Abstract
Aims
To compare the acute effects of alcohol on set-shifting task performance (relative to sober baseline performance) during ascending and descending limb breath alcohol concentration (BrAC), as well as possible moderation of these effects by baseline individual differences.
Design
Shifting performance was tested during an initial baseline and a subsequent drinking session, during which participants were randomly assigned to one of three beverage conditions (alcohol, placebo, or control) and one of two BrAC limb conditions (ascending and descending [A/D], or descending-only [D-only]).
Setting
A human experimental laboratory on the University of Missouri campus in Columbia, MO, USA.
Participants
A total of 222 moderate-drinking adults (ages 21–30 years) recruited from Columbia, MO and tested between 2010- and 2013.
Measurements
The outcome measure was performance on set-shifting tasks under the different beverage and limb conditions. Shifting performance assessed at baseline was a key moderator.
Findings
Although performance improved across sessions, this improvement was reduced in the alcohol compared with no-alcohol groups (post-drink latent mean comparison across groups, all Ps≤.05) and this effect was more pronounced in individuals with lower pre-drink performance (comparison of pre-to-post drink path-coefficients across groups, all Ps≤.05). In the alcohol group, performance was better on descending compared with ascending limb (p≤.001), but descending limb performance did not differ across the A/D and D-only groups.
Conclusions
Practising tasks before drinking moderates the acute effects of alcohol on the ability to switch between tasks. Greater impairment in shifting ability on descending compared with ascending breath alcohol concentration is not related to task practice.
Keywords: Acute alcohol, baseline EF performance, executive functioning, latent-variable approach, limbs of the BrAC, set-shifting
INTRODUCTION
A large literature describes an important role for higher-order cognitive abilities, such as planning, problem solving, and flexibility, in addiction (1,2). This broad class of cognitive abilities is often referred to as executive functioning (EF). Many EF-related abilities are known to be impaired under acute alcohol intoxication both in the lab (3–5) and in naturalistic settings (6–8). This is a major concern, given that impairment in the regulatory processes represented by these EF abilities can promote protracted alcohol and drug use despite their health risks and contribute to a range of acute alcohol-related problems.
To date, most studies investigating acute effects of alcohol on EF have focused on abilities related to response inhibition or working memory; relatively few investigations have tested alcohol’s effects on the ability to switch between tasks (9). This ability, often termed shifting (10), represents the ability to perform a new operation in the face of proactive interference (see, (11)). Along with inhibition and working memory updating, shifting is considered one of core EF abilities supporting self-regulatory control (10), and although these three aspects of EF share some common features, they are also theoretically and empirically distinct (10). Addressing this imbalance in the literature on alcohol and EF is important because although alcohol’s effects on EF are extensive, they are far from uniform (9). The purpose of the current study was to provide the most thorough test to date of alcohol’s effects on shifting.
Set-shifting paradigms involve a sequence of trials that require either transitioning to a new task set or maintaining the current one on successive trials. Outcome measures in set-shifting paradigms include the switch cost—the additional time (represented in response time [RT]) required to successfully reconfigure a new task set relative to maintaining the current one—and perseverative errors—responding to a stimulus according to a prior task set configuration that is inappropriate in the current one. Earlier studies showed that alcohol increases perseverative errors both in laboratory (4,12,13) and in naturalistic (bar) settings (7), but no previous studies have tested acute alcohol effects on switch costs.
Extant studies in this literature have suffered from several shortcomings, leading to uncertainty over their conclusions. First, in most studies alcohol’s effects on shifting have been modeled using only a single behavioral task (9). Moreover, the tasks used in most of those studies (e.g., Wisconsin Card Sort; Tower of London; Trails-making Test part B) are cognitively complex, meaning they tap multiple EF abilities as well as other, non-EF processes (language; visuospatial), introducing variability in performance attributable to task stimuli but unrelated to shifting ability (10,14). Previous work (15) demonstrates how alcohol’s effects can vary according to such stimulus-driven factors. The current study overcame this problem by adopting a latent variable approach in which three exemplar tasks selected to have different nonexecutive requirements but to tap the same underlying shifting ability were administered. The resulting latent variable represents an estimate of the latent variable of shifting ability with no measurement error (16).
Two additional shortcomings of the extant literature were addressed in this study. First, previous studies of alcohol’s effects on shifting, indeed on cognitive functions more generally, have failed to investigate potential differences associated with ascending and descending breath alcohol concentration (BrAC). This issue is important in that reduced impairment during descending relative to ascending BrAC has been reported for some EF abilities (17–22); for reviews, see (23,24). However, this apparent recovery on the descending limb of the BrAC curve has been observed mostly in within-subjects comparisons, where the same individuals are tested during both limbs (17–22). This design makes it difficult to infer whether improvements during descending BrAC merely reflect practice effects (7,25), but also see (26). Disentangling practice from acute tolerance effects (23) requires a design in which some participants complete EF tasks under both ascending and descending BrAC (A/D group), whereas others complete the tasks only under descending BrAC (D-only group). Better descending-limb (DL) performance in the A/D group relative to the D-only group would provide evidence of a practice effect; equivalent DL performance in the two groups would suggest that acute tolerance occurs.
Finally, very few studies have considered the moderating influence of individual differences in sober (baseline) performance on the magnitude of alcohol’s effects on EF. Low EF ability represents a dual hazard for harmful drinking outcomes because not only does poor performance on EF tasks predict escalation of alcohol involvement (27–29) and risk-taking behaviors in youth (30), but individuals with poor EF ability often experience greater impairment from alcohol than their higher-EF peers (31,32). The current study included a sober baseline testing session to permit modeling effects of individual differences in shifting ability.
The primary aim of this study was to provide the most comprehensive test to date of alcohol’s acute effects on shifting, using a latent variable approach to isolate alcohol’s effects on shifting from non-EF processes (see (14)). Based on the findings of earlier acute alcohol studies on EF, we focused on the following questions: (a) Is post-drink performance in the alcohol group worse than post-drink performance in the no-alcohol group; (b) Is performance on the DL significantly less impaired than performance on the ascending limb (AL) in the alcohol group, which might suggest acute tolerance or practice effects; and (c) Does post-drink performance vary according to both pre-drink performance levels and beverage group, such that individuals with poorer baseline shifting ability experience greater impairment from alcohol than those with better baseline ability. We also wished to test (d) whether any ostensible performance recovery on the DL appears due to practice; a difference in DL performance between the A/D and D-only groups would suggest a practice effect.
METHOD
Design
The study consisted of a baseline session and a drinking session; during the drinking session participants consumed alcohol, a placebo beverage or a control beverage. This design permitted comparison of shifting performance (measured as a latent variable derived from performance in three set-shifting tasks) as a function of alcohol pharmacology (alcohol vs. placebo and control) and alcohol expectancy (alcohol and placebo vs. control) on the ascending and descending limb of the breath alcohol curve, as well as moderation of these effects by baseline individual differences. Prior to data collection, the statistical power analysis of the structural models was assessed using the Monte Carlo component of Mplus, and for each combination of three effect size magnitudes (low, medium, high) for both alcohol and expectancy effects based on previously published estimates for the updating construct and assuming a sample size of 216 individuals. The power to detect small, medium and large effects for either construct were .19–.20, .89–.90, .99; respectively.
Sample
Two-hundred fifty-eight participants between the ages of 21-to-30 were recruited from the Columbia, MO community for a study examining effects of alcohol on cognition. Interested individuals were screened for their eligibility. (See Supplementary Materials for exclusion criteria.) Participants were paid $35 for completion of the first and $14/hr. for the second session (and a $10 bonus for completing both sessions). Demographic characteristics of the sample are given in Table 1.
Table 1.
Demographic characteristics of study sample.
| Experimental Group (n=222) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Placebo | Alcohol | ||||
|
| ||||||
| D-only N=40 |
A/D N=40 |
D-only N=35 |
A/D N=37 |
D-only N=37 |
A/D N=33 |
|
| % Male | 57.5 | 42.5 | 57.14 | 56.75 | 43.24 | 39.39 |
| Age (mean(SD)) | 22.57(2.57) | 22.3(2.44) | 22.71(3.1) | 22.73(2.39) | 22.97(2.75) | 23.24(3.74) |
| % Caucasian | 97.44 | 87.18 | 94.12 | 94.59 | 97.3 | 96.77 |
| Smoking† (Never/occasional/ex-/current smoker) | 19/16/2/3 | 14/18/4/3* | 17/16/2/0 | 19/15/2/1 | 10/18/3/6 | 15/13/0/5 |
| Drinks per week (mean(SD)) | 6.54(5.24) | 9.52(8.65) | 7.16(8.61) | 5.79(4.95) | 7.97(5.46) | 6.19(4.99) |
Note. D-only: participants tested only on the descending limb of the breath alcohol concentration (BrAC) curve; A/D: participants tested on both the ascending and the descending limbs of the BrAC curve.
Units of measurement: total number of subjects.
In the Control A/D limb group, one subject’s smoking data was missing.
Procedure
The baseline session started at 9:00 a.m. and took approximately 3–4 hours; drinking sessions, which took place 1–3 weeks (M=19.1 days) later, started between 12:00–1:00 p.m. and lasted approximately 4 hours. At the beginning of the baseline session, participants completed demographics and other self-report measures, followed by completion of the three set-shifting as well as nine additional tasks assessing other EF abilities (not reported here). When participants returned to the lab for the drinking session they were randomly assigned to one of three beverage conditions by a research assistant using a computerized randomizer algorithm: a no-alcohol control beverage (n = 80), an active placebo beverage (n=72; .04 g/kg ethanol), or an alcohol beverage (n=70; .80 g/kg ethanol for men, .72 g/kg ethanol for women) (for alcohol administration procedure, see Supplementary Materials). Participants in the control condition were told their drink contained no alcohol; those in the placebo and alcohol conditions were told their drink contained “a moderate amount of alcohol.”
To permit separate assessment of practice effects from acute alcohol tolerance on the DL, a missing by design method was employed (33,34) in which participants were randomly assigned to one of two task completion conditions. Participants in the A/D group completed the shifting tasks on both the AL and DL, whereas those in the D-only group completed the tasks only on the DL. All participants completed the set-shifting tasks in a predetermined order (category-switch, color-shape, number-letter), followed by two unrelated tasks. Following previous research (35), D-only participants watched episodes of a popular television sitcom (The Office) during ascending BrAC (in the alcohol condition, or until an equivalent amount of time had elapsed post-drinking in the placebo and control conditions). The order of the tasks was reversed on the DL so that each task would be completed at equivalent breath alcohol concentrations on the AL and DL.
Measures
Subjective Effects of Alcohol
At baseline and each post-drinking BrAC measurement, participants completed measures of self-reported stimulation, sedation and subjective intoxication. Stimulation and sedation were assessed using the Biphasic Alcohol Effects Scale (BAES; 36), and subjective intoxication (“How intoxicated do you feel right now?”) was assessed using a 10-point scale (1 = not at all intoxicated, 10 = more intoxicated than I’ve ever been).
Set-shifting Paradigms
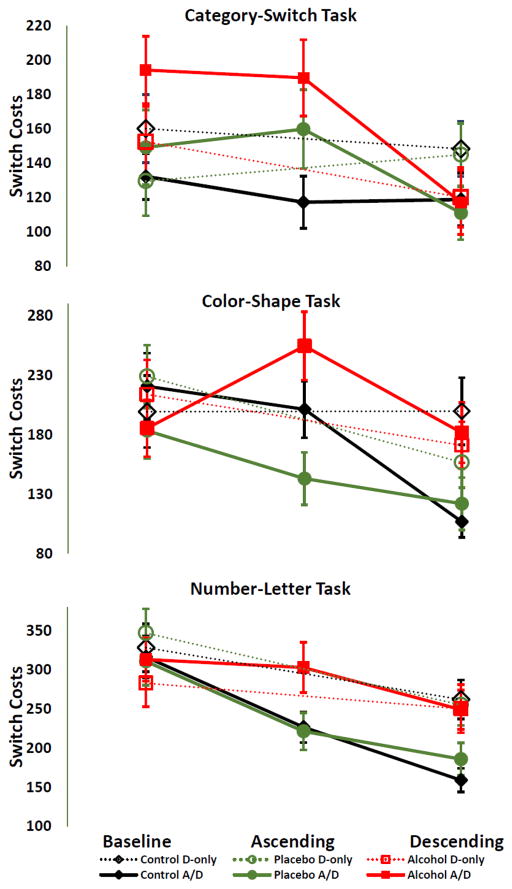
Participants performed three cued set-shifting tasks (Number-letter, color-shape, and category-switch) previously used in the study by Friedman and colleagues (14). For brevity, here we provide brief descriptions; complete details are provided in the Supplementary Materials. In all tasks, each trial was preceded by an informative cue indicating which one of the two subtasks should be performed on that trial. Subjects were required to switch between the two task-sets if the cue presented in the current trial differed from the cue presented in the previous trial. In the number-letter task (11), participants were presented with a number–letter or letter–number pair (e.g., 7G) and were expected to make an ‘even/odd’ or ‘consonant/vowel’ judgment depending on the pre-trial cue. In the color-shape task (37), participants were presented with a circle or a triangle in either red or green. They were expected to make a ‘red/green’ or ‘circle/triangle’ judgment depending on the cue. In the category switch task (38), participants were presented with a word and were asked either to make a ‘living/nonliving’ or ‘smaller/bigger than a soccer ball’ judgment based on a symbol presented above the word. The dependent measure in each task was the switch cost, calculated as the difference between the average RTs of the trials that required a task switch and the average RTs of the trials in which no switch occurred (see Fig. 1). Each task consisted of an equal number of switch and no-switch trials. The cue and the target were displayed on the screen until the participant responded, followed by a 350 ms. response-to-cue interval. An auditory feedback (“beep”) was presented if subjects responded incorrectly. Each target type and cue-target combination appeared equally often in each block. No more than four switch trials occurred in a row.
Figure 1.
Mean switch costs in the Alcohol, Placebo and Control Groups. Vertical bars indicate standard errors.
Statistical Methods
For the analysis, a set of Structural Equation Models (SEM) were applied because (i) within these models, latent factors (i.e. shifting ability) can be formed from multiple indicators (three set-shifting paradigms), this way measurement error can be reduced, resulting in increased reliability; (ii) they allow us to estimate how these latent variables are affected by other (latent) variable indices across multiple groups.
Data preparation was performed as in previous research (14). After exclusion of outliers/dropouts, Model A-B and Model C-D (for models, see Figure 3) were estimated with 218 and 222 participants’ data, respectively (see Supplementary Materials for data preparation and exclusion/outliers criteria). SEMs were estimated with Mplus7.2 (39) by using multi-group covariance, longitudinal factor (40) and multiple-indicator-multiple-cause (MIMIC) models (41). In baseline models, all parameters were constrained to be equal across groups (strict invariance models). Next, parameters of interest were freed across groups and the difference likelihood ratio test (Δχ2) was used to examine if nested models were significantly better than the strict invariance models as described in (42). Models with incomplete data on the ascending limb were tested by using missing data analysis with full information maximum likelihood (FIML) estimation procedures (43). All other models were tested with maximum likelihood estimation procedures. In addition, Root Mean Square Error of Approximation (RMSEA), Comparative Fit Index (CFI), and Tucker-Lewis Index (TLI) were used to judge the fit of individual models. All strict invariance models fit the data relatively well (for a list of models estimated and statistics, see Tables 3 and 5).
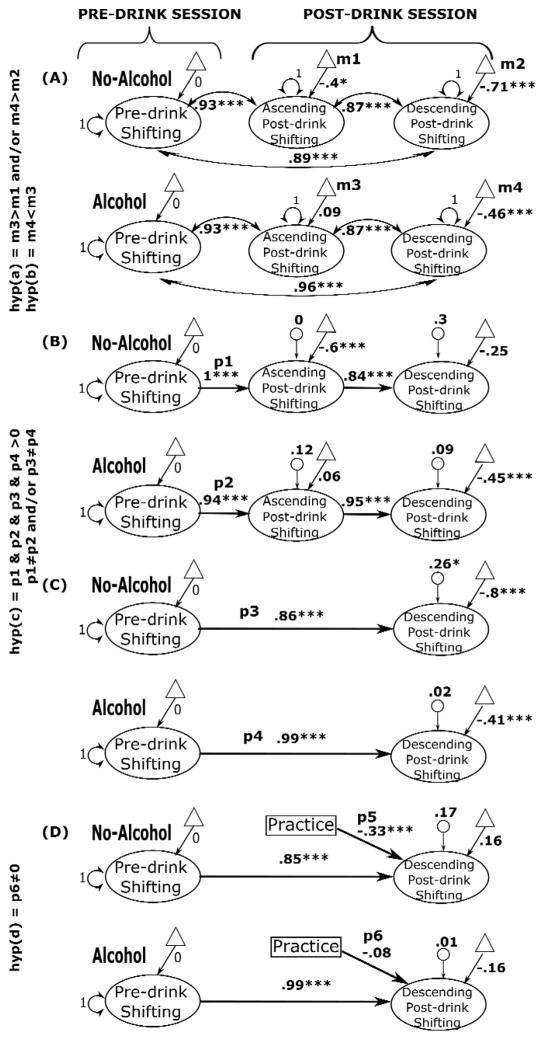
Figure 3.
Standardized parameter estimates for the multi-group covariance, longitudinal factor, and MIMIC models for alcohol and no-alcohol groups. For the parameter estimates: ***p < .001, *p < .05 (one-sample t-tests). Pre-drink and post-drink latent variables (ovals) are constructed from scores on three set-shifting tasks (category-switch, color-shape, and number-letter). For simplicity, individual indicators are not presented. Triangles represent latent means (m), double-headed arrows on latent variables represent variances, double-headed arrows between latent variables represent co-variances, single-headed arrows between latent variables represent path-coefficients (p), and small circles with single-headed arrows represent disturbances; m: mean, p: path coefficient. Larger factor scores represent greater switch costs (i.e., worse performance).
Table 3.
Results from the models with baseline, ascending and descending limb data together (n=218).
| Models | χ2 | df | RMSEA (95% CI) | CFI | TLI | Δχ2 | p |
|---|---|---|---|---|---|---|---|
| Multi-group (alcohol/no-alcohol) Covariance Models | |||||||
|
| |||||||
| A1: Strict invariance | 125.320*** | 76 | .077 (.052–.101) | .917 | .921 | ||
| A2: Asc. & desc. post-drink factor mean | 114.351** | 74 | .071 (.044–.095) | .932 | .934 | 10.969 | <.01 |
|
| |||||||
| A3: Factor loadings † | 198.863*** | 66 | .136 (114–158) | .776 | .755 | ||
|
| |||||||
| Multi-group (alcohol/no-alcohol) Longitudinal Models | |||||||
|
| |||||||
| B1: Strict invariance | 130.153*** | 78 | .078 (.054–.101) | .912 | .919 | ||
| B2: Asc. & desc. post-drink factor mean | 110.406** | 76 | .064 (.035–.090) | .942 | .945 | 43.476 | <.001 |
| B3: Asc. & desc. post-drink factor var. | 109.898** | 75 | .065 (036–.090) | .941 | .943 | 23.793 | <.001 |
| B4: Base-to-asc. & asc.-to-desc. path coefficients | 102.497* | 73 | .061 (029–.087) | .95 | .951 | 33.841 | <.001 |
Note. Under the Models column, parameters are listed which are freely estimated across groups. Note that the free parameters in each successive model also include the parameters freed in previous steps. For example, in model B3, ascending and descending post-drink factor variance means that, in addition to the parameters freed earlier (i.e. factor means in model B2), the ascending and descending limb factor variances were also estimated freely across groups. Likelihood ratio difference tests (Δχ2) were reported in comparison to the strict invariance model. Base = baseline; asc. = ascending limb; desc. = descending limb; var. = variance; ns = non-significant; RMSEA = root mean square of approximation; CI = confidence interval; CFI = comparative fit index; TLI = Tucker–Lewis index.
p<.05,
p<.01,
p<.001.
For model identification, the ascending and descending post-drink latent mean and variance were set to zero and one, respectively.
Table 5.
Results from the models with baseline and descending limb data together (n=222).
| Models | χ2 | df | RMSEA (95% CI) | CFI | TLI | Δχ2 | p |
|---|---|---|---|---|---|---|---|
| Multi-group (alcohol/no-alcohol) Longitudinal Factor Models | |||||||
|
| |||||||
| C1: Strict invariance | 49.801* | 36 | .059 (0–.096) | .960 | .966 | - | - |
| C2: Desc. post-drink factor mean | 44.840 | 35 | .050 (0–.090) | .971 | .975 | 5.0586 | <.025 |
| C3: Desc. post-drink factor var. | 45.097 | 34 | .054 (0–.093) | .968 | .971 | 3.8219 | >.1 |
| C4: Pre-to-post path coefficient | 39.656 | 33 | .043 (0–.085) | .981 | .982 | 8.1597 | <.05 |
|
| |||||||
| Multi-group (alcohol/no-alcohol) MIMIC models (with Practice variable) | |||||||
|
| |||||||
| D1: Strict invariance | 62.951* | 47 | .055 (0–.088) | .959 | .963 | - | - |
| D2: Limb path coefficient | 55.580 | 46 | .043 (0–.080) | .975 | .978 | 7.001 | <.01 |
| D3: Desc. post-drink factor mean & var. | 53.836 | 44 | .045 (0–.082) | .975 | .976 | 8.233 | <.05 |
| D4: Pre-on-post path coefficient | 46.635 | 43 | .028 (0–.071) | .991 | .991 | 14.692 | <.01 |
Note. Names of parameters in the Models column were freely estimated across groups in each successive model are listed. Note that free parameters in each successive model also include the parameters that are freed in previous steps; E.g., in Model C4, pre-to-post path coefficient means that, in addition to the parameters freed earlier (i.e. post-drink factor mean and variance in model C2 and C3), the path coefficient from pre- to post-drink latent variable was also estimated freely across groups. Likelihood ratio tests (Δχ2) were reported in comparison to the strict invariance models. Desc. = descending limb; var. = variance; RMSEA = root mean square of approximation; CI = confidence interval; CFI = comparative fit index; TLI = Tucker–Lewis index.
p<.05,
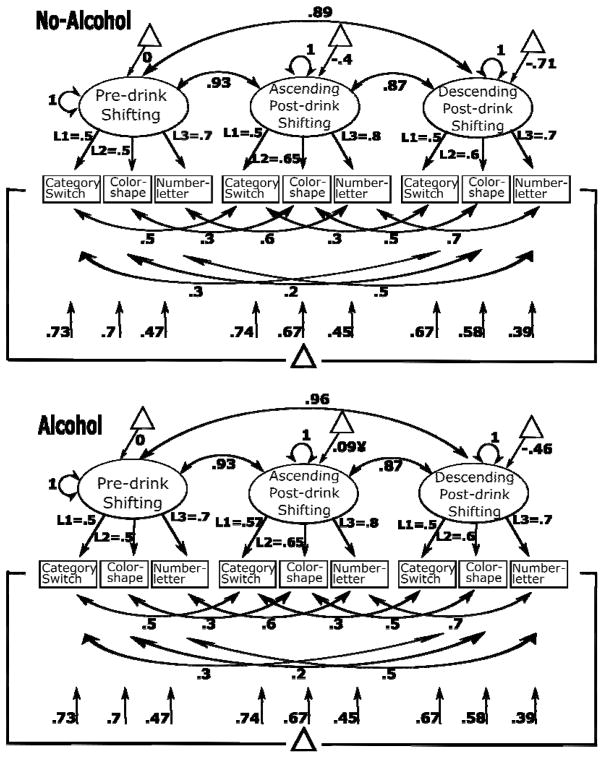
The following specifications were the same in all SEMs presented here (Figure 2 presents measurement model for Model-A2 as an example): The RT switch costs derived from the three set-shifting tasks were used to construct the unobserved latent variable, shifting, separately at pre-drink (baseline) and post-drink AL and DL. A dummy-coded alcohol variable, comparing the control and placebo groups (coded 0) with the alcohol group (coded 1), was created to test for pharmacological effects. For model identification purposes, on the pre-drink shifting factor the mean and variance were set to zero and one, respectively, in both groups. Note that freeing the pre-drink shifting latent mean in the alcohol group resulted in a mean of zero for the latent variable in the alcohol group, confirming equal latent means in alcohol and no-alcohol groups at the pre-drink baseline. The co-variances between the three indicators at pre- and post-drink were allowed, but constrained to be equal across alcohol and no-alcohol groups. The three factor loadings, intercepts and residual variances for the three indicators were freely estimated, but were constrained to be equal for pre- and post-drink shifting factors and across alcohol and no-alcohol groups. Also note that freeing factor loadings for the three indicators across time and groups significantly worsened the fit (Model-A3 in Table 3), corroborating that the same latent construct was measured before and after beverage administration. This provided a sufficient condition of equal units of measurement and origin of scale to test latent means across groups.
Figure 2.
Standardized parameter estimates for the multi-group covariance model (Model A2), presented as an example of measurement models that are simplified in Figure 3. Values under vertical arrows pointing to measurement variables (category switch, color shape etc.) represent residual variances, variance not explained by the latent variable. L1, L2, and L3 represent loadings constrained to be equal across time and groups. Triangles represent latent means, double-headed arrows on latent variables represent variances, double-headed arrows between latent variables represent co-variances. Larger factor scores represent greater switch costs (i.e., worse performance). All parameters were significant at p<.05, except one (indicated by ¥).
Note that, we also tested whether performance on the DL was affected by the expectancy that alcohol was consumed, by using expectancy (0: control group, 1: placebo and alcohol groups) instead of alcohol dummy coding for grouping. Nested models testing expectancy effect were not significantly better than the strict invariance models (see Supplementary Materials), suggesting that expectancy effects did not appear to influence performance; therefore not discussed in the remainder of this paper.
MODEL RESULTS
To determine whether post-drink performance in the alcohol group was worse than in the no-alcohol group and whether performance on the AL in the alcohol group was significantly less impaired than performance on the DL, a covariance-model was estimated in which the covariances among the three shifting latent factors were allowed, but constrained to be equal across the alcohol and no-alcohol groups. Testing hypothesis (a) and (b) requires comparison of group means across time and groups, by freeing the post-drink shifting latent means (Figure 3A), which fit the data significantly better than the baseline model (Table 3, Model A). Comparing the shifting latent mean across groups revealed that post-drink performance (both on the AL and DL) was worse (i.e., larger switch costs) in the alcohol group compared to the no-alcohol group (also see Table 4). Comparing the latent means across the limbs of the BrAC in the alcohol group revealed an improvement in performance from the AL to the DL in the alcohol group, suggesting an acute tolerance effect on the DL. These findings indicate support for hypotheses (a) and (b).
Table 4.
Standardized parameters from the models with baseline, ascending and descending limb data together in the No-alcohol and Alcohol groups
| Models | Latent mean Parameter Estimates | Path Coefficient Estimates | ||
|---|---|---|---|---|
|
|
|
|||
| No-Alcohol | Alcohol | No-Alcohol | Alcohol | |
| Multi-group (alcohol/no-alcohol) Covariance Factor Models | Ascending | |||
|
| ||||
| A1: Strict invariance | −.21 | −.21 | - | - |
| A2: Asc. & desc. post-drink factor mean | −.4**b | .089b | - | - |
|
| ||||
| Descending | ||||
|
| ||||
| A1: Strict invariance | −.61*** | −.61*** | - | - |
| A2: Asc. & desc. post-drink factor mean | −.71***a | −.46***a | - | - |
|
| ||||
| A2: Asc. vs. desc. limb | a | b | - | - |
|
| ||||
| Multi-group (alcohol/no-alcohol) Longitudinal Factor Models | Ascending | Base-to-Ascending | ||
|
| ||||
| B1: Strict invariance | −.2 | −.2 | .98*** | .98*** |
|
| ||||
| B2: Asc. & desc. post-drink factor mean | −.48***b | .09b | 1*** | .85*** |
|
| ||||
| B4: Base-to-asc. & asc.-to-desc. path coefficient | −.61***b | .06b | 1***a | .94***a |
|
| ||||
| Descending | Ascending-to-Descending | |||
|
| ||||
| B1: Strict invariance | −.44*** | −.44*** | .91*** | .91*** |
|
| ||||
| B2: Asc. & desc. post-drink factor mean | −.35* | −.51*** | .88*** | .91*** |
|
| ||||
| B4: Base-to-asc. & asc.-to-desc. path coefficient | −.25 | −.45*** | .84*** | .95*** |
Note. Under the Models column, parameter are listed which are freely estimated across groups. Note that the parameters listed in each successive model also include parameters that are freed in previous steps. Significance levels represent whether estimated parameters are significantly different than zero:
p≤.05,
p≤.01,
p≤.001.
Significance levels for the t-tests comparing parameter estimates for the no-alcohol vs. alcohol groups and for the t-test comparing ascending versus descending limb factor means are indicated as,
p≤.05;
p≤.01.
Base = baseline; asc.= ascending limb; desc.= descending limb.
To address research question (c), whether post-drink performance varies according to pre-drink performance levels and beverage groups (specifically whether individuals with poorer baseline shifting ability experience greater impairment from alcohol than those with better baseline ability), a multi-group longitudinal factor model was estimated where the pre-drink component was regressed on the post-drink component. The post-drink latent means and variances, as well as the pre-drink-to-AL and AL-to-DL path coefficients were freely estimated (Figure 3B). In the no-alcohol group, pre-drink performance fully explained AL performance (path coefficient=1) leaving no residual variance to be explained by the AL data (disturbance=0). This is to be expected given that no alcohol was administered to participants in this group. In the alcohol group, pre-drink performance also predicted AL performance but to a lesser extent than in the no-alcohol group; this difference across groups was significant (Table 3&4, Model B). We then tested whether pre-drink performance predicted DL performance differently in the alcohol and no-alcohol groups. To do so, the same model was tested without the AL data (Figure 3C; Table 5, Model C). Comparing the path coefficients across groups revealed that pre-drink shifting ability explained the variance in DL performance more in the no-alcohol group than in the alcohol group (also see Figure 4).
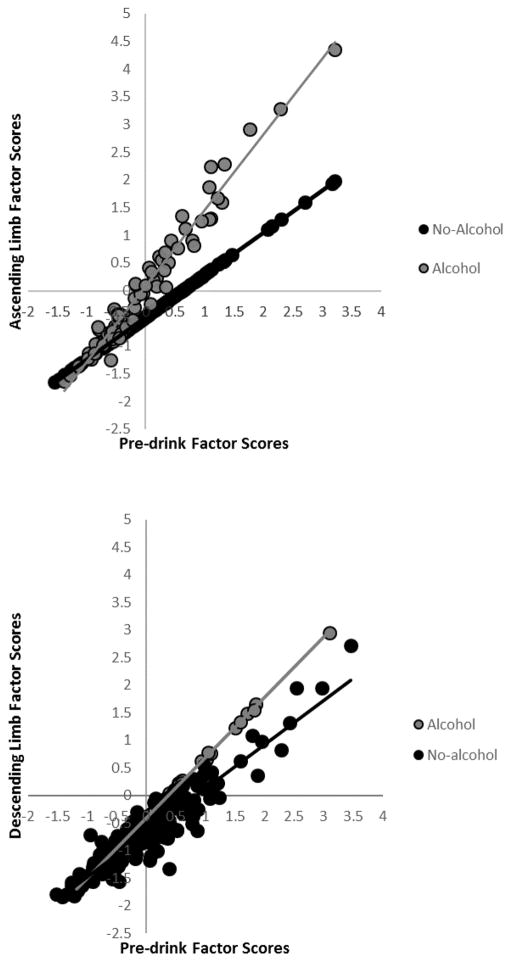
Figure 4.
Post-drink shifting latent variable factor scores for the ascending limb (from ModelB4) and descending limb (from ModelC4) as a function of beverage group (alcohol vs. no alcohol) and baseline task performance (pre-drink shifting latent variable factor scores). Larger factor scores represent greater switch costs (i.e., worse performance).
To address research question (d), whether performing the tasks on the AL affected performance on the DL in the alcohol group, a MIMIC model was estimated by including a practice dummy variable (1=D-only group, 2=A/D group) to the previous model and regressing on the DL component (Figure 3D). Freely estimating the regression from the practice variable to the DL shifting variable resulted in a significant improvement over the baseline model (Table 5, Model D). Significant negative path coefficients from practice to the DL post-drink shifting ability in the no-alcohol group (−.33, p<.001) imply that the switch cost at DL post-drink was smaller (i.e., better shifting performance) in the A/D group compared to the D-only group; however, the analogous path for the alcohol group was not significant (−.08, p=.23), indicating that practice did not improve DL performance in the alcohol group. Also, as shown in Table 6, these coefficients across groups were significantly different from each other. This finding is inconsistent with hypothesis (d).
Table 6.
Standardized parameters from the models with baseline and descending limb data together.
| Models | Parameter Estimates
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Latent Mean | Latent Variance | Pre-Post path | Practice-Post path | |||||
|
| ||||||||
| No-alcohol | Alcohol | No-alcohol | Alcohol | No-alcohol | Alcohol | No-alcohol | Alcohol | |
| Multi-group (alcohol/no-alcohol) Longitudinal Factor Models | ||||||||
|
| ||||||||
| C1: Strict invariance | −.660*** | −.660*** | .166 | .166 | .913*** | .913*** | - | - |
| C2: Des. post-drink factor mean | −.751***a | −.476***a | .174 | .174 | .909*** | .909*** | - | - |
| C3: Des. post-drink factor variance | −.749***a | −.477***a | .176 | .166 | .908*** | .913*** | - | - |
| C4: Pre-to-post path coefficient | −.808***b | −.396***b | .260* | .019 | .860***a | .988***a | - | - |
|
| ||||||||
| Multi-group (alcohol/no-alcohol) MIMIC models (with Practice variable) | ||||||||
|
| ||||||||
| D1: Strict invariance | .029 | −.029 | .115 | .115 | .912*** | .912*** | −.230*** | −.230*** |
| D2: Limb path coefficient | .009 | .009 | .121 | .126 | .902*** | .921*** | −.257*** b | −.159** b |
| D3: Des. post-drink factor mean & var. | .143 | −.265 | .097 | .181 | .901*** | .902*** | −.602*** a | −.150a |
| D4: Pre-on-post path coefficient | .162 | −.157 | .166 | .010 | .853*** b | .992***b | −.326*** a | −.081a |
Note. Names of parameters freely estimated across groups in each successive model are listed under the Models column. These parameters are successive in that they include parameters freed in previous steps. E.g., in Model C4, pre-to-post path coefficient means that, in addition to the parameters freed earlier, the path coefficient from pre- to post-drink latent variable was also estimated freely across groups. Significance levels represent whether estimated parameters are significantly different than zero:
p≤.05,
p≤.01,
p≤.001.
Significance levels for the t-tests comparing parameter estimates for the no-alcohol vs. alcohol groups are indicated as:
p≤.05;
p≤.01.
Desc, = descending limb; var. = variance; Pre-Post path = pre to post-drink (descending limb) path coefficient; Practice-Post path = practice to post-drink (descending limb) path coefficient.
DISCUSSION
This experiment investigated the acute effect of alcohol on shifting. The study addressed limitations in the extant literature in several ways, including the use of a latent variable framework informed by multiple indicators of set-shifting ability, examining whether individual differences in baseline shifting ability moderate alcohol effects on shifting performance, and testing whether alcohol expectancy or practice (performing the tasks on both BrAC limbs) would explain ostensible acute tolerance on the DL of the BrAC curve. Several findings from this study advance understanding of alcohol’s effects on shifting. First, the good fit of our model with three indicators of shifting provided evidence for a latent shifting factor tapped by the three tasks we administered. Moreover, the SEM in which factor loadings for the three indicators were freed across time and groups significantly worsened fit (Model-A3 in Table 3), corroborating that the same latent construct was measured at baseline and after beverage administration.
In both alcohol and no-alcohol groups, performance was better at post-drink (lower switch costs and latent mean), possibly due to performing the tasks a second time (or third time for the A/D group). However, this improvement in performance was greater in the no-alcohol group than the alcohol group, suggesting that pharmacological effects of alcohol limited the effects of practice. Yet, compared to the AL performance, DL performance was improved in the alcohol group, a finding similar to that seen in previous studies using within-subjects comparisons.
Additionally, baseline performance differentially predicted post-drink performance according to beverage condition: Pre-drink performance better explained AL performance in the no-alcohol group than in the alcohol group. Presumably, this occurred because alcohol interfered with shifting performance on the AL, reducing the extent of improvement from pre-drink levels in the alcohol group. More interestingly, the relative reduction in performance enhancement from pre-to-post drink in the alcohol condition was more pronounced in individuals with lower pre-drink performance (see Figure 4). This could mean that individuals with low EF ability not only have relatively weak EF when sober but they are also more susceptible to the detrimental effects of acute alcohol exposure. In the long run, these individuals might carry greater risk for alcohol abuse and, ultimately, addiction.
The current study also investigated whether practice and/or expectancy effects influence shifting performance while BrAC is falling. There has been limited research on whether alcohol differentially affects shifting under ascending and descending BrAC. Previous studies have reported impaired EF performance on the AL and a recovery from this impairment on the DL, a finding that has been interpreted as a sign of acute tolerance. Possibly to eliminate individual variability in response to acute alcohol and increase statistical power, most studies examining such limb effects have utilized within-subjects designs in which participants were tested both under ascending and descending BrAC (17–22). On the other hand, one study compared participants tested on the AL to those tested on the DL of BrAC and reported no difference in shifting performance (7). In the current study, we scrutinized this issue by (a) testing whether performing the tasks on the AL improves DL performance (i.e., a within-subjects comparison), and (b) testing whether DL performance differs according to whether or not the tasks were performed on the AL (i.e., a between-subjects comparison across the A/D and D-only groups). Results showed a general performance improvement on the DL compared to the AL. Moreover, performing the tasks on the AL improved DL performance, but only in the no-alcohol group. A lack of difference between the performance of the A/D and D-only groups in the alcohol condition suggests that the acute tolerance observed on the DL cannot be explained as merely a practice effect. Regarding the expectancy factor, the findings were inconclusive as to whether or not expectancy influenced EF performance.
In summary, our results indicate that individuals low in a key aspect of EF, switching, might experience loss of executive control after initiation of a drinking episode. However, whether individuals low or high in EF would be more prone to initiate a drinking episode is a question for future research. Limitations of the current study should be noted, however. First, although this sample is large by the standards of alcohol challenge studies, it was not large enough to permit estimation of numerous parameters in our models. Future studies with larger samples could test this possibility. Second, the alcohol effect on shifting reported here is related to a broad, latent shifting construct and may not generalize to specific tasks. However, our use of a latent variable approach to capture an underlying shifting ability measured during both a sober baseline and a subsequent drinking session represents a significant advance over typical designs utilizing only a single shifting task and measuring shifting at only one (drinking) session, and stands as the most comprehensive examination to date of the acute effect of alcohol on set-shifting performance.
Supplementary Material
Table 2.
Means and Standard Deviations of Switch Costs in the Alcohol, Placebo and Control Groups.
|
Experimental Group (n=222)
|
||||||
|---|---|---|---|---|---|---|
| Control | Placebo | Alcohol | ||||
| D-only | A/D | D-only | A/D | D-only | A/D | |
| Baseline | N =40 | N = 40 | N =35 | N = 37 | N = 37 | N = 33 |
|
| ||||||
| Number-Letter | 328.34 (193.92) | 316.45 (170.73) | 347.01 (182.37) | 311.02 (186.41) | 282.84 (182.81) | 313.21 (159.34) |
| Color-Shape | 199.28 (192.03) | 220.61 (175.86) | 228.79 (116.00) | 183.19 (140.52) | 213.64 (177.60) | 185.31 (137.62) |
| Category-switch | 160.17 (125.98) | 132.33 v(85.05) | 129.55 (119.52) | 149.26 (132.96) | 152.49 (125.06) | 194.22 (112.87) |
|
| ||||||
| Ascending | ||||||
|
| ||||||
| Number-Letter | - | 226.67 (124.77) | - | 221.29 (144.95) | - | 303.05 (184.76) |
| Color-Shape | - | 201.16 (150.71) | - | 142.92 (133.63) | - | 254.46 (165.47) |
| Category-Switch | - | 117.32 v(96.14) | - | 159.85 (139.29) | - | 189.71 (127.62) |
|
| ||||||
| Descending | ||||||
|
| ||||||
| Number-Letter | 262.02 (158.50) | 158.96 (95.41) | 255.25 (155.58) | 185.81 (126.28) | 250.29 (187.57) | 249.04 (143.73) |
| Color-Shape | 199.61 (178.40) | 106.66 (83.17) | 156.45 (125.65) | 121.75 (133.62) | 170.93 (120.53) | 181.55 (146.25) |
| Category-Switch | 148.39 (101.77) | 118.88 v(96.52) | 144.93 (107.59) | 110.97 v(94.11) | 120.17 (105.31) | 117.17 (107.22) |
Note. A/D = completed tasks on the ascending and descending limb; D-only = completed tasks on the descending limb only.
Footnotes
Conflict of interest
All authors declare no conflict of interest or relevant financial disclosure regarding the submitted manuscript. This research was supported by grant P60 AA011998 (Heath/B.D.B), K05 AA017242 (K.J.S.) and R01 AA020970 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
References
- 1.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93(3):237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moselhy H, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36(5):357–68. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 3.Dry MJ, Burns NR, Nettelbeck T, Farquharson AL, White JM. Dose-related effects of alcohol on cognitive functioning. PLoS One. 2012;7(11):e50977. doi: 10.1371/journal.pone.0050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casbon TS, Curtin JJ, Lang AR, Patrick CJ. Deleterious effects of alcohol intoxication: Diminished cognitive control and its behavioral consequences. J Abnorm Psychol. 2003;112(3):476–87. doi: 10.1037/0021-843x.112.3.476. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy DM, Niculete ME, Treloar HR, Morris DH, Bartholow BD. Acute alcohol effects on impulsivity: associations with drinking and driving behavior. Addiction. 2012;107(12):2109–14. doi: 10.1111/j.1360-0443.2012.03974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day A, Celio M, Lisman S, Johansen G, Spear L. Acute and chronic effects of alcohol on trail making test performance among underage drinkers in a field setting. J Stud Alcohol Drugs. 2013;74:635–41. doi: 10.15288/jsad.2013.74.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyvers M, Tobias-Webb J. Effects of acute alcohol consumption on executive cognitive functioning in naturalistic settings. Addict Behav. 2010;35(11):1021–8. doi: 10.1016/j.addbeh.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Scholey A, Benson S, Neale C, Owen L, Tiplady B. Neurocognitive and mood effects of alcohol in a naturalistic setting. Hum Psychopharmacol Clin Exp. 2012;27:514–6. doi: 10.1002/hup.2245. [DOI] [PubMed] [Google Scholar]
- 9.Day A, Kahler C, Ahern D, Clark U. Executive functioning in alcohol use studies: A brief review of findings and challenges in assessment. Curr Drug Abuse Rev. 2015;8:26–40. doi: 10.2174/1874473708666150416110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 11.Rogers R, Monsell S. Costs of a predictible switch between simple cognitive tasks. J Exp Psychol. 1995;124(2):207–31. [Google Scholar]
- 12.Guillot C, Fanning J, Bullock J, McCloskey M, Berman M. Effects of alcohol on tests of executive functioning in men and women: a dose response examination. Exp Clin Psychopharmacol. 2010;18(5):409–17. doi: 10.1037/a0021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyvers M, Maltzman I. Selective effects of alcohol on Wisconsin card sorting test performance. Br J Addict. 1991;86(4):399–407. doi: 10.1111/j.1360-0443.1991.tb03417.x. [DOI] [PubMed] [Google Scholar]
- 14.Friedman N, Miyake A, Young S, Defries J, Corley R, Hewitt J. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137(2):201–25. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández OH, Vogel-Sprott M, Huchín-Ramirez TC, Aké-Estrada F. Acute dose of alcohol affects cognitive components of reaction time to an omitted stimulus: differences among sensory systems. Psychopharmacology. 2006;184(1):75–81. doi: 10.1007/s00213-005-0237-7. [DOI] [PubMed] [Google Scholar]
- 16.Loehlin JC. Latent variable models: An introduction to factor, path, and structural equation analysis. 4. Mahwah, NJ: Erlbaum; 2011. [Google Scholar]
- 17.Gilbertson R, Ceballos Na, Prather R, Nixon SJ. Effects of acute alcohol consumption in older and younger adults: perceived impairment versus psychomotor performance. J Stud Alcohol Drugs. 2009;70(2):242–52. doi: 10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Söderlund H, Parker ES, Schwartz BL, Tulving E. Memory encoding and retrieval on the ascending and descending limbs of the blood alcohol concentration curve. Psychopharmacology (Berl) 2005;182(2):305–17. doi: 10.1007/s00213-005-0096-2. [DOI] [PubMed] [Google Scholar]
- 19.Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31(6):1301–9. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- 20.Schreckenberger M, Amberg R, Scheurich A, Lochmann M, Tichy W, Klega A, et al. Acute alcohol effects on neuronal and attentional processing: striatal reward system and inhibitory sensory interactions under acute ethanol challenge. Neuropsychopharmacology. 2004;29(8):1527–37. doi: 10.1038/sj.npp.1300453. [DOI] [PubMed] [Google Scholar]
- 21.Hiltunen A. Acute alcohol tolerance in cognitive and psychomotor performance: influence of the alcohol dose and prior alcohol experience. Alcohol. 1997;14(2):125–30. doi: 10.1016/s0741-8329(96)00115-2. [DOI] [PubMed] [Google Scholar]
- 22.Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66(5):663–72. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- 23.Vogel-Sprott M. Is behavioral tolerance learned? Alcohol Health Res World. 1997;21(2):161–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacol. 2008;16(3):240–50. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- 25.Pihl RO, Paylan SS, Gentes-Hawn A, Hoaken PNS. Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcohol Clin Exp Res. 2003;27(5):773–9. doi: 10.1097/01.ALC.0000065434.92204.A1. [DOI] [PubMed] [Google Scholar]
- 26.Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend. 2009;100(1–2):91–9. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peeters M, Janssen T, Monshouwer K, Boendemaker W, Pronk T, Wiers R, et al. Weaknesses in executive functioning predict the initiating of adolescents’ alcohol use. Dev Cogn Neurosci. 2015;16:139–46. doi: 10.1016/j.dcn.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry The American Academy of Child and Adolescent Psychiatry. 2006;45(4):468–75. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 29.Khurana A, Romer D, Betancourt L, Brodsky N, Giannetta J, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction. 2013;108(3):506–15. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pharo H, Sim C, Graham M, Gross J, Hayne H. Risky business: executive function, personality, and reckless behavior during adolescence and emerging adulthood. Behav Neurosci. 2011;125(6):970–8. doi: 10.1037/a0025768. [DOI] [PubMed] [Google Scholar]
- 31.Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112(3):424–36. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- 32.Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146(4):465–72. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- 33.Rhemtulla M, Little TD. Tools of the trade: Planned missing data designs for research in cognitive development. J Cogn Dev. 2012;13(4):425–38. doi: 10.1080/15248372.2012.717340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham JW, Taylor BJ, Olchowski AE, Cumsille PE. Planned missing data designs in psychological research. Psychol Methods. 2006;11(4):323–43. doi: 10.1037/1082-989X.11.4.323. [DOI] [PubMed] [Google Scholar]
- 35.Roehrich L, Goldman MS. Implicit priming of alcohol expectancy memory processes and subsequent drinki ngbehavior. Exp Clin Psychopharmacol. 1995;3(4):402–10. [Google Scholar]
- 36.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcoholism: Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 37.Miyake A, Emerson MJ, Padilla F, Ahn J. Inner speech as a retrieval aid for task goals: the effects of cue type and articulatory suppression in the random task cuing paradigm. Acta Psychol. 2004;115(2–3):123–42. doi: 10.1016/j.actpsy.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Mayr U, Kliegl R. Task-set switching and long-term memory retrieval. J Exp Psychol Learn Mem Cogn. 2000;26(5):1124–40. doi: 10.1037//0278-7393.26.5.1124. [DOI] [PubMed] [Google Scholar]
- 39.Muthén LK, Muthén BO. Mplus Version 7.2. 2013. [Google Scholar]
- 40.Cudeck R, MacCallum RC. Factor analysis at 100: Historical developments and future directions. Mahwah; New Jersey: 2003. [DOI] [PubMed] [Google Scholar]
- 41.Jöreskog K, Goldberger A. Estimation of a model with multiple indicators and multiple causes of a single latent variable. J Am Stat Assoc. 1975;70(351):631–9. [Google Scholar]
- 42.Byrne BM. Structural Equation Modeling with Mplus: Basic concepts, applications, and programming. New York, NY: Routledge; 2012. [Google Scholar]
- 43.Enders CK, Bandalos DL. The relative performance of full Information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model. 2001;8(3):430–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.