Summary
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in humans. However its mechanisms are poorly understood and AF therapy is often sub-optimal. This article reviews recent experimental, numerical and clinical data on dynamics of wave propagation during AF and its mechanistic link to ionic and structural properties of the atria. At the outset, we review numerical and optical mapping data suggesting that the sustained activity of periodic drivers (rotors) with exceedingly high dominant frequencies (DF) of excitation underlies the spatial dispersion and irregularity of activation rates that characterize AF. We show how optical mapping of the fibrillating atria in isolated normal sheep hearts has revealed that the rotors tend to localize in the left atrium (LA). We then discuss exciting new data in the sheep model of tachypacing induced AF demonstrating that the DF of paroxysmal AF episodes increases progressively over days or weeks during the transition to persistent AF (PeAF) and that the rate of the DF increase predicts the onset of PeAF. The data show that the DF increase during the transition may be explained by rotor acceleration secondary to upregulation of IK1 and downregulation of ICaL. The article concludes with a discussion on how translation of experimentally derived knowledge on the behavior and frequency of rotors may find its way into the clinic. We focus on studies in which the analysis of the spatial patterns of DF distribution in the atria of patients with paroxysmal vs persistent has increased our understanding of human AF and how that knowledge might contribute to improve the outcome of AF ablation procedures.
Keywords: Atrial fibrillation, dominant frequency, rotors, remodeling
Introduction
AF is associated with increased morbidity and mortality in cardiovascular disease patients and its prevalence in the general population continues to increase.1 Yet, despite more than 100 years of basic and clinical research, the fundamental mechanisms of AF initiation and maintenance are poorly understood, which has likely contributed to our inability to treat it effectively. A commonly accepted mechanism, the multiple wavelet hypothesis,2,3 assumes that cardiac fibrillation results from randomly propagating waves with intermittent blockades, annihilation and re-generation of discrete waves. A more recent variant posits that AF depends on longitudinal/transmural dissociation,4 with the fibrillatory waves lacking any kind of hierarchical organization. However, multiple theoretical,5,6 experimental7 and clinical8,9 studies have repeatedly demonstrated that wave propagation during AF is not totally random but contains deterministic components that depend on self-organized drivers (rotors) that spin at exceedingly high frequency. The spiraling waves emerging from such rotors give rise to the characteristically complex patterns of fibrillatory conduction as they propagate through the atria. Remarkably, the heterogeneous distribution of ion channels in the atria enables rotors to dwell at specific areas whose structure, electrical properties and relatively short refractory periods promote rotor attachment.10 One emergent property of such complex spatio-temporal dynamics was the hierarchical distribution of local cycle lengths (CLs), that was first reported in early experimental studies by Morillo et al.11 and by Harada et al.12 This was followed by the combined use of phase mapping13 and dominant frequency (DF) mapping to quantitate the dynamics and the spatial organization of AF activation rates in both atria and by the demonstration that the highest DF corresponded to the location of the rotor that was driving the arrhythmia.14–17 This article has three major objectives: first, to discuss the electrophysiological significance of spectral analysis in AF; second to discuss data that strongly support the hypothesis that remodeling in atrial ionic properties contributes to the transition from paroxysmal to persistent AF; and third to review and discuss clinical data showing a distribution of DFs across the atria during AF and how that distribution may be used to guide ablation procedures.
1.1 Frequency-dependent breakdown of wave propagation
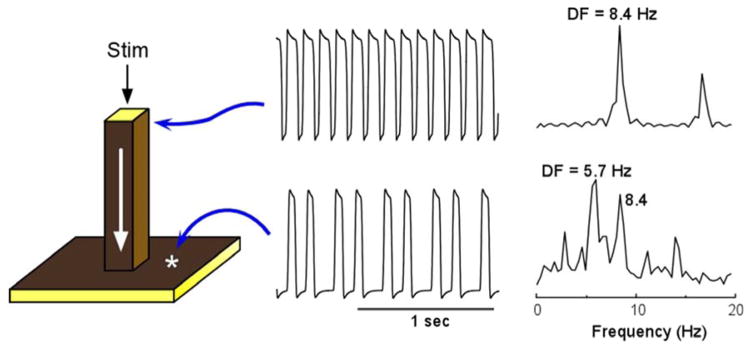
To gain insight into the distinct spatial distribution of CLs in the study of Morillo et al,11 we used spectral analysis of each of the time series recorded at specific locations in both atria to determine their DFs. Most notably, the activation frequencies in certain areas of the left atrium (LA) were always faster than any other region.15,16 In subsequent studies we confirmed the hierarchical organization of the DFs.14 In addition, we demonstrated that such an organization was the result of the exceedingly high frequency at which the spiraling waves emerging from the spinning rotor propagated haphazardly through the heterogeneous atria, undergoing spatially distributed intermittent Wenckebach-like patterns that are typical of fibrillatory conduction.18,19 In retrospect this is not too surprising since it is well known that the atria are very heterogeneous in both their anatomical structure and electrophysiological properties, and waves that propagate at high frequency in such an environment are likely to encounter obstacles in their path. To illustrate how we investigate the mechanism of fibrillatory conduction we used a simplified mathematical model of a heterogeneous substrate consisting of a large pectinate muscle connected to a small sheet representing the thin atrial wall (see Figure 1).10 Periodic stimulation was applied to the top free edge of the pectinate bundle (25 mm2) and the impulse was allowed to propagate downstream to invade the two-dimensional sheet.10 The traces on the right show the action potentials and corresponding power spectra of sites in the bundle and in the sheet. As shown by the top and bottom time series, stimulation at a constant period of 0.119 sec resulted in a 3:2 propagation pattern across the boundary between the thin bundle and the sheet. This is reflected also in the corresponding power spectra: While the source region (i.e., the pectinate bundle) displayed a DF of 8.4 Hz, the geometrical expansion into the sheet imposed a spectral transformation whereby the DF shifted to 5.7 Hz. The two power spectra display additional peaks originating from the combined effect of the sharp action potential deflections and the inter-beat cycle length variations. The constant cycle length at the thin bundle results in a narrow peak at a DF that is the exact inverse of the cycle length (CL, 1/0.119 = 8.4 Hz) with an additional smaller peak at about 16.8 Hz, which is an integer multiple of the DF (i.e., a harmonic). The CL of the activity in the sheet on the other hand is not constant and can be seen to alternate between short and long values. This in turn gives rise to a more complex profile: Several peaks are seen in the power spectrum, consequent of the combination of various intervals in the time series including not only the long and short CLs, but also their sum and difference. The Fourier algorithm, nevertheless, considers the most stationary combination of the CLs to be the DF at 5.7 Hz, which is the average number of local activations per second,18,20 corresponding to the 3:2 ratio of the input cycle length (frequency) of 0.119 sec (8.4 Hz) represented by a smaller peak.
Figure 1.
Computer model of action potential propagation from a pectinate muscle to the atrial wall. A 3-dimensional (60×60×60 elements) model includes a 1-dimensional bundle attached to a 2-dimensional sheet (left panel). Periodic stimulation (Stim) was applied at the top edge of the bundle and the impulse was allowed to propagate downward with conduction velocity of ~0.29 m/sec and to invade the two dimensional sheet. The voltage time series and corresponding power spectra are shown for a site near the stimulation point and a site at the sheet. Comparison between the points indicates a 3:2 pattern of propagation into the sheet with a concomitant spectral transformation and a DF shift from 8.4 to 5.7 Hz. From Jalife J, Berenfeld O, Skanes A, Mandapati R. Mechanisms of atrial fibrillation: Mother rotors or multiple daughter wavelets, or both? J.Cardiovasc.Electrophysiol. 1998;9:S2–S12; with permission.
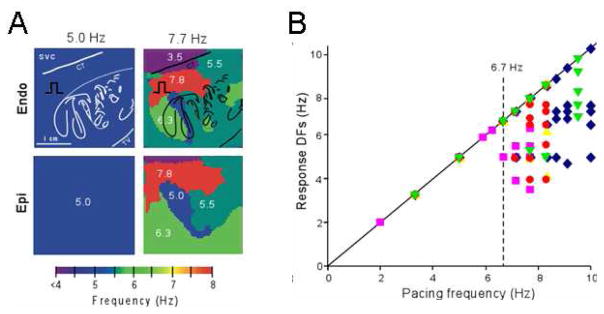
Figure 2 shows results from an optical mapping experiment in which we used DF analysis in the isolated right atrium (RA) of a sheep heart. In this experiment, the numerical predictions discussed above (Figure 1) are clearly borne out by the complexity of the responses of the RA to high-frequency periodic stimuli. The preparation was subjected to periodic pacing by a bipolar electrode on Bachmann’s Bundle (BB) to simulate activity arriving from a periodic LA source across BB into the RA.21 In panel A, stimulation at 5.0 Hz resulted in 1:1 activation of the entire RA and thus the output DF was also 5.0 Hz everywhere on the endocardial (top) and epicardial (bottom) surfaces. However, when pacing at 7.7 Hz, propagation into the RA was no longer 1:1. Instead, a heterogeneous distribution of DF domains was established both in the endocardium and the epicardium, with frequencies ranging between 3.5 and 7.7 Hz. Composite data from five experiments are presented in panel B. The DFs measured on the endocardium, are plotted as a function of the pacing frequency. Clearly, below 6.7 Hz the response DF showed no dispersion in any of the experiments, which meant that activation was 1:1 everywhere in the RA. Above the ‘breakdown frequency’ of 6.7 Hz, there was a large DF dispersion manifested as multiple domains whose individual frequencies were either equal or lower than the pacing frequency. In addition, we found that intermittent block patterns occur primarily at branching sites of the pectinate bundles of the atria. Such structurally related blockades led to a significant loss of consistency in the beat-to-beat direction of wave propagation and provided a direct explanation for the difficulty in tracing back the origin of the activation during fibrillatory conduction.21 We also found that the spatial distribution of action potential duration at a low pacing rate of 3.3 Hz was different from the distribution of DF domains, which led us to suggest that dispersion of refractoriness at normal frequencies is a poor predictor of the spatial distribution of intermittent block patterns that characterize AF outside the source region.21
Figure 2.
The ‘breakdown frequency’ in a sheep heart. A. Endocardial and epicardial DF maps of same isolated RA preparation paced at 5.0 and 7.7 Hz. Note appearance of heterogeneous DF domains at 7.7 Hz. B. Response DFs versus the pacing rate (n=5). Each symbol represents one experiment. Pacing BB at rates below ~6.7 Hz, results in 1:1 activation. At higher rates, the number of domains increases but the DFs’ value decrease. SVC, superior vena cava; CT, crista terminalis. From Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM, Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ.Res. 2002;90:1173–1180; with permission.
1.2 The Relationship between the Dynamic Patterns of Rotors and DFs
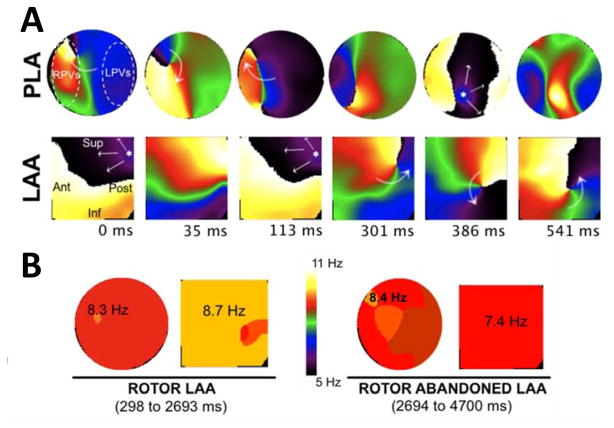
In a set of 5 sheep experiments we studied patterns of surface wave propagation during persistent AF (PeAF).22 To establish the patterns of activation underlying the DFmax values in the LA we used phase movies13 to correlate those with reentries in the PLA and LAA.23 In Figure 3A a dynamic combination of breakthroughs (BTs) and reentries is seen in the PLA and LAA during AF, with a rotor drifting from the PLA towards the LAA (See Video). As soon as the rotor enters the LAA field of view, the patterns of activation switch from BTs to reentry and the drifting rotor becomes the main source driving the AF. In Panel B, concomitant transition in the DFmax values from the PLA to the LAA when the rotor appears in the field of view of the LAA further confirms the essential role of rotors in this AF model.22 Further analysis of the DF distribution in our sheep PeAF revealed that despite a transient back and forth drift, the longest sojourn of the rotor was in the PLA, as suggested by the DF map demonstrating a statistically significant DF gradient from PLA to LAA and RAA (9.1±1.0 vs. 7.9±0.7 and 6.9±0.9 Hz, respectively, p<0.05).
Figure 3.
Patterns of activation in the PLA and LAA of isolated hearts during AF. A. snapshots from a phase movie show a rotor appearing in the field of view of the LAA. The patterns of activation switch from breakthroughs (0-to-113 ms) to a meandering rotor (301-to-541 ms). B. the DFmax is in the LAA when the rotor stays in the field of view and goes back to PLA when the rotor drifts outside the LAA. Adapted from Filgueiras-Rama D, Price NF, Martins RP, Yamazaki M, Avula UM, Kaur K, Kalifa J, Ennis SR, Hwang E, Devabhaktuni V, Jalife J, Berenfeld O. Long-term frequency gradients during persistent atrial fibrillation in sheep are associated with stable sources in the left atrium. Circulation. Arrhythmia and electrophysiology. 2012;5:1160–1167; with permission.
2.1 Transition From Paroxysmal to Persistent AF is Reflected by DF changes
We took advantage of the availability of a sheep model of long-term PeAF to characterize the transition from paroxysmal to PeAF in the frequency domain.24 Animals were chronically instrumented with a transvenous pacemaker implanted in the RA for intermittent tachypacing (30 sec) at a rate of 20 Hz whenever the heart was determined to be in sinus rhythm. As expected, with time, the paroxysmal AF episodes induced by the tachypacing became progressively longer until becoming at least 7 days, which fit the current clinical definition of PeAF.25 In seven animals, The DF of the first AF episode recorded from the intracardiac RA lead was relatively slow at 7.5±0.1 Hz (range 6.5–8.25 Hz). Simultaneous DFs from the surface ECG and an implanted loop recorder (ILR) after QRST subtraction were 7.7±0.2 Hz (range 6.5–9.25 Hz) and 9.0±0.1 Hz (range 8.9–9.4 Hz), respectively. Thus at the outset there was a significant DF difference between RA and LA (p<0.001).22 Thereafter, DF increased progressively in both atria. At the transition time to PeAF DFs recorded on the RA, surface ECG and LA were higher than during the first episode (p<0.001). However, the last DFs recorded after 1 year of uninterrupted PeAF were not significantly different from the transition. Thus, the major increase in DF occurred during paroxysmal-to-PeAF transition and not during self-sustained PeAF.24 Additionally, while a significant LA-to-RA frequency gradient was present during the early episodes, this gradient diminished at both the transition (p=0.06) and after 12 months of PeAF (p=0.1), likely reflecting remodeling of refractory periods in both atria. In any given animal, once maximum DF values were achieved, they remained relatively stable even after one year follow up; there was no significant difference between maximum DF at transition vs. DF at ~350 days.24
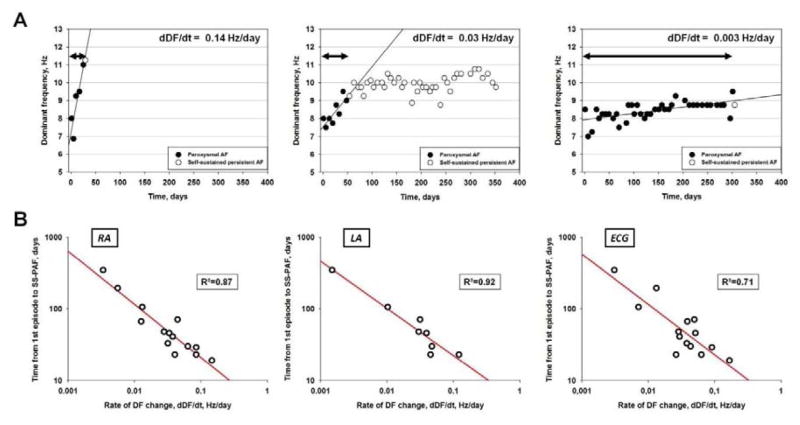
2.2 The Rate of DF Increase Predicts the Onset of Persistent AF
We analyzed several parameters to determine whether or not the time of transition to self-sustained persistent AF could be predicted.24 We first surmised that a critical DF should be reached before self-sustained PeAF developed, but the data did not support this hypothesis. Not only did maximal DF vary among animals, but the rate of DF increase during transition was also highly variable, ranging between 0.003 and 0.15 Hz/day in the RA and between 0.001 and 0.12 Hz/day in the LA. However, sheep that developed uninterrupted PeAF early, also had a steep slope of DF increase with time (dDF/dt), regardless of the DF value during the first episode, whereas those with a delayed onset of persistent AF had a shallower DF slope (Figure 4A). There was a strong nonlinear relationship between time to persistent AF onset and dDF/dt regardless of whether DF was determined in the RA, LA or surface ECG (R²= 0.87, 0.92 and 0.71, respectively, Figure 4B). The faster the DF increase, the quicker the animal developed self-sustained persistent AF. Furthermore, non-invasive measurement of dDF/dt (surface ECG lead I) correlated strongly with RA and LA dDF/dt.24
Figure 4.
Rate of increase in DF during paroxysmal AF predicts transition to persistent AF. A. representative graphs for three animals. Left, sheep with the highest dDF/dt (0.14 Hz/day, time to transition 19 days); middle, intermediate dDF/dt (0.03 Hz/day, time to transition 46 days); right, lowest dDF/dt (0.003 Hz/day, time to transition 346 days); left and right from transition group, middle from LS-PAF group. B. log-log plots of time from first episode to onset of self-sustained persistent AF versus dDF/dt for the RA (intracardiac electrode), LA (loop recorder) and ECG (surface Lead 1). Each point represents an animal. dDF/dt correlated with time to develop self-sustained persistent AF. N=14 for RA and ECG, N=8 for LA. From Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129:1472–1482; with permission.
2.3 Electrophysiological Remodeling in Ionic Currents and DF Increase
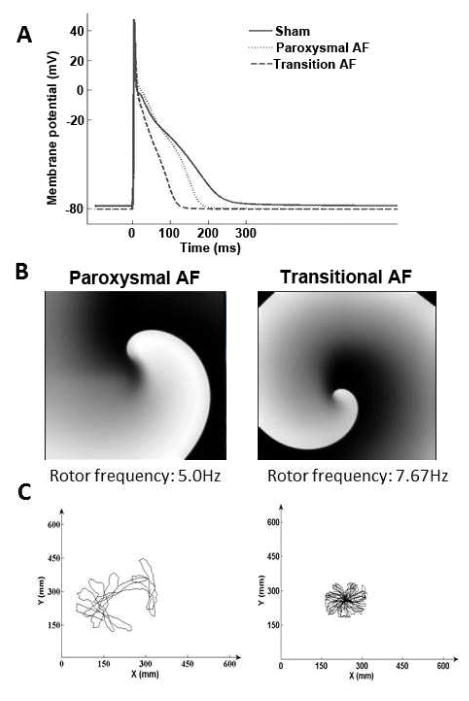
We conducted computer simulations to address the question of whether differential changes in ion currents could explain DF increase during transition from paroxysmal to the onset of PeAF. We generated action potentials (APs) for control, paroxysmal, and transition AF conditions using the Grandi-Pandit computer model26 for the human atrial AP (Figure 5A). The membrane ionic current changes were based on our experimental patch-clamp recordings that demonstrated a 65% reduction in the L-type calcium current (ICaL); 50% reduction in the sodium inward current (INa); 75% reduction in the transient outward current; and 100% increase in the inward rectifier potassium current (IK1).24 To represent paroxysmal AF, we retained the ionic changes recorded at the end of the transition to PeAF, but reduced the magnitude of ICaL by only 30%, such that the simulated APD90 was shortened significantly by 17% in paroxysmal AF, compared to 51% at the transition to PeAF (Figure 5A).24
Figure 5.
Simulations predict consequences of ion channel remodeling on rotor frequency. A. Action potential traces for sham, paroxysmal and transition AF predicted by experimentally derived ion channel changes. APD90 was abbreviated in both paroxysmal and transition AF compared to sham. Resting membrane potential was hyperpolarized -2 mV. B. Rotor in paroxysmal (left) had lower frequency than transition AF. C. Rotors in paroxysmal AF meandered considerably and eventually self-terminated upon collision with boundary. In transition AF, the rotor was stable, had higher frequency and persisted throughout the simulation. From Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129:1472–1482; with permission.
We conducted simulations on a 2D sheet model of reentry to investigate whether AP differences between paroxysmal and transition to PeAF would explain the progressive DF increase demonstrated in vivo. Sustained functional reentry (rotor) dynamics showed differential properties. The rotor in paroxysmal AF (Figure 5B, left) was short lived, and exhibited low rotation frequency (5.0 Hz) and considerable meandering (Figure 5C, left), eventually self-terminating upon collision with boundary edges. In contrast, in the transition to PeAF model, the rotor was stable and persisted throughout the length of the simulation (Figure 5B, right) with significantly less rotor meander (Figure 5C, right) and higher DF (7.67 Hz) compared to the transition case. When reduction in INa density was not incorporated, the DF increased only slightly to 8.67 Hz, but the rotor was unstable and eventually stopped.24
We also investigated the roles of individual ionic changes in a subset of models where rotors were simulated in 2D sheets and individual ionic currents were changed, compared to controls. The simulation results confirmed that changes in IK1 and ICaL are key determinants of rotor acceleration in paroxysmal and transition AF.24
3.1 Translation to patients: The High DF Sites and Maintenance of AF
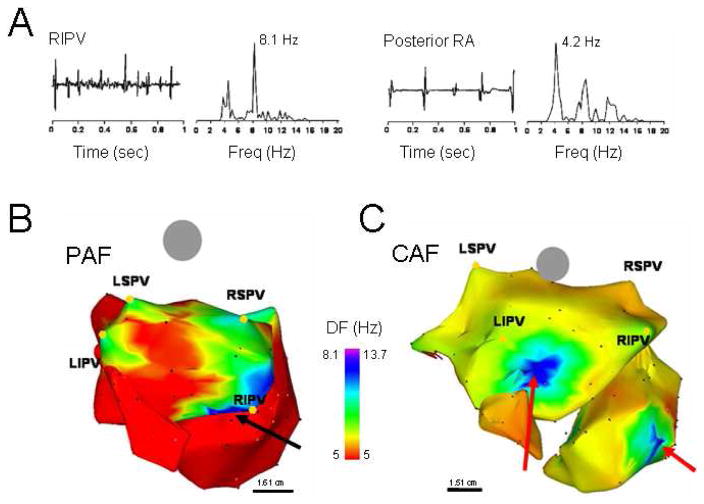
AF mapping studies in humans have recognized the presence of temporally and spatially periodic activity17,27–29 emanating from the PV region with regularity,16 suggesting that these structures may have a role in maintaining AF,17 by harboring either localized short cycle length reentrant sources and/or focal automatic activity.30,31 Indeed, in the patient whose DF map is presented in Figure 6B, focal radiofrequency ablation applied to the high DF (HDF) site near the right inferior PV (RIPV, red arrow) effectively terminated AF.32 A recent study utilizing a morphologically accurate computer model of the atria has demonstrated that the PV region is a preferential site for anchoring rotors.33 In the clinic, paroxysms of short cycle length activity have been observed in the PVs of patients undergoing AF ablation.34–36 In addition, sequential ablation of sites showing the shortest cycle length has been associated with a progressive slowing of AF frequency culminating in termination in 75% of patients with paroxysmal AF.37
Figure 6.
DF analysis in AF patients. A. Bipolar electrograms and corresponding power spectra obtained from the RIPV (left) and posterior RA in a patient with spontaneous paroxysmal AF. Each site shows distinct DF (8.1 and 4.2 Hz in RIPV and RA, respectively) demonstrating the utility of spectral analysis. B. DF map in a patient with paroxysmal AF (Posterior-anterior view; 6 hours). Note HDF sites in each of the PVs. Ablation sequence in this patient was LSPV, LIPV RSPV and RIPV (site of AF termination, black arrow); AFCL increased by 10 ms, 25 ms, 9 ms and 75 ms, respectively, before termination. C. DF map in a patient with permanent AF (24 months). The maximal DF and atrial frequency are higher than the patient in panel A. In addition, HDF sites are located outside the PVs (red arrows). Ablation sequence in this patient was RIPV, RSPV, LSPV and LIPV; AFCL increased by 5 ms, 2 ms, 0 ms and 5 ms respectively. Color-bar, DF scale in Hz. PAF: paroxysmal AF, CAF: permanent AF, LSPV, LIPV, RSPV, RIPV: Left/right superior/inferior pulmonary veins (PVs). From Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797; with permission.
3.2 DF Mapping in Patients to Guide AF Ablation
Using a blind correlation between atrial DF distribution and ablation, without any attempt at identifying potentially arrhythmogenic sites at the time of the procedure, Sanders et al32 found that ablation at PVs harboring HDF sites resulted in an increase in the atrial fibrillation cycle length (AFCL; ≥5 ms) within the CS in 89% of cases. The latter was true in patients with either paroxysmal or permanent AF. However, eventual arrhythmia termination occurred during ablation in 15 (88%) out of 17 patients with paroxysmal but none with permanent AF (p<0.0001). In 13 (87%) of the 15 paroxysmal AF patients arrhythmia termination was associated with ablation at a HDF site; 11 localized to a PV and 2 to the LA roof and the fossa ovalis.
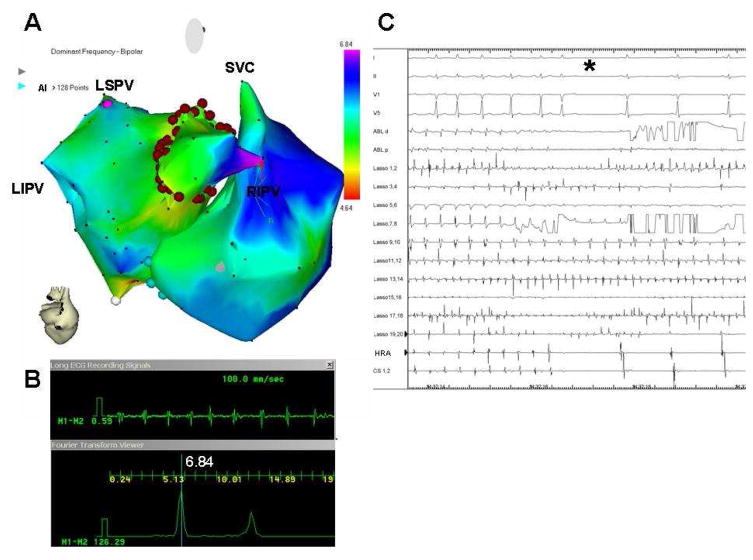
The aforementioned data, together with previous studies by Atienza et al8,38 and by Lazar et al,39 clearly indicate that the HDF sites play a role in the maintenance of AF in a significant number of patients. Atienza et al used DF mapping of AF in real time in drug-refractory paroxysmal and persistent AF patients admitted for RF ablation. After the catheters were in place and sustained AF was induced in paroxysmal AF patients, 3-dimensional reconstruction of the atrial chambers and real-time DF determination were conducted using the CARTO navigation system with embedded spectral analysis capabilities.8 Color-coded DF maps in real time during ongoing AF were superimposed on the atrial shell geometry, displaying low frequencies in red and high frequencies in purple (Figure 7). Once a maximal DF site was identified, ablation started at that site, creating a circumferential set of lesions (Figure 7). For ethical reasons, because of the observational nature of the study and the a priori unknown outcome of HDF sites ablation, CPVI was performed in all patients after HDF sites ablation. The study of Atienza et al demonstrated that the real-time spectral analysis of AF was safe and that it enabled identification and elimination of sources responsible for AF maintenance (Figure 7).8 Most important, Atienza et al showed that targeting such sources followed by circumferential PV isolation resulted in long-term sinus rhythm maintenance in 75% of paroxysmal and 50% of persistent AF patients. Our study led to the conclusion that radiofrequency ablation leading to the elimination of pre-existing DF gradients between the LA and the RA predicts long-term sinus rhythm maintenance in both paroxysmal and persistent AF patients.8
Figure 7.
A, Real-time atrial DF map (posterior view; CARTO system) in a paroxysmal AF patient. Purple, primary DFmax site (red arrow) on right intermediate PV (RIPV). Red dots, circumferential ablation line. B, bipolar recording (top) of primary DFmax site and its power spectrum (bottom) prior to ablation. C, surface ECG leads and intracardiac lasso catheter electrograms within RIPV; ablation catheter in the encircled area, CS and HRA catheter during isolation of right-sided PVs. Catheters recording outside the encircled area (CS, HRA) show conversion to SR (star) whereas the lasso catheter inside RIPV demonstrates ongoing AF. From Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernandez-Aviles F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6:33–40; with permission.
Concluding Remarks
The experimental and numerical studies on AF discussed here were conducted using sheep and simplified computer models, respectively. Obviously, results obtained from animal hearts should be cautiously extrapolated to humans. Similarly, one must be extremely cautious when attempting to generalize the applicability of theoretical concepts derived from numerical experiments. Nevertheless, as discussed above, knowledge derived from spectral analysis in the sheep atria may be used to derive important predictions on ionic and structural mechanisms of AF in patients. In general, strong evidence that rotor-like activity is the driving force that maintains human atrial fibrillation in patients is emerging at an accelerating pace.40,41
DF mapping in combination with phase mapping, patch clamping, molecular biology and numerical simulations has contributed to the recognition that inward rectifying K+ currents play an important role in the dynamics of rotors in both paroxysmal and persistent AF.23,38 This finding has opened the possibility of developing new and effective antiarrhythmic therapies targeting rotor formation or termination.42,43 For instance, our numerical simulations on AF demonstrate that sustained rotors in the atria depend on the conductance level the inward-rectifiers IK1 and IK,ACh.44–46 IK,ACh activation induces rotor acceleration and stabilization in acute AF.23,38 However, in persistent AF the increase in IK1 plays a greater role accelerating and stabilizing rotors.24 Increasing the degree of rectification of IK1 can either slow or abolish rotor activity and AF.42,43 Hence, one could envision that, in the foreseeable future, development of a new generation of safe and effective antifibrillatory K+ channel modifying drugs could lead to protection against AF in patients.
An alternative approach for drug-refractory AF in patients is ablation. However this approach can be challenging in persistent AF patients.47 In the future, the combined use of time, frequency and phase domain measures, including electrogram fractionation,48 principal value decomposition and DF mapping,49 should help elucidate mechanism of impulse propagation and the identification of the drivers and characterization of their dynamics to provide an efficient mean to facilitate patient-specific ablation procedures with well-defined end-points,8,50,51 potentially leading to increased efficacy and safety.52,53
Key points.
When a stable, self-sustained rotor forms in the left or right atrium its high frequency spinning results in the complex patterns of fibrillatory conduction that characterize AF.
While two or more rotors co-exist in the atria, the rotor with the highest dominant frequency (DF) predominates and maintains the overall activity.
In a sheep model of tachypacing-induced AF, the rate of weekly DF increase predicts the time for the transition from paroxysmal to persistent AF.
The increase in DF during the paroxysmal-to-persistent AF transition is explained by a reduction in the L-type Ca2+ current (ICaL) density, which together with an increase in the density of the inward rectifier potassium current (IK1), shortens the refractory period to accelerate and stabilize rotors.
The distribution of DF gradients in patients with paroxysmal AF is different from patients in persistent AF; abolishing DF gradients by RF ablation of high DF sites predicts freedom of AF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References List
- 1.Chen LY, Shen WK. Epidemiology of atrial fibrillation: A current perspective. Heart Rhythm. 2007;4:S1–S6. doi: 10.1016/j.hrthm.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Archives Internationales de Pharmacodynamie et de Therapie. 1962;CXL:183–188. [Google Scholar]
- 3.Allessie MA, Lammers WJEP, Bonke FIM, Hollen J. Experimental evaluation of moe's wavelet hypothesis of atrial fibrillation. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology and arrhythmias. Orlando: Grune & Stratton; 1985. pp. 265–275. [Google Scholar]
- 4.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 5.Krinskii VI. Excitation propagation in nonhomogenous medium (actions analogous to heart fibrillation) Biofizika. 1966;11:676–683. [PubMed] [Google Scholar]
- 6.Winfree AT. Suppressing drosophila circadian rhythm with dim light. Science. 1974;183:970–972. doi: 10.1126/science.183.4128.970. [DOI] [PubMed] [Google Scholar]
- 7.Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Iii. The “leading circle” concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernandez-Aviles F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan SM, Krummen DE, Donsky A, Swarup V, Miller JM. Precise rotor elimination without concomitant pulmonary vein isolation for the successful elimination of paroxysmal atrial fibrillation. Precise-paf. Heart Rhythm. 2013;10:LBCT4. [Google Scholar]
- 10.Jalife J, Berenfeld O, Skanes A, Mandapati R. Mechanisms of atrial fibrillation: Mother rotors or multiple daughter wavelets, or both? J Cardiovasc Electrophysiol. 1998;9:S2–S12. [PubMed] [Google Scholar]
- 11.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing: Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 12.Harada A, Sasaki K, Fukushima T, Ikeshita M, Asano T, Yamauchi S, Shoji T. Atrial activation during chronic atrial fibrillation in patients with isolated mitral valve disease. Ann Thorac Surg. 1996;61:104–112. doi: 10.1016/0003-4975(95)00824-1. [DOI] [PubMed] [Google Scholar]
- 13.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 14.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 15.Berenfeld O, Mandapati R, Dixit S, Skanes AC, Chen J, Mansour M, Jalife J. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the langendorff-perfused sheep heart. J Cardiovasc Electrophysiol. 2000;11:869–879. doi: 10.1111/j.1540-8167.2000.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 16.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 17.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 18.Berenfeld O, Ennis S, Hwang E, Hooven B, Grzeda K, Mironov S, Yamazaki M, Kalifa J, Jalife J. Time- and frequency-domain analyses of atrial fibrillation activation rate: The optical mapping reference. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1758–1765. doi: 10.1016/j.hrthm.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovasc Res. 2011;89:766–775. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuessler RB, Kay MW, Melby SJ, Branham BH, Boineau JP, Damiano RJ., Jr Spatial and temporal stability of the dominant frequency of activation in human atrial fibrillation. J Electrocardiol. 2006 doi: 10.1016/j.jelectrocard.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM, Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ Res. 2002;90:1173–1180. doi: 10.1161/01.res.0000022854.95998.5c. [DOI] [PubMed] [Google Scholar]
- 22.Filgueiras-Rama D, Price NF, Martins RP, Yamazaki M, Avula UM, Kaur K, Kalifa J, Ennis SR, Hwang E, Devabhaktuni V, Jalife J, Berenfeld O. Long-term frequency gradients during persistent atrial fibrillation in sheep are associated with stable sources in the left atrium. Circulation. Arrhythmia and electrophysiology. 2012;5:1160–1167. doi: 10.1161/CIRCEP.111.969519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarmast F, Kolli A, Zaitsev A, Parisian K, Dhamoon AS, Guha PK, Warren M, Anumonwo JMB, Taffet SM, Berenfeld O, Jalife J. Cholinergic atrial fibrillation: I-k,i-ach gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res. 2003;59:863–873. doi: 10.1016/s0008-6363(03)00540-6. [DOI] [PubMed] [Google Scholar]
- 24.Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129:1472–1482. doi: 10.1161/CIRCULATIONAHA.113.004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 26.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and ca2+ model: Sinus rhythm and chronic atrial fibrillation. Circulation research. 2011;109:1055–1066. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu TJ, Doshi RN, Huang HLA, Blanche C, Kass RM, Trento A, Cheng W, Karagueuzian HS, Peter CT, Chen PS. Simultaneous biatrial computerized mapping during permanent atrial fibrillation in patients with organic heart disease. J Cardiovasc Electrophysiol. 2002;13:571–577. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 28.Sih HJ, Zipes DP, Berbari EJ, Adams DE, Olgin JE. Differences in organization between acute and chronic atrial fibrillation in dogs. J Am Coll Cardiol. 2000;36:924–931. doi: 10.1016/s0735-1097(00)00788-9. [DOI] [PubMed] [Google Scholar]
- 29.Wu TJ, Ong JJC, Chang CM, Doshi RN, Yashima M, Huang HLA, Fishbein MC, Ting CT, Karagueuzian HS, Chen PS. Pulmonary veins and ligament of marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation. 2001;103:1157–1163. doi: 10.1161/01.cir.103.8.1157. [DOI] [PubMed] [Google Scholar]
- 30.Arora R, Verheule S, Scott L, Navarrete A, Katari V, Wilson E, Vaz D, Olgin JE. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation. 2003;107:1816–1821. doi: 10.1161/01.CIR.0000058461.86339.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, Berenfeld O, Nattel S. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 32.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 33.Vigmond EJ, Tsoi V, Kuo S, Arevalo H, Kneller J, Nattel S, Trayanova N. The effect of vagally induced dispersion of action potential duration on atrial arrhythmogenesis. Heart rhythm : the official journal of the Heart Rhythm Society. 2004;1:334–344. doi: 10.1016/j.hrthm.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 34.Kumagai K, Yasuda T, Tojo H, Noguchi H, Matsumoto N, Nakashima H, Gondo N, Saku K. Role of rapid focal activation in the maintenance of atrial fibrillation originating from the pulmonary veins. Pacing Clin Electrophysiol. 2000;23:1823–1827. doi: 10.1111/j.1540-8159.2000.tb07029.x. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell D, Furniss SS, Bourke JP. Paroxysmal cycle length shortening in the pulmonary veins during atrial fibrillation correlates with arrhythmogenic triggering foci in sinus rhythm. J Cardiovasc Electrophysiol. 2002;13:124–128. doi: 10.1046/j.1540-8167.2002.00124.x. [DOI] [PubMed] [Google Scholar]
- 36.Oral H, Ozaydin M, Tada H, Chugh A, Scharf C, Hassan S, Lai S, Greenstein R, Pelosi F, Jr, Knight BP, Strickberger SA, Morady F. Mechanistic significance of intermittent pulmonary vein tachycardia in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2002;13:645–650. doi: 10.1046/j.1540-8167.2002.00645.x. [DOI] [PubMed] [Google Scholar]
- 37.Haissaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavee C, Takahashi Y, Rotter M, Pasquie JL, Garrigue S, Clementy J, Jais P. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 38.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 39.Lazar S, Dixit S, Callans DJ, Lin D, Marchlinski FE, Gerstenfeld EP. Effect of pulmonary vein isolation on the left-to-right atrial dominant frequency gradient in human atrial fibrillation. Heart Rhythm. 2006;3:889–895. doi: 10.1016/j.hrthm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: Confirm (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. Journal of the American College of Cardiology. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigo M, Guillem MS, Climent AM, Pedron-Torrecilla J, Liberos A, Millet J, Fernandez-Aviles F, Atienza F, Berenfeld O. Body surface localization of left and right atrial high frequency rotors in atrial fibrillation patients: A clinical-computational study. Heart rhythm : the official journal of the Heart Rhythm Society. 2014 doi: 10.1016/j.hrthm.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filgueiras-Rama D, Martins RP, Mironov S, Yamazaki M, Calvo CJ, Ennis SR, Bandaru K, Noujaim SF, Kalifa J, Berenfeld O, Jalife J. Chloroquine terminates stretch-induced atrial fibrillation more effectively than flecainide in the sheep heart. Circulation. Arrhythmia and electrophysiology. 2012;5:561–570. doi: 10.1161/CIRCEP.111.966820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noujaim SF, Stuckey JA, Ponce-Balbuena D, Ferrer-Villada T, Lopez-Izquierdo A, Pandit S, Calvo CJ, Grzeda KR, Berenfeld O, Sanchez Chapula JA, Jalife J. Specific residues of the cytoplasmic domains of cardiac inward rectifier potassium channels are effective antifibrillatory targets. FASEB J. 2010 doi: 10.1096/fj.10-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandit SV, Berenfeld O, Anumonwo J, Zaritski R, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-d model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005 doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 46.Calvo CJ, Deo M, Zlochiver S, Millet J, Berenfeld O. Attraction of rotors to the pulmonary veins in paroxysmal atrial fibrillation: A modeling study. Biophysical journal. 2014;106:1811–1821. doi: 10.1016/j.bpj.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 48.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 49.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2008;5:846–854. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida K, Chugh A, Good E, Crawford T, Myles J, Veerareddy S, Billakanty S, Wong WS, Ebinger M, Pelosi F, Jongnarangsin K, Bogun F, Morady F, Oral H. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7:295–302. doi: 10.1016/j.hrthm.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 51.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: Extended follow-up of the confirm trial (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) Journal of the American College of Cardiology. 2014;63:1761–1768. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertaglia E, Zoppo F, Tondo C, Colella A, Mantovan R, Senatore G, Bottoni N, Carreras G, Coro L, Turco P, Mantica M, Stabile G. Early complications of pulmonary vein catheter ablation for atrial fibrillation: A multicenter prospective registry on procedural safety. Heart Rhythm. 2007;4:1265–1271. doi: 10.1016/j.hrthm.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 53.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]