1. Introduction
Ablation strategies to treat atrial fibrillation (AF) have evolved from the initial focal pulmonary vein ablation approach of Haïssaguerre et al.1 to new technical developments aimed at simplifying the procedure and increasing its safety.2 However, ablation procedures are still associated with unsatisfactory rates of long-term success, particularly in persistent and long-term AF.3 We submit that such disappointing outcomes are the result of the intrinsic limitations of a strategy that has relied mainly on anatomical landmarks during the last two decades, and paid scant attention to the underlying mechanisms of the arrhythmia.
Very recently, approaches aimed at localizing and targeting AF sources based on careful analysis of the fibrillatory patterns often obtained via multiple electrode mapping systems (basket-catheter mapping, body-surface mapping and electrocardiographic imaging) have resulted in an increased specificity of ablation procedures, and long-term freedom from AF.4–7 Such mechanistically based ablation techniques have the potential to enable translational insights from experimental animal models into further improved AF ablation therapy. Indeed, they are a direct result of insights into the dynamic behavior of AF drivers, obtained from experimental optical mapping studies and computer simulations of AF dynamics.
The purpose of this article is to briefly review the following: 1) competing theories attempting to explain mechanisms underlying AF maintenance; 2) different methodologies to map electrical activity during AF; 3) the most recent mechanistic ablation approaches aimed at targeting AF drivers and their outcomes; and 4) the controversies and near-future directions in the quest to increase the understanding of AF mechanisms and improve ablation outcomes.
2. Mechanisms Underlying AF Maintenance
2.1 Historical perspective: Reentry, Ectopic Foci, Random Wavelets, CFAEs and Endo-Epicardial Breakthroughs
AF mechanisms have a long history of controversy.8 During the first half of the 20th century two theories were prevalent: the concept of reentry around an anatomical obstacle (circus movement reentry),9 and the “ectopic focus theory”.10 Focus isolation in the latter terminated the arrhythmia.10 In 1959, Moe and Abildskov postulated that fibrillation was self-sustained by multiple independent, electrical wavelets propagating randomly throughout the atria.11 Clinical support for this hypothesis seemed to come from the MAZE procedure, a surgical procedure that effectively creates an electrical maze in the atrium, allowing sinus impulses to activate the entire atrial myocardium and AV node, but disallowing maintenance of the randomly propagating wavelets that, allegedly, would sustain AF.12 Importantly, this procedure isolated the pulmonary veins that were later recognized as important triggers of AF.1
In 1977, Allessie et al. described the concept of functional reentry via the leading-circle hypothesis.13 Years later, leading-circle reentry was observed during pacing-induced AF in humans,14 and both foci and reentrant mechanisms were found during cardiac surgery in patients with permanent AF and mitral valve disease.15
A decade ago, Nademanee et al. proposed that areas with complex fractionated atrial electrograms (CFAEs) may be critical for maintaining AF and their ablation might result in better clinical outcomes.16 CFAEs were described as fractionated or continuous electrical activity at short cycle lengths.16 CFAEs may colocalize with potential drivers of AF (rotor cores).17 However, most CFAEs are passive, consequence of fibrillatory conduction, wavefront collision, drifting/aceleration of rotors,18 fibrosis,19 or might be even considered an artifact of the bipolar recording methodology since they are a function of the inter-electrode distance.20
More recently, the so-called “double layer hypothesis” based on high-density atrial data has been put forth,21 suggesting that persistent AF is maintained by endo-epicardial breakthroughs acting as randomly distributed “multiplication sites” of fibrillation waves. Such breakthroughs are envisioned to continuously generate new breakthroughs emerging at the contralateral layer, sometimes annihilating other preexistent waves in the new layer.21 A double layer mechanism of persistent AF would have important practical consequences since it would make it impossible for a limited ablation to successfully restore sinus rhtyhm.21 However, it has been reported that acute termination of AF can be achieved after ablation at specific target sites in most paroxysmal and persistent AF patients,4–7, 22 which supports the idea of driving sources underlying AF.
2.2 Rotors and Scroll Waves
The use of voltage-sensitive probes and high-resolution video imaging in isolated animal hearts has provided support for the hypothesis that AF may be driven by one or a small number of high-frequency rotors.23–29 Rotors are 3D electrical objects (scroll waves) but current technical limitations restrict their detection to 2 dimensions (Figure 1A) either from the epicardial or the endocardial surface. In theory, a typical scroll wave rotates around a linear I-shaped filament (Figure 1B) that spans the atrial wall, although filaments may also acquire more complex shapes (L-shape, U-shape, O-shape).31 Often, one can only observe indirect footprints of scroll waves in the form of breakthrough patterns resembling focal activation.23 In addition, the true drivers that maintain AF are those with the highest rotation frequency, and whose domain of 1:1 activation is the narrowest, which makes it extremely difficult to find them even at the highest spatio-temporal resolution provided by optical mapping. The waves generated by such rotors propagate through the cardiac muscle and interact with anatomic and functional obstacles leading to fibrillatory conduction, fragmentation and new wavelet formation.30 Narayan et al. have recently reported evidence of stable rotors in human patients with either paroxysmal, persistent or long-lasting AF.7 Haïssaguerre et al. have also recently reported that 80% of the drivers during persistent AF were reentrant. Most rotors and focal breakthroughs co-localized and ~70% of them were in the left atrium (Figure 1C).6 Therefore, not only experimental but also growing clinical evidence indicates that rotors can be key players in AF maintenance.
Figure 1.
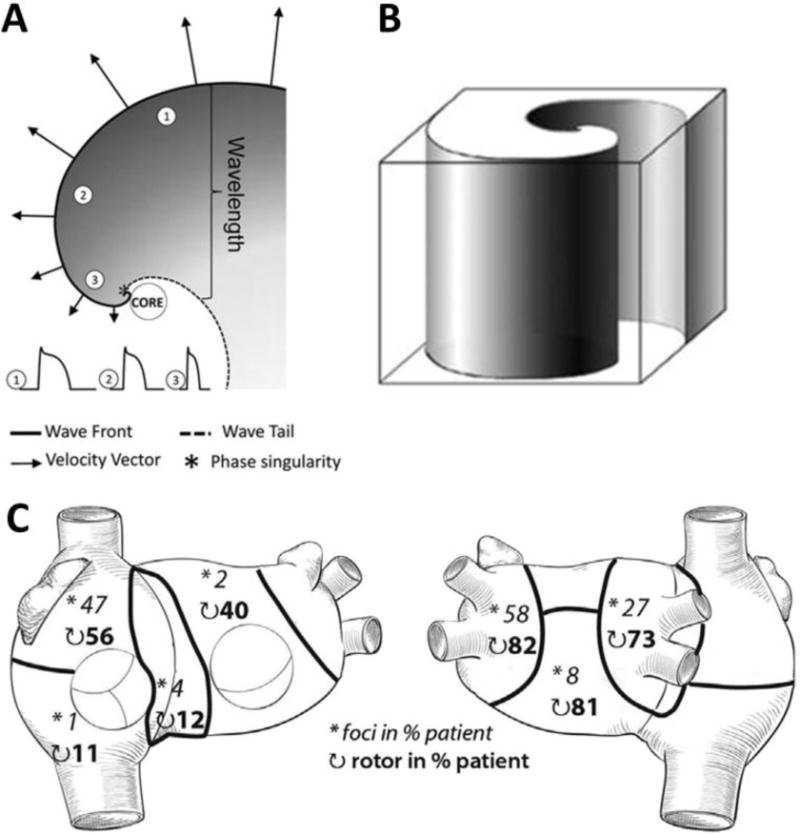
A. Wavefront (solid line) and wavetail (dashed line) near a rotor core. Wavefront velocity (arrows) decreases when proximity to the core increases. The wave is blocked at the tip of the rotor as a result of the high curvature of the wavefront which creates a great imbalance between the depolarizing charge of the wave and the surrounding unexcited tissue (sink-to-source mismatch). Thus, the wave pivots around a singularity point in the excitable but unexcited core of the rotor. Wavelength is defined as the distance between the depolarizing front and the repolarizing tail. B. Schematic representation of a three-dimensional scroll wave. Its epicardial and endocardial manifestation would be a two-dimensional rotor as the one depicted in A. C. Spatial distribution of drivers (asterisks: focal breakthroughs, curved arrows: reentry events) reported as the percentage of patients who presented such drivers in persistent AF (A and B reproduced from Pandit & Jalife,30 C reproduced from Haïssaguerre et al.6)
3. Mapping Methods to Localize Sources and Understand AF Dynamics
3.1 Spectral Analysis and Phase Mapping
Both spectral analysis and phase mapping of cardiac potentials can be applied to any of the approaches described in the next sub-sections. Both algorithms derive originally from optical mapping experiments conducted in isolated hearts.23, 32
Spectral analysis is used to map the distribution of AF frequencies and localize the areas with the highest activation frequencies (i.e., the shortest cycle lengths),23 which usually coincide with the location of AF sources (foci or rotors) that maintain AF.4, 5 A good tutorial for clinicians on spectral analysis of AF electrograms may be found elsewhere.33 Briefly, the power spectrum of a signal displays its energy/power distribution in the frequency domain. The frequency of the highest peak in the spectrum at one location is called dominant frequency (DF), which is often used as a surrogate for the average activation rate (i.e., 1/cycle length) at that location (Figure 2A).33 Spectral analysis becomes particularly useful when the activation rate is difficult to measure in the time domain, as may happen during AF. Figure 2B–C illustrates examples of DF maps obtained during optically mapped AF, where areas of maximum DF (DFmax) thought to drive the fibrillation process are displayed. These areas are surrounded by others that are incapable of activating at such exceedingly high frequencies and depict fibrillatory conduction.30
Figure 2.
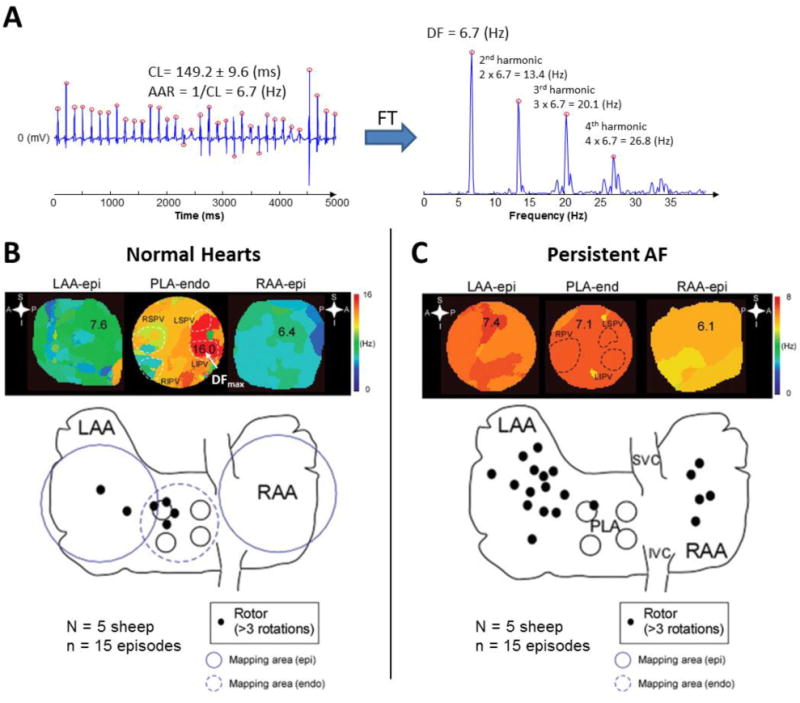
A. Bipolar electrogram obtained from the left atrial appendage of a patient with AF. Red circles display activation times. CL: cycle length, AAR: average activation rate, FT: Fourier Transform, DF: Dominant Frequency. Note that the fundamental peak of the spectrum is located at a frequency (DF) that matches the AAR. The peaks located at the frequencies multiple of the DF are called harmonics. A harmonic peak should not be selected as DF since this would lead to wrong conclusions. B. Top: DF maps in a normal sheep heart during stretch-induced AF showing fibrillatory conduction from the highest frequency domain (DFmax, 16 Hz, in red) located at the PLA, specifically at the LSPV and LIPV. Colors in each map indicate atrial areas (domains) with different DFs. The color bar on the right indicates frequency in Hz. A large frequency gradient between the fastest (red) and the slowest (blue) domains is usually found in acute episodes of AF. Bottom: Aggregated data from normal sheep hearts during stretch-induced AF showed that rotors were mainly located at the PLA and LAA. C. Top: DF maps of atria from sheep with persistent AF where a more homogeneous distribution of DFs (lower gradient) was found. The DFmax domain was located at the LAA (in red, 7.4 Hz). Colors in each map indicate atrial areas (domains) with different DFs. The color bar on the right indicates frequency in Hz. As shown, in persistent AF, the frequency gradient between the fastest and the slowest domains is usually smaller than in paroxysmal episodes. Bottom: Aggregated data from hearts showed that, in persistent AF, the role of the pulmonary veins becomes less prevalent in terms of harboring rotors. Endo: endocardial view, Epi: epicardial view, LAA: left atrial appendage, LIPV: left inferior pulmonary vein, LSPV: left superior pulmonary vein, PLA: posterior left atrium, RAA: right atrial appendage, RIPV: right inferior pulmonary vein, RPV: right pulmonary vein (B and C adapted from Yamazaki et al.28 by permission of Oxford University Press).
Phase mapping is a complementary approach that enables visualization of the spatiotemporally distributed patterns of propagation during cardiac fibrillation by determining the local phase of the activation/recovery cycle at each time point. It makes it possible to detect the phase singularities (i.e., the rotor pivots) that organize reentry and fibrillation (Figure 3). It can also be used to analyze electrograms,34 although optical mapping (the gold standard) provides much higher resolution and accuracy in space and time when tracking rotor formation and maintenance.35 A tutorial on phase mapping during cardiac fibrillation can be found elsewhere.34
Figure 3.
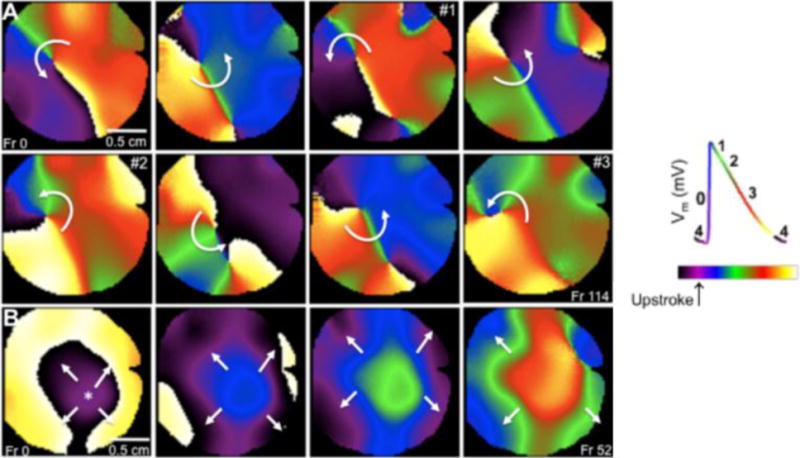
Varying activation patterns identified in phase movies from the left atrium of the sheep heart. A. Sequential snapshots show a rotor pivoting around a phase singularity (point where all phases converge). B. Breakthrough activation pattern. The wave appears on the center of the field of view and propagates outward. Right: key for the different phases of the action potential is color-coded (reproduced from Filgueiras-Rama et al.24)
3.2 Simultaneous Epicardial and Endocardial Optical Mapping
The introduction of optical mapping over two decades ago enabled the characterization of wave propagation with sub-millimeter resolution and postulated rotors as potential drivers of cardiac fibrillation in animal models.23, 26, 29, 32, 36–38 More recently, recording voltage-sensitive dye fluorescence simultaneously from the endocardial and epicardial surfaces (Figure 4A)24, 25, 27, 28 enabled interpretation of the 3D behavior of scroll waves. Generation of phase movies identified several patterns of activation, including rotors (Figure 3A) and breakthroughs (Figure 3B). Interestingly, these experiments identified I-shaped scroll waves in the left atrium spanning from epicardium to endocardium.28 As expected, each mapped surface showed synchronized two-dimensional rotors (Figure 4B). Other recorded patterns also suggested the presence of L- or U-shaped intramural scroll waves by the presence of differing activation patterns at each surface (Figure 4B).27 Very recently, simultaneous subendo-subepicardial optical mapping of ex-vivo diseased human right atria has shown that pinacidil-induced fibrillatory activity was driven by spatially and temporally stable intramural reentry anchored to fibrosis-insulated and twisted atrial bundles. Radiofrequency ablation of these localized micro-reentrant tracks terminated the fibrillatory activity.39 However, current optical mapping strategies do not allow panoramic endocardial mapping and require the use of toxic voltage-sensitive dyes that preclude their use for human in-vivo studies.
Figure 4.
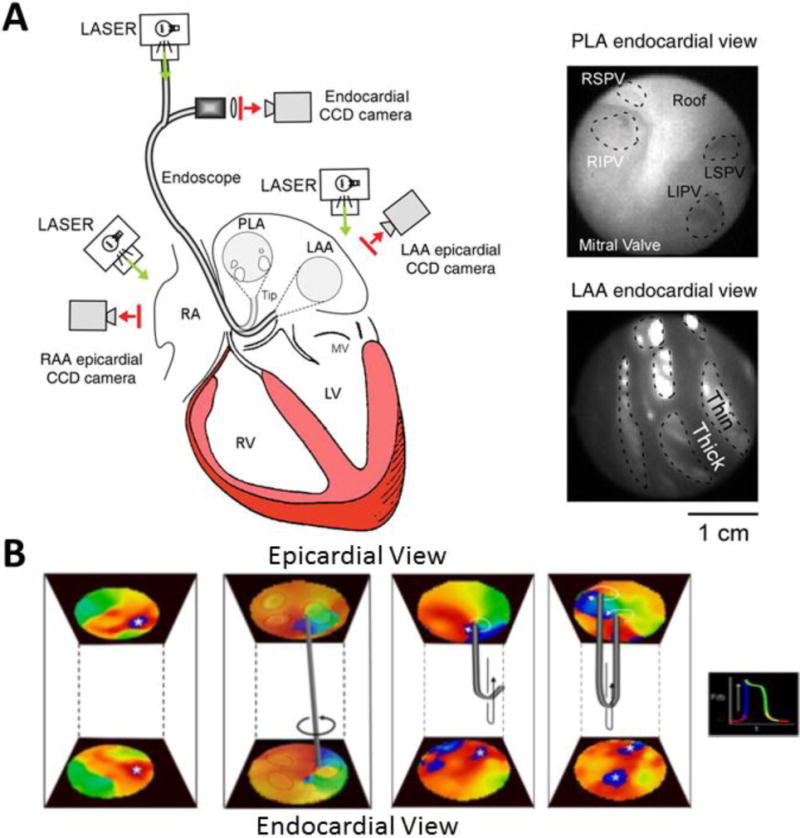
A. Optical mapping setup for simultaneous endo-epicardial mapping. An endoscope is focused on the endocardial surface of the posterior left atrium (PLA) or left atrial appendage (LAA). A CCD camera is coupled to the endoscope and laser illumination is provided. Epicardial mapping of PLA or LAA is simultaneously performed. B. From left to right: examples of simultaneous endo-epicardial breakthrough activation and potential intramural I-shaped, L-shaped and U-shaped scroll waves (A reproduced from Yamazaki et al.28 by permission of Oxford University Press; B adapted from Yamazaki et al.27 with permission from Elsevier).
3.3 Multielectrode Plaque Epicardial Electrical Mapping
Over the years, many studies have used high-density multielectrode plaques to record local epicardial electrical activity from patients undergoing cardiac surgery.14, 15, 21, 40 These approaches have the advantage of not requiring the use of toxic voltage sensitive dyes, which makes the technique suitable for in-vivo mapping in humans. However, placing multielectrode plaques on the epicardium requires open chest surgery and is limited to small areas with limited spatial resolution when compared to optical mapping, though occasionally it is possible to identify rotational activation sequences which might be compatible with rotors (Figure 5).40
Figure 5.
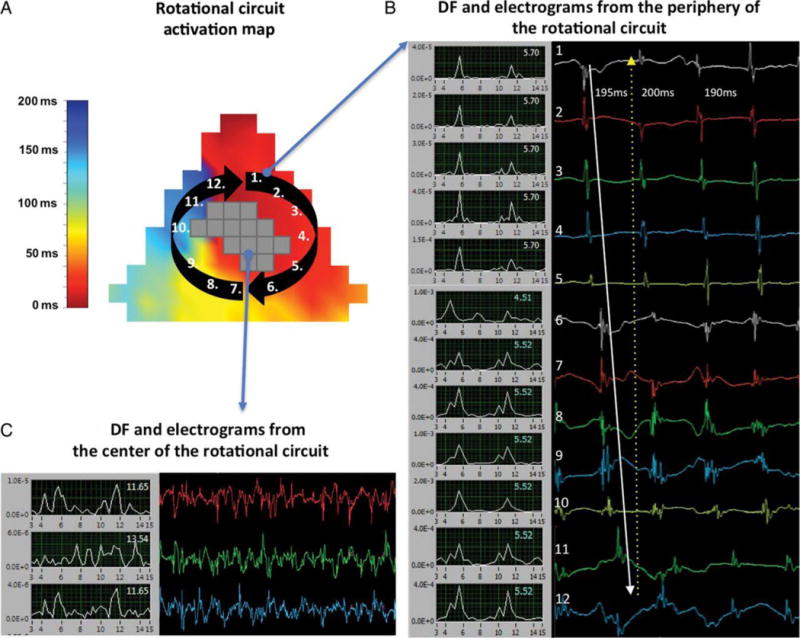
A. Activation map of a clock-wise revolution of a rotational circuit at the posterior left atrial wall. Electrodes registering CFAEs at the core are shown in grey (no local activation times were assigned given their ambiguity). B. Frequency domain (left, 3–15 Hz) and time domain (right) electrograms from sites just outside the core (1–12). The blue numbers in the frequency domain signals display dominant frequencies. The white arrow in the time domain displays the clock-wise sequence of activation. C. Frequency domain (left, 3–15 Hz) and time domain (right) CFAEs from sites inside the core (reproduced from Lee et al.40 by permission of Oxford University Press).
3.4 Basket-Catheter Endocardial Electrical Mapping
Recently, Narayan et al. used two 64-pole basket-catheters to obtain simultaneous unipolar endocardial electrograms from 128 locations in both atria of patients undergoing AF ablation (Figure 6A).7 A computational mapping system (RhythmView™, Topera, Inc.) processed these electrograms to generate activation movies of the atrial electrical activity after considerable processing and interpolation.41 Local activation times were calculated as the times when each unipolar electrogram crosses a certain voltage threshold. Monophasic action potentials (MAPs) were used to establish the minimum repolarization interval and obtain physiologically feasible sequential activation paths. Movies of activation patterns and isochronal maps from individual cycles were obtained after using bi-linear interpolation of the “phase state” between each electrode and its nearest neighbors (Figure 6B).41 Rotational activities (early-meets-late, i.e. red-meets-blue) around a center of rotation were identified as rotors. Focal (centrifugal) activations were also identified. Both were considered drivers only if they sustain for ≥50 rotations or focal discharges (arbitrary limit).7 The main advantages of this approach are that, in contrast to the electrocardiographic imaging (ECGI) method described in section 3.6, i) it does not use estimated but real cardiac potentials and ii) enables real-time assessment of changes in driver activity during the ablation procedure. More arguments in favor of this mapping approach can be found elsewhere in a recent theoretical study.41
Figure 6.
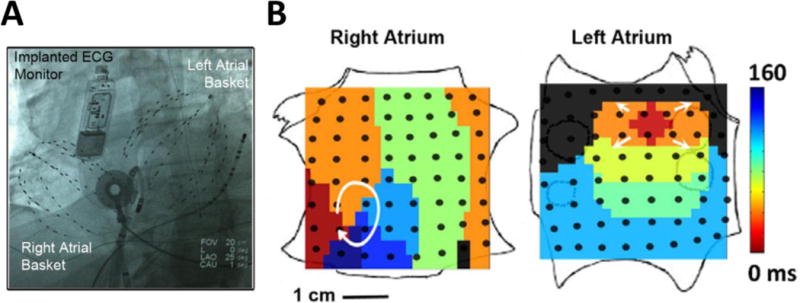
A. Two 64-pole basket-catheters deployed in both atria. B. Maps showing a stable clockwise right atrial rotor and a simultaneous left atrial breakthrough in a patient with persistent AF. One year after ablating these two “drivers” the patient remained AF-free (reproduced from Narayan et al.7 with permission from Elsevier).
However, there are a number of limitations that need to be considered: i) the system uses a proprietary algorithm which makes the methodology difficult to evaluate since the electrograms from which the color maps are obtained are not usually shown; ii) like any other electrode recording system, extracellular signals are subject to artifacts,35 and ventricular activity often contaminates atrial recordings; thus an appropriate QRST subtraction technique is critical; iii) basket-catheters often offer suboptimal electrode-tissue contact at many poles; iv) the splines are sometimes not equidistantly separated once they are deployed in the atria and the raw inter-spline spatial resolution offered by basket-catheters is poor; v) in the presence of scarce or poor quality data the amount of extrapolation is difficult to determine; vi) interpolation of phases is inherently biased toward detection of rotors as the algorithm is devised to demonstrate rotational activity. Thus, focal activation might be displayed as rotational activity if the wavefront reaches the surrounding electrodes sequentially.35 Although the approach is not universally accepted, it is one of the first mechanistically based approaches to AF ablation that has reported promising results in the long-term42 and during its first multicenter validation.22
3.5 Body Surface Mapping (BSM)
The non-invasive BSM methodology has recently gained visibility for the analysis of activation patterns during AF.43, 44 Guillem et al. used a custom-made 67-electrode vest that covered the whole torso of the patient (Figure 7A–B); intracardiac signals at several locations were simultaneously recorded.43 They selected either segments without ventricular activity after adenosine infusion, or applied complex subtraction of QRST if such intervals were not found. After computing and performing comparisons between intracadiac and surface DF maps, they demonstrated that high-frequency sources could be reflected on a small area of the body surface close to the atrium harboring the highest DF (Figure 7C).43 More recently, Rodrigo et al. used phase-mapping and filtered the unipolar signals with a narrow 2-Hz bandpass around the highest DF (HDF filtering) to improve the detection of stable rotors (8.3% of the time before filtering, 73% after filtering, Figure 7D–E).44 Prior to HDF band-pass filtering, phase maps displayed unstable reentries, likely as a result of superposition of the disorganized electrical activity coming from the rest of atrial tissue. HDF filtering accentuated the organized activity of scroll waves, after which rotors were the main pattern of activation during AF (median of 2.8 rotations, present 73% of the time). Also, computer simulations showed that epicardial propagation is spatially smoothened when projected on the torso. For example, nearby epicardial rotors with opposing chirality may not be detected on the torso. This fact and the possibility of temporal intervals in which AF activity may be affected by ectopic foci, could explain the lack of detected rotors during the remaining 27% of the time.44
Figure 7.
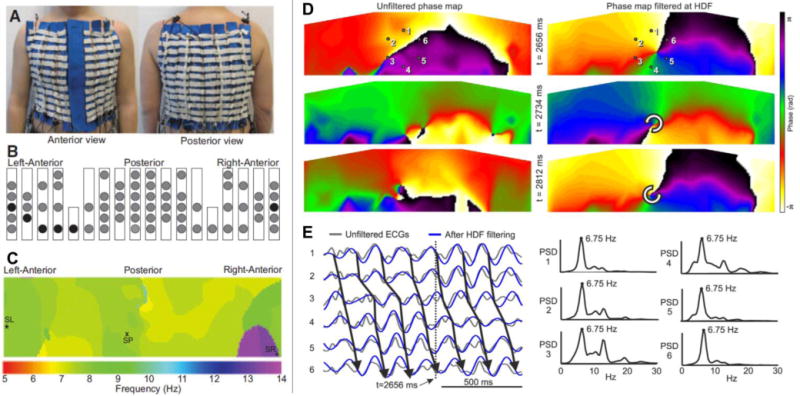
A. Custom-made vest with 67 electrodes. B. Location of surface electrodes (circles). C. DF map of the torso during AF with right atrium faster than left atrium. D. Phase movie snapshots before and after HDF bandpass-filtering to improve detection of stable reentries during AF. E. Left: Unipolar electrograms in the 6 points around the core of rotation in D before (black) and after (blue) HDF filtering. Right: Their power spectrum before HDF filtering showing the rotor activity at 6.75 Hz and residual disorganized activity coming from the rest of the atria (A–C reproduced from Guillem at al.43, D–E reproduced from Rodrigo et al.44 with permission from Elsevier).
3.6 Electrocardiographic Imaging (ECGI)
The output of BSM is potentials on the body surface, not on the heart. In contrast, the ECGI mapping system developed by Rudy’s laboratory (Figure 8A) provides estimated epicardial electrograms in relation to the heart’s anatomy. The approach utilizes over 250 electrodes distributed throughout the patient’s torso, which in combination with computed tomography (CT) imaging enables the positioning of the heart’s epicardial geometry in relation with the electrode positions. The body surface potentials recorded by the electrodes and the geometric information provided by CT are combined by complex mathematical algorithms that solve the electrocardiographic inverse problem in order to noninvasively obtain estimated epicardial electrograms.45, 46
Figure 8.
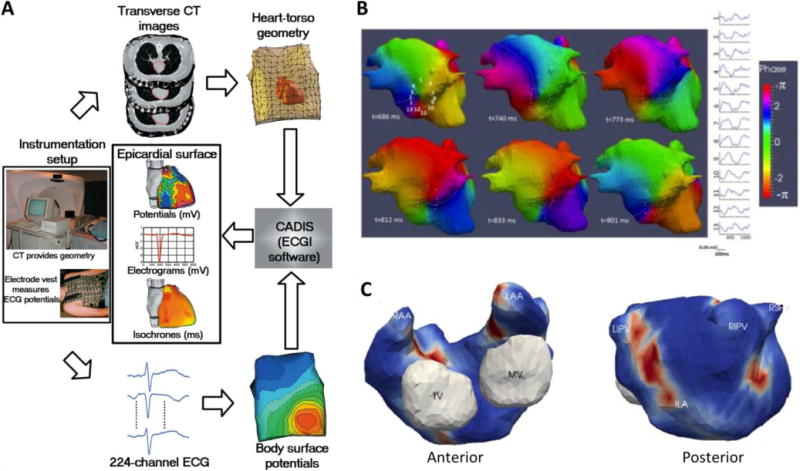
A. Block diagram of the electrocardiographic imaging (ECGI) procedure. B. Phase mapping of ECGI data in a persistent AF patient. A full rotation of a rotor in the inferior left atrium is displayed. Electrograms around its core are also shown (1–12). C. Example of a driver density map used to guide the ablation process in a patient with drivers clustered in 3 regions: inferoposterior left atrium (ILA) and right and left pulmonary veins (A reprinted from Ramanathan et al.45 by permission from Macmillan Publishers Ltd; B–C reproduced from Haïssaguerre et al.6).
The main advantage of this approach is that it is non-invasive and it does not require surgery or sedation, and could be used to provide detailed data on follow-ups and AF recurrence.45, 46 However, limitations similar to the BSM approach apply, since AF body surface potentials often have low voltages and are smoothed by the torso volume-conductor.44, 46 These include poor sensitivity for detecting very low amplitude signals (e.g. from scarred and/or previously ablated areas) or highly localized sources.44, 46 Indeed, ECGI does not discern among epicardial breakthroughs, spontaneous depolarizations and microreentry (subcentimeter), which are seen as focal activity.46 Also, ECGI is limited to providing virtual electrograms of the atrial epicardium; activity on the interatrial septum, pulmonary vein-left atrial appendage ridge, etc, is not recorded. Importantly, CT imaging is required to obtain the torso geometry, so the patient is exposed to a considerable dose of radiation.45, 46
Haïssaguerre et al. have recently employed the ECGI approach,45, 46 combined with phase mapping to detect AF drivers during persistent AF and guide the ablation procedure.6 Intervals (≥1s) free from ventricular activity were selected for analysis to avoid QRST subtraction.6 Phase mapping movies were displayed over the biatrial geometry. AF sources were classified as either focal or reentry. In the latter, raw local electrograms were visualized to confirm their truly reentrant nature (sequential activation around the core, Figure 8B). The authors reported that these rotors moved over wide regions of the atria, but recurred periodically in the same region. The median and maximum number of rotations per rotor was 2.6 and 8, respectively.
4. Outcomes of New Mapping and Mechanistic Approaches for AF Ablation
PVI remains the ‘gold standard’ approach to treat drug-refractory AF. However, its success rate remains suboptimal especially in patients with persistent AF in whom more extensive ablation techniques have been proposed.3 Targeting CFAEs and/or linear ablation are some of the complementary strategies after PVI.3 Recent results from the STAR-AF 2 trial have shown that the addition of further ablation (lines or CFAEs) to PVI increased ablation time but did not reduce the recurrence of AF in 589 persistent AF patients.3 At 18 months, the percentage of patients who were free from AF recurrence after one procedure without anti-arrhythmic medication did not significantly differ among groups (PVI alone 48%; PVI+CFAEs 37%; and PVI+lines 33%).3 This confirms that CFAE and extensive ablation has clearly lost momentum in favor of more specific and mechanistically based approaches for AF ablation.
4.1 Ablation of High Dominant Frequency Sites
Since atrial regions with the highest activation frequencies (DFmax) may host the drivers that maintain AF,4, 5, 23 targeting DFmax areas may effectively terminate AF. Atienza et al.4 first used such a strategy in AF patients.4 They used an electroanatomical mapping system with embedded real-time spectral analysis capabilities to generate DF maps during sequential acquisition of bipolar signals.4 PVI was complemented with ablation of DFmax sites. This protocol achieved 88% and 56% long-term freedom of AF in paroxysmal and persistent AF patients, respectively, in all cases a DF gradient was abolished by the ablation.4 Even though sequential point-by-point acquisition of bipolar signals is limited in its ability to identify high frequency sources that are often dynamic, and may drift and/or change over time, the recent multicenter RADAR-AF trial conducted in 232 patients demonstrated that targeting DFmax sites in paroxysmal AF was not inferior to, and was associated with a lower incidence of severe adverse events than conventional PVI at 1-year follow-up.5 Although a pure DFmax mapping strategy has not been compared with PVI in persistent AF cases, DFmax ablation after PVI did not improve ablation outcomes and significantly increased the risk of adverse events.5 These results have been interpreted by some as “more evidence favoring antral PVI in patients with either paroxysmal or persistent AF”.47 However, an alternative interpretation is that i) PVI is not the “cure” for all AF; ii) extensive ablation procedures that include PVI predict a poor long-term outcome;3 and iii) there is an urgent need for more mechanistically based mapping to guide limited ablation strategies.
4.2 Focal Impulse and Rotor Modulation (FIRM) Ablation
The novel computational mapping approach described in section 3.4 has recently shown localized sources (long-standing rotors or foci) sustaining the arrhythmia in 98% of AF patients.7 The patients (71% with persistent AF) were prospectively treated by FIRM-guided ablation followed by PVI versus PVI alone. In each case a median of 2 sources were classified as re-entrant. The number of sources was significantly higher in persistent than in paroxysmal AF patients. Interestingly, AF acutely terminated in 56% of cases after brief ablation of the primary source without performing PVI. Conversely, AF termination was only achieved in 20% of cases that underwent conventional PVI. Recently, an extended 3-year follow-up results of the trial have been reported.42 Patients in the FIRM-guided ablation group maintained higher rates of freedom from AF (78% vs. 36%) after a single procedure. However, it is important to note that FIRM-guided ablation protocol included not just the ablation of potential drivers, but also, PVI and other standard procedures.42 The initial independent outcomes for FIRM ablation at 10 experienced centers have been also reported.22 Each patient exhibited a similar mean of rotors/focal sources (≈2.3) as previouly reported (25% in the right atrium). After a single-procedure and 1-year follow-up freedom from AF was 87.5%.22
4.3 Ablation of Driver Domains in Persistent AF
Recently, Haïssaguerre et al. have reported the results of their mechanistic approach in persistent AF ablation.6 They used ECGI (section 3.6) combined with phase mapping (section 3.1) to identify the drivers of persistent AF in 103 patients undergoing AF ablation. They observed incessantly changing wavefronts and a varying spatiotemporal behavior of drivers.6 Reentrant drivers were unsustained and meandered substantially, but recurred repetitively in the same region. Aggregated driver-density maps (Figure 8C) were computed over a cumulative registering period to guide the ablation procedure (median: 4 driver domains per patient). Interestingly, the number of driver regions increased with the continuous duration of AF. Ablation of driver domains alone terminated AF in 63% of patients presenting in AF (75% persistent and 15% long-lasting persistent AF), which supports the presence of stable high-frequency sources at certain regions of the atria. The onset or extinction of drivers during ablation was not assesed, so there is room for improving the ablation results if real-time data are used.6 After a 12-month follow-up, 83% and 75% of patients with paroxysmal or persistent/long-lasting AF were free from AF, respectively.6
5. Controversies and Future Directions
Multiple experimental and clinical studies using widely different technologies are creating a growing body of evidence in favor of organized sources underlying AF maintenance. It remains unresolved whether such sources are: i) one or a few stationary and stable rotors or micro-anatomic intramural reentries, ii) multiple short-lived but iteratively created rotors at restricted locations, iii) stable ectopic foci, or iv) short-lived but repetitively triggered focal bursts. Interestingly, some of these seemingly different underlying mechanisms might converge if one considers the different mapping, filtering and interpolation approaches that are being used to display the atrial electrical data, the 3D nature of the atrial tissue, and the imperfections of the recording systems being used. For example, although apparently different, rotors/intramural reentry and foci may be dynamic manifestations of the same mechanism within the 3D structure of the atria. Thus, so-called foci might actually represent breakthroughs generated by intramural scroll-waves with nonlinear filament shapes (U-shape, L-shape, or even O-shape, Figure 4B),27, 28, 31 or micro-anatomic intramural reentries.39
The non-invasive ECGI approach by Cuculich et al. showed foci, but only rarely demonstrated reentry in AF.46 Rotors seemed short-lived but their location was reproducible through several ECGI movies.46 However, ECGI lacks the ability to discern among epicardial breakthroughs, spontaneous depolarizations and (subcentimeter) microreentry, which are seen as focal activity.46 In addition, in BSM (and very likely in ECGI), appropriate filtering enables detection of more stable reentrant high-frequency sources by suppressing secondary influences from disorganized activity.44 Although not fully-disclosed, the ECGI/phase mapping approach used by Haïssaguerre et al.6 might have included similar filtering, since similar stabilities of rotors were reported in both studies (median: 2.8 rotations in Rodrigo et al.44, 2.6 rotations in Haïssaguerre et al.6). In both studies, rotors were detected most of the time.6, 44 The presence of fast reentrant sources and the concept that targeting limited areas may be enough to terminate AF is supported by the outcomes of recent studies.4–7, 39, 42 In contrast, it is now clear that targeting CFAEs and/or performing additional linear ablation add unnecessary ablation due to lack of specificity.3
Even though low-density basket-catheter endocardial electrical mapping7 and non-invasive BSM/ECGI approaches6, 44 provide seemingly different descriptions of the drivers maintaining human AF, both coincide in their mostly reentrant nature. The former describe long-standing drivers.7 The latter observe substantial drifting and repetitive short-lived reentries requiring statistical driver density maps.6 Although the interaction of very stable high frequency sources (e.g. intramural scroll waves) can produce the onset of repetitive drifting rotors,37 these differences most likely rely on the filtering methods in BSM/ECGI approaches and the extent of interpolation used in basket-catheter mapping. Thus, excessive interpolation/filtering or lack thereof may lead to differing conclusions about the actual stability of the sources driving AF.
From the foregoing it can be surmised that rotors are an important mechanism for sustaining AF in humans, although other mechanisms might certainly be involved in initiation and specific cases of AF maintenance.48 Nowadays, experimental data and ablation outcomes are making it increasingly apparent that sequential acquisition of epicardial electrical data over small areas of the atria during cardiac surgery21 may be insufficient as it might simply show epiphenomena, reflecting disorganized activity (fibrillatory conduction).
There is still substantial room for improvement in detecting AF drivers. New technological breakthroughs to obtain experimental and clinical panoramic data will certainly help in tracking drifting rotor trajectories over wide areas of the atria. In the clinic, a panoramic, direct-contact endocardial mapping system with higher density of electrodes than currently available would offer enhanced spatial resolution and help increase accuracy. This would provide more and better signals to compute, minimizing interpolation in the maps used to guide the ablation procedure. In this regard, new basket-catheters with more electrodes might be very useful. In both experimental and clinical scenarios, robust algorithms to track drifting, meandering or stationary rotors over long periods of time would be of interest.37 We recently showed that “beat phenomenon” and “amplitude modulation” patterns in cardiac potentials can be useful to track rotors.37 Potentially, this strategy might be used during AF even with a single mapping catheter. In addition, a combined activation, phase and frequency mapping approach might prove extremely useful. Areas allegedly hosting rotors or foci in endocardial basket-catheter or BSM/ECGI maps detected by any of these mapping approaches could be locally re-mapped with much higher spatial-resolution using a novel 64-pole mini-basket catheter (IntellaMap Orion™, Boston Scientific) to confirm or exclude the existence of a potential driver.
6. Concluding Remarks
Recent studies using differing novel mapping approaches specifically targeting potential AF sources have been reported with very promising results. Such mechanistically based ablation techniques evolved thanks to insights into the dynamic patterns of AF provided by optical mapping studies and computer-based simulations of AF dynamics,23–29, 32, 36, 38 which yielded algorithms that are now being used in point-by-point sequential mapping,4, 5 basket-catheter mapping,7, 22 BSM43, 44 and ECGI.6 Interestingly, all data generated to date are compatible with the concept that a small number of localized high-frequency sources can maintain AF for relatively long periods of time. It is our expectation that, in the near future, 1) simultaneous panoramic endocardial and epicardial optical mapping in the experimental laboratory, 2) higher density electrical mapping combined with activation, frequency and phase mapping in the clinical laboratory, and 3) new algorithms to accurately detect and track rotors and foci in both scenarios will provide a more complete understanding of AF driver dynamics. Hopefully the increase in knowledge will improve the acute and long-term success rates of AF ablation to the benefit of the patient.
Acknowledgments
Funding Supported by grants from Fondo Europeo de Desarrollo Regional (FEDER) and Instituto de Salud Carlos III [RD12/0042/0036 (RIC), RD06/0003/0009 (REDINSCOR)]. The CNIC is supported by the Spanish Ministry of Economy and Competitiveness and the Pro-CNIC Foundation. Supported also by the Salud 2000 Foundation (DFR); the Leducq Foundation (JJ and OB), the University of Michigan Health System and Peking University Health Sciences Center Joint Institute for Translational and Clinical Research; grants R01-HL122352 (JJ) and R01-HL118304 (OB) form the US NIH.
Footnotes
Disclosures
JJ serves on the Scientific Advisory Board of Topera, Inc. OB is cofounder and scientific officer of Rhythm Solutions, Inc.
Journal Subject Codes: [5], [22], [106], [132]
References
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Dewire J, Calkins H. Update on atrial fibrillation catheter ablation technologies and techniques. Nat Rev Cardiol. 2013;10:599–612. doi: 10.1038/nrcardio.2013.121. [DOI] [PubMed] [Google Scholar]
- 3.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P, Star-AF II Investigators Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 4.Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernández-Avilés F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atienza F, Almendral J, Ormaetxe JM, Moya A, Martínez-Alday JD, Hernández-Madrid A, Castellanos E, Arribas F, Arias MA, Tercedor L, Peinado R, Arcocha MF, Ortiz M, Martínez-Alzamora N, Arenal A, Fernández-Avilés F, Jalife J, Radar-AF Investigators Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: A noninferiority randomized multicenter radar-af trial. J Am Coll Cardiol. 2014;64:2455–2467. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 6.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 7.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovasc Res. 2011;89:766–775. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis T, Fell H, Stroud W. Observations upon flutter and fibrillation. Heart. 1920;7:191–233. [Google Scholar]
- 10.Scherf D. Studies on auricular tachycardia caused by aconitine administration. Proc Soc Exp Biol Med. 1947;64:233–239. doi: 10.3181/00379727-64-15754. [DOI] [PubMed] [Google Scholar]
- 11.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 12.Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg. 1991;101:584–592. [PubMed] [Google Scholar]
- 13.Allessie MA, Bonke FI, Schopman FJ. The “leading circle” concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 14.Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 15.Nitta T, Ishii Y, Miyagi Y, Ohmori H, Sakamoto S, Tanaka S. Concurrent multiple left atrial focal activations with fibrillatory conduction and right atrial focal or reentrant activation as the mechanism in atrial fibrillation. J Thorac Cardiovasc Surg. 2004;127:770–778. doi: 10.1016/j.jtcvs.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 17.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–854. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atienza F, Calvo D, Almendral J, Zlochiver S, Grzeda KR, Martínez-Alzamora N, González-Torrecilla E, Arenal A, Fernández-Avilés F, Berenfeld O. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081–1092. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashihara T, Haraguchi R, Nakazawa K, Namba T, Ikeda T, Nakazawa Y, Ozawa T, Ito M, Horie M, Trayanova NA. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: Implications for electrogram-based catheter ablation. Circ Res. 2012;110:275–284. doi: 10.1161/CIRCRESAHA.111.255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correa de Sa DD, Thompson N, Stinnett-Donnelly J, Znojkiewicz P, Habel N, Muller JG, Bates JH, Buzas JS, Spector PS. Electrogram fractionation: The relationship between spatiotemporal variation of tissue excitation and electrode spatial resolution. Circ Arrhythm Electrophysiol. 2011;4:909–916. doi: 10.1161/CIRCEP.111.965145. [DOI] [PubMed] [Google Scholar]
- 21.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 22.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: Multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berenfeld O, Mandapati R, Dixit S, Skanes AC, Chen J, Mansour M, Jalife J. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the langendorff-perfused sheep heart. J Cardiovasc Electrophysiol. 2000;11:869–879. doi: 10.1111/j.1540-8167.2000.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 24.Filgueiras-Rama D, Martins RP, Mironov S, Yamazaki M, Calvo CJ, Ennis SR, Bandaru K, Noujaim SF, Kalifa J, Berenfeld O, Jalife J. Chloroquine terminates stretch-induced atrial fibrillation more effectively than flecainide in the sheep heart. Circ Arrhythm Electrophysiol. 2012;5:561–570. doi: 10.1161/CIRCEP.111.966820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filgueiras-Rama D, Price NF, Martins RP, Yamazaki M, Avula UM, Kaur K, Kalifa J, Ennis SR, Hwang E, Devabhaktuni V, Jalife J, Berenfeld O. Long-term frequency gradients during persistent atrial fibrillation in sheep are associated with stable sources in the left atrium. Circ Arrhythm Electrophysiol. 2012;5:1160–1167. doi: 10.1161/CIRCEP.111.969519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki M, Filgueiras-Rama D, Berenfeld O, Kalifa J. Ectopic and reentrant activation patterns in the posterior left atrium during stretch-related atrial fibrillation. Prog Biophys Mol Biol. 2012;110:269–277. doi: 10.1016/j.pbiomolbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki M, Mironov S, Taravant C, Brec J, Vaquero LM, Bandaru K, Avula UM, Honjo H, Kodama I, Berenfeld O, Kalifa J. Heterogeneous atrial wall thickness and stretch promote scroll waves anchoring during atrial fibrillation. Cardiovasc Res. 2012;94:48–57. doi: 10.1093/cvr/cvr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 30.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–862. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wellner M, Berenfeld O, Jalife J, Pertsov AM. Minimal principle for rotor filaments. Proc Natl Acad Sci U S A. 2002;99:8015–8018. doi: 10.1073/pnas.112026199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 33.Ng J, Goldberger JJ. Understanding and interpreting dominant frequency analysis of af electrograms. J Cardiovasc Electrophysiol. 2007;18:680–685. doi: 10.1111/j.1540-8167.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 34.Umapathy K, Nair K, Masse S, Krishnan S, Rogers J, Nash MP, Nanthakumar K. Phase mapping of cardiac fibrillation. Circ Arrhythm Electrophysiol. 2010;3:105–114. doi: 10.1161/CIRCEP.110.853804. [DOI] [PubMed] [Google Scholar]
- 35.Berenfeld O, Oral H. The quest for rotors in atrial fibrillation: Different nets catch different fishes. Heart Rhythm. 2012;9:1440–1441. doi: 10.1016/j.hrthm.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray RA, Jalife J, Panfilov AV, Baxter WT, Cabo C, Davidenko JM, Pertsov AM. Mechanisms of cardiac fibrillation. Science. 1995;270:1222–1223. author reply 1224–1225. [PubMed] [Google Scholar]
- 37.Quintanilla JG, Moreno J, Archondo T, Chin A, Pérez-Castellano N, Usandizaga E, García-Torrent MJ, Molina-Morúa R, González P, Rodríguez-Bobada C, Macaya C, Pérez-Villacastín J. KATP channel opening accelerates and stabilizes rotors in a swine heart model of ventricular fibrillation. Cardiovasc Res. 2013;99:576–585. doi: 10.1093/cvr/cvt093. [DOI] [PubMed] [Google Scholar]
- 38.Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992;355:349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- 39.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM, Kalman JM. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: Transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 41.Rappel WJ, Narayan SM. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos. 2013;23:023113. doi: 10.1063/1.4807098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: Extended follow-up of the CONFIRM trial. J Am Coll Cardiol. 2014;63:1761–1768. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillem MS, Climent AM, Millet J, Arenal A, Fernández-Avilés F, Jalife J, Atienza F, Berenfeld O. Noninvasive localization of maximal frequency sites of atrial fibrillation by body surface potential mapping. Circ Arrhythm Electrophysiol. 2013;6:294–301. doi: 10.1161/CIRCEP.112.000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigo M, Guillem MS, Climent AM, Pedrón-Torrecilla J, Liberos A, Millet J, Fernández-Avilés F, Atienza F, Berenfeld O. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: A clinical-computational study. Heart Rhythm. 2014;11:1584–1591. doi: 10.1016/j.hrthm.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santangeli P, Marchlinski FE. Pulmonary vein isolation for atrial fibrillation: Forever young. J Am Coll Cardiol. 2014;64:2468–2470. doi: 10.1016/j.jacc.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 48.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]


