.12.015. doi: 10.1016/j.jhep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, et al. TGF-ß1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. doi: 10.1371/journal.ppat.1002594. doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]