Abstract
Children and adolescents who present with neuroendocrine tumors are at extremely high likelihood of having an underlying germline predisposition for the Multiple Endocrine Neoplasia (MEN) syndromes, including MEN1, MEN2A and B, MEN4, and Hyperparathyroid-Jaw Tumor (HPT-JT) Syndromes. Each of these autosomal dominant syndromes results from a specific germline mutation in unique genes: MEN1 is due to pathogenic MEN1 variants (11q13), MEN2A and B are due to pathogenic RET variants (10q11.21), MEN4 is due to pathogenic CDKN1B variants (12p13.1), and the HPT-JT Syndrome is due to pathogenic CDC73 variants (1q25). Although each of these genetic syndromes share the presence of neuroendocrine tumors, each syndrome has a slightly different tumor spectrum with specific surveillance recommendations based upon tumor penetrance, including the age and location for which specific tumor types most commonly present. Although the recommended surveillance strategies for each syndrome contain similar approaches, important differences do exist among them. Therefore, it is important for caregivers of children and adolescents with these syndromes to become familiar with the unique diagnostic criteria for each syndrome, and also to be aware of the specific tumor screening and prophylactic surgery recommendations for each syndrome.
Keywords: Multiple Endocrine Neoplasia (MEN) 1; 2A, 2B, 4; Hyperparathyroid-Jaw Tumor Syndromes; MEN1; RET; CDKN1B; CDC73; surveillance; germline
Introduction
Tumors of hormone-producing tissues are common among patients with hereditary cancer syndromes. Signs and symptoms of hormone excess are the frequent initial findings among patients with endocrine tumors, although mass-effect may be the presenting complaint, particularly in the case of non-functional tumors. Pathogenic germline variants in different tumor suppressor genes and oncogenes are responsible for distinct hereditary endocrine tumor syndromes. Each of the so-called Multiple Endocrine Neoplasia (MEN) syndromes is characterized by a distinct spectrum of clinical manifestations, both benign and malignant with unique underlying genetic predisposition. The focus of this article is the pre-symptomatic screening of at-risk patients which has allowed for earlier detection and intervention, with a resultant decrease in mortality and morbidity associated with these tumors.
Expert or consensus guidelines currently exist for many of the clinical entities described herein. In the course of reviewing existing guidelines, effort was made to balance the financial, personal and psychosocial burdens of surveillance with the benefits of early detection and intervention with respect to consequences of hormonal hypersecretion. This was particularly pertinent among those histologically benign neoplasms, where morbidity is primarily paraneoplastic. Experiences with other hereditary tumor syndromes has also demonstrated reduced surgical morbidity when tumors are identified at earlier stages as the result of pre-symptomatic surveillance(1). It is also recognized that patient preference plays an important role in determining the onset of early tumor surveillance and intervention wherein data regarding optimal timing are imprecise. Such patient preferences should be ascertained and implemented by the clinical team, wherever medically appropriate.
Multiple Endocrine Neoplasia Type 1 (MEN1)
Multiple Endocrine Neoplasia Type 1 (MEN1, OMIM 131100) was initially identified as early as 1903 (2), and formally defined by Underhal and Werner nearly 50 years later (3,4) as an autosomal-dominant familial disorder characterized by 1) primary hyperparathyroidism (PHPT) and hypercalcemia resulting from parathyroid adenomas (generally multi-glandular); 2) hormone-secreting or non-secreting pancreatic islet tumors (commonly gastrinomas and rarely insulinomas, VIPomas, glucagonomas or other neoplasms); and 3) anterior pituitary neuroendocrine tumors (PitNETs), predominantly prolactinomas, less frequently, growth hormone secreting adenomas (5,6) and rarely, other PitNETs (which constitute <5% of pituitary tumors in MEN1)(7). Angiofibromas, lipomas and collagenomas (8–10) are common dermatologic manifestations of MEN1, and adrenocortical adenomas are identified in 35% of patients with MEN1 (11). Other minor manifestations of MEN1 include leiomyomas (12) and CNS neoplasms (including ependymomas and meningiomas) (13,14). The prevalence of MEN1 has been estimated at 1:20,000–40,000(15,16). Pedigrees expressing PHPT in the absence of other MEN1 manifestations (familial isolated hyperparathyroidism, FIHP) have also been described and may simply reflect PHPT as the most penetrant manifestation of MEN1 (17). Although originally thought to be an adult-onset disorder, tumors have been diagnosed as young as age five years (18) and continue to manifest through older adulthood. Seventeen percent of MEN1-associated tumors are diagnosed under the age of 21 years (19). Recent data suggest that 42% of MEN1 patients in the second decade of life may possess clinically occult (non-functioning) pancreatic NETs(20). Disease penetrance for a first manifestation of MEN1 among MEN1 carriers is estimated at 45%, 82% and 96% at 30, 50 and 70 years (21).
Although MEN1 may present with any of its major constituent manifestations, PHPT is the most common presenting feature and manifests in 95% of MEN1 patients (5,7). Pancreatic neuroendocrine tumors occur in 40–75% of MEN1 patients (7,20), whereas PitNETs are identified in 30–55% (22,23). Most commonly, these PitNETs are microadenomas <1cm, but they may be larger and retain potential to compromise visual fields. Clinical diagnosis of MEN1 is predicated on the identification of at least two of the major constituent tumors (e.g., parathyroid tumor, pancreatic islet cell tumor, PitNET ). Specific indications for genetic testing are articulated below. Patients with MEN 1 are at increased risk of premature death (24,25) with malignant pancreatic neuroendocrine tumors (including non-functioning tumours) the leading cause of death (19,20,26), although a secular trend has suggested decreasing mortality over the past 20 years, presumably the result of more prevalent screening for pre-symptomatic abdominal tumors (19).
Molecular Genetics of MEN1
MEN1 is associated with pathogenic variants in the MEN1 tumor-suppressor gene on 11q13, which encodes the Menin protein, a scaffold protein with suspected functions including cell-cycle control, transcriptional regulation and maintenance of genomic stability (27,28). Inactivating pathogenic variants in MEN1 in affected individuals occur throughout the gene and include truncation, missense, splice-site, and insertions/deletions (29). A limited number of large deletions also have been observed. Pathogenic germline variants in MEN1 are identified in 80–95% of familial cases (7,30) and 65–70% of de novo cases (31). Individuals meeting clinical diagnostic criteria for MEN1 without an identifiable pathogenic gene variant tend to develop tumors at an older age. This may, in fact, reflect carriers of other pathogenic gene variants such as CDKN1B (see MEN4, below), although these are rare (32). There are no apparent genotype-phenotype correlations in MEN1, and no mutational hotspots.
Comprehensive MEN1 genetic testing is indicated in 1) any person with two or more constituent MEN1 tumors; 2) any person with one MEN1 tumor and a first-degree relative with MEN1; or 3) any individual under the age of 30 with PHPT, pancreatic precursor lesions (33) or pancreatic islet tumor, regardless of family history. The prevalence of pathogenic MEN1 variants among individuals with isolated PitNETs is much lower (in some series 0–5%(34,35)) and in patients <30 years, the prevalence of pathogenic MEN1 variants may be as low as 3.4%(36). Thus, the role for genetic screening of MEN1 in patients with isolated PitNET is less clear(37).
In addition, genetic testing of the specific MEN1 pathogenic variant identified in the proband (familial mutation testing) should be offered to all at risk first-degree relatives. Finally, suspicion for “atypical” MEN1 has been proposed as an indication for testing, and comprises any of: parathyroid adenoma prior to age 45, gastrinoma (at any age), multiple pancreatic NETs, or either multiglandular/recurrent parathyroid adenomas or four-gland parathyroid hyperplasia (38).
Pre-symptomatic Surveillance for MEN1 manifestations
Recommendations for pre-symptomatic screening in MEN1 carriers have been established and are based on the youngest age at which disease manifestations have been reported (5,7,21)Table 1 defines a recommended surveillance paradigm, modified from that described by Thakker et al. (5). Delays in diagnosis and/or the onset of tumor surveillance among MEN1-carriers are associated with increases in both morbidity and mortality (39). For first-degree relatives of MEN1 carriers with unknown mutational status, we advocate annual serum prolactin from age five and annual serum calcium (corrected for albumin) from age 10 years. While treatment approaches for most manifestations of MEN1 are beyond the scope of this report, approaches to treatment of PHPT are addressed below.
Table 1.
Surveillance for MEN1 mutation carriers
| MEN1 manifestation | Screen Starting at Age | Clinical Screening | Annual Biochemical Tests | Imaging |
|---|---|---|---|---|
| Insulinoma | 5 yrs | Syncope, light-headedness, documented hypoglycemia, ⇑growth | Fasting Glucose & Insulin | None |
| Pituitary neuroendocrine tumor | 5 yrs+ | Headaches, visual changes, galactorrhea, ⇑growth | Prolactin, IGF-1 | Brain MRI (q3 years) |
| Parathyroid Adenoma/ 1o HyperPTH | 8 yrs | Back pain, bone pain, weakness, fatigue, psychiatric changes, kidney stones, nausea, vomiting, constipation. Multiple or pathologic fractures. | Calcium++ | None |
| Pancreatic NET | 10 yrs | Generally not identified symptomatically. VIPoma can cause profuse diarrhea. Glucagonoma assoc. with hyperglycemia, nausea, polyuria, thirst. | (Chromogranin A, glucagon, proinsulin, pancreatic polypeptide, VIP)+++ | Abdominal MRI (annually) |
| Adrenal adenoma | 10 yrs | None | None | MRI (contemporaneous with pancreatic imaging) |
| Gastrointestinal, Bronchial and Thymic NETs | 20 yrs | Frequently asymptomatic, but h/o flushing, diarrhea, wheezing, edema or abdominal pain should arouse suspicion | CT/MRI Chest and Abdomen (q1–2 years) | |
| Gastrinoma (duodenal and pancreatic) | 20 yrs | Abdominal pain, gastric ulcers. Proton-pump inhibitor usage. | Fasting Gastrin | None |
MRI surveillance is to begin once patient is able to tolerate a non-sedated MRI. In the authors’ experience, this is generally at about the age of 5 years, but may be deferred on an individualized basis.
Hypercalcemia on screening should prompt assessment with contemporaneous serum calcium and iPTH to establish a diagnosis of PHPT.
Pancreatic tumours may be non-secretory, therefore the added sensitivity contributed by biochemical screening has not been demonstrated.
(Data from Thakker et al. Ref: (5))
Multiple Endocrine Neoplasia 2A, 2B and Familial Medullary Thyroid Carcinoma
Multiple endocrine neoplasia type 2 (MEN2) was initially described by Sipple in 1961 (40). It results from pathogenic germline variants in the RET proto-oncogene. MEN2 is characterized by risk for both medullary thyroid carcinoma (MTC) and pheochromocytoma (PHEO) and can be further subdivided, clinically and genetically, into MEN2A (OMIM 171400) and MEN2B (OMIM 162300). MEN2A can also manifest PHPT, whereas MEN2B has earlier onset of tumors and may present with mucosal neuromas, intestinal ganglioneuromatosis and characteristic physical features. The prevalence of MEN2 is estimated at 1:35,000 to 1:40,000 (41). Familial medullary thyroid carcinoma (FMTC), once considered a separate subtype from MEN2A, is now widely considered to be a variant of MEN2A with decreased penetrance of PHEO and PHPT. MEN2A accounts for 91% of MEN2 patients (35% with isolated FMTC) and MEN2B, the remaining 9%.
MEN2 is inherited in an autosomal dominant fashion. Pathogenic germline RET variants are associated with striking genotype-phenotype correlations and the clinical phenotype has characteristic and predictable features based on the affected codon (Table 2). The risk for developing each of the three pathognomonic tumors in MEN2 is based on the codon-specific variant, with the most penetrant forms [American Thyroid Association (ATA) “Highest” and “High” risk (42)] characterized by lifetime risks of >95% risk to develop MTC, 50% risk to develop PHEO, and, for those with “High” risk alleles, a 20–30% risk to develop PHPT. Of note, carriers of the M918T variant do not develop PHPT. There is a lower prevalence of PHEO (10–50%) among “moderate” risk allele carriers, with risk conferred by the specific allele (42).
Table 2.
Genotype-Phenotype Associations with Specific RET Mutations
| ATA MTC Risk Category | RET Codon | MTC | PHEO | PHPT | CLA | HD |
|---|---|---|---|---|---|---|
| Highest (HST) | 918 | +++ | +++ | − | − | |
| High (H) | 634, 883 |
+++ | +++ | + − |
+ − |
|
| Moderate (MOD) | All others | +++ | +/++ | + | + (only codon 804) | + (only codons 609, 611, 618, 620) |
Abbreviations: American Thyroid Association (ATA) Risk Categories based on RET allele. MTC: medullary thyroid carcinoma; PHEO: pheochromocytoma; PHPT: primary hyperparathyroidism; CLA: cutaneous lichen amyloidosis; HD: Hirschsprung’s Disease.
Data from Wells et al. (66)
The age of onset of MTC also varies by genotype, occurring in early childhood in those with MEN2B, and adolescence or early adulthood in MEN2A, whereas pedigrees with isolated MTC tend to present with disease onset in middle-age. This difference in age of onset is very relevant clinically with regard to surveillance and surgery recommendations (below). Typically, PHEO presents in the 4th or 5th decade of life in pathogenic RET variant carriers, but it has been described in carriers as young as 8 years of age (43). MEN2A can be further classified into “classical MEN2A”, MEN2A with cutaneous lichen amyloidosis (CLA), and MEN2A with Hirschsprung disease (HD).
MEN2B is characterized by a 100% risk of developing MTC (which often presents in infancy and can be more aggressive) and a 50% risk for PHEO. PHPT does not occur in MEN2B. Roughly 50% of MEN2B occurs de novo (44), and presence of common clinical features, including alacrima, mucosal neuromas, constipation (secondary to intestinal ganglioneuromatosis) and marfanoid habitus should raise suspicion for MEN2B (44–46). Early diagnosis and treatment within the first year of life, by a high-volume thyroid surgeon, is critical to increase the potential for surgical prevention or curative treatment of MEN2B-associated MTC (47).
PHEO is associated with both MEN2A and MEN2B, presenting in ~10–50% of pathogenic RET-variant carriers, depending on genotype (42,48). Familial PHEO may also be reflective of pathogenic germline variants at other loci including VHL (von Hippel-Lindau disease), NF1 (Neurofibromatosis type 1/von Recklinghausen disease), SDHx (A-D), TMEM127 or MAX (hereditary PHEO/paraganglioma syndrome) (all described in detail elsewhere in this issue) (49).
Molecular Genetics of MEN2A, 2B and FMTC
The RET proto-oncogene is a receptor tyrosine kinase on 10q11.21, and pathogenic variants have been reported in exons 5, 8, 10, 11, 13, 14, 15 and 16 (with variants in exons 10 and 11 comprising 95% of individuals with MEN2A). The RET protein has an extracellular binding domain, a trans-membrane domain, and an intracellular segment with a split tyrosine kinase domain (50). Variants that strongly activate the intracellular tyrosine kinase domain are associated with early age of onset and more aggressive disease progression. The p.M918T variant in exon 16 disrupts the tyrosine kinase domain. It is considered to be the highest risk variant in the RET gene and is associated with the majority of cases of MEN2B. It is also the most common somatic pathogenic variant occurring in sporadic MTC (51). The p.A883F variant has rarely been reported in individuals with phenotypic features associated with MEN2B, including mucosal neuromas and PHEO (42). MTC presentation in these rare patients has ranged from metastatic disease in early adolescence to later onset and less aggressive disease (52,53). Similarly, specific double-variants in RET occurring on the same allele result in an atypical form of MEN2B with onset around 20–30 years of age (42,54–57). Codon 634 variants are associated with higher risks of PHEO and PHPT than other RET variants (58–60). Variants in codon 634 and 804 are associated with CLA (42,61). MEN2A with HD only occurs in patients with variants in codons 609, 611, 618, 620 (62,63). Lastly, rare patients can be found with combined, heterozygous variants involving A883F, but these occurrences are presently too uncommon to define a predictable phenotype. The de novo mutation rate in MEN2A has been estimated at 9% (64), while that of MEN2B is as high as 50%(44).
Pre-symptomatic Surveillance for MEN2A and MEN2B and Treatment Strategies
Medullary Thyroid Cancer
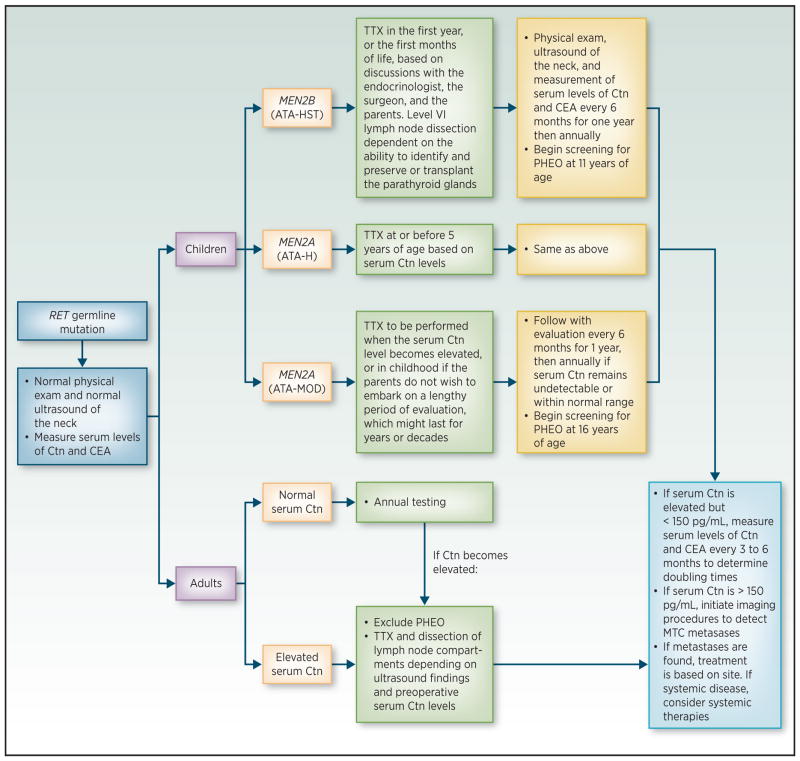
Routine surveillance for MTC comprises both serial ultrasounds and biochemical monitoring of serum calcitonin levels. Total thyroidectomy, when performed by surgeons experienced in the MEN2 syndromes, is the primary preventive strategy for managing the risk for MTC in individuals with pathogenic RET variants, and it has been shown to be effective in preventing subsequent biochemical evidence of disease (65). However, genotype and calcitonin levels may also allow the clinician to tailor the age at which thyroidectomy is indicated (Figure 1) (66). For the “highest” risk allele, p.M918T, thyroidectomy is advised within the first year of life (42). MTC is preceded by C-cell hyperplasia and hypercalcitoninemia, however, physiological calcitonin levels in infancy may be as high as 50 pg/ml with a decreasing trend over the first three years of life, limiting the utility of this marker to gauge disease status pre-operatively in this age group (67,68). Since the variant arises de novo in approximately 50% of those with p.M918T, these affected individuals are unlikely to have been diagnosed as carriers and typically present with metastatic disease if diagnosis occurs after 5 years of age. Once MTC has metastasized, there is low likelihood of achieving surgical cure. Thus, prophylactic thyroidectomy is standard-of-care whenever achievable (69). Preoperative staging with ultrasound or cross-sectional imaging (e.g., contrast-enhanced neck CT or MRI) is essential to identify regional lymphadenopathy and to facilitate adequate surgical planning. For those with progressive or symptomatic metastatic MTC, two drugs, cabozatanib and vandetinib, have been approved in adults, although neither has been demonstrated to impact survival (70).
Figure 1. Management of patients with a pathogenic RET germline variant detected on genetic screening.
ATA, American Thyroid Association risk categories for aggressive medullary thyroid carcinoma (MTC) (HST, highest risk, H, high risk, MOD, moderate risk); Ctn, calcitonin; CEA, carcinoembryonic antigen; HPTH, hyperparathyroidism; PHEO, pheochromocytoma; RET, REarranged during Transfection; TTX, total thyroidectomy; US, ultrasound. (Reprinted with permission from Wells et al. (42); the publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers).
For children with “high”-risk alleles (codons 634 and 883), thyroidectomy may be delayed to balance risks of surgical complications. These children should undergo annual ultrasound and screening for increased calcitonin levels starting at 3 years of age and proceed to thyroidectomy when elevated levels are detected or at 5 years of age. Metastatic disease is rare if serum calcitonin levels are <40 pg/ml, and neck dissection can often be avoided if cervical adenopathy is not detected by imaging or clinical examination (42,69).
Timing of surgery for individuals with “moderate” risk alleles is most challenging, because this category represents a wide range of penetrance and age of onset associated with different alleles, and there is considerable variability even within families harboring the same variant. For example, a comparison of individual alleles in the moderate risk category (previously ATA classifications A and B) found a 7-fold higher risk for MTC in individuals with a codon 620 variant compared with individuals carrying a codon 611 variant. The median time to MTC was 19 years for codon 620 variant carriers and 56 years for individuals with a variant of codon 611 (71). Therefore, individualization based on variant-specific data and family history may be considered for reasonable surgical planning. Thyroidectomy may be delayed if the family is willing to pursue annual surveillance with serum calcitonin and thyroid ultrasound with prophylactic surgery offered when there is suspicion for progression. Current guidelines for carriers of “moderate” risk alleles recommend thyroidectomy when calcitonin level demonstrates an upward trend or, if a biopsy has been performed and shows cytological evidence of MTC in a thyroid lesion (66). It is critical that families understand the importance of adhering to routine follow-up in these circumstances. Families may elect to pursue thyroidectomy at an earlier time to decrease anxiety or the burden of annual surveillance. Finally, although ultrasound has multiple applications in evaluation of the thyroid and cervical lymph nodes (it can be used to document a normal gland in at-risk individuals with a family history or positive genetic test and for pre-operative staging), ultrasound appears to be less sensitive then calcitonin for diagnosis of MTC, and so it should not be used to exclude malignancy or delay surgery (72).
Pheochromocytoma
Prior clinical guidelines had advocated initiation of PHEO surveillance at 8 years (43), although according to the more recent ATA guidelines, screening for PHEO should commence at 11 years for carriers of the “high” and “highest” risk alleles, and at 16 years for patients with “moderate” risk alleles (42). As genetically at-risk patients undergo routine bloodwork for MTC surveillance, some clinicians elect to also initiate PHEO screening at an earlier age, when performing MTC screening. Plasma free metanephrines and normetanephrines, or 24-hour urinary fractionated metanephrines, are the currently recommended screening tests (42). Additionally, biochemical screening for PHEO should be performed prior to any planned surgery and pregnancy, regardless of age (42,69). Pre-operative alpha-adrenergic blockade is essential for patients with catecholamine-secreting PHEOs to mitigate risk of intra-operative hypertensive crisis. Imaging in the absence of biochemical evidence of disease is not advised in MEN2. In the pediatric population, if biochemical tests are abnormal, MRI of the abdomen and pelvis, with and without intravenous contrast, is preferred over contrast-enhanced CT, due to the lack of ionizing radiation with MRI.
Hyperparathyroidism
Screening for PHPT with serum calcium (if normo-albuminemic) should begin at 11 years and 16 years of age for “high” and “moderate” risk allele carriers respectively (42). Development of hypercalcemia during surveillance is suggestive of hyperparathyroidism and should be followed-up with contemporaneous measurement of calcium and intact parathyroid hormone (iPTH). Many providers include 25-OH Vitamin D levels [either routinely or in response to elevated intact parathyroid hormone (iPTH)] to exclude hypovitaminosis D as a concomitant secondary cause of hyperparathyroidism. Hypercalcemia in the context of normal or elevated iPTH is diagnostic of hyperparathyroidism and should prompt referral to an endocrinologist and surgeon with experience in managing parathyroid disease to determine indications for and timing of surgery (73).
Surgical excision of abnormal parathyroid tissue is the only definitive cure for PHPT. The surgical approach may include resection of a solitary enlarged gland or total four-gland parathyroidectomy with autotransplantation of parathyroid tissue to the neck or forearm. Transcervical thymectomy is often performed at the same time as parathyroidectomy because of the increased risk of supernumerary (or intrathymic) parathyroid glands (and, in the case of MEN1, to reduce the risk of thymic NETs) (74,75). The parathyroid glands may also be removed and autotransplanted during prophylactic thyroidectomy in individuals harboring a RET genotype associated with a high risk for PHPT. However, for patients with “moderate” risk alleles who have both a lower risk for metastatic disease (in cases of non-elevated calcitonin levels) and a low risk for PHPT, central neck dissection should be avoided during thyroidectomy, to preserve the parathyroid glands (76). PHPT is not a feature of “highest” risk codon 918 variants, and so preserving the parathyroid glands when performing surgery in infancy should be a priority. Given the inherent surgical challenges associated with thyroidectomy in infancy, the importance of an experienced surgeon cannot be overstated.
Preoperative imaging of a parathyroid adenoma typically includes ultrasound and/or dual-phase 99mTc-sestamibi scintigraphy with SPECT/CT (if available), referred to as a “parathyroid scan”. The combination of these two tests is highly sensitive for the localization of parathyroid adenoma. The parathyroid scan offers the additional opportunity to detect developmental variants including intrathyroidal or mediastinal parathyroid tissue (77).
Individualization of Care
Pathogenic RET variants afford a significant opportunity to prevent metastatic disease by adopting prophylactic management and individualization of care based on a patient’s variant status. Ongoing collection of data and outcomes will be necessary to develop the most accurate, variant-specific risk estimates to guide families and clinicians, particularly among carriers of “moderate” risk alleles, in whom disease manifestations can be widely varied. We also recognize the importance of patient preference in determining age at prophylactic thyroidectomy (early thyroidectomy vs. active biochemical and radiographic surveillance) and advocate a mutual decision-making process shared by clinician and patient, balancing disease risk with those of intervention for those with “High” and “Moderate” risk alleles. Among patients at risk for non-adherence to surveillance, appropriate care mandates that strong consideration be given to early prophylactic thyroidectomy to reduce risk of late-presentation with metastatic disease (which is incurable). Patients with pathogenic RET variants should ideally be followed at centers with expertise in interpretation of genetic risk, biochemical analyses, surgical expertise and resources for supporting long-term follow-up. Ongoing molecular research should be complemented with studies aimed at mitigating risk, as well as enhancing communication and adherence to screening, in order to optimize outcomes for these patients.
Multiple Endocrine Neoplasia 4 (MEN 4) or “CDKN1B-related MEN”
MEN 4 (OMIM 610755) was first described as a unique clinical entity in 2006 (78), although a paucity of affected patients to date has thus far limited the ability to generate a complete picture of the syndrome. It was originally identified in the context of a spontaneous CDKN1B variant arising in a rat colony leading to progressive parathyroid adenomas and bilateral PHEO/paraganglioma in 100% of affected animals, with occasionally noted thyroid C-cell hyperplasia and pancreatic islet hyperplasia (78). Humans with inactivating pathogenic variants in CDKN1B appear to have a more restricted phenotype, most similar to MEN1, with a high incidence predominantly of PHPT (in 100% of affected individuals) (79) and PitNETss (somatotroph, corticotroph and non-functioning adenomas). Despite the animal data, to date, no MEN2-spectrum tumors (MTC, PHEO) have been identified among human carriers. Other manifestations described in affected individuals include gastro-entero-pancreatic neuroendocrine tumors, uterine neoplasms, adrenocortical masses and thyroid tumors (79), although none at high enough frequency to mandate surveillance.
Molecular Genetics of MEN4
Nine pedigrees have been reported thus far with CDKN1B-associated MEN1-like tumor syndrome (78,80–83), with 13 pathogenic germline variants described in humans, including frameshift, nonsense and missense variants (84,85). CDKN1B variants have also been associated with familial isolated parathyroid adenoma (83) and isolated PitNET (86). At present, testing for CDKN1B may be considered for those with PHPT in whom MEN1 variant testing is negative.
CDKN1B encodes the p27kip1 inhibitor of cell cycle progression. In tumor tissues, pathogenic CDKN1B variants appear to be associated with normal transcript levels, but low or undetectable protein levels, suggesting reduced translation or protein half-life in pathogenesis. Other variants seem to be associated with functional defects that interrupt binding to interacting partners or disrupt nuclear localization (78,80–82,87).
Pre-symptomatic Surveillance for MEN4
Given the paucity of pedigrees with MEN 4, surveillance guidelines have yet to be established. To the best of our knowledge, the youngest patient with CDNK1B-attributable disease presented at age 30 with acromegaly (78). Thus, surveillance is primarily clinical and should concentrate on evidence of growth hormone excess (gigantism/acromegaly) (88) and glucocorticoid excess (Cushing’s syndrome) (89), with concern for either prompting endocrine consultation. In children, routine anthropometric assessment with review of growth charts is relevant, as PitNETs may present with growth acceleration (GH hypersecretion) or growth retardation with weight gain (ACTH hypersecretion, causing Cushing’s Disease). In the absence of data, and based solely on expert consensus, we recommend annual bloodwork for PHPT (total calcium adjusted for serum albumin), as well as biochemical surveillance for secretory pituitary somatotroph adenomas (annual IGF-1) beginning at adolescence. A role for baseline or serial pre-symptomatic CNS imaging has not been established.
CDC73-Related (Hyperparathyroid-Jaw Tumor) Syndrome
Rare individuals or families with PHPT develop ossifying fibromas of the jaw (distinct from the “brown tumors” of hyperparathyroidism) and occasionally, parathyroid carcinoma (90). Linkage analysis in such pedigrees excluded loci associated with MEN1 or MEN2 and ultimately, linkage was established at chromosomal location 1q31.2 and the term Hyperparathyroid-Jaw Tumor Syndrome (OMIM 145001) was coined (91).
Molecular Genetics of CDC73-Related Tumor Syndrome
CDC73-related (Hyperparathyroid Jaw Tumor, HPT-JT) Syndrome results from truncating (~80%) or missense variants in the CDC73 gene (also known as HRPT-2), which encodes the parafibromin protein (92–95). Loss of nuclear parafibromin staining by immunohistochemistry (IHC) in any parathyroid adenoma or carcinoma should prompt consideration of germline analysis of CDC73(96,97), and, for this reason we advocate parafibromin IHC for all parathyroid neoplasms in young patients. Additionally, while controversial, parafibromin immunoreactivity may help distinguish adenomatous from carcinomatous nodules and may influence prognosis (98–101).
Pathogenic germline variants in CDC73 typically result in single-gland parathyroid adenomas (>70% of affected individuals) (102), ossifying maxillary or mandibular fibromas (25–50%) or infrequently parathyroid carcinoma (~15%) (103,104). Other manifestations of pathogenic germline CDC73 variants include a high rate of benign and malignant uterine tumors (~75% of patients) (102,105) and renal anomalies (~20%) including cysts, hamartomas and, rarely, Wilms tumor (106).
Disease is inherited in an autosomal dominant pattern with high, but incomplete penetrance, estimated at 70–90% (102,105,107). Pathogenic CDC73 variants are identified in 50–75% of patients with HPT-JT and 14% of patients with familial isolated hyperparathyroidism (108). Among pathogenic variant-carriers, hyperparathyroidism may be diagnosed as young as 7 years (109) while fibromas have been described in patients as young as 10 years (93). Thus far, there are no clear genotype-phenotype associations, although carriers of missense variants seem to have a higher likelihood of isolated hyperparathyroidism (92,103).
Surveillance for carriers of pathogenic germline variants in CDC73 should begin at age 5–10 years and should include: 1) annual biochemical screening for hyperparathyroidism (total calcium, corrected for serum albumin); 2) dental panoramic films every 5 years; and 3) renal ultrasound every 5 years. Women of reproductive age are advised to undergo routine gynecologic assessment with uterine ultrasound as clinically indicated (i.e., with menorrhagia or abnormal uterine bleeding) (103).
Patients with hypercalcemia should evaluated for PHPT as described above (MEN2) and, if confirmed, PHPT should be managed in consultation with an endocrinologist, and consideration given to referral to a high-volume parathyroid surgeon (110). Biopsy of suspicious neck lesions in these patients is discouraged due to risk of seeding carcinomatous cells through the biopsy track. Management of other manifestations of CDC73-tumor syndrome should take place under the direction of a relevant subspecialist.
Conclusion
Multiple Endocrine Neoplasia syndromes offer both a challenge and an opportunity to identify disease at early stages and to intervene to mitigate morbidity and mortality by instituting appropriate management of disease manifestations in the context of multidisciplinary care. Clinical and biochemical markers offer clinicians the ability to detect disease and to follow evolution through the course of observation and treatment. An evolving understanding of genotype-phenotype relationships and progression of disease are altering our approach to management of affected patients, with an emphasis on definitive management as early as necessary, within a framework of limiting surgical and medical complications and disease-related mortality.
Acknowledgments
Funding support: NCI-5P30CA054174-21 to G.E. Tomlinson.
Footnotes
The authors declare no potential conflicts of interest
Contributor Information
Jonathan D. Wasserman, Division of Endocrinology, The Hospital for Sick Children, Toronto, Ontario.
Gail E. Tomlinson, Department of Pediatrics, Division of Hematology and Oncology and Greehey Children’s Cancer Research Institute, University of Texas Health Science Center at San Antonio, San Antonio, TX.
Harriet Druker, Division of Haematology-Oncology, The Hospital for Sick Children, Toronto, Ontario.
Junne Kamihara, Division of Hematology-Oncology, Children’s Hospital, Boston and Dana-Farber Cancer Institute, Boston, MA.
Wendy K. Kohlmann, Huntsmann Cancer Institute, Salt Lake City, Utah
Christian P. Kratz, Pediatric Hematology and Oncology, Hannover Medical School, Hannover, Germany
Katherine L. Nathanson, Department of Medicine, Division of Translational Medicine and Human Genetics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA
Kristian W. Pajtler, Department of Pediatric Oncology, Hematology and Immunology, University Hospital, Heidelberg, Germany. Division of Pediatric Neuro-Oncology, German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany
Andreu Parareda, Division of Oncology. Predisposition and Survivorship Units. Sant Joan de Déu - Barcelona Children’s Hospital, Barcelona, Catalonia.
Surya P. Rednam, Department of Pediatrics, Baylor College of Medicine, Texas Children’s Cancer Center, Texas Children’s Hospital, Houston, TX
Lisa J. States, Department of Radiology, Children’s Hospital of Philadelphia, Philadelphia, PA
Anita Villani, Division of Haematology-Oncology, The Hospital for Sick Children, Toronto, Ontario.
Michael F. Walsh, Departments of Medicine and Pediatrics, Memorial Sloan Kettering Cancer Center, New York, NY
Kristin Zelley, Division of Oncology, Children’s Hospital of Philadelphia, Philadelphia, PA.
Joshua D. Schiffman, Department of Pediatrics and Huntsman Cancer Institute, University of Utah, Salt Lake City, Utah
References
- 1.Villani A, Shore A, Wasserman JD, Stephens D, Kim RH, Druker H, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncology. 2016;17(9):1295–305. doi: 10.1016/S1470-2045(16)30249-2. [DOI] [PubMed] [Google Scholar]
- 2.Erdheim J. Zur normalen und pathologischen Histologie der Glandula thyreoidea, parathyroidea und Hypophysis. Beit Z Path Anat Z Allg Path. 1903;33:158–236. [Google Scholar]
- 3.Underdahl LO, Woolner LB, Black BM. Multiple endocrine adenomas; report of 8 cases in which the parathyroids, pituitary and pancreatic islets were involved. J Clin Endocrinol Metab. 1953;13(1):20–47. doi: 10.1210/jcem-13-1-20. [DOI] [PubMed] [Google Scholar]
- 4.Wermer P. Genetic aspects of adenomatosis of endocrine glands. Am J Med. 1954;16(3):363–71. doi: 10.1016/0002-9343(54)90353-8. [DOI] [PubMed] [Google Scholar]
- 5.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97(9):2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 6.Asa SL, Casar-Borota O, Chanson P, Delgrange E, Earls P, Ezzat S, et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an International Pituitary Pathology Club proposal. Endocrine-Related Cancer. 2017;24(4):C5–C8. doi: 10.1530/erc-17-0004. [DOI] [PubMed] [Google Scholar]
- 7.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–71. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 8.Asgharian B, Turner ML, Gibril F, Entsuah LK, Serrano J, Jensen RT. Cutaneous tumors in patients with multiple endocrine neoplasm type 1 (MEN1) and gastrinomas: prospective study of frequency and development of criteria with high sensitivity and specificity for MEN1. J Clin Endocrinol Metab. 2004;89(11):5328–36. doi: 10.1210/jc.2004-0218. [DOI] [PubMed] [Google Scholar]
- 9.Darling TN, Skarulis MC, Steinberg SM, Marx SJ, Spiegel AM, Turner M. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133(7):853–7. [PubMed] [Google Scholar]
- 10.Vortmeyer AO, Boni R, Pak E, Pack S, Zhuang Z. Multiple endocrine neoplasia 1 gene alterations in MEN1-associated and sporadic lipomas. Journal of the National Cancer Institute. 1998;90(5):398–9. doi: 10.1093/jnci/90.5.398. [DOI] [PubMed] [Google Scholar]
- 11.Skogseid B, Rastad J, Gobl A, Larsson C, Backlin K, Juhlin C, et al. Adrenal lesion in multiple endocrine neoplasia type 1. Surgery. 1995;118(6):1077–82. doi: 10.1016/s0039-6060(05)80117-5. [DOI] [PubMed] [Google Scholar]
- 12.Ikota H, Tanimoto A, Komatsu H, Ozawa Y, Matsushita H. Ureteral leiomyoma causing hydronephrosis in Type 1 multiple endocrine neoplasia. Pathol Int. 2004;54(6):457–9. doi: 10.1111/j.1440-1827.2004.01642.x. [DOI] [PubMed] [Google Scholar]
- 13.Kato H, Uchimura I, Morohoshi M, Fujisawa K, Kobayashi Y, Numano F, et al. Multiple endocrine neoplasia type 1 associated with spinal ependymoma. Intern Med. 1996;35(4):285–9. doi: 10.2169/internalmedicine.35.285. [DOI] [PubMed] [Google Scholar]
- 14.Asgharian B, Chen YJ, Patronas NJ, Peghini PL, Reynolds JC, Vortmeyer A, et al. Meningiomas may be a component tumor of multiple endocrine neoplasia type 1. Clin Cancer Res. 2004;10(3):869–80. doi: 10.1158/1078-0432.ccr-0938-3. [DOI] [PubMed] [Google Scholar]
- 15.Marx SJ. Multiple endocrine neoplasia type 1. In: CRS, ALB, WSS, DV, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York, NY: Mcgraw-Hill; 2001. pp. 943–66. [Google Scholar]
- 16.DeLellis RA International Agency for Research on Cancer., World Health Organization., International Academy of Pathology., International Association for the Study of Lung Cancer. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press; 2004. p. 320. [Google Scholar]
- 17.Miedlich S, Lohmann T, Schneyer U, Lamesch P, Paschke R. Familial isolated primary hyperparathyroidism--a multiple endocrine neoplasia type 1 variant? Eur J Endocrinol. 2001;145(2):155–60. doi: 10.1530/eje.0.1450155. [DOI] [PubMed] [Google Scholar]
- 18.Stratakis CA, Schussheim DH, Freedman SM, Keil MF, Pack SD, Agarwal SK, et al. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2000;85(12):4776–80. doi: 10.1210/jcem.85.12.7064. [DOI] [PubMed] [Google Scholar]
- 19.Goudet P, Murat A, Binquet C, Cardot-Bauters C, Costa A, Ruszniewski P, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34(2):249–55. doi: 10.1007/s00268-009-0290-1. [DOI] [PubMed] [Google Scholar]
- 20.Goncalves TD, Toledo RA, Sekiya T, Matuguma SE, Maluf Filho F, Rocha MS, et al. Penetrance of functioning and nonfunctioning pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 in the second decade of life. J Clin Endocrinol Metab. 2014;99(1):E89–96. doi: 10.1210/jc.2013-1768. [DOI] [PubMed] [Google Scholar]
- 21.Glascock MJ, Carty SE. Multiple endocrine neoplasia type 1: fresh perspective on clinical features and penetrance. Surg Oncol. 2002;11(3):143–50. doi: 10.1016/s0960-7404(01)00031-7. [DOI] [PubMed] [Google Scholar]
- 22.Trouillas J, Labat-Moleur F, Sturm N, Kujas M, Heymann MF, Figarella-Branger D, et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am J Surg Pathol. 2008;32(4):534–43. doi: 10.1097/PAS.0b013e31815ade45. [DOI] [PubMed] [Google Scholar]
- 23.Verges B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87(2):457–65. doi: 10.1210/jcem.87.2.8145. [DOI] [PubMed] [Google Scholar]
- 24.Dean PG, van Heerden JA, Farley DR, Thompson GB, Grant CS, Harmsen WS, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg. 2000;24(11):1437–41. doi: 10.1007/s002680010237. [DOI] [PubMed] [Google Scholar]
- 25.Geerdink EA, Van der Luijt RB, Lips CJ. Do patients with multiple endocrine neoplasia syndrome type 1 benefit from periodical screening? Eur J Endocrinol. 2003;149(6):577–82. doi: 10.1530/eje.0.1490577. [DOI] [PubMed] [Google Scholar]
- 26.Doherty GM, Olson JA, Frisella MM, Lairmore TC, Wells SA, Jr, Norton JA. Lethality of multiple endocrine neoplasia type I. World J Surg. 1998;22(6):581–6. doi: 10.1007/s002689900438. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 27.Matkar S, Thiel A, Hua X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends in biochemical sciences. 2013;38:394–402. doi: 10.1016/j.tibs.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29(1):22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 29.Concolino P, Costella A, Capoluongo E. Multiple endocrine neoplasia type 1 (MEN1): An update of 208 new germline variants reported in the last nine years. Cancer genetics. 2016;209:36–41. doi: 10.1016/j.cancergen.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjold M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332(6159):85–7. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- 31.Guo SS, Sawicki MP. Molecular and genetic mechanisms of tumorigenesis in multiple endocrine neoplasia type-1. Mol Endocrinol. 2001;15(10):1653–64. doi: 10.1210/mend.15.10.0717. [DOI] [PubMed] [Google Scholar]
- 32.de Laat JM, Tham E, Pieterman CR, Vriens MR, Dorresteijn JA, Bots ML, et al. Predicting the risk of multiple endocrine neoplasia type 1 for patients with commonly occurring endocrine tumors. Eur J Endocrinol. 2012;167(2):181–7. doi: 10.1530/EJE-12-0210. [DOI] [PubMed] [Google Scholar]
- 33.Mete O, Asa SL. Precursor lesions of endocrine system neoplasms. Pathology. 2013;45(3):316–30. doi: 10.1097/PAT.0b013e32835f45c5. [DOI] [PubMed] [Google Scholar]
- 34.Corbetta S, Pizzocaro A, Peracchi M, Beck-Peccoz P, Faglia G, Spada A. Multiple endocrine neoplasia type 1 in patients with recognized pituitary tumours of different types. Clinical Endocrinology. 1997;47(5):507–12. doi: 10.1046/j.1365-2265.1997.3311122.x. [DOI] [PubMed] [Google Scholar]
- 35.Andersen HO, Jorgensen PE, Bardram L, Hilsted L. Screening for multiple endocrine neoplasia type 1 in patients with recognized pituitary adenoma. Clinical Endocrinology. 1990;33(6):771–5. doi: 10.1111/j.1365-2265.1990.tb03914.x. [DOI] [PubMed] [Google Scholar]
- 36.Cuny T, Pertuit M, Sahnoun-Fathallah M, Daly A, Occhi G, Odou MF, Tabarin A, et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don’t forget MEN1 genetic analysis. doi: 10.1530/EJE-12-0763. (1479–683X (Electronic)) [DOI] [PubMed] [Google Scholar]
- 37.Lecoq A-L, Kamenicky P, Guiochon-Mantel A, Chanson P. Genetic mutations in sporadic pituitary adenomas[mdash]what to screen for? Nature reviews Endocrinology. 2015;11(1):43–54. doi: 10.1038/nrendo.2014.181. http://www.nature.com/nrendo/journal/v11/n1/abs/nrendo.2014.181.html - supplementary-information. [DOI] [PubMed] [Google Scholar]
- 38.Newey PJ, Thakker RV. Role of multiple endocrine neoplasia type 1 mutational analysis in clinical practice. Endocr Pract. 2011;17(Suppl 3):8–17. doi: 10.4158/EP10379.RA. [DOI] [PubMed] [Google Scholar]
- 39.van Leeuwaarde RS, van Nesselrooij BP, Hermus AR, Dekkers OM, de Herder WW, van der Horst-Schrivers AN, et al. Impact of Delay in Diagnosis in Outcomes in MEN1: Results From the Dutch MEN1 Study Group. J Clin Endocrinol Metab. 2016;101(3):1159–65. doi: 10.1210/jc.2015-3766. [DOI] [PubMed] [Google Scholar]
- 40.Sipple JH. The association of pheochromocytoma with carcinoma of the thyroid gland. The American journal of medicine. 1961;31(1):163–6. [Google Scholar]
- 41.DeLellis RA International Agency for Research on Cancer., World Health Organization., International Academy of Pathology. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press; 2004. p. 320. [Google Scholar]
- 42.Wells SAAS, Jr, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, et al. Revised American Thyroid Association guidelines for the management of medullary thryoid cancer. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid : official journal of the American Thyroid Association. 2009;19(6):565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 44.Marquard JEC. GeneReviews. University of Washington; 2015. Nov 15, [Accessed 2016 15 Nov]. Multiple endocrine neoplasia type 2. [Google Scholar]
- 45.Smith VVEC, Milla PJ. Intestinal ganglioneuromatosis and multiple endocrine neoplasia type 2B: implications for treatment. Gut. 1999;45(1):143–6. doi: 10.1136/gut.45.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen MSPJ, Albison C, DeBenedetti MK, Skinner MA, Lairmore TC, Doherty GM, Balfe DM, Wells SA, Jr, Moley JF. Gastrointestingal manifestations of multiple endocrine neoplasia type 2. Ann Surg. 2002;235(5):648–54. doi: 10.1097/00000658-200205000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brauckhoff MMA, Lorenz K, Bjoro T, Varhaug JE, Dralle H. Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2B: a changing perspective. Ann Surg. 2014;259(4):800–6. doi: 10.1097/SLA.0b013e3182a6f43a. [DOI] [PubMed] [Google Scholar]
- 48.Iihara M, Yamashita T, Okamoto T, Kanbe M, Yamazaki K, Egawa S, et al. A nationwide clinical survey of patients with multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma in Japan. Jpn J Clin Oncol. 1997;27(3):128–34. doi: 10.1093/jjco/27.3.128. [DOI] [PubMed] [Google Scholar]
- 49.Karasek D, Shah U, Frysak Z, Stratakis C, Pacak K. An update on the genetics of pheochromocytoma. J Hum Hypertens. 2013;27(3):141–7. doi: 10.1038/jhh.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raue FF-RK. Genotype-phenotype correlation in multiple endocrine neoplasia type 2. Clinics (Sao Paulo) 2012;67(Suppl 1):69–75. doi: 10.6061/clinics/2012(Sup01)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eng C, Mulligan Lm, Smith DP, Healey CS, Frilling A, Raue F, Neumann HP, et al. Mutation of the RET protooncogene in sporadic medullary thyroid carcinoma. Genes, chromosomes & cancer. 1995;12(3):209–12. doi: 10.1002/gcc.2870120308. [DOI] [PubMed] [Google Scholar]
- 52.Jasim SYA, Waguespack SG, Rich TA, Grubbs EG, Jimenez C, et al. Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma comparted with RET M918T mutation. Thyroid. 2011;21(2):189–92. doi: 10.1089/thy.2010.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathiesen JSSK, Loegstrup Poulsen P, Vestergaard EM, et al. Aggressive medullary thyroid carcinoma in a ten-year-old patient with multiple endocrine neoplasia 2B due to the A883F mutation. Thyroid. 2015;25(1):139–40. doi: 10.1089/thy.2014.0177. [DOI] [PubMed] [Google Scholar]
- 54.Miyauchi AFH, Hai N, Yokozawa T, Kuma K, Aoki N, Kosugi S, Sugano K, Yamguchi K. Two germline missense muations at codon 804 and 806 of the RET proto-oncogene in the same allele in a patient with multiple endocrine neoplasia type 2B without codon 918 mutations. Jpn J Cancer Res. 1999;90(1):1–5. doi: 10.1111/j.1349-7006.1999.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwashita TMH, Kurokawa K, Kawai K, Miyauchi A, Futami H, Qiao S, Ichihara M, Takahashi M. A two-hit model for development of multiple endocrine neoplasia type 2B by RET mutations. Biochemical and biophysical research communications. 2000;268(3):804–8. doi: 10.1006/bbrc.2000.2227. [DOI] [PubMed] [Google Scholar]
- 56.Kamayama KOH, Takami H. RET oncogene mutations in 75 cases of familial medullary thyroid carcinoma in Japan. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2004;58(6–7):345–7. doi: 10.1016/j.biopha.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Nakao KTUT, Ikeda M, Mori Y, Yamamoto T, Kawashima ST, Nanba K, Yuno A, Tamanaha T, Tagami T, Naruse M, Asato R, Shimatsu A. Novel tandem germline RET proto-oncogen mutations in a patient with multiple endocrine neoplasia type 2B: report of a case and a literature review of tandem RET mutations with in silico analysis. Head Nexk. 2013;35(12):E363–8. doi: 10.1002/hed.23241. [DOI] [PubMed] [Google Scholar]
- 58.Imai TUS, Okamoto T, Suzuki S, Kosugi S, Kikumori T, Sakurai A MEN Consortium of Japan. High penetrance of pheochromocytoma in multiople endocrine neoplasia 2 cuased by germ line RET codon 634 mutation in Japanese patients. Eur J Endocrinol. 2013;168(5):683–7. doi: 10.1530/EJE-12-1106. [DOI] [PubMed] [Google Scholar]
- 59.Frank-Raue KRL, Erlic Z, Schweizer H, Winter A, Milos I, Toledo SP, Toledo RA, Tavares MR, et al. Risk profiles and penetrance estimations in mulitple endocrine neoplasia type 2A cuased by germline RET mutations located in exon 10. Hum Mutat. 2011;32(1):51–8. doi: 10.1002/humu.21385. [DOI] [PubMed] [Google Scholar]
- 60.Herfarth KKBD, Doherty GM, Wells SA, Jr, Lairmore TC. Surgical management of hyperparathyroidism in patients with multiple endocrine neoplasia type 2A. Surger. 1996;120(6):973–4. doi: 10.1016/s0039-6060(96)80042-0. [DOI] [PubMed] [Google Scholar]
- 61.Ceccherini IRC, Barone V, Pacini F, Martino E, Loviselli A, Pinchera A, Romeo G. Identification of the Cys634-->Try mutation of the RET proto-oncogene in a pedigree with multiple endocrine neoplasia type 2A and localized cutaneous lichen amyloidosis. J Endocrinol Invest 1994. 1994;17(3):201–4. doi: 10.1007/BF03347719. [DOI] [PubMed] [Google Scholar]
- 62.Mulligan LMEC, Attie T, Lyonnet S, Marsh DJ, Hyland VJ, Robinson BG, Frilling A, Verellen-Dumoulin C, Safar A, et al. Diverse phenotypes associated with exon 10 mutations in the RET proto-oncogene. Hum Mol Genet. 1994;3(12):2163–7. doi: 10.1093/hmg/3.12.2163. [DOI] [PubMed] [Google Scholar]
- 63.Mulligan LMPB. Genetic basis of endocrine disease: multiple endocrine neoplasia type 2. J Clin Endocrinol. 1995;80(7):1989–95. doi: 10.1210/jcem.80.7.7608246. [DOI] [PubMed] [Google Scholar]
- 64.Schuffenecker I, Ginet N, Goldgar D, Eng C, Chambe B, Boneu A, et al. Prevalence and parental origin of de novo RET mutations in multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma. Le Groupe d’Etude des Tumeurs a Calcitonine. Am J Hum Genet. 1997;60(1):233–7. [PMC free article] [PubMed] [Google Scholar]
- 65.Skinner MA, Moley JA, Dilley WG, Owzar K, Debenedetti MK, Wells SA. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. 2005;353(11):1105–13. doi: 10.1056/NEJMoa043999. [DOI] [PubMed] [Google Scholar]
- 66.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basuyau JPME, Leroy M, Brunelle P. Reference intervals for serum calcitonin in men, women, and children. Clin Chem. 2004;50(10):1828–30. doi: 10.1373/clinchem.2003.026963. [DOI] [PubMed] [Google Scholar]
- 68.Castagna MGFL, Maino F, Covelli D, Memmo S, Sestini F, Fioravanti C, et al. Reference ranges of serum calcitonin in pediatric population. J Clin Endocrinol Metab. 2015;100(5):1780–4. doi: 10.1210/jc.2014-4508. [DOI] [PubMed] [Google Scholar]
- 69.Haddad RILW, Busaidy NL, Byrd D, Callender G, Duh Q-Y, Ehya H, Haymart M, et al. NCCN Guidelines Thyroid Carcinoma: Version 1.2016. 2016 < nccn.org>.
- 70.Maia AL, Wajner SM, Vargas CV. Advances and controversies in the management of medullary thyroid carcinoma. Current opinion in oncology. 2017;29(1):25–32. doi: 10.1097/cco.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 71.Rich TAFL, Busaidy N, Cote GJ, Gagel RF, Hu M, Jimenez C, Lee JE, Perrier N, Sherman SI, et al. Prevalence by age and predictors of medullary thyroid cancer in patients with lower risk germline RET proto-oncogene mutations. Thyroid. 2014;24(7):1096–106. doi: 10.1089/thy.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris LFWS, Edeiken-Monroe BS, Lee JE, Rich TA, Ying AK, Warneke CL, Evans DB, Perrier ND, Grubbs EG. Ultrasounography should not guide the timing of thyroidectomy in pediatric patients diagnosed with multiple endocrine neoplasia syndrome 2A through genetic screening. Annals of surgical oncology. 2013;20(1):53–9. doi: 10.1245/s10434-012-2589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bilezikian JP, Khan Aa, Potts JT, Jr, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab 2009. 2009;94(2):335–9. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powell AC, Alexander HR, Pingpank JF, Steinberg SM, Skarulis M, Bartlett DL, et al. The utility of routine transcervical thymectomy for multiple endocrine neoplasia 1-related hyperparathyroidism. Surgery. 2008;144(6):878–83. doi: 10.1016/j.surg.2008.08.031. discussion 83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goudet P, Murat A, Cardot-Bauters C, Emy P, Baudin E, du Boullay Choplin H, et al. Thymic neuroendocrine tumors in multiple endocrine neoplasia type 1: a comparative study on 21 cases among a series of 761 MEN1 from the GTE (Groupe des Tumeurs Endocrines) World J Surg. 2009;33(6):1197–207. doi: 10.1007/s00268-009-9980-y. [DOI] [PubMed] [Google Scholar]
- 76.Moley JFSM, Gillanders WE, Lairmore TC, Rowland KJ, Traugott AL, Jin LX, Wells SA., Jr Management of the parathyroid glands during preventive thryoidectomy in patients with multiple endocrine neoplasia type 2. Ann Surg. 2015;262(4):641–6. doi: 10.1097/SLA.0000000000001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson NATM, Ogilvie JB. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR. 2007;188:1706–15. doi: 10.2214/AJR.06.0938. [DOI] [PubMed] [Google Scholar]
- 78.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Hofler H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A. 2006;103(42):15558–63. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellegata NS. MENX and MEN4. Clinics (Sao Paulo) 2012;67(Suppl 1):13–8. doi: 10.6061/clinics/2012(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92(8):3321–5. doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- 81.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94(5):1826–34. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molatore S, Marinoni I, Lee M, Pulz E, Ambrosio MR, degli Uberti EC, et al. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum Mutat. 2010;31(11):E1825–35. doi: 10.1002/humu.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Costa-Guda J, Marinoni I, Molatore S, Pellegata NS, Arnold A. Somatic mutation and germline sequence abnormalities in CDKN1B, encoding p27Kip1, in sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2011;96(4):E701–6. doi: 10.1210/jc.2010-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee M, Pellegata NS. Multiple endocrine neoplasia syndromes associated with mutation of p27. J Endocrinol Invest. 2013;36(9):781–7. doi: 10.3275/9021. [DOI] [PubMed] [Google Scholar]
- 85.Georgitsi M. MEN-4 and other multiple endocrine neoplasias due to cyclin-dependent kinase inhibitors (p27(Kip1) and p18(INK4C)) mutations. Best practice & research Clinical endocrinology & metabolism. 2010;24(3):425–37. doi: 10.1016/j.beem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Tichomirowa MA, Lee M, Barlier A, Daly AF, Marinoni I, Jaffrain-Rea ML, et al. Cyclin-dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocr Relat Cancer. 2012;19(3):233–41. doi: 10.1530/ERC-11-0362. [DOI] [PubMed] [Google Scholar]
- 87.Malanga D, De Gisi S, Riccardi M, Scrima M, De Marco C, Robledo M, et al. Functional characterization of a rare germline mutation in the gene encoding the cyclin-dependent kinase inhibitor p27Kip1 (CDKN1B) in a Spanish patient with multiple endocrine neoplasia-like phenotype. Eur J Endocrinol. 2012;166(3):551–60. doi: 10.1530/EJE-11-0929. [DOI] [PubMed] [Google Scholar]
- 88.Katznelson L, Laws ER, Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–51. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 89.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson CE. Hereditary hyperparathyroidism associated with recurrent pancreatitis. Ann Intern Med. 1954;49(4):829–36. doi: 10.7326/0003-4819-49-4-829. D - CLML: 5935:13158:429:433 OTO - NLM. [DOI] [PubMed] [Google Scholar]
- 91.Szabo J, Heath B, Hill VM, Jackson CE, Zarbo RJ, Zarbo Rj, Mallette LE, Chew SL, et al. Hereditary hyperparathyroidism-jaw tumor syndrome: the endocrine tumor gene HRPT2 maps to chromosome 1q21–q31. Am J Hum Genet. 56(4):944–50. NLM: PMC1801214 EDAT- 1995/04/01 MHDA- 1995/04/01 00:01 CRDT- 1995/04/01 00:00 PST - ppublish. [PMC free article] [PubMed] [Google Scholar]
- 92.Masi G, Barzon L, Iacobone M, Viel G, Porzionato A, Macchi V, et al. Clinical, genetic, and histopathologic investigation of CDC73-related familial hyperparathyroidism. Endocr Relat Cancer. 2008;15(4):1115–26. doi: 10.1677/ERC-08-0066. [DOI] [PubMed] [Google Scholar]
- 93.Newey PJ, Bowl MR, Cranston T, Thakker RV. Cell division cycle protein 73 homolog (CDC73) mutations in the hyperparathyroidism-jaw tumor syndrome (HPT-JT) and parathyroid tumors. Hum Mutat. 2010;31(3):295–307. doi: 10.1002/humu.21188. [DOI] [PubMed] [Google Scholar]
- 94.Starker LF, Akerstrom T, Long WD, Delgado-Verdugo A, Donovan P, Udelsman R, et al. Frequent germ-line mutations of the MEN1, CASR, and HRPT2/CDC73 genes in young patients with clinically non-familial primary hyperparathyroidism. Hormones & cancer. 2012;3(1–2):44–51. doi: 10.1007/s12672-011-0100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carpten JD, Robbins Cm, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, Simonds WF, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32(4):676–80. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- 96.Gill AJ, Clarkson A, Gimm O, Keil J, Dralle H, Howell VM, et al. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor (HPT-JT) syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. American Journal of Surgical Pathology. 2006;30(9):1140–9. doi: 10.1097/01.pas.0000209827.39477.4f. [DOI] [PubMed] [Google Scholar]
- 97.Cetani F, Ambrogini E, Viacava P, Pardi E, Fanelli G, Naccarato AG, et al. Should parafibromin staining replace HRTP2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma? EUR J ENDOCRINOL. 2007;156(5):547–54. doi: 10.1530/EJE-06-0720. [DOI] [PubMed] [Google Scholar]
- 98.Kruijff S, Sidhu SB, Sywak MS, Gill AJ, Delbridge LW. Negative parafibromin staining predicts malignant behavior in atypical parathyroid adenomas. Annals of surgical oncology. 2014;21(2):426–33. doi: 10.1245/s10434-013-3288-8. [DOI] [PubMed] [Google Scholar]
- 99.Juhlin CC, Villablanca A, Sandelin K, Haglund F, Nordenstrom J, Forsberg L, et al. Parafibromin immunoreactivity: its use as an additional diagnostic marker for parathyroid tumor classification. Endocrine-Related Cancer. 2007;14(2):501–12. doi: 10.1677/ERC-07-0021. [DOI] [PubMed] [Google Scholar]
- 100.Tan MH, Morrison C, Wang P, Yang X, Haven CJ, Zhang C, et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clinical Cancer Research. 2004;10(19):6629–37. doi: 10.1158/1078-0432.CCR-04-0493. [DOI] [PubMed] [Google Scholar]
- 101.Mangray S, Delellis RA. Parafibromin in the Diagnosis of Parathyroid Carcinoma. Advances in Anatomic Pathology. 2007;14(4):299–301. doi: 10.1097/PAP.0b013e3180ca8ad0. [DOI] [Google Scholar]
- 102.Iacobone M, Masi G, Barzon L, Porzionato A, Macchi V, Ciarleglio FA, et al. Hyperparathyroidism-jaw tumor syndrome: a report of three large kindred. Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2009;394(5):817–25. doi: 10.1007/s00423-009-0511-y. [DOI] [PubMed] [Google Scholar]
- 103.Jackson MA, Rich TA, Hu MI, Perrier ND, Waguespack SG. CDC73-Related Disorders. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al., editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 104.Kutcher MR, Rigby MH, Bullock M, Trites J, Taylor SM, Hart RD. Hyperparathyroidism-jaw tumor syndrome. Head & neck. 2013;35(6):E175–7. doi: 10.1002/hed.22918. [DOI] [PubMed] [Google Scholar]
- 105.Bradley KJ, Hobbs MR, Buley ID, Carpten JD, Cavaco BM, Fares JE, et al. Uterine tumours are a phenotypic manifestation of the hyperparathyroidism-jaw tumour syndrome. J Intern Med. 2005;257(1):18–26. doi: 10.1111/j.1365-2796.2004.01421.x. [DOI] [PubMed] [Google Scholar]
- 106.Tan MH, Teh BT. Renal neoplasia in the hyperparathyroidism-jaw tumor syndrome. Current molecular medicine. 2004;4(8):895–7. doi: 10.2174/1566524043359719. [DOI] [PubMed] [Google Scholar]
- 107.Teh BT, Farnebo F, Kristoffersson U, Sundelin B, Cardinal J, Axelson R, et al. Autosomal dominant primary hyperparathyroidism and jaw tumor syndrome associated with renal hamartomas and cystic kidney disease: linkage to 1q21–q32 and loss of the wild type allele in renal hamartomas. J Clin Endocrinol Metab. 1996;81(12):4204–11. doi: 10.1210/jcem.81.12.8954016. [DOI] [PubMed] [Google Scholar]
- 108.Bricaire L, Odou MF, Cardot-Bauters C, Delemer B, North MO, Salenave S, et al. Frequent Large Germline HRPT2 Deletions in a French National Cohort of Patients With Primary Hyperparathyroidism. J Clin Endocrinol Metab. 2013;98(2):E403–8. doi: 10.1210/jc.2012-2789. [DOI] [PubMed] [Google Scholar]
- 109.Pichardo-Lowden AR, Manni A, Saunders BD, Baker MJ. Familial hyperparathyroidism due to a germline mutation of the CDC73 gene: implications for management and age-appropriate testing of relatives at risk. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17(4):602–9. doi: 10.4158/ep10337.ra. [DOI] [PubMed] [Google Scholar]
- 110.Iacobone M, Barzon L, Porzionato A, Masi G, Macchi V, Viel G, et al. The extent of parathyroidectomy for HRPT2-related hyperparathyroidism. Surgery. 2009;145(2):250–1. doi: 10.1016/j.surg.2008.06.027. author reply 49. [DOI] [PubMed] [Google Scholar]