Abstract
IFN regulatory factor 3 (IRF3), a constitutively expressed protein localizing largely to the cytoplasm, is a primary effector of the innate immune response. Infection can trigger the phosphorylation, dimerization, and nuclear translocation of IRF3, where the factor stimulates the expression and release of IFN. In this study, we determined that the rotavirus gene 5 product, nonstructural protein 1 (NSP1), interacts with IRF3 in the infected cell. To understand the importance of the interaction, we compared IRF3 activation by rotaviruses expressing wild-type and C-truncated forms of NSP1. The analysis showed that IRF3 underwent dimerization and nuclear translocation and stimulated IFN promoter activity in infected cells expressing truncated NSP1. In contrast, infected cells expressing wild-type NSP1 were characterized by the rapid degradation of IRF3 during the replication cycle, severe decreases in IRF3 dimerization and nuclear translocation, and lack of IFN promoter activity. The implication of these results, that wild-type NSP1 is an antagonist of the IFN-signaling pathway, was confirmed in transient expression assays, which showed that wild-type NSP1, but not the C-truncated protein, induced the degradation of IRF3 fusion proteins. Related experiments indicated that NSP1 mediates IRF3 degradation through a proteasome-dependent pathway. The critical role of NSP1 in promoting cell-to-cell spread of rotavirus was demonstrated by using gene 5-specific short interfering RNAs in plaque assays. Although several viruses have been described that subvert the innate immune response by preventing IRF3 activation, rotavirus is identified as one that accomplishes this task by inducing the degradation of IRF3.
Keywords: innate immunity, antiviral state, double-stranded RNA virus, reovirus
Virus infection triggers a cascade of cellular events culminating in the expression and secretion of immunomodulatory proteins. The interaction of such secreted proteins, including the IFNs, with neighboring cells induces the establishment of the antiviral state. The capacity of the antiviral state to suppress virus replication is a critical element by which the host can control the spread of infection (1).
Events associated with virus infection that can initiate the innate immune response include entry, production of double-stranded RNA, and expression of viral proteins (2, 3). These events promote activation by inducing expression of IFN-stimulated gene (ISG) products. Induction of the ISGs is mediated by IFN regulatory factor 3 (IRF3), a ubiquitously expressed 55-kDa protein that accumulates as an inactive monomer in the cytoplasm (4). Viral infection stimulates the phosphorylation of IRF3 by IκB or IκB kinase-related kinases, a modification initiating structural changes in IRF3 that result in its dimerization (5). This activated form of IRF3 is translocated to the nucleus, where it interacts with specific promoter elements, stimulating expression of IFN-α and IFN-β (6). The secreted IFNs signal the production and activation of antiviral proteins in neighboring cells. The turnover of the nuclear, activated form of IRF3 is mediated by proteasomal degradation (7).
Viruses have evolved diverse methods for subverting establishment of the antiviral state. Among RNA viruses, VP35 of Ebola virus, the NS1/NS2 proteins of respiratory syncytial virus, the NS3/4A serine proteases of hepatitis C virus, and the NS1 protein of influenza virus suppress IFN induction by interfering with the phosphorylation and nuclear translocation of IRF3 (7–10). Given that these viral proteins do not interact directly with IRF3, they are likely to effect IRF3 activation through secondary processes, such as suppressing the activity of the IκB kinase-related kinases. Among DNA viruses, the E6 protein of human papilloma virus suppresses innate immunity by interacting with IRF3, thus rendering the factor unable to stimulate transcription of IFN genes (11). Finally, herpes simplex virus 1 inhibits IFN expression by interfering with the activation and nuclear translocation of IRF3, likely through the action of the immediate early protein, ICPO (12).
Rotaviruses, members of the Reoviridae, are the primary cause of acute dehydrating gastroenteritis in infants and young children, leading to >500,000 deaths annually (13). Rotaviruses can replicate to high titer in cell lines capable of expressing IFN, but their replication is restricted if the cells are first exposed to IFN (14). The behavior of rotavirus under these conditions is consistent with the idea that the virus encodes proteins that suppress the activation of ISGs or that inhibit the activity of the ISG products.
The recently identified interaction between the rotavirus nonstructural protein 1 (NSP1) and IRF3 by using a yeast two-hybrid system suggests that NSP1 may be involved in modulating the innate immune response (15). NSP1, the product of the rotavirus gene 5 RNA, is a 55-kDa RNA-binding protein that accumulates in the cytoplasm of the infected cell. Rotavirus mutants encoding C-truncated forms of NSP1 (NSP1-deletion mutants) can replicate efficiently in cell lines that are highly permissive for virus growth, a finding indicating that the protein has nonessential function (16). Indeed, knockdown experiments using RNA interference have confirmed that the protein is not needed for virus replication (17). However, the NSP1-deletion mutants share a characteristic small plaque phenotype, a property suggesting that their inability to encode wild-type NSP1 renders the mutants defective in cell-to-cell spread (16, 18).
Herein, we describe experiments addressing the possible role of rotavirus NSP1 as a modulator of the innate immune response. Our results demonstrate that the protein represents an antagonist of the IFN signaling pathway, functioning to induce the rapid degradation of IRF3 in the infected cell. The capacity of NSP1 to interfere with establishment of the antiviral state suggests that the protein represents a critical virulence determinant of the virus.
Materials and Methods
Cell Culture and Viruses. MA104 cells were grown in medium 199 (Invitrogen) supplemented with 5% FBS, and 293T, FRhL2, and Caco-2 cells were grown in DMEM supplemented with 10% FBS. Stocks of SA11-4F, SA11-5S, SA11-30-1A, and SA11-30-19 strains of rotavirus were propagated and titered in MA104 cells (17).
Expression Plasmids. Sequences encoding full-length wild-type NSP1 or forms with C-truncations of 17 (NSP1ΔC17) or 71 (NSP1ΔC71) residues were produced by using PCR with the vector pT7-gene 5 (16) as template, the forward primer 5′-GCTCTAGAATGGCTACTTTTAAAGATGCA-3′, and the reverse primers 5′-GCTCTAGATTACTCATTGTCATCTTCTGAGTT-3′, 5′-GCTCTAGATTAAGTTCCAGAATTCTTCATTTC-3′, or 5′-GCTCTAGATTACAATCCAAGAATCAACATGATG-3′, respectively. The PCR products were ligated into the XbaI site of the mammalian expression plasmid pCI (Promega), yielding pCI-NSP1, pCI-NSP1ΔC17, and pCI-NSP1ΔC71. The plasmid p55C1BLuc expresses luciferase under the control of positive regulatory domain I, a portion of the promoter element of the INF-β gene recognized by IRF3 (19). The plasmid pEGFP-IRF3 contains a human IRF3 cDNA in the vector pEGFP-C1 (Clontech) (1).
Immunoprecipitation. Caco-2 cells were mock-infected or infected with SA11-4F at a multiplicity of infection (moi) of 3. At 6 h after infection, the cells were lysed in immunoprecipitation buffer [20 mM Tris·HCl, pH 8.0/0.5 M NaCl/5 mM MgCl2/0.5% Triton X-100 and complete protease inhibitor mixture (Roche)]. After sonication, the lysates were clarified by centrifugation at 10,000 × g for 10 min. Immunoprecipitation assays were performed by using the ProFound Mammalian Co-Immunoprecipitation kit (Pierce). Briefly, the clarified lysates were precleared by incubation with the control gel component for 4 h at 4°C, then incubated with gel-immobilized anti-IRF3 antiserum (SC-9082, Santa Cruz Biotechnology) for 16 h at 4°C. Immunoprecipitates were washed four times with the immunoprecipitation buffer and once with reduced-salt immunoprecipitation buffer (125 mM NaCl) before elution.
Western Blot Assay. Cells were lysed in 3 mM Tris·HCl, pH 8.0/3 mM NaCl/0.5 mM MgCl2/0.5% Triton X-100 and protease inhibitor. After sonication, the lysate proteins were electrophoresed on 10% Tris/glycine gels (Invitrogen), transferred to nitrocellulose membranes, and analyzed with antisera specific for IRF3 (1:500), proliferating cell nuclear antigen, actin, rotavirus VP6, or NSP1(C19) (20). Primary antibodies were detected by using horseradish peroxidase-conjugated secondary antibody (1:10,000) and chemiluminescent substrate (SuperSignal West Pico, Pierce).
IRF3 Dimerization Assay. Caco-2 cells were mock-infected or infected with rotavirus at a moi of 3. At 12 h after infection, the cells were lysed in buffer containing 50 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM sodium orthovanadate, 100 nM okadaic acid, 5 mM NaF, and protease inhibitor. After clarification by centrifugation at 10,000 × g for 10 min, the lysates were electrophoresed on 7% polyacrylamide gels containing 1% deoxycholate (2). IRF3 monomers and dimers were detected by using Western blot assay.
Luciferase Assays. FRhL2 cells were grown to confluency in poly(l-lysine)-coated six-well plates. After rinsing the cells with Leibovitz L-15 medium, 2 ml of the same medium supplemented with 1% l-glutamine, 1% nonessential amino acid solution, and 1 mM sodium pyruvate were added to each well. Afterward, transfection mixtures consisting of 500 μl of Opti-MEM I (Invitrogen), 1.6% Lipofectamine 2000 (Invitrogen), and 1.5 μg of p55C1BLuc plasmid were combined with the overlay. At 3 h after transfection, 2.5 ml of supplemented Leibovitz L-15 medium containing 20% FBS was placed into each well. At 6 h after transfection, the medium was removed and the cells were mock-infected or infected with rotavirus at a moi of 10. At 10 h after infection, the cells were analyzed for luciferase activity by using a Luciferase Assay System kit (Promega).
IRF3 Nuclear Translocation Assay. FRhL2 cells were grown to confluency on poly(l-lysine)-coated glass coverslips in six-well plates. After transfection with 1 μg of pEGFP-IRF3 per well, cells were mock-infected or infected with rotavirus at a moi of 10. Cells were fixed with paraformaldehyde, permeabilized with Triton X-100, and stained with DAPI (Pierce) to detect nuclei. Fluorescence was detected by using a confocal microscope (TCS-NT, Leica Microsystems, Exton, PA).
FACS Analysis. Monolayers of 293T cells in six-well plates were transfected by using Lipofectamine 2000 with 4-μg combinations of equivalent amounts of appropriate plasmid DNAs. At 16 h after infection, the cells were detached by incubating in a 3:1 mixture of EDTA (0.1%) and trypsin (0.05%). The cells were then collected by using low-speed centrifugation, suspended in Sorter medium (Quality Biological, Gaithersburg, MD), and subjected to flow cytometry by using a Becton Dickinson FACScan instrument. The collected data were analyzed by using flowjo software (Tree Star, Ashland, OR).
Plaque Assay on Cell Monolayers Transfected with Short Interfering RNAs (siRNAs). Monolayers of MA104 cells in six-well plates were rinsed with medium 199, then overlaid with 2 ml of the same medium per well. A 500-μl volume of Opti-MEM I containing 2% Lipofectamine 2000 and 0.6 μM duplex siRNA g5E or an irrelevant (Ir) siRNA was added to the medium contained in each well (17). After incubation for 4 h, 2.5 ml of medium 199 containing 20% FBS was added to each well. After incubation for 12 h, monolayers were washed with Medium 199 and infected with 50 plaque-forming units of SA11-4F. Inocula were replaced with 1 ml of Eagle's minimal essential medium containing 0.6% agarose, 1% GASP, and 1 μg of trypsin. Plaques were detected at 4 days after infection by staining cells with a solution of 20% ethanol containing 0.2% crystal violet.
Results
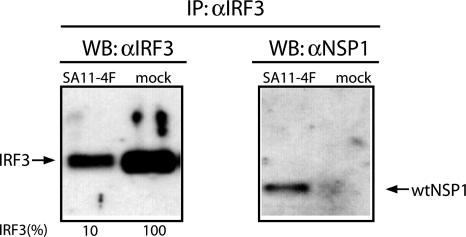
Interaction of NSP1 and IRF3. Yeast two-hybrid interaction screens using NSP1 as bait provided the original evidence of the possible interaction between NSP1 and IRF3 (15). Subsequent pull-down assays performed by incubating recombinant IRF3 with NSP1 prepared from infected cells provided further support of such an interaction (15). To test for the interaction of NSP1 and IRF3 in vivo, we prepared lysates at 6 h after infection from Caco-2 cells that were mock-infected or infected with rotavirus SA11-4F. Immunoprecipitates recovered from the lysates by using IRF3-specific antisera were then analyzed for the presence of IRF3 and NSP1 by using Western blot assay (Fig. 1). Detection of NSP1 in the IRF3 precipitate from SA11-4F-infected cells indicates that NSP1 forms complexes with IRF3 during the viral replication cycle. Unexpectedly, the analysis also indicated that the amount of IRF3 present in SA11-4F-infected cells was severalfold less than that contained in uninfected cells.
Fig. 1.
NSP1–IRF3 complexes formed in infected cells. IRF3-specific antiserum was used to prepare immunoprecipitates from SA11-4F- or mock-infected Caco-2 cells. The precipitates were analyzed by using Western blot assay with IRF3 or NSP1(C19)-specific antiserum. Intensities (percent) of IRF3 bands were determined with a PhosphorImager and normalized to 100% for IRF3 from mock-infected cells.
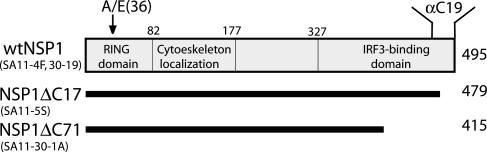
Linkage Between NSP1 Expression and IRF3 Degradation. To gain further insight into the impact of rotavirus and its NSP1 gene product on IRF3 levels in infected cells, we took advantage of the existence of rotavirus strains differing in their coding capacity for NSP1. Notably, whereas the parental strains SA11-4F and SA11-30-19 encode wild-type NSP1, their respective daughter strains SA11-5S and SA11-30-1A encode forms of NSP1 with C-truncations of 17 (NSP1ΔC17) or 71 (NSP1ΔC71) residues (Fig. 2) (16). The truncated species expressed by SA11-5S and SA11-30-1A are stable, as judged from their accumulation to levels approximating that of the wild-type forms expressed by SA11-4F and SA11-30-19 (Fig. 3C) (ref. 16 and unpublished data). Yeast two-hybrid assays have indicated that the C terminus of NSP1 plays an essential role in NSP1–IRF3 interactions (15), thus the truncated forms encoded by SA11-5S and SA11-30-1A are likely defective in interacting with IRF3.
Fig. 2.
Full-length (wt) and C-truncated (ΔC17 and ΔC71) NSP1 species encoded by SA11 isolates. The NSP1(C19) antiserum was made by using a peptide corresponding to the C-terminal 19 residues of NSP1. Residue 36 of NSP1 is Ala for SA11-4F but Glu for SA11-30-19. The NSP1 National Center for Biotechnology Information protein database accession numbers are AAK14071 (SA11-4F), AAK14072 (SA11-5S), AAK14069 (SA11-30-19), and AAK14070 (SA11-30-1A).
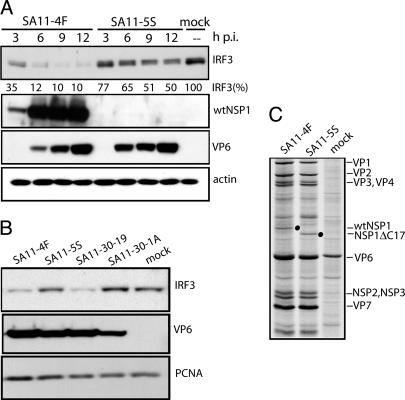
Fig. 3.
IRF3 levels in infected cells. (A) Lysates were prepared at 3-h intervals from Caco-2 cells infected with SA11-4F or SA11-5S and assayed for IRF3, full-length NSP1, VP6, and actin by Western blot analysis. The NSP1(C19) antiserum does not react with the C-truncated NSP1 species of SA11-5S and SA11-30-19. Intensities (percent) of IRF3 bands were determined with a PhosphorImager and normalized to 100% for IRF3 from mock-infected cells. (B) Lysates were prepared at 10 h after infection from FRhL2 cells that were mock-infected or infected with SA11-4F or SA11-5S. Lysates were assayed for IRF3, VP6, and proliferating cell nuclear antigen (PCNA) by Western blot analysis. (C) Lysates were prepared at 8 h after infection from MA104 cells infected at a moi of 20 with SA11-4F or SA11-5S and maintained in 35S-labeled amino acids beginning at 3 h after infection (18). Radiolabeled proteins in lysates were detected by SDS/PAGE and autoradiography (18). Positions of wtNSP1 and NSP1ΔC17 are indicated with dots.
As shown in Fig. 3A, infection of Caco-2 cells with SA11-4F resulted in a reduction in IRF3 as a function of time, such that by 9–12 h after infection, IRF3 levels were considerably lower than those levels present in mock-infected cells. The decrease in IRF3 levels correlated inversely with the intracellular levels of viral proteins, suggesting that viral gene products were responsible for the IRF3 reduction.
In comparison with SA11-4F, SA11-5S caused a lesser reduction in IRF3 levels in infected cells (Fig. 3A). Given that the only known difference in the expression of viral proteins by the SA11-4F and SA11-5S strains is that the former produces wild-type NSP1 and that the latter produces C-truncated NSP1 (NSP1ΔC17), the results may be interpreted to mean that full-length NSP1 is responsible for causing the reduction of IRF3 in infected cells. A similar effect was seen in a comparison of the impact of SA11-30-19 and SA11-30-1A on IRF3 levels in Caco-2 cells (data not shown).
The effect of rotavirus encoding wild-type or C-truncated NSP1 on IRF3 in the semicontinuous diploid FRhL2 cell line also was analyzed. FRhL2 cells have been used in propagating attenuated strains of rotavirus for vaccine formulation and are known to impose a heightened selective pressure favoring retention of NSP1-gene function (21). Consistent with the results presented above, infection of FRhL2 cells with viruses encoding wild-type NSP1 (SA11-4F and SA11-30-19) led to decreases in the intracellular levels of IRF3, compared with mock-infected cells (Fig. 3B). In contrast, viruses encoding C-truncated NSP1 (SA11-5S and SA11-30-1A) had lesser or no effect on IRF3 levels. The mutant virus (SA11-30-1A) expressing the severely truncated form of NSP1 (NSP1ΔC71) (16) was phenotypically a slow grower in FRhL2 cells, displaying lower levels of viral protein expression (e.g., VP6 in Fig. 3B), double-stranded RNA synthesis, and progeny virus formation than did its parental virus SA11-30-19.
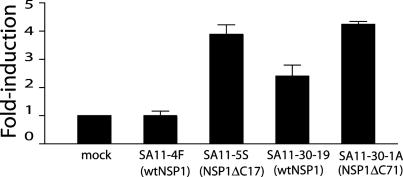
NSP1 Reduces IFN-Promoter Activation. The correlation between expression of wild-type NSP1 and reduction of IRF3 in infected cells suggests that rotavirus may subvert IFN induction by a mechanism in which NSP1 triggers IRF3 degradation. To examine the possibility that NSP1 can suppress activation of ISGs, we transfected FRhL2 cells with p55C1BLuc, a vector expressing luciferase under the control of an IRF3-stimulated IFN-β promoter element. The cells were subsequently infected with rotaviruses encoding wild-type or truncated forms of NSP1, and then at 10 h after infection, assayed for luciferase activity. The results showed that, although SA11-4F failed to induce the expression of luciferase, compared with mock-infected cells, SA11-5S caused a nearly 4-fold induction in its expression (Fig. 4). Thus, the IFN-β promoter element was not up-regulated in infected cells producing wild-type NSP1 but was activated in infected cells producing NSP1ΔC17.
Fig. 4.
Impact of viral infection on IFN-pathway activation. FRhL2 cells were transfected with p55C1BLuc, a plasmid containing an IRF3-activated IFN-β promoter element that drives luciferase expression. The cells were mock-infected or infected with the indicated virus, and, at 10 h after infection, cellular lysates were assayed for luciferase activity. The values are reported as fold increase in luciferase activity relative to mock-infected cells.
Similar, but not identical, results were obtained when the p55C1BLuc-transfected cells were infected with SA11-30-19 and SA11-30-1A. Unlike SA11-4F, which did not stimulate luciferase expression, SA11-30-19 caused an ≈2-fold increase in luciferase expression (Fig. 4), despite the fact that both these viruses (SA11-4F and SA11-30-19) encode full-length forms of NSP1 (wild-type NSP1). Importantly, though, NSP1 of SA11-4F and SA11-30-19 differ in that the protein of the former contains an A → E substitution within the highly conserved ring-finger domain (Fig. 1). It may be that this substitution generates a form of the protein that is less efficient in preventing the IRF3-dependent activation of the IFN-β promoter element. Despite the potential decreased efficiency of its wild-type NSP1 product, SA11-30-19 induced luciferase expression in p55C1BLuc-transfected cells to levels approximately one-half that of SA11-30-1A, the virus encoding NSP1ΔC71 (Fig. 4). Thus, like the results obtained by using SA11-4F and SA11-5S, a lower level of IRF3-dependent activation of the IFN promoter element occurred in cells expressing the wild-type NSP1 product of SA11-30-19 than in cells expressing the C-truncated NSP1 of SA11-30-1A. Taken together, these data suggest a role for NSP1 in suppressing the induction of ISGs in rotavirus-infected cells.
NSP1 Suppresses IRF3 Nuclear Translocation. Transactivation of the IFN genes requires translocation of IRF3 from the cytoplasm to the nucleus. To assess the potential impact of NSP1 on IRF3 translocation, FRhL2 cells were transfected with a plasmid expressing the fusion protein EGFP-IRF3. The cells were subsequently infected with rotaviruses encoding wild-type or C-truncated NSP1, and then at 10 h after infection, the localization of EGFP-IRF3 was evaluated by confocal immunofluorescence. The analysis showed that EGFP-IRF3 accumulated in the cytoplasm of cells infected with viruses encoding wild-type NSP1 (SA11-4F and SA11-30-19), with little or none of the fusion protein detected in the nuclei (Fig. 5). In contrast, EGFP-IRF3 accumulated in the nuclei of cells infected with viruses encoding C-truncated NSP1 (SA11-5S and SA11-30-1A). Thus, EFGP-IRF3 failed to undergo nuclear translocation in cells containing wild-type NSP1 but underwent nuclear translocation in cells containing C-truncated NSP1. These data indicate that NSP1 interferes with induction of ISGs by preventing the nuclear translocation of IRF3.
Fig. 5.
Localization of EGFP-IRF3 in rotavirus-infected cells. FRhL2 cells were transfected with the plasmid pCI-EGFP-IRF3, and, at 6 h after transfection, the cells were infected with the indicated virus. At 10 h after infection, EGFP-IRF3 (green) and the DAPI-nuclear (red) signals were detected by confocal immunofluorescence microscopy.
NSP1 Reduces Accumulation of IRF3 Dimers. Activation of IRF3 requires the phosphorylation and dimerization of the monomer form of the protein. To understand the impact of rotavirus infection on IRF3 activation, we infected Caco-2 cells with viruses encoding wild-type NSP1 (SA11-4S) or NSP1ΔC17 (SA11-5S) and then analyzed lysates from the infected cells by using nondenaturing PAGE and Western blot assay for IRF3 monomers and dimers. The results showed that, unlike mock-infected cells, cells infected with SA11-4F and SA11-5S contained IRF3 dimers (Fig. 6). Thus, rotavirus infection does lead to IRF3 activation. However, the level of the IRF3 dimer in SA11-4F-infected cells was much less than the level in SA11-5S-infected cells. This difference suggests that wild-type NSP1 suppresses the accumulation of the activated form of IRF3 in infected cells.
Fig. 6.
Levels of dimerized IRF3 in rotavirus-infected cells. Lysates prepared from infected Caco-2 cells were electrophoretically resolved under nondenaturing or denaturing conditions. Western blot analysis was used to locate monomer and dimer forms of IRF3 in the nondenaturing gel and IRF3, wtNSP1, and proliferating cell nuclear antigen (PCNA) in the denaturing gel.
A substantial fraction of Caco-2 cells is resistant to rotavirus infection even at high moi. For example, immunofluorescence assay indicates that ≈35% of the cells contained in a Caco-2 monolayer infected at a moi of 25 lack viral antigen (data not shown). Thus, the origin of the IRF3 monomer detected in the lysates of the SA11-4F- and SA11-5S-infected cells is not clear (Fig. 6); possibly the monomer arises from either resistant or infected Caco-2 cells in the population.
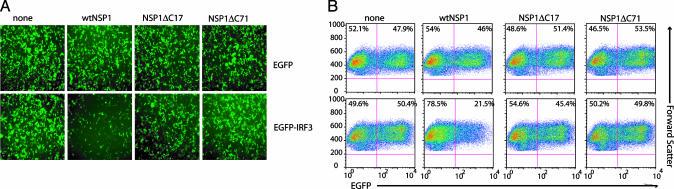
NSP1 Promotes Loss of IRF3. To address the possibility that wild-type NSP1 induces IRF3 degradation in rotavirus-infected cells, 293T cells were cotransfected with plasmids expressing EGFP or EGFP-IRF3 and wild-type or C-truncated NSP1. The fluorescence signal of EGFP in the transfected cells was monitored by using epifluorescence microscopy, and the number of fluorescent cells was quantified by using flow cytometry (Fig. 7). The results showed that coexpression of wild-type NSP1 or NSP1-ΔC17 or NSP1-ΔC71 with EGFP had no effect on the percentage of fluorescent cells in the population. Likewise, coexpression of the truncated forms, NSP1-ΔC17 and NSP1-ΔC71, with EGFP-IRF3 did not change the percentage of fluorescent cells. In contrast, expression of wild-type NSP1 with EGFP-IRF3 reduced the number of fluorescent cells by 50%. Thus, the presence of wild-type NSP1, but not C-truncated NSP1, led to a decrease in the accumulation of the IRF3-containing target proteins. This result is consistent with the suggestion that the wild-type NSP1 product of SA11-4F and SA11-30-19 mediates the degradation of IRF3 in infected cells, whereas the truncated forms encoded by SA11-5S and SA11-30-1A are deficient in such activity.
Fig. 7.
Effect of NSP1 on EGFP-IRF3 levels. Equivalent amounts of pEGFP or pEGFP-IRF3 were cotransfected with pCI, pCI-NSP1, pCI-NSP1ΔC17, or pCI-NSP1ΔC71 into 293T cells to drive the expression of either EGFP or EGFP-IRF3 alone or together with wtNSP1 or NSP1ΔC proteins. (A) Epifluorescence microscopy (×10) was used to analyze the EGFP signal (green). (B) Flow cytometry analysis was used to calculate percentage of total cells that were fluorescent. The data are represented in density plot graphics where the upper left quadrant corresponds to nonfluorescent cells and the upper right quadrant corresponds to fluorescent cells. The percentage of cells in each group is given in the corresponding quadrant.
Proteasome Inhibitor Interferes with IRF3 Degradation. To determine whether the NSP1-dependent loss of IRF3 in rotavirus-infected cells occurred via the proteasome pathway, 293T cells were co-transfected with plasmids expressing wild-type NSP1 and EGFP-IRF3. Afterward, some cells were maintained in media containing the proteasome inhibitor, MG132. The analysis showed that the percentage of fluorescent cells in the total population of cells expressing wild-type NSP1 and EGFP-IRF3 and maintained in MG132 was the same as that in the total population of cells expressing no recombinant protein but maintained in MG132 (Fig. 8, compare A with B). In contrast, the percentage of fluorescent cells was reduced by approximately one-half when the population of wild-type NSP1- and EGFP-IRF3-expressing cells was maintained in the absence of MG132 (Fig. 8, compare B with D). These data show that the inhibitor completely overcame the NSP1-dependent degradation of the IRF3-containing target protein, suggesting that the turnover of IRF3 in rotavirus-infected cells is mediated by proteasomes.
Fig. 8.
Reduction in IRF3 degradation by a proteasome inhibitor. Appropriate plasmids were transfected into 293T cells to drive the expression of EGFP-IRF3 alone or together with wtNSP1. The transfected cells or mock-transfected cells were then incubated with media containing 2.5 μM protease inhibitor MG132 dissolved in DMSO or DMSO alone. After 16 h, the percentage of luminescent cells in the population was determined by flow cytometry.
Silencing of NSP1 Inhibits Virus Spread. IRF3 activation induces the establishment of the antiviral state in uninfected cells, an event that can suppress virus spread (22). Our results indicate that NSP1 antagonizes this process by causing IRF3 degradation, and thus, NSP1 should have the effect of promoting the cell-to-cell spread of the virus. To test this possibility, SA11-4F was plaque-assayed on monolayers of MA104 cells that had been transfected with an siRNA specific for the SA11 gene encoding NSP1 (g5E siRNA) or with a control siRNA specific for the gene of the Wa strain of rotavirus encoding NSP1 (Ir siRNA) (17). Sequence differences preclude the Ir siRNA from affecting the stability or expression of the NSP1 gene in SA11-4F-infected cells (17). The results of using the g5E siRNA in the plaque assay showed that knockdown of NSP1 expression caused a 2- to 4-fold decrease in the size of the plaques generated by SA11-4F, compared with using the control Ir siRNA (Fig. 9A). This finding is consistent with the proposed role of NSP1 in promoting rotavirus spread. Remarkably, we observed a similar difference in the size of plaques produced when MA104 cells were infected with SA11-4F and the NSP1-defective virus, SA11-5S, a result also suggesting that NSP1 promotes virus spread.
Fig. 9.
Impact of NSP1 knockdown on plaque phenotype. (A) MA104 cells were transfected with an siRNA specific to the gene encoding NSP1 (g5E) or with an Ir siRNA. Subsequently, the cells were infected with 50 plaque-forming units of SA11-4F per well. (B) Plaques formed by plating SA11-4F and SA11-5S on MA104 cells.
Discussion
From analysis of cells infected with rotavirus NSP1-deletion mutants, it is apparent that the virus has the potential to trigger the activation of IRF3, the nuclear translocation of IRF3, and the induction of promoter elements controlling IFN expression. Yet, these events are suppressed in cells infected with rotaviruses encoding wild-type NSP1, an indication that a primary function of the protein is to act as an antagonist of the innate immune response. Our experiments indicate that wild-type NSP1 mediates this function by directly interacting with IRF3. Although several other viral antagonists of the innate immune response have been identified, only the E6 protein of human papilloma virus 16 (11) is known to interact with IRF3. However, the interaction of E6 with IRF3 does not cause changes to the intracellular levels of the transcription factor, unlike NSP1, which promotes the rapid degradation of IRF3 in rotavirus-infected cells. Thus, the mechanism by which NSP1 subverts the innate immune response is one that has not been recognized previously.
The linkage between NSP1 and the degradation of IRF3 was demonstrated by transient expression experiments, which showed that wild-type and not C-truncated NSP1 caused a loss of an IRF3-containing reporter protein. An outcome of the effect that NSP1 has on IRF3 is to suppress the accumulation of the activated (homodimerized) form of the transcription factor in the infected cell. Because it is this form of IRF3 that is actively translocated to the nucleus, it follows that the lack of activated IRF3 in the cell would prevent the accumulation of IRF3 in the nucleus and the induction of IFN gene expression. Our experiments have not resolved whether NSP1 preferentially targets the inactive monomer or activated dimer of IRF3 for degradation. Because the monomer is the precursor of the dimer, degradation of either form would produce the lower dimer levels that are seen in the infected cell. Given that NSP1 can interact with IRF3 expressed as a fusion protein in bacteria (GST-tagged IRF3) and yeast (GAL1 fragment-IRF3) (15), we can presume that NSP1 has the capacity to bind to the inactive IRF3 monomer. In addition to subverting the innate immune response by causing IRF3 degradation, just the binding of NSP1 to IRF3 may be sufficient to suppress IFN induction. That is, the association of NSP1 with IRF3 may impede the necessary phosphorylation, structural rearrangement, or monomer–monomer interaction required to produce activated IRF3.
The critical event brought on by NSP1 that causes IRF3 degradation remains to be determined; however, there are no features of NSP1 to indicate that it has proteolytic activity. Instead, assays performed with the MG132 inhibitor suggest that NSP1 causes the degradation of IRF3 through a proteasome-dependent pathway. From an earlier study showing that rotavirus infection induces expression of genes encoding proteasome-associated proteins (23), levels of proteasome activity may be stimulated as rotavirus replicates, further increasing the effectiveness of the IRF3-degradation process. Unlike rotavirus infection, Sendai virus infection is characterized by the accumulation of activated IRF3 in the nucleus (1). Turnover of the nuclear IRF3, likely critical for cells to step-down from an antiviral state, is also proteasome-dependent. Because rotavirus infection is characterized by IRF3 degradation in the absence of nuclear accumulation, the cytoplasm would seem to be the likely site of IRF3 degradation.
Among the various strains of rotavirus, NSP1 is noted for its high degree of sequence variability, particularly within its C-terminal portion. The protein is much more conserved among rotaviruses infecting the same host. For example, NSP1 sequences of the human rotaviruses are highly conserved (unpublished data). Given the critical nature of the C terminus of NSP1 in its interaction with IRF3, we can predict that NSP1 has evolved such that it is species-specific in its ability to suppress host innate immunity. This specificity may explain in part why rotavirus strains causing severe gastroenteritis in a homologous animal model are usually less infectious and asymptomatic in a heterologous animal model (24).
Viruses defective in the expression of INF-antagonist proteins can replicate within the cell, but their capacity to spread is diminished (22). Although NSP1 does not affect replication (17), we have demonstrated that it affects plaque phenotype and thus plays a role in rotavirus spread. The effect of NSP1 on innate immunity and virus spread leads to the conclusion that gene 5 is an important virulence determinant of rotavirus. This conclusion is supported by an earlier study of the reassortants made from murine and rhesus rotaviruses that showed a strong correlation between gene 5 and virus virulence and spread (25). Analyses of other RNA viruses containing gene deletions that prevent the expression of IRF3 antagonists have shown that these viruses are phenotypically less virulent than their wild-type counterparts. This finding has led to the pursuit of a new generation of attenuated virus isolates suitable for vaccine development (26, 27). Similarly, we propose that the generation of NSP1-defective human rotaviruses may be used to create a new class of more effective live rotavirus vaccines.
Acknowledgments
We thank Drs. John Hiscott (McGill University, Montreal) and Takashi Fujita (Tokyo Metropolitan Institute of Medical Science, Tokyo) for providing us with the plasmids pEGFP-IRF3 and p55C1Bluc, respectively. Taka Hoshino's contributions to this project are greatly appreciated.
Author contributions: M.B. and J.T.P. designed research; M.B. performed research; M.B. and J.T.P. analyzed data; and M.B. and J.T.P. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: IRF3, IFN regulatory factor 3; ISG, IFN-stimulated gene; NSP, nonstructural protein; moi, multiplicity of infection; siRNA, short interfering RNA; Ir siRNA, irrelevant siRNA.
References
- 1.Lin, R., Heylbroeck, C., Pitha, P. M. & Hiscott, J. (1998) Mol. Cell. Biol. 18, 2986–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwamura, T., Yoneyama, M., Yamaguchi, K., Suhara, W., Mori, W., Shiota, K., Okabe, Y., Namiki, H. & Fujita, T. (2001) Genes Cells 6, 375–388. [DOI] [PubMed] [Google Scholar]
- 3.tenOever, B. R., Servant, M. J., Grandvaux, N., Lin, R. & Hiscott, J. (2002) J. Virol. 76, 3659–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiscott, J., Pitha, P., Genin, P., Nguyen, H., Heylbroeck, C., Mamane, Y., Algarte, M. & Lin, R. (1999) J. Interf. Cytok. Res. 19, 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Sharma, S., tenOever, B. R., Grandvaux, N., Zhou, G. P., Lin, R. & Hiscott, J. (2003) Science 300, 1148–1151. [DOI] [PubMed] [Google Scholar]
- 6.Au, W. C., Moore, P. A., Lowther, W., Juang, Y. T. & Pitha, P. M. (1995) Proc. Natl. Acad. Sci. USA 92, 11657–11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basler, C. F., Mikulasova, A., Martinez-Sobrido, L., Paragas, J., Muhlberger, E., Bray, M., Klenk, H. D., Palese, P. & Garcia-Sastre, A. (2003) J. Virol. 77, 7945–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spann, K. M., Tran, K. C., Chi, B., Rabin, R. L. & Collins, P. L. (2004) J. Virol. 78, 4363–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foy, E., Li, K., Wang, C., Sumpter, R., Jr., Ikeda, M., Lemon, S. M. & Gale, M., Jr. (2003) Science 300, 1145–1148. [DOI] [PubMed] [Google Scholar]
- 10.Talon, J., Horvath, C. M., Polley, R., Basler, C. F., Muster, T., Palese, P. & Garcia-Sastre, A. (2000) J. Virol. 74, 7989–7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronco, L.V., Karpova, A. Y., Vidal, M. & Howley, P. M. (1998) Gene Dev. 12, 2061–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eidson, K. M., Hobbs, W. E., Manning, B. J., Carlson, P., DeLuca, N. A. (2002) J. Virol. 76, 2180–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapikian, A. Z. (2001) Novart. Fdn. Symp. 238, 153–171. [DOI] [PubMed] [Google Scholar]
- 14.Bass, D. M. (1997) Gastroenterology 113, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graff, J. W., Mitzel, D. N., Weisend, C. M., Flenniken, M. L. & Hardy, M. E. (2002) J. Virol. 76, 9545–9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patton, J. T., Taraporewala, Z., Chen, D., Chizhikov, V., Jones, M., Elhelu, A., Collins, M., Kearney, K., Wagner, M., Hoshino, Y., et al. (2001) J. Virol. 75, 2076–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvestri, L. S., Taraporewala, Z. F. & Patton, J. T. (2004) J. Virol. 78, 7763–7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taniguchi, K., Kojima, K. & Urasawa, S. (1996) J. Virol. 70, 4125–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneyama, M., Suhara, W., Fukuhara, Y., Sato, M., Ozato, K. & Fujita, T. (1996) J. Biochem. (Tokyo) 120, 160–169. [DOI] [PubMed] [Google Scholar]
- 20.Hua, J., Chen, X. & Patton, J. T. (1994) J. Virol. 68, 3990–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearney, K., Chen, D., Taraporewala, Z. F., Vende, P., Hoshino, Y., Tortorici, M. A., Barro, M. & Patton, J. T. (2004) EMBO J. 23, 4072–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredericksen, B. L., Smith, M., Katze, M. G., Shi, P. Y. & Gale, M., Jr. (2004) J. Virol. 78, 7737–7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuadras, M. A., Feigelstock, D. A., An, S. & Greenberg, H. B. (2002) J. Virol. 76, 4467–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn, S. J., Cross, T. L. & Greenberg, H. B. (1994) Virology 203, 178–183. [DOI] [PubMed] [Google Scholar]
- 25.Broome, R. L., Vo, P. T., Ward, R. L., Clark, H. F. & Greenberg, H. B. (1993) J. Virol. 67, 2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talon, J., Salvatore, M., O'Neill, R. E., Nakaya, Y., Zheng, H., Muster, T., Garcia-Sastre, A. & Palese, P. (2000) Proc. Natl. Acad. Sci. USA 97, 4309–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins, P. L. & Murphy, B. R. (2002) Virology 296, 204–211. [DOI] [PubMed] [Google Scholar]