Abstract
There is a paucity of research examining the relationships between dietary patterns (DPs) and risk of developing pre-cancerous lesions as well as biomarkers associated with such DPs. The purpose of the current study was to identify DPs that are associated with higher grades of cervical intraepithelial neoplasia (CIN 2+) and to determine whether these DPs are associated with the degree of DNA methylation in the long interspersed nucleotide elements (L1s) of peripheral blood mononuclear cells (PBMCs), a biomarker associated with risk of developing CIN 2+. Study population consisted of 319 child-bearing age women. DPs were derived by factor analysis. The degree of PBMC L1 methylation was assessed by pyrosequencing. Logistic regression models were used to evaluate the associations between DPs and CIN 2+. Similar models were used to evaluate the association between DPs and degree of PBMC L1 methylation in women free of CIN 2+. Women with the unhealthiest DP were 3.5 times more likely to be diagnosed with CIN 2+ compared to women with the healthiest DP (OR=3.5; 95% CI, 1.2–10.1; P=0.02). Women at risk for developing CIN 2+ with the healthiest DP were 3.3 times more likely to have higher PBMC L1 methylation compared to women with the unhealthiest DP (OR=3.3; 95% CI, 1.0–10.6; P=0.04). Our findings suggest that HPV associated risk of developing CIN 2+ may be reduced by improving DPs. The degree of PBMC L1 methylation may serve as a biomarker for monitoring the effectiveness of dietary modifications needed for reducing the risk of CIN 2+.
Keywords: Dietary patterns, Biomarkers, Pre-neoplasia
Introduction
The importance of diet for health was emphasized more than a quarter century ago when Doll and Peto reported that ~ 35% (10–70%) of all cancers might be attributable to dietary factors and ~ 90% of colorectal cancer may be preventable through dietary modifications (1). During the early 1990s, we had high expectations that a higher consumption of fruits and vegetables would reduce the risk of many cancers (2). However, this evidence was based primarily on results generated by case-control studies. The results generated by prospective cohort studies completed in more recent years did not confirm these findings (3). Several researchers responded to these inconsistent results between case-control and cohort studies by pointing out that those case-control studies were biased by differences in recall of fruit and vegetable intake by individuals diagnosed with cancer and healthier controls. Further, even if both cases and non-cases reported their intakes similarly, non-cases who participated in these studies could have been more health conscious than non-cases who didn’t participate, leading to an exaggerated benefit of fruits and vegetables in cancer prevention. However, we still don’t exclude the possibility that specific groups of fruits and vegetables, specific substances in some fruits and vegetables or overall dietary patterns (DPs) have important cancer protective effects. Recent studies have shown that a higher consumption of dark green and deep yellow vegetables and fruits was associated with lower risk of having cervical intraepithelial neoplasia (CIN), precursor lesions for developing cervical cancer, especially among smokers (4). Frequent consumption of fruits high in anti-oxidant nutrients was also shown to be associated with lower risk of CIN (5). Studies also support a role for fruit and vegetable consumption in reducing the risk of CIN, especially in women infected with a higher load of human papillomaviruses, the main causative factor for CIN and cervical cancer (6). A study also suggested that diets rich in plant-based nutrients may lower the risk of cervical cancer (7).
Because of the obesity epidemic and its associated chronic disease risk, assessment of overall DPs and their link to chronic disease risk (8) and diet-related alterations in the epigenome are becoming increasingly recognized as important. To our knowledge, only a few studies have focused on pre-cancerous stages in relation to DPs, a point where the development of cancers could be prevented by dietary modifications. Even though it is logical to assume that dietary recommendations focused on promoting a healthier overall DP rather than encouraging consumption of certain foods or food categories should be the first line of intervention for prevention of many different types of cancers, use of biomarkers to monitor the effectiveness of these interventions should be an integral part of such efforts. To our knowledge, there have been no systematic studies conducted to derive biomarkers of DPs which are also associated with higher risk of developing pre-cancerous lesions. We have recently documented that a higher degree of DNA methylation in the long interspersed nucleotide elements (L1s) of peripheral blood mononuclear cells (PBMCs) was associated with 56% lower risk of being diagnosed with higher grades of cervical intraepithelial neoplasia (CIN 2+), a common pre-cancerous lesion found among sexually active women exposed to carcinogenic or high-risk (HR) types of human papillomaviruses (HPVs) (9). The main purpose of the current study was to identify overall DPs that are associated with CIN 2+ and to determine whether these DPs are associated with the degree of L1 methylation in PBMCs.
Materials and Methods
Patient population
The present analysis is based on 319 women enrolled in an ongoing prospective follow-up study funded by the National Cancer Institute (R01 CA105448, Prognostic Significance of DNA & Histone Methylation). The study has been described in a previous publication (10). Briefly, all women were diagnosed with abnormal cervical cells in clinics of the Health Departments in Alabama and were referred to the University of Alabama at Birmingham (UAB) for further examination by colposcopy and biopsy. Women were 19–50 years old, had no history of cervical cancer or other cancers of the lower genital tract, no history of hysterectomy or destructive therapy of the cervix; were not pregnant, were not using antifolate medications such as methotrexate, sulfasalazine, or phenytoin and were non-vitamin supplement users. Of the 319 women, 93 women were diagnosed with CIN 2+ (cases, including CIN 2 [n=57], CIN 3 [n=33] or carcinoma in situ [CIS, n=3]) and 226 women were diagnosed with ≤CIN 1 (non-cases, including normal cervical epithelium [n=12], HPV cytopathic effect [HCE, n=26], reactive nuclear enlargement [RNE, n=39] or CIN 1 [n=149]). Both cases and controls tested positive for HR-HPV (any one of 13 types of HR-HPV, HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 based on Roche Diagnostics Linear Array results). All women included in this analysis participated in an interview that assessed socio-demographic variables and lifestyle risk factors (age, race, level of education, smoking status, use of vitamin supplements and oral/hormonal contraceptives), physical activity (CDC questionnaire) and dietary intake (Block’s food frequency questionnaire, version 98.2). The healthy eating index (HEI) (Block scale of 0–100) was obtained from Block questionnaire data. Height, weight and waist circumference (WC) measurements were obtained using standard protocols. The body mass index (BMI) was calculated as weight (kilograms) divided by height (meters squared). The study protocol and procedures were approved by the UAB Institutional Review Board.
Laboratory Methods
DNA was extracted from buffy coat samples using a standard phenol-chloroform extraction method. As described below, methylation of the L1 promoter (GenBank accession no.x58075) in PBMCs was investigated using a pyrosequencing-based methylation analysis.
Bisulfite-pyrosequencing L1 analysis
Bisulfite treatment of 1 μg of DNA extracted from buffy coat was completed using the EZ DNA methylation kit (Zymo Research, CA) and the converted DNA was eluted with 30 μl TE buffer. PCR reactions were carried out using forward (5′-TTTTTTGAGTTAGGTGTGGG-3′) and reverse-biotinylated (5′-biotin-TCTCACTAAAAAATACCAAACAA-3′) primers, as described (11). The biotinylated PCR product, purified and made single-stranded to act as a template, was annealed to the pyrosequencing primer (5′-GGGTGGGAGTGAT-3′) (0.4μM final concentration), and then was subjected to sequencing using an automatically generated nucleotide dispensation order for sequences to be analyzed corresponding to each reaction. The pyrograms were analyzed using allele quantification (AQ) mode to determine the proportion of C/T, and hence methylated and unmethylated cytosines at the targeted position(s). The degree of methylation was evaluated at three CpG methylation sites (11). The reproducibility of the assay was satisfactory with a CV of 2.0–2.2%.
Dietary patterns derived by factor analysis
Usual dietary intake assessed with the Block food frequency questionnaire (FFQ), version 98.2, which includes intake of phytochemicals, was used to derive DPs in this population. Women with daily calorie intakes of <1000 kcal and >5000 Kcal were excluded prior to deriving the DPs. Food consumption frequencies were standardized into frequencies of intake per week. To reduce the number of patterns generated and to increase interpretability, we assigned each food item into a defined food group based on similarity of nutrients in a given food item, source (plant vs. animal) and how they are commonly consumed. This resulted in 35 food groups. The frequencies of intake of these foods in a given group were summed up to give the total intake per week for the food group. Some food groups contain only one food item and were entered in the model for deriving patterns as individual foods because of their unique nutrient profiles (example, water) or because their consumption reflects a distinct DP. We used PROC FACTOR in SAS v.9.2 (SAS Institute, Cary NC; 2008) to derive DPs and corresponding factor loadings for each food group. We generated the SCREE plots and examined Eigen values for each food or food group and determined the number of factors to keep. After determining the number of factors to keep, we refitted the model but with NFACTOR option to limit the number of factors generated to two factors. An orthogonal transformation was done using the VARIMAX rotation option to produce uncorrelated DPs. Factor loadings for each food group were generated to reflect the contribution of each food or food group to the DP. Food items with a factor loading of 0.30 or more were considered important components of each pattern and were used to identify and name the DPs.
Analysis of dietary data
We observed two main distinct DPs in our study population. As shown in Table 1, the unhealthiest DP or DP 1 mainly consisted of food items considered to be unhealthy (high sugar beverages, pasta and starchy foods, margarine, butter, refined grains, desserts and sweets, snacks, high fat dairy, fatty meat, sausages and bacon, condiments, pizza, macaroni and cheese). Each of these food items had a factor loading ≥0.30 for the first factor. DP 2 mainly consisted of healthier food items (seafood, beans and lentils, tofu and meat substitutes, whole grains, fresh fruits, canned fruits, vegetables, peanut butter, low fat dairy, chicken and turkey, cereals, water, yogurt, dressings and gravy, and phytochemical rich foods) each with a factor loading ≥0.30 for the second factor. This pattern was named as the healthy DP. The study participants were then ranked in ascending order according to pattern 1 or pattern 2 scores. Using PROC RANK in SAS, two groups per DP were derived (i.e., above or below median for a given DP). Those with a factor score rank above median for the unhealthy pattern (factor 1) and a factor score rank below median for the healthy pattern (factor 2) were classified as unhealthiest (n=76). Those with a factor score rank above median for the healthy pattern and a factor score rank below median for the unhealthy pattern were classified as the healthiest (n=63). These two extremes of the patterns (healthiest and unhealthiest) comprised 44% of the women in our population. 56% of women had intermediary dietary patterns (i.e. higher factor score ranks for both pattern 1 and pattern 2 [intermediary DP 1, n=96] or lower factor score ranks for both pattern 1 and pattern 2 [intermediary DP 2, n=84]).
Table 1.
Factor Loading Matrix for the Dietary Patterns Identified
| Food Categories | Dietary Patterns | |
|---|---|---|
| Unhealthiest | Healthiest | |
| Real fruit Juice | 0.04 | 0.22 |
| Higher sugar beverages | 0.53 | −0.23 |
| Tea and coffee | 0.15 | 0.22 |
| Pasta and starchy foods | 0.61 | 0.26 |
| Eggs | 0.28 | 0.10 |
| Seafood | 0.13 | 0.32 |
| Beans and lentils | 0.21 | 0.35 |
| Tofu and meat substitute | −0.08 | 0.31 |
| Margarines | 0.36 | 0.10 |
| Butter | 0.40 | 0.03 |
| Refined grains | 0.65 | 0.01 |
| Whole grains | 0.01 | 0.42 |
| Fresh fruits | −0.03 | 0.60 |
| Canned fruits | 0.03 | 0.42 |
| Vegetables | −0.01 | 0.78 |
| Desserts and sweets | 0.67 | 0.03 |
| Snacks | 0.61 | −0.04 |
| Peanut butter | 0.14 | 0.31 |
| High fat dairy | 0.34 | 0.02 |
| Low fat dairy | −0.18 | 0.39 |
| Fatty meat | 0.66 | 0.04 |
| Chicken and turkey | 0.08 | 0.36 |
| Cereals | −0.02 | 0.33 |
| Sausage and bacon | 0.50 | −0.08 |
| Condiments | 0.42 | 0.21 |
| Alcohol | 0.05 | 0.12 |
| Pizza | 0.38 | −0.08 |
| Macaroni cheese | 0.39 | 0.13 |
| Ensure | −0.14 | 0.26 |
| Water | −0.18 | 0.40 |
| Yogurt | −0.27 | 0.43 |
| Dressings and gravy | 0.26 | 0.52 |
| Soups | 0.17 | 0.22 |
| Chinese food | 0.17 | 0.19 |
| Phytochemical rich foods | 0.27 | 0.55 |
Statistical analysis
We tested whether participant characteristics such as age, race, level of education, BMI, smoking status, physical activity, use of oral/hormonal contraceptives, total dietary calorie intake, PBMC L1 methylation and case status varied by the four DP groups (healthiest, intermediary DP1, intermediary DP2 and the unhealthiest). We also tested whether energy adjusted intakes of “cancer protective micronutrients” (total folate DFE, vitamins B12, B6, B2, alpha-carotene, beta-carotene, vitamins A, C and E) and the HEI differ by the four DPs in the entire population and among non-cases only. We used ANOVA and the two-sided χ2 to test the statistical significance for continuous variables and categorical variables respectively. Using the “healthiest” DP as the referent in unconditional logistic regression models, we estimated the odds ratios (95% CI) for having CIN2+ (yes or no) for each of the DP groups. These analyses were adjusted for age (≥median vs. <median), race (Caucasian American [CA] vs. African American [AA])/level of education (less than high school education vs. high school education or greater), BMI (>25 vs. ≤25 kg/m2), smoking status (ever vs. never), physical activity (>150 vs. ≤150 minutes/week), use of oral/hormonal contraceptives (ever vs. never) and total dietary calorie intake (≥median vs. <median).
We then examined the association between DPs and PBMC L1 methylation among non-cases using unconditional logistic regression models. Exclusion of the cases was necessary to avoid the possibility of reverse causation (i.e. CIN 2+ status influencing dietary habits). If women were above the 50th percentile of the percent PBMC L1 methylation distribution they were classified as having higher methylation; otherwise they were classified as having lower methylation. In this analysis, three unconditional logistic regression models were run to test the association between PBMC LI methylation and DP keeping the unhealthiest, intermediary DP1 or intermediary DP2 as the referent groups. All models were adjusted for age, race/level of education, BMI, smoking status, physical activity, use of oral/hormonal contraceptives and total dietary calorie intake. Since race and level of education were highly correlated we did not include both variables as covariates in the same model. We tested all models separately with each of these variables.
Results
The characteristics of the study population based on the four DPs are reported in Table 2. Race, use of oral/hormonal contraceptives and median total dietary calorie intake per day were significantly different among the four DP groups (P=0.01, P<0.01 and P<0.001 respectively). None of the other variables were statistically different among the four DPs. Results from a similar univariate analysis between the healthiest DP and the unhealthiest DP showed that the unhealthiest DP was more common among AA women (78%) compared to CA women (22%) (P=0.0009). Women with the unhealthiest DP were significantly less likely to be engaged in >150 min/week physical activity and more likely to use oral/hormonal contraceptives compared to women with the healthiest DP (P=0.043 and 0.001 respectively). We also observed that women with the unhealthiest DP had significantly higher median total dietary calorie intake compared to women with healthiest DP (P<0.001). The median PBMC L1 methylation was significantly lower in women with the unhealthiest DP compared to women with the healthiest DP (P=0.021). Further, 34% of women with the unhealthiest DP were cases while only 19% of women with the healthiest DP were cases (P=0.046). None of the other variables were statistically different between women with the healthiest DP and women with the unhealthiest DP.
Table 2.
Characteristics of the study population based on dietary patterns (DPs)
| Variables | Healthiest DP a |
Intermediary DP1 b |
Intermediary DP2 c |
Unhealthiest DP d |
P-value |
|---|---|---|---|---|---|
| Total number (N) | 63 | 96 | 84 | 76 | |
| Age (years) (Mean ± SD) | 25.8 ± 5.9 | 25.4 ± 5.4 | 23.7 ± 4.3 | 24 ± 3.3 | 0.09 |
| Level of education (% less than high school education)* | 10 (16%) | 21 (22%) | 17 (20%) | 23 (30%) | 0.22 |
| Race (% African American [AA])* | 32 (51%) | 62 (65%) | 60(71%) | 59 (78%) | 0.01 |
| BMI e (% > 25kg/m2)* | 37 (62%) | 61 (64%) | 53 (64%) | 41(54%) | 0.54 |
| Smoking status (% Ever smoker)* | 25 (40%) | 42 (44%) | 39 (46%) | 29 (38%) | 0.71 |
| Physical activity (% > 150 min/week)* | 19 (30%) | 20 (21%) | 16 (19%) | 12 (16%) | 0.20 |
| Oral/hormonal contraceptive use (% ever user)* | 18 (29%) | 52 (55%) | 38 (46%) | 43 (57%) | <0.01 |
| Median total dietary calorie intake (Kcal/day) | 1603.5 | 3069.1 | 1383.2 | 2818.6 | <0.001 |
| Median PBMC L1 methylation (%) | 66.7 | 62.0 | 61.4 | 61.8 | 0.44 |
| Case status (% with CIN2+)* | 12 (19%) | 29 (30%) | 26 (31%) | 26 (34%) | 0.24 |
Lower factor score for pattern 1 and higher factor score for pattern 2
Higher factor scores for both pattern 1 and pattern 2
Lower factor scores for both pattern 1 and pattern 2
Higher factor score for pattern 1 and lower factor score for pattern 2
Body mass index
Number and (%) of women
Table 3 shows the median energy adjusted intakes of cancer protective micronutrients and HEI among the four DPs. The intake of all micronutrients (except for vitamin C) and HEI were significantly different among the four DPs. We observed that the unhealthiest DP had significantly lower HEI and intakes of all micronutrients (except for vitamin C) compared to the healthiest DP (P<0.05 for HEI and all micronutrients except for vitamin C). Among the non cases, all micronutrients (including vitamin C) and HEI were significantly higher in women with the healthiest DP compared to unhealthiest DP (P<0.05 for all comparisons, data not shown).
Table 3.
Energy adjusted median intakes of “cancer protective” micronutrients and median HEI among women with four dietary patterns (DPs)
| Micronutrient Intake | Healthiest DP a |
Intermediary DP1 b |
Intermediary DP2 c |
Unhealthiest DP d |
P-value |
|---|---|---|---|---|---|
| Total number (N) | 63 | 96 | 84 | 76 | |
| Total Dietary Folate (DFE) | 229.7 | 192.9 | 183.9 | 159.9 | <0.0001 |
| Vitamin B12 (μg) | 1.8 | 1.6 | 1.4 | 1.3 | <0.0001 |
| Vitamin B6 (mg) | 0.8 | 0.7 | 0.7 | 0.6 | <0.0001 |
| Vitamin B2 (mg) | 0.8 | 0.7 | 0.7 | 0.6 | <0.0001 |
| Alpha-carotene (mcg) | 233 | 96.7 | 80.3 | 47.7 | <0.0001 |
| Beta-carotene (mcg) | 1282.4 | 684.8 | 839.4 | 587.2 | <0.0001 |
| Vitamin A (IU) | 3335.7 | 2113.9 | 2176.7 | 1376.3 | <0.0001 |
| Vitamin C (mg) | 78.8 | 59.9 | 61.2 | 64 | 0.1080 |
| Vitamin E (a-TE) | 4.54 | 4.07 | 3.6 | 3.6 | <0.0001 |
| HEI index (Block scale 0–100) | 62 | 53 | 51 | 49 | <0.0001 |
Lower factor score for pattern 1 and higher factor score for pattern 2
Higher factor scores for both pattern 1 and pattern 2
Lower factor scores for both pattern 1 and pattern 2
Higher factor score for pattern 1 and lower factor score for pattern 2
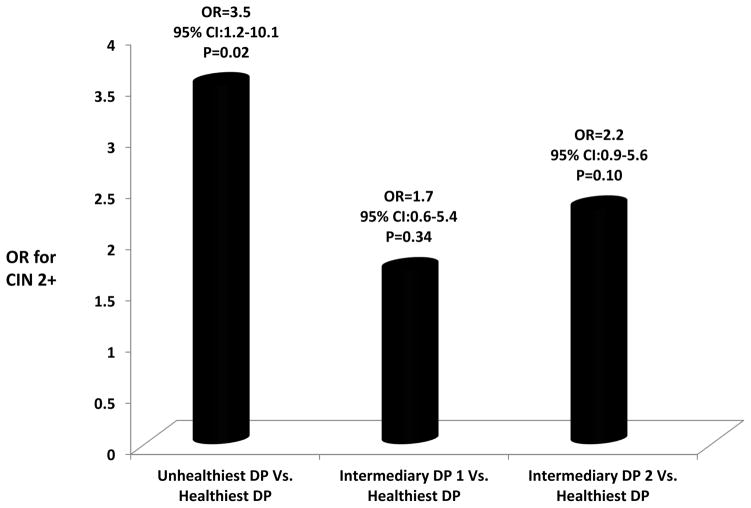
As shown in Figure 1, women with the unhealthiest DP were 3.5 times more likely to be diagnosed with CIN 2+ compared to women with the healthiest DP (OR=3.5; 95% CI, 1.2–10.1; P=0.02). Compared to the healthiest DP, women with the intermediary DP 1 (OR=1.7, 95% CI, 0.6–5.4, P=0.34) and intermediary DP 2 (OR=2.2, 95% CI, 0.9–5.6, P=0.10) showed a non-significant positive association with CIN 2+ status.
Figure 1.
As shown in Table 4, pre-cancer free women with the healthiest DP were 3.3 times more likely to have higher PBMC L1 methylation compared to women with the unhealthiest DP (OR=3.3; 95% CI: 1.0–10.6 P=0.04) in a model that adjusted for age, race, BMI, smoking status, physical activity, use of oral/hormonal contraceptives and total dietary calorie intake. Women with the healthiest DP compared to the intermediary DP 1 and DP 2 were 1.5 and 1.7 times more likely to have higher PBMC L1 methylation respectively, but these associations were statistically non-significant (OR=1.5, 95% CI, 0.5–4.1, P=0.45; OR=1.7, 95% CI, 0.8–3.8, P=0.20, respectively). Models yielded similar results when race was replaced with the level of education.
Table 4.
The association between dietary patterns (DPs) and PBMC L1 methylation
| Models* | PBMC L1 methylation | |
|---|---|---|
| OR (95%CI) | P-Value | |
| Healthiest DP vs. unhealthiest DP | 3.3 (1.0–10.6) | 0.04 |
| Healthiest DP vs. intermediary DP1 | 1.5 (0.5–4.1) | 0.45 |
| Healthiest DP vs. intermediary DP2 | 1.7 (0.8–3.8) | 0.20 |
Models adjusted for age, race, BMI, smoking status, physical activity, use of oral/hormonal contraceptives and total dietary calorie intake
Discussion
Even though it is logical to assume that dietary recommendations focused on promoting a healthier overall diet rather than encouraging consumption of certain foods or food categories should be the first line of intervention for prevention and control of cancer, we are currently in a weak position to do so because of lack of data on biologically meaningful overall DPs that are specific for cancer prevention and control.
The most common health outcomes examined in relation to DPs for some time have been all-cause mortality and cardiovascular disease risk (12). The use of a DP approach in the setting of cancer research has become more common, but largely limited to cross-sectional studies of cancer risk (13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23) or survival from cancer (24, 25). A recent follow-up study demonstrated that a DP rich in fruit and salad might protect against invasive breast cancer (26). A prospective follow-up study on prostate cancer, however, failed to identify any DP associated with risk of prostate cancer (27).
The limited number of studies which evaluated the effects of DPs on pre-cancer risk has focused largely on colorectal adenomas. A DP consisting of a higher consumption of dairy products and fruits and vegetables with low alcohol consumption was associated with lower risk of colorectal adenomas in Japanese men (28). A high-fruit, low-meat diet was shown to be protective against colorectal adenomas compared to a DP of higher vegetable and meat consumption (29). A recent study demonstrated that AA women may be able to reduce their risk of developing colorectal adenomas by following a prudent DP (30). Our study was able to identify an unhealthy DP which might put women at higher risk for developing HR-HPV related CIN 2+, precursor lesions for developing cervical cancer. The unhealthiest DP identified in our study is similar to a Western DP. Further, women with the unhealthiest DP were significantly more likely to use oral/hormonal contraceptives and had lower HEI, factors that may be associated with lower cancer protective micronutrient status. In fact, intakes of several micronutrients with cancer protective effects were significantly lower in women with the unhealthiest DP, demonstrating the biological plausibility of the observed association between unhealthiest DP and higher risk of CIN 2+. AAs were found to consume lower amounts of micronutrients and were reported to be at higher risk for some cancers than CAs (31). Interestingly, we observed that the lower micronutrient containing unhealthiest DP identified in our study was significantly more common among AAs compared to CAs. We also observed that women with the unhealthiest DP consumed significantly higher amount of calories and were physically less active compared to women with the healthiest DP. Even though the association between physical activity and cancer risk is inconsistent, higher calorie consumption is associated with higher risk of some cancers (32).
Even though only the unhealthiest DP was associated with statistically significant higher risk of CIN 2+, both intermediary DPs were associated with approximately 2 fold higher risk of CIN 2+, indicating that 80% of this population do not have DPs which may exert cervical cancer protective effects. Therefore, these observations suggest that the consumption of higher amounts of healthier food items along with higher amounts of unhealthier food items or the consumption of lower amounts of both healthier and unhealthier food items are unlikely to be beneficial for reducing cancer risk. Therefore, modifications toward the healthiest DP based on food categories identified by our study may exert the most beneficial effects on the prevention of cervical cancer in this population.
Our study has limitations inherent to factor analysis used to derive DPs, i.e, subjective judgment in deriving 35 food groups, in determining the number of patterns and possibly the interpretation of these patterns. However, evaluation of a pre-cancer related biomarker in relation to DPs is a unique aspect of this study. The biomarker we have chosen to associate with DPs (L1 methylation) is a validated surrogate biomarker of genome-wide methylation changes (33). Studies have shown that methylation levels measured in L1 regions, which are easy to characterize by pyrosequencing technology, do not vary significantly with time within an individual, and therefore changes in their methylation levels could potentially be attributed to dietary or lifestyle factors or interventions with such factors (9). A recent study has demonstrated that a prudent DP was associated with a lower prevalence of PBMC L1 hypomethylation in a dose dependent manner suggesting the beneficial effects of a healthier DP on L1 methylation in a cancer-free population (34). We evaluated the association between DPs and PBMC L1 methylation in women free of cervical pre-cancer, but they are at higher risk for developing cervical pre-cancer or cancer because they are diagnosed with abnormal pap and tested positive for HR-HPVs. In these women, we observed that those with the healthiest DP were significantly more likely to have higher PBMC L1 methylation compared to those with the unhealthiest DP. We observed that the intakes of several methyl donor micronutrients (folate, vitamins B12, B2 and B6) were significantly higher in the healthiest DP compared to the unhealthiest DP identified in our study, indicating the biological plausibility for higher PBMC L1 methylation observed in women with the healthiest DP identified in our study population. Our results also showed that the PBMC L1 methylation was 1.5–1.7 fold higher in the healthiest DP compared to the two intermediary DPs. Even though these differences were statistically non-significant, the observed results suggest that 80% of the population may not have DPs which provide adequate L1 methylation. We have previously shown that higher PBMC L1 methylation is associated with lower risk of CIN 2+ (5). Therefore, intervening to change the unhealthiest and the two intermediary DPs toward the healthiest DP may result in lower risk of developing cervical pre-cancer in this population.
PBMC L1 methylation may serve as a unique epigenetic marker for monitoring the effectiveness of such dietary interventions. A higher degree of PBMC L1 methylation was also shown to be associated with lower risk of other cancers such as head and neck (35) and bladder (36) and also pre-cancerous conditions such as colorectal adenomas (37). Therefore, L1 methylation may serve as a biomarker for DP interventions that are targeted to reduce the risk of these cancerous and pre-cancerous conditions. To our knowledge, this is the first study to demonstrate that a pre-cancer related biomarker is associated with a DP. Future studies are needed to confirm whether the association between DP and risk of developing CIN 2+ holds in longitudinal studies and whether L1 methylation serves as a biomarker for monitoring the effectiveness of DP-based interventions for cancer prevention.
Acknowledgments
Supported by R01 CA105448 funded by the National Cancer Institute.
The staff of the Molecular Epidemiology Laboratory of Piyathilake assisted with the dietary data collection and Ilene Brill and David Helms assisted with the statistical analysis.
References
- 1.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 2.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 3.Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, van Duijnhoven FJ, Büchner FL, Key T, et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2010;102:529–37. doi: 10.1093/jnci/djq072. [DOI] [PubMed] [Google Scholar]
- 4.Tomita LY, Roteli-Martins CM, Villa LL, Franco EL, Cardoso MA BRINCA Study Team. Associations of dietary dark-green and deep-yellow vegetables and fruits with cervical intraepithelial neoplasia: modification by smoking. Br J Nutr. 2011;105:928–37. doi: 10.1017/S0007114510004447. [DOI] [PubMed] [Google Scholar]
- 5.Siegel EM, Salemi JL, Villa LL, Ferenczy A, Franco EL, Giuliano AR. Dietary consumption of antioxidant nutrients and risk of incident cervical intraepithelial neoplasia. Gynecol Oncol. 2010;118:289–94. doi: 10.1016/j.ygyno.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang JH, Lee JK, Kim TJ, Kim MK. The association between fruit and vegetable consumption and HPV viral load in high-risk HPV-positive women with cervical intraepithelial neoplasia. Cancer Causes Control. 2010;21:51–9. doi: 10.1007/s10552-009-9433-9. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh C, Baker JA, Moysich KB, Rivera R, Brasure JR, McCann SE. Dietary intakes of selected nutrients and food groups and risk of cervical cancer. Nutr Cancer. 2008;60:331–41. doi: 10.1080/01635580701861769. [DOI] [PubMed] [Google Scholar]
- 8.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Piyathilake CJ, Macaluso M, Alvarez RD, Chen M, Badiga S, Siddiqui NR, et al. A higher degree of LINE-1 methylation in peripheral blood mononuclear cells, a one-carbon nutrient related epigenetic alteration is associated with a lower risk of developing cervical intraepithelial neoplasia. Nutrition. 2011;27:513–9. doi: 10.1016/j.nut.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piyathilake CJ, Maurizio M, Alvarez RD, Bell WC, Heimburger DC, Partridge EE. Lower Risk of Cervical Intraepithelial Neoplasia in Women with High Plasma Folate and Sufficient Vitamin B12 in the Post-Folic Acid Fortification Era. Cancer Prev Res. 2009;2:658–64. doi: 10.1158/1940-6207.CAPR-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estécio MR, Gharibyan V, Shen L, Ibrahim AEK, Doshi K, He R, Jelinek J, Yang AS, Yan PS, et al. LINE-1 Hypomethylation in Cancer Is Highly Variable and Inversely Correlated with Microsatellite Instability. PLoS ONE. 2007;2:e399. doi: 10.1371/journal.pone.0000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–35. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN. Eating patterns and risk of colon cancer. Am J Epidemiol. 1998;148:4–16. doi: 10.1093/aje/148.1.4-a. [DOI] [PubMed] [Google Scholar]
- 14.Rashidkhani B, Akesson A, Lindblad P, Wolk A. Major dietary patterns and risk of renal cell carcinoma in a prospective cohort of Swedish women. J Nutr. 2005;135:1757–62. doi: 10.1093/jn/135.7.1757. [DOI] [PubMed] [Google Scholar]
- 15.Bahmanyar S, Ye W. Dietary Patterns and Risk of Squamous-Cell Carcinoma and Adenocarcinoma of the Esophagus and Adenocarcinoma of the Gastric Cardia: A Population-Based Case-Control Study in Sweden. Nutr Cancer. 2006;54:171–8. doi: 10.1207/s15327914nc5402_3. [DOI] [PubMed] [Google Scholar]
- 16.Buck K, Vrieling A, Flesch-Janys D, Chang-Claude J. Dietary patterns and the risk of postmenopausal breast cancer in a German case-control study. Cancer Causes Control. 2011;22:273–82. doi: 10.1007/s10552-010-9695-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CX, Ho SC, Fu JH, Cheng SZ, Chen YM, Lin FY. Dietary patterns and breast cancer risk among Chinese women. Cancer Causes Control. 2011;22:115–24. doi: 10.1007/s10552-010-9681-8. [DOI] [PubMed] [Google Scholar]
- 18.Bravi F, Edefonti V, Bosetti C, Talamini R, Montella M, Giacosa A, et al. Nutrient dietary patterns and the risk of colorectal cancer: a case-control study from Italy. Cancer Causes Control. 2010;21:1911–18. doi: 10.1007/s10552-010-9619-1. [DOI] [PubMed] [Google Scholar]
- 19.Kurotani K, Budhathoki S, Joshi AM, Yin G, Toyomura K, Kono S, et al. Dietary patterns and colorectal cancer in a Japanese population: the Fukuoka Colorectal Cancer Study. Br J Nutr. 2010;104:1703–11. doi: 10.1017/S0007114510002606. [DOI] [PubMed] [Google Scholar]
- 20.Toledo AL, Koifman RJ, Koifman S, Marchioni DM. Dietary patterns and risk of oral and pharyngeal cancer: a case-control study in Rio de Janeiro, Brazil. Cad Saude Publica. 2010;26:135–42. doi: 10.1590/s0102-311x2010000100014. [DOI] [PubMed] [Google Scholar]
- 21.De Stefani E, Ronco AL, Deneo-Pellegrini H, Boffetta P, Aune D, Acosta G, et al. Dietary patterns and risk of advanced prostate cancer: a principal component analysis in Uruguay. Cancer Causes Control. 2010;21:1009–16. doi: 10.1007/s10552-010-9527-4. [DOI] [PubMed] [Google Scholar]
- 22.Hajizadeh B, Rashidkhani B, Rad AH, Moasheri SM, Saboori H. Dietary patterns and risk of oesophageal squamous cell carcinoma: a case-control study. Public Health Nutr. 2010;13:1107–12. doi: 10.1017/S1368980010000145. [DOI] [PubMed] [Google Scholar]
- 23.Bastos J, Lunet N, Peleteiro B, Lopes C, Barros H. Dietary patterns and gastric cancer in a Portuguese urban population. Int J Cancer. 2010;127:433–41. doi: 10.1002/ijc.25013. [DOI] [PubMed] [Google Scholar]
- 24.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 25.Dolecek TA, McCarthy BJ, Joslin CE, Peterson CE, Kim S, Freels SA, et al. Prediagnosis food patterns are associated with length of survival from epithelial ovarian cancer. J Am Diet Assoc. 2010;110:369–82. doi: 10.1016/j.jada.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Baglietto L, Krishnan K, Severi G, Hodge A, Brinkman M, English DR, McLean C, Hopper JL, Giles GG. Dietary patterns and risk of breast cancer. Br J Cancer. 2011;104:524–31. doi: 10.1038/sj.bjc.6606044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller DC, Severi G, Baglietto L, Krishnan K, English DR, Hopper JL, et al. Dietary patterns and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:3126–9. doi: 10.1158/1055-9965.EPI-09-0780. [DOI] [PubMed] [Google Scholar]
- 28.Mizoue T, Yamaji T, Tabata S, Yamaguchi K, Shimizu E, Mineshita M, et al. Dietary patterns and colorectal adenomas in Japanese men: the Self-Defense Forces Health Study. Am J Epidemiol. 2005;161:338–45. doi: 10.1093/aje/kwi049. [DOI] [PubMed] [Google Scholar]
- 29.Austin GL, Adair LS, Galanko JA, Martin CF, Satia JA, Sandler RS. A diet high in fruits and low in meats reduces the risk of colorectal adenomas. J Nutr. 2007;137:999–1004. doi: 10.1093/jn/137.4.999. [DOI] [PubMed] [Google Scholar]
- 30.Makambi KH, Agurs-Collins T, Bright Gbebry M, Rosenberg L, Palmer JR, Adams-Campbell LL. Dietary patterns and the risk of colorectal adenomas: the Black Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2011;20:818–25. doi: 10.1158/1055-9965.EPI-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol. 2009;15:3734–43. doi: 10.3748/wjg.15.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan SY, DesMeules M, Morrison H, Wen SW Canadian Cancer Registries Epidemiology Research Group. Obesity, high energy intake, lack of physical activity, and the risk of kidney cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2453–60. doi: 10.1158/1055-9965.EPI-06-0616. [DOI] [PubMed] [Google Scholar]
- 33.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang FF, Morabia A, Carroll J, Gonzalez K, Fulda K, Kaur M, et al. Dietary patterns are associated with levels of global genomic DNA methylation in a cancer-free population. J Nutr. 2011;141:1165–71. doi: 10.3945/jn.110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ting Hsiung D, Marsit CJ, Houseman EA, Eddy K, Furniss CS, McClean MD, Kelsey KT. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:108–14. doi: 10.1158/1055-9965.EPI-06-0636. [DOI] [PubMed] [Google Scholar]
- 36.Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol. 2009;112:293–9. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim U, Flood A, Choi SW, Albanes D, Cross AJ, Schatzkin A, et al. Genomic methylation of leukocyte DNA in relation to colorectal adenoma among asymptomatic women. Gastroenterology. 2008;134:47–55. doi: 10.1053/j.gastro.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]