Abstract
Boiling histotripsy (BH) is a high-intensity focused ultrasound (HIFU)–based method of mechanical tissue fractionation that utilizes millisecond-long bursts of HIFU shock waves to cause boiling at the focus in milliseconds. The subsequent interaction of the incoming shocks with the vapor bubble mechanically lyses surrounding tissue and cells. The acoustic parameter space for BH has been investigated previously and an inverse dependence between the HIFU frequency and the dimensions of a BH lesion has been observed. The primary goal of the present study was to investigate in more detail the ablation rate and reliability of BH in the frequency range relevant to treatment of deep abdominal tissue targets (1–2 MHz). The second goal was to investigate the effect of focal peak pressure levels and shock amplitude on BH lesion formation, given a constant duty factor, a constant ratio of the pulse duration to the time to reach boiling and a constant number of BH pulses. A custom-built 12-element sector array HIFU transducer with F-number = 1.05 was used in all experiments. BH pulses at 5 different frequencies (1, 1.2, 1.5, 1.7 and 1.9 MHz) were delivered to optically transparent polyacrylamide gel phantoms and ex vivo bovine liver and myocardium tissue to observe cavitation and boiling bubble activity with high-speed photography and B-mode ultrasound imaging, correspondingly. In gel phantoms, a cavitation bubble cloud was shown to form prefocally and to shield the focus in all exposures at 1 and 1.2 MHz and in the highest amplitude exposures at 1.5–1.7 MHz; shielding was not observed at 1.9 MHz. In ex vivo tissue, this shielding effect was observed in 25% of exposures when peak negative in situ pressure exceeded 10.2 MPa at 1 MHz and 14.5 MPa at 1.5 MHz. When shielding occurred, the exposures resulted in mild tissue disruption in the prefocal region, but not liquefaction. The dimensions of liquefied lesions followed the inverse proportionality trend with frequency; consequently, the frequency range of 1.2–1.5 MHz appeared to be preferable for BH exposures in terms of the compromise between the ablation rate and reliability. The lesion size was independent of the duration of the BH pulses (or the total “HIFU on” time), provided that the number of pulses was constant and boiling was induced within each pulse. Thus, the use of shorter (1 ms vs. 10 ms), higher amplitude BH pulses allowed up to 10-fold reduction in treatment time for a given duty factor.
Keywords: High-intensity focused ultrasound, HIFU, Histotripsy, Boiling histotripsy
INTRODUCTION
Boiling histotripsy (BH) is a high-intensity focused ultrasound (HIFU) regime that utilizes non-linear propagation effects and formation of shocks at the focus (Khokhlova et al. 2011). As a result of enhanced absorption of ultrasound energy at the shocks in tissue, a large vapor bubble is induced at the focus of a HIFU transducer within milliseconds (Canney et al. 2010) and its interaction with the incoming shocks mechanically lyses tissue into subcellular debris via atomization and acoustic micro-fountain mechanisms (Simon et al. 2012). Multiple clinical applications for BH have been suggested, primarily in oncology, to mechanically ablate unwanted soft tissues. The most relevant applications, in which traditional HIFU is faced with substantial problems are deep abdominal organ malignancies, including the liver, pancreas and kidney (Khokhlova et al. 2015). In order to access these organs, thermal HIFU treatments utilize the frequencies within 1–2 MHz range to balance the penetration depth with heating efficiency and precision (Wu et al. 2004). In BH, the dependence of treatment outcome on HIFU frequency has also been observed within 1–3 MHz range: The lesion size increased with decreasing frequency (Khokhlova et al. 2011), given the same number of BH pulses delivered. Therefore, the ablation of tissue volumes at 1 MHz would be much faster than at higher frequencies. Thus, using the frequency of 1 MHz or lower for deep abdominal applications would seem like a natural choice, since it combines the treatment efficiency and allows appropriate penetration depth.
However, it was occasionally observed that lesion formation became less reliable at 1 MHz compared with higher frequencies (2 MHz and 3 MHz), most probably because of the formation of prefocal cavitation bubbles and their shielding effect (Khokhlova et al. 2011). The primary goal of this work thus was to investigate the effect of HIFU frequency within 1–2 MHz range on the formation of BH lesions and to identify the optimal frequency range for performing BH treatment of deep abdominal targets in terms of ablation rate, reliability and requirements to the driving electronics. The secondary objective was to investigate the dependence of BH lesion formation on the amplitude of the shock in the HIFU waveform. The increase in the shock amplitude leads to a shorter time to reach boiling temperature. Therefore pulse duration can be reduced and pulse repetition frequency (PRF) increased to maintain the same duty cycle. The motivation for using higher PRF is to accelerate the treatment: according to our preliminary evidence, the size of the BH lesion is dictated by the number of delivered pulses, provided millisecond boiling is reached in each of the pulse, not by the total “HIFU on” duration (Wang et al. 2013). The overall treatment duration would thus be inversely proportional to the PRF used.
MATERIALS AND METHODS
Experimental procedure
Two series of experiments were performed first using transparent 5% polyacrylamide (PA) gel phantoms (Lafon et al. 2005) and second using ex vivo bovine myocardium and liver. The PA phantom mixtures were degassed in a desiccant chamber for one h before being poured into rectangular molds of 55- × 55- × 70-mm size and polymerized. The ex vivo tissues were obtained from a local slaughterhouse and kept on ice before the start of the experiments. Samples of 70- × 70- × 30-mm size were cut from the tissues, placed into degassed room-temperature saline and degassed for one h in the desiccant chamber. As confirmed by thermocouple measurements in a separate set of tests, the tissue samples equilibrated to room temperature during this degassing step. The gel phantoms or tissue samples were then transferred into a custom-built holder attached to a 3-D positioning system and placed in a degassed room-temperature (21–23°C) water tank for BH exposures (Fig. 1a). The treatments were performed using a custom-built 1.5-MHz 12-element transducer array (Fig. 1b) with a 75-mm aperture and a 79-mm focal length (F-number = 1.05). The transducer was manufactured from flat trapezoid lead, zirconate, titanate (PZT-8) modules distributed on a flat surface and bonded by a tungsten-epoxy matching layer to an acoustic lens manufactured with a stereolithography system following Kim et al. (2014). The transducers were powered by a custom-built amplifier (up to 26 kW peak power in a pulsed regime and up to 10 ms-long bursts) and a field-programmable gate array (FPGA) board controlled by a personal computer (Maxwell et al. 2014). To facilitate efficient operation within the frequency range of 1–2 MHz, 5 matching networks were built to adjust the electrical impedance of the transducer array at the frequencies of 1 MHz, 1.2 MHz, 1.5 MHz, 1.7 MHz and 1.9 MHz. The treatments in transparent gel phantoms were targeted and monitored by Photron Fastrax APS-RX high-speed monochrome camera (Photron, San Diego, CA, USA) at a pixel resolution of 256 × 512 with a Nikon 105-mm lens and a bellows extension to obtain a field of view of 7.5 × 3.7 mm, 14 μm/pixel (Supplemental Videos 1–3) or a field of view of 11.6 × 5.8 mm, 22 μm/pixel (Supplemental Videos 4,5). All PA gel exposures were filmed with 4-μs shutter speed and 20,000 frames per second. We used the Photogenic PowerLight 2500 DR—a continuous, disperse light source (Photogenic Professional Lighting, Bartlett, IL, USA)—positioned at an angle slightly off-axis from the camera lens to backlight the gel phantom. In ex vivo tissue samples, the treatments were observed with B-mode ultrasound (Sonix RP, Ultrasonix) with the imaging probe (P4-2) positioned in the tank perpendicularly to the transducer axis (Fig. 1a).
Fig. 1.
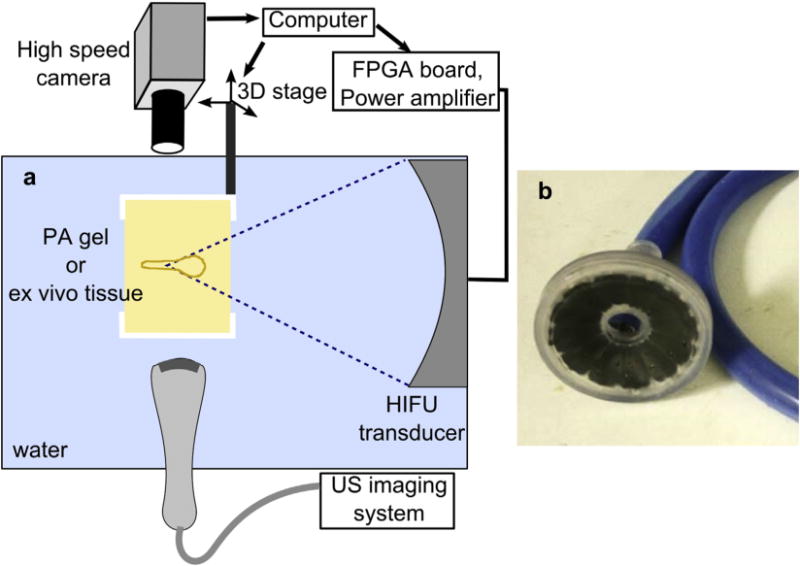
(a) Schematic diagram of the experimental arrangement; (b) photograph of the custom-built 12-element HIFU transducer array. The center frequency of the array was 1.5 MHz, and the range of operable frequencies was 1.0–1.9 MHz. HIFU = high-intensity focused ultrasound.
Focal pressure waveforms
The focal pressure waveforms produced by the transducer at different output power levels and different frequencies were measured in water by a fiber optic probe hydrophone (FOPH 2000, RP Acoustics, Leutenbach, Germany). The representative waveforms are shown in Figure 2. In order to equilibrate the exposure levels across different frequencies, the direct current voltage of the power source, V0, corresponding to the condition of fully developed shocks, was determined for each frequency. The condition of fully developed shock was defined following Rosnitskiy et al. (2015), as the output level when the peak positive pressure, p+, and the shock amplitude, As, are equal; i.e., when the lower pressure value at the shock = 0 (Fig. 2, black lines). This condition corresponded to the lowest output power used in this work and will therefore be referred to as “low output level”. In accord with the findings in Rosnitskiy et al. (2015), the shock amplitude under the condition of developed shocks was only weakly dependent on frequency, provided the F-number of the transducer is the same, and ranged within 67–72 MPa for 1.9–1 MHz, correspondingly, for the transducer used in this study (F-number = 1.05). A slightly lower shock amplitude at higher frequency is explained by stronger accumulation of non-linear effects on the way to the focus. For higher frequencies, non-linear effects accumulate over longer distance, measured in wavelengths of the fundamental frequency. Therefore, lower transducer surface pressure levels are necessary for forming the shock and the shock itself has a lower amplitude when formed.
Fig. 2.
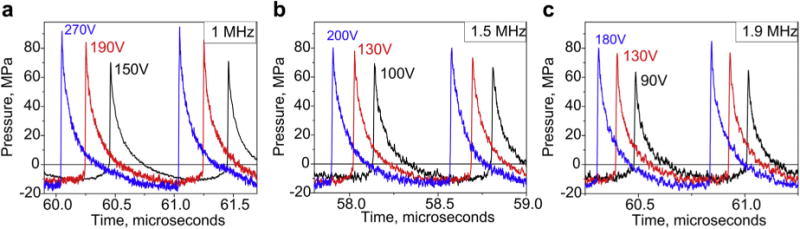
Focal waveforms measured in water by FOPH at 3 of the operating frequencies (1 MHz, 1.5 MHz and 1.9 MHz) at three different output levels. The corresponding power supply DC voltages are listed next to each waveform. The lowest shock amplitude sufficient to achieve boiling within a 10 ms pulse (black line) corresponded to the condition of fully developed shock (Rosnitskiy et al. 2015) in which the shock amplitude is equal to the peak positive pressure. The highest shock amplitude (blue line) corresponded to the non-linear saturation condition, in which shock amplitude was equal to the sum of the peak positive and peak negative pressures. The red line corresponds to the intermediate output level. FOPH = fiber optic probe hydrophone; DC = direct current.
The highest output level corresponded to a two-fold increase in the power source voltage, 2 V0, and will be referred to as “high” (Fig. 2, blue lines) or the condition of saturated shocks, in which shock amplitude is equal to the sum of peak negative and peak positive pressures. Again, in accordance with theoretical predictions, at these output levels the shock amplitude for each frequency increased by 41%–48% compared with the condition of the developed shock (Rosnitskiy et al. 2015, 2017). The output level corresponding to the shock amplitude in the middle between “low” and “high” levels will be referred to as “intermediate.”
BH exposure parameters
In the first series of experiments with transparent gel phantoms, isolated 10-ms BH pulses were used to induce cavitation and boiling bubble activity at 2-cm depth in the gel. Two to three pulses separated by 10 s were delivered to a single focal spot in the gel and then the focal spot was moved by 5 mm in the transverse direction. Transducer driving voltage was varied across the focal spots, the minimum level being just enough to produce a boiling bubble at the focus within a 10-ms pulse. At least three focal spots were considered at a given frequency and driving voltage level. For each exposure, the high-speed camera recordings were used to measure the time to reach boiling (defined as the appearance of the large boiling bubble at the focus) and to observe prefocal cavitation bubble behavior.
In the second series of experiments with ex vivo bovine myocardium and liver tissue, two sets of measurements were performed, in which the effect of HIFU frequency in the regime of fully developed shocks or the effect of shock amplitude at a given frequency were investigated. The diagram illustrating the choice of BH treatment parameters in these cases is shown in Figure 3a. In the first case, given a certain HIFU frequency, isolated 10-ms long BH pulses at gradually increasing amplitude were delivered to the tissue sample until a hyperechoic region was observed on the B-mode image (Fig. 3b). This minimal output amplitude was then used to deliver 30 pulses of 10-ms duration at PRF of 1 Hz per single focal spot. The focus was then moved laterally by 5–10 mm and the process repeated, with 5–10 focal spots treated per frequency. In the second case, the HIFU frequency was fixed at either 1.5 MHz, 1.2 MHz or 1.0 MHz. Isolated pulses with a set duration shorter than 10 ms (in particular, 5 ms, 2 ms and 1 ms) were delivered with gradually increasing amplitude until the hyperechoic region was observed. Then 30 pulses of a set duration and PRF resulting in 1% duty factor (2, 5 and 10 Hz, correspondingly) were delivered (Fig. 3a). After all the exposures were performed, the tissue sample was imaged with a higher frequency probe (L7-4), bisected and photographed. The shape, color (whitened or reddened vs. same color as surrounding tissue) and consistency (liquid vs. cohesive soft tissue) of the lesion and its maximum axial and longitudinal dimensions were the outcome measures.
Fig. 3.
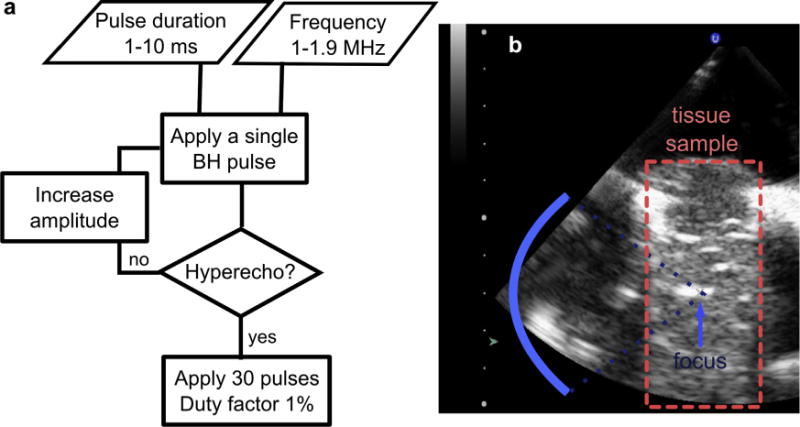
BH pulsing–protocol selection in the ex-vivo tissue exposures. (a) In the first series of experiments pulse duration was fixed at 10 ms and the HIFU frequency was selected within the range 1.0–1.9 MHz. A single BH pulse was delivered and, if the bright hyperechoic region was observed on B-mode ultrasound (b), 30 more pulses were delivered at the PRF of 1 Hz (duty factor 1%). The appearance of the hyperechoic region served as the indicator of boiling bubble formation. If the hyperechoic region was unobserved, the output power was increased and a single pulse was delivered again. The process was repeated until the hyperecho was seen. In the second series of experiments the HIFU frequency was fixed at either 1 MHz, 1.2 MHz or 1.5 MHz and the pulse duration was selected at 1 ms, 2 ms or 5 ms. The output power was selected similarly to the first scenario, by firing test pulses with increasing power until hyperecho was observed. Thereafter 30 pulses at 1% duty factor were delivered. BU = boiling histotripsy; HIFU = high-intensity focused ultrasound; PRF = pulse repetition frequency.
The time to reach boiling temperature in both PA gel and ex vivo tissue samples was predicted according to weak shock theory as follows (Canney et al. 2010; Hamilton and Blackstock 1998):
| (1) |
where ΔT is the change from ambient temperature to 100°C, cv is the volumetric heat capacity of tissue, AS is the shock amplitude, β is the coefficient of non-linearity in tissue or gel, fo is the fundamental HIFU frequency, ρ is the gel/tissue density and c is the sound speed in gel or tissue. The physical parameters of PA gel and tissue used to calculate the time to reach boiling are listed in Table 1. The in situ pressures in PA gel were considered the same as in water. In tissue, we used a modified derating procedure developed in previous work (Khokhlova et al. 2011), which accounts for both tissue attenuation, α, and coefficient of non-linearity. In that method, the focal HIFU waveforms are first measured in water at each output electric voltage setting. The in situ focal waveform at a depth l in tissue, corresponding to a voltage level V is then calculated as follows: (i) Take the focal waveform measured in water at the voltage setting, Vʹ:
| (2) |
Table 1.
Physical parameters of the PA gel phantoms and ex vivo bovine liver and myocardium tissues
| Material | Density, kg/m3 | Sound speed, m/s | Volumetric heat capacity, J/C/m3 | Non-linearity | Attenuation*, Np/cm |
|---|---|---|---|---|---|
| PA gel | 1044 | 1544 | 4460 × 103 | 3.5 | 0.01–0.019 |
| Bovine liver | 1050 | 1580 | 3540 × 103 | 4.1 | 0.043–0.097 |
| Bovine heart | 1060 | 1570 | 3940 × 103 | 4.1 | 0.06–0.114 |
PA = polyacrylamide; HIFU = high-intensity focused ultrasound.
Attenuation is given for the frequency range of 1–1.9 MHz (Duck 1990; Khokhlova et al. 2011; Lafon et al. 2005).
(ii) Multiply the corresponding waveform by the ratio βwater/β
Here βwater = 3.5 and the values for attenuation in heart and liver tissues were taken from the literature (Duck 1990; Khokhlova et al. 2011; Lafon et al. 2005) and are summarized in Table 1.
RESULTS
Characterization of bubble activity in transparent PA gel phantoms
Examples of high-speed camera frames recorded during isolated 1.5 MHz 10-ms–long BH pulses are shown in Figure 4 and represent three types of bubble behavior noted in this study. Full videos of these insonations are available online. The first type of bubble behavior, further referred to as “boiling”, is illustrated in Figure 4a and Supplemental Video 1 and was observed at low output levels, close to the condition of fully developed shock. In accordance with observations in our previous studies (Khokhlova et al. 2011), heating in the focal area appeared as a narrow dark region because of light refraction. Little to no cavitation bubble activity was observed prefocally before a large boiling bubble appeared just in front of the focus, in this case at 9.3 ms. The time to reach boiling corresponded closely to the predictions of weak shock theory (9.2 ms). Figure 4b and Supplemental Video 2 show the second type of bubble behavior, further referred to as “cavitation/boiling” that occurred at higher driving voltage or lower HIFU frequency. The presence of prefocal cavitation bubbles was noticeable during sonication, and the onset of boiling was delayed in comparison with the predictions of weak shock theory (in this case to 3.8 ms vs. 2.7 ms), presumably as a result of the shielding or distortion of the BH pulse by the cavitation. Further increase of the driving voltage led to pronounced prefocal cavitation bubble presence, as illustrated in Figure 4c and Supplemental Video 3. The cavitation bubble cloud almost completely occupied the prefocal region from the beginning of the pulse. Under this condition, heating at the focus was not optically observed and boiling did not occur.
Fig. 4.
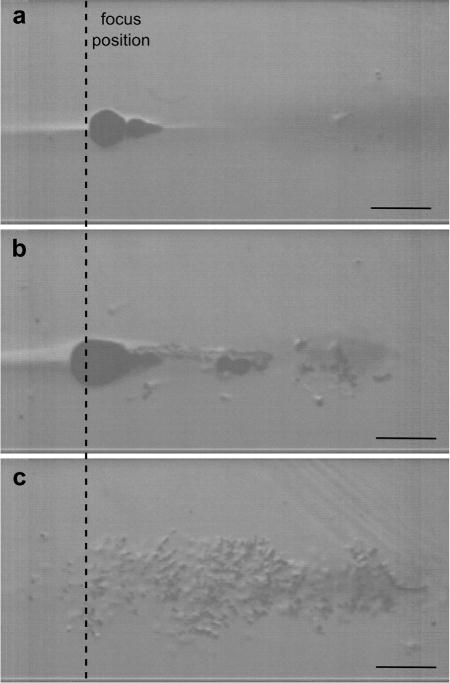
Selected high-speed camera frames from recordings of PA gel insonations by a single 10-ms pulse at the frequency of 1.5 MHz at 3 different output levels (1 MHz, 1.5 MHz and 1.9 MHz). HIFU is incident from the right. (a) At the low output level, close to the condition of fully developed shock, boiling starts as predicted (frame corresponds to the beginning of boiling at 9.3 ms) and the appearance of only a few prefocal cavitation bubbles do not appear to disrupt the incident field. (b) At higher output levels, a larger number of cavitation bubbles appear prefocally and act as a shield, thus delaying the onset of boiling (frame taken at 3.8 ms, at the beginning of boiling). (c) Further increase in the output power leads to the immediate formation of the dense cavitation bubble cloud that completely shields the focus; boiling does not occur within 10 ms (frame taken at 5.2 ms). (Scale bar in all images is 1 mm.) PA = polyacrylamide; HIFU = high-intensity focused ultrasound.
The peak negative pressure ranges at which the different types of bubble behavior were observed in the PA gel phantoms are summarized in Table 2. Note that these levels are dependent on frequency. In particular, at 1 and 1.2 MHz boiling was not achieved within a single 10-ms pulse, regardless of the focal pressure amplitude. Thus, it was found that when the shock amplitude was large enough to cause boiling within 10 ms, the peak negative pressure was also large enough to cause substantial shielding of the focus by cavitation bubbles. At 1.5 and 1.7 MHz, all three types of bubble activity were observed as the focal pressure amplitudes were gradually increased (Table 2). At 1.9 MHz cavitation was not observed at any of the attainable output levels and boiling was regularly observed at sufficient shock amplitudes, in agreement with weak shock-theory predictions.
Table 2.
Range of peak negative focal pressures at which the corresponding effects were observed in transparent PA gel phantom upon delivering two 10-ms BH pulses (Figs. 4 and 5)
| Frequency
|
|||||
|---|---|---|---|---|---|
| Observed effects* | 1 MHz | 1.2 MHz | 1.5 MHz | 1.7 MHz | 1.9 MHz |
| Boiling* | —————— | —————— | 11.5–13 MPa | 11–14.5 MPa | 11–15.5 MPa |
| Cavitation/boiling† | —————— | —————— | 13–14.5 MPa | 14.5–15 MPa | ————— |
| Cavitation/boiling 2nd pulse‡ | 13.5–14 MPa | —up to 13 MPa | 14.5–15.5 MPa | 15–15.5 MPa | ————— |
Boiling bubble appeared in accordance with the time predicted by weak shock theory.
Boiling bubble appeared later than predicted because of the prefocal shielding by cavitation bubbles.
Boiling did not occur within the first 10-ms pulse because of prefocal shielding, but did occur within the second 10-ms pulse. A dash indicates that the corresponding effect was not observed at any output level up to either the “high” level or the maximum attainable pressure level (for 1.2 frequency).
In the exposures at intermediate output power levels, the observed bubble behavior was different for the first 10-ms pulse previously described and for the second pulse delivered 10 s later. Figure 5 and Supplemental Videos 4 and 5 (first and second pulses, correspondingly) illustrate this phenomenon for the case of 2 consecutive 10-ms pulses delivered at 1.5-MHz frequency and intermediate output power (150 V, corresponding to p+ = 77 MPa, p− = 14.5 MPa). The first pulse immediately induced a bubble cloud that became denser during the 10-ms pulse through growth (up to 200μm) and coalescence of individual bubbles and precluded heating of the focal region. After HIFU was turned off, the bubbles dissolved within milliseconds. During the second pulse, prefocal cavitation activity was not observed and boiling started at 4.8 ms— close to the theoretical prediction (3.7 ms). The explanation for this effect may be “liquid strengthening” observed before for liquids, gels and tissue (Li et al. 2014; Wang et al. 2011): Most of the cavitation nuclei present in the focal and prefocal area underwent inertial cavitation and then dissolved after the first pulse. This behavior was only observed in a narrow window of amplitudes and frequencies (Table 2, last row). If the output pressure was further increased, an equally dense bubble cloud formed prefocally during every pulse (up to 4 pulses tested). The bottom plot in Figure 5 illustrates the position of the bubbles relative to the axial HIFU pressure distribution. As seen, bubbles formed prefocally within the main focal lobe, up to 6-mm axial distance from the focus, where peak negative pressure was the highest (0.86 of the maximum), and peak positive pressure dropped considerably over that distance (down to ~1/e of the peak level).
Fig. 5.
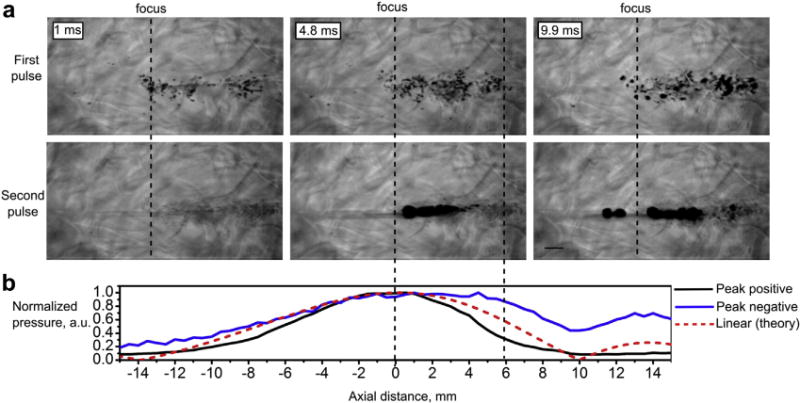
A representative set of high-speed camera frames of the PA gel corresponding to the first and second 10-ms pulses, separated by 10 s at 1.5-MHz frequency and high output level. (HIFU is incident from the right, the scale bar is 1 mm.) Boiling does not occur within the first pulse because of prefocal shielding by the cavitation bubbles. However, the first pulse depletes most cavitation nuclei and boiling starts within the second pulse at 4.8 ms. The normalized axial distribution of peak positive (black line) and peak negative (blue line) pressures recorded by FOPH in water in the shocked regime and the theoretically predicted axial pressure distribution in the linear regime (bottom plot, dashed red line). The distributions are shown in the same scale as the high-speed camera images (0 corresponds to the focus of the transducer). PA = polyacrylamide; HIFU = high-frequency focused ultrasound; FOPH = fiber optic probe hydrophone.
Another noteworthy detail of the bubble dynamics seen in the Supplemental Videos 1–5 is the motion of the gel toward the transducer immediately following the end of the 10-ms HIFU pulse because of the cessation of the acoustic radiation force. The total displacement of the gel material and the associated bubbles in the focal area ranges were within 0.4–1.4 mm, depending on the HIFU power.
Dependence of BH lesion formation on frequency
On the basis of the observations in the PA gel phantoms, the 1-MHz, 1.2-MHz and 1.5-MHz frequencies were selected for further investigation in ex vivo tissue, as they included the range that demonstrated the presence or absence of prefocal shielding by cavitation, as well as likely providing the largest ablation rate. For comparison, the 1.9-MHz frequency was also included in some of the experiments, because it was the closest to the 2.1-MHz frequency which we used extensively in our previous studies (Canney et al. 2010; Khokhlova et al. 2011). Figure 6a shows representative photographs of bisected BH lesions induced in bovine liver and myocardium at the frequencies of 1 MHz, 1.2 MHz, 1.5 MHz and 1.9 MHz, at low output power level. Table 3 lists the width and the length of the lesions in bovine liver averaged in the number of samples tested, with variabilities mostly caused by inaccuracies in the tissue bisection rather than the natural variability of the process, on the basis of our prior experience (Wang et al. 2013). As expected, the overall trend of the sizes being inversely proportional to the HIFU frequency was observed. Both transverse and longitudinal sizes of the lesions at 1.9 MHz, to within the measurement error, were consistent with those reported in Khokhlova et al. (2011) at 2.1 MHz.
Fig. 6.
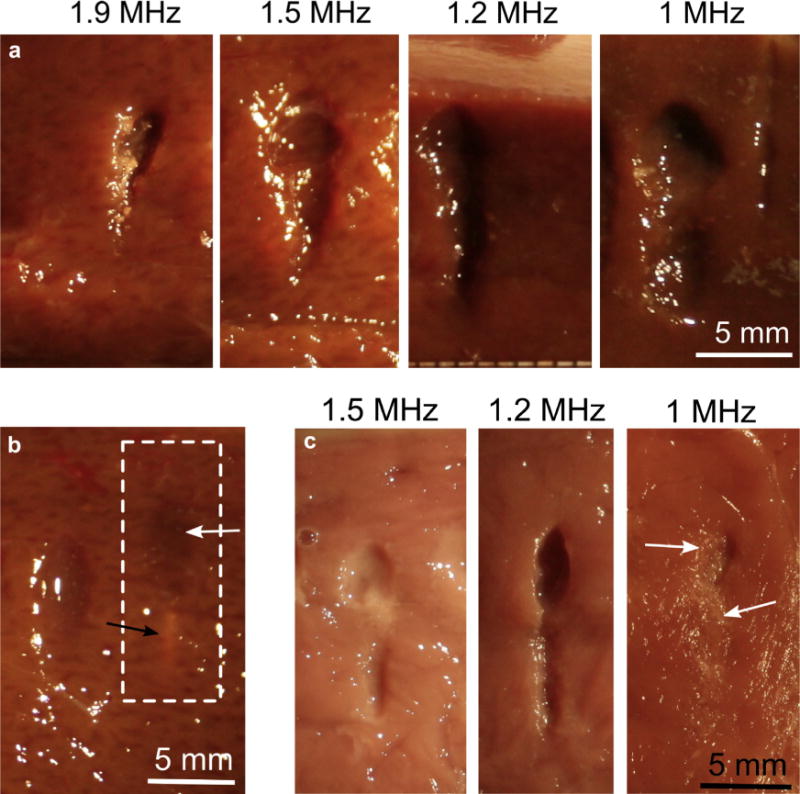
BH lesions produced in bovine liver and cardiac muscle tissues at different frequencies, under low output power level—shock amplitude minimally sufficient to induce boiling within a 10-ms pulse. PRF was 1 Hz, 30 pulses were delivered in all cases (ultrasound was incident from the top of the images). (a) Comparison of the lesions produced using different HIFU frequencies in bovine liver (lesion sizes listed in Table 2). (b) Occasionally, exposures at 1 MHz formed “ghost” lesions in liver (dashed line), a small thermally denatured region around the focus (black arrow) and a prefocal region of tissue that appeared to be mechanically disrupted, but not liquefied, i.e., could not be irrigated out (white arrow). (c) Comparison of the lesions produced at different frequencies in bovine cardiac muscle tissue. At 1 MHz, only ghost lesions were observed (white arrows point out mechanically disrupted areas of tissue). BH = boiling histotripsy; PRF = pulse repetition frequency; HIFU = high-intensity focused ultrasound.
Table 3.
BH lesion sizes in bovine liver, mm*
| Frequency | 1 MHz | 1.2 MHz | 1.5 MHz | 1.9 MHz |
|---|---|---|---|---|
| Length | 11.6 ± 1.1 | 10.4 ± 1.7 | 10 ± 2 | 6.4 ± 1.2 |
| Width | 4.6 ± 0.7 | 3 ± 0.2 | 3.0 ± 0.7 | 2.5 ± 0.3 |
| Focal zone dimensions | 37 × 3.6 | 29.5 × 3 | 24 × 2.4 | 19 × 2 |
BH = boiling histotripsy.
Average over 4–12 samples ± standard deviation. Focal zone dimensions for each frequency are given for comparison.
Another observation (illustrated in Figure 6b) is the formation of “ghost” lesions instead of liquefied voids in the liver at 1 MHz (dashed white box), which was observed in 25% of these exposures. The ghost lesion consisted of a prefocal region of tissue that appeared mechanically disrupted, on the basis of darkened coloration, but not liquefied (i.e., could not be irrigated out), and a small region of thermally denatured tissue at the focus. However, hyperechoes on B-mode images corresponding to ghost lesions were not distinguishable from hyperechoes observed when boiling at the focus yielded a typical liquefied lesion. For ghost lesions, B-mode hyperechoes most likely corresponded to the prefocal cavitation bubble cloud also observed in PA gel at 1 MHz that shielded the focal area and prevented the onset of boiling. In bovine myocardium, such ghost lesions were formed at 1 MHz in all cases (Fig. 6c). The liquefied lesions formed at 1.5 MHz in bovine myocardium were somewhat smaller than those at 1.2 MHz, which follows the same trend observed in liver tissue.
Dependence of BH lesion formation on the shock amplitude
In this set of BH exposures of ex vivo tissue, the HIFU frequency was fixed at either 1 MHz, 1.2 MHz or 1.5 MHz, and the focal pressure amplitude was gradually increased while reducing the pulse duration accordingly, and keeping the duty factor and the number of pulses per spot the same (1% and 30 pulses, correspondingly). Figure 7 shows the bisected BH lesions in bovine liver that were produced at 1.5 MHz using pulse durations of 10 ms, 5 ms, 2 ms and 1 ms, with corresponding driving voltages of 110 V, 120 V, 150 Vand 190 V. The rightmost photograph shows a ghost lesion that formed at 200 V with pulse duration of 1 ms. Note that the derated in situ pressures at 110 V correspond to the low output level (i.e., the condition of fully developed shocks) and those at 200 V correspond to high output (i.e., saturated shocks). Table 4 summarizes the peak in situ pressures across different frequencies and different amplitudes derated for propagation in ex vivo bovine liver, as well as the predicted times to reach boiling. For all frequencies, the lowest output level corresponded to the regime of developed shocks in situ, and the highest at 1 MHz was close to the regime of saturated shocks in situ. Each exposure was repeated at least 4 times in a given tissue sample for at least 2 different samples.
Fig. 7.
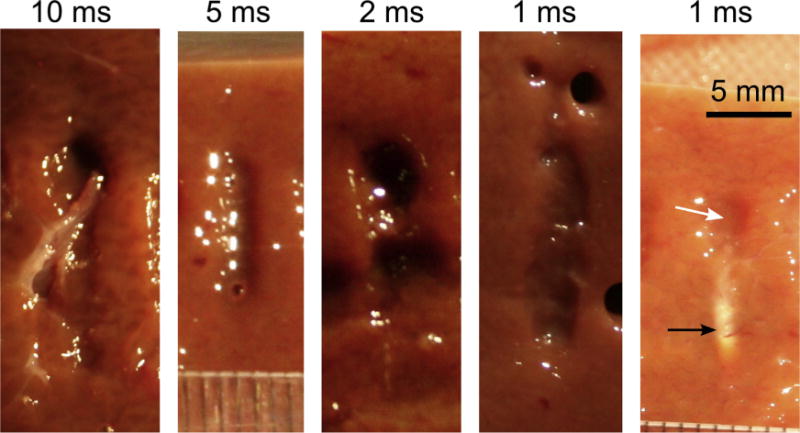
BH lesions produced in bovine liver at the frequency of 1.5 MHz and different pulse durations, while keeping the number of pulses delivered per focal spot and duty factor the same (30 and 1%, correspondingly). The smaller pulse duration implies higher shock amplitude at the focus, so that boiling occurs at each pulse. Peak negative in situ focal pressure is also increased, and at 1-ms pulse duration it is estimated as 14.5 MPa. At 1-ms pulse duration, ghost lesions are occasionally formed (rightmost panel) indicating the onset of prefocal cavitation that shields the focus. As also shown in Fig. 6b, the ghost lesion consists of a narrow thermally denatured area around the focus (black arrow) and an area of mechanically disrupted tissue prefocally (white arrow). (HIFU is incident from the top of the images.) BH = boiling histotripsy; HIFU = high-intensity focused ultrasound.
Table 4.
Formation of ghost lesions in bovine liver at different frequencies and pulse durations
| Frequency (MHz)
|
||||||
|---|---|---|---|---|---|---|
| 1 MHz
|
1.2 MHz
|
1.5 MHz
|
||||
| Pulse duration* (ms) | p− | tb | p− | tb | p− | tb |
| 10 | 10.2 | 8.3 | 10 | 7.9 | 10.3 | 8.3 |
| 5 | 13 | 3.9 | 11 | 6 | 11 | 4.8 |
| 2 | 13.5 | 3.3 | ———————— | 12.8 | 4 | |
| 1 | 13.5 | 3.3 | ———————— | 14.5 | 2.8 | |
Note: No shading in a table cell indicates that ghost lesions were never observed. Shading indicates that ghost lesions were observed: in 25% cases or fewer (light grey), in 25–75% cases (darker grey) and in 100% cases (black). At 1.2 MHz the amplitudes necessary to reach boiling within 1–2 ms in tissue were unattainable because of insufficient output of the transducer at that frequency.
Values for derated in situ peak negative pressure p− (MPa) and predicted time to reach boiling tb (ms) are provided for each exposure. Shorter pulse duration implies higher peak focal pressures (i.e., shorter time to reach boiling temperature), but with a higher probability for inducing prefocal cavitation.
Considering uncertainties associated with tissue structure variability and bisection-related inaccuracies, no difference was found in the size of the BH lesions produced at different pulse durations for a given ultrasound frequency. However, the probability of ghost lesion formation increased for shorter pulse durations. For example, at 1.5 MHz all exposures formed liquefied lesions except for the ones with 1-ms pulses, which produced ghost lesions in 25% of cases. This effect may be attributed to the prefocal shielding by cavitation bubbles, similar to that observed in the PA gels. The corresponding derated peak negative pressure was 14.5 MPa, which corresponded to substantial shielding in the gel phantom (as seen in Tables 2 and 4). Similar dependencies were observed in myocardium (data not provided), but the incidence of ghost lesions was 100% for 2-ms pulses and shorter.
Another important observation of exposures at the highest output levels (corresponding to 1-ms and 2-ms pulses) is that the theoretically estimated times to reach boiling temperature exceeded the corresponding pulse durations for all frequencies. That is, the tissue temperature at the focus was not expected to reach 100 C within a single pulse, according to weak shock theory predictions, yet experimentally the hyperechoes were observed, and the liquefied BH lesions formed in some (at 1 MHz) or most (at 1.5 MHz) cases.
DISCUSSION
Some of the most promising potential clinical applications of BH involve ablation of tumors in the liver, kidney and pancreas. The considerable depth of these targets within the body warrant the use of relatively low HIFU frequencies in the range of 1–2 MHz, as are used in most commercial HIFU ablation instruments. Historically, most studies in optimization of BH, both in vivo and ex vivo, were performed at 2 MHz, and the acoustic parameter space within the 1–2 MHz frequency range and focal pressure amplitude was not thoroughly explored. In this study, we investigated the efficiency of BH ablation within this frequency range using a broadband HIFU transducer in conjunction with a 26 kW power amplifier. In accord with previous findings, the size of the lesion and, therefore, the ablation rate generally increased with decreasing frequencies. At the higher frequency of 1.9 MHz the lesion dimensions in ex vivo tissue agreed with those produced in our previous studies at 2.1 MHz to within the experimental error.
The use of lower frequencies (1–1.2 MHz) resulted in a higher incidence rate of ghost lesions—a prefocal area of tissue that appears to be mechanically disrupted, with a narrow thermally denatured area at the focus. We speculate that this effect can be attributed to the lower cavitation threshold at lower frequencies, and the increased probability of forming a prefocal bubble cloud that distorts the acoustic field and prevents the onset of boiling at the focus. The heating at the focus may still be sufficient to form the narrow denatured area in ghost lesions, but not to reach boiling temperature. Indeed, the formation of a cavitation bubble cloud that visibly distorted the heating pattern within the 10-ms BH pulse was directly observed in all exposures at 1 MHz in transparent gel phantoms. In tissue, the activity of the prefocal cavitation cloud alone appeared to be insufficient for full liquefaction within the 30 pulses delivered, only for partial tissue disruption. It is unclear however, whether the activity of this cavitation cloud would eventually produce a fully liquefied void provided it was given a longer exposure duration, i.e., a larger number of delivered pulses. This is an intriguing question that warrants further exploration.
On real-time B-mode imaging, the appearance of a cavitation cloud was indistinguishable from that for a boiling bubble that produced a liquefied BH cavity— both were seen as a transient hyperechoic region. The confusion between these two phenomena may be deceiving in BH treatment planning. Such confusion could be avoided by operating in a parameter range that is unlikely to produce cavitation-induced shielding. Above 1.5 MHz, we observed no cloud cavitation, which suggests the use of a higher frequency. However, the use of lower frequencies is still desirable, because the greater ablation volume would allow the treatment to proceed much more quickly. In this case, other parameters could be altered to minimize the incidence of ghost lesions, e.g., using longer (10–20 ms at 1.2 MHz or 2–10 ms at 1.5 MHz) pulses with lower focal pressures, so that the prefocal negative pressure does not induce cavitation shielding effects. On the basis of the observed efficacy of longer pulses (between 1.2–1.5 MHz), this frequency range appears to be a good compromise between treatment reliability and ablation rate for BH ablation of deep tissues such as the kidney, liver and pancreas.
An important question in the investigation of BH parameter space that has been overlooked in past experiments is whether the size of the BH lesion is determined by the total “HIFU on” time or by the number of pulses delivered to a given focal spot, provided that each pulse induces boiling. The BH exposures of ex vivo tissues performed here at pulse durations of 1–10 ms and the same number of pulses per spot produced lesions of the same size. This indicates that the number of pulses delivered per spot can be considered as an appropriate metric for BH exposure “dose.” In addition, we have demonstrated that the exposure time can be reduced 10-fold by decreasing the pulse duration (and increasing the PRF and pressure amplitude at the same time) and still produce the same effect on tissue. In using shorter pulse durations and higher amplitudes, care must be taken to avoid the occurrence of the cavitation cloud shielding the focus that leads to the formation of the ghost lesion. This effect is also frequency-dependent: at 1.5 MHz, ghost lesions were occasionally observed only for the highest output power tested (corresponding to the shortest pulses of 1-ms duration), at 1.2 MHz—at the intermediate output power, but at 1 MHz—already at the lowest power (corresponding to the longest pulse duration of 10 ms).
As shown in Table 4, the estimated time to reach boiling temperature was in good agreement with the experimental observation of hyperechoic region on B-mode ultrasound for 10-ms and 5-ms pulse durations, but not for the shorter pulses (1 ms and 2 ms). For the latter cases, the estimated time to reach boiling temperature was consistently longer than the pulse duration, yet liquefied lesions still formed. Several factors may contribute to this effect. The tissue at the focus may, indeed, fail to reach the boiling temperature within the first pulse, yet the focal pressure is large enough to produce a cavitation cloud that appears similar to a boiling bubble on B-mode ultrasound. However, the larger PRFs (5–10 Hz) used in these exposures can lead to some heat accumulation between pulses, and, according to the simple estimation of heat diffusion between pulses (following Parker 1983), a combination of 2–3 pulses may lead to 100°C temperatures.
In this work, B-mode ultrasound imaging was used for treatment monitoring with no optimization or synchronization, similar to our previous studies. With this experimental procedure, we were unable to distinguish between the formation of prefocal cavitation clouds and the onset of boiling, while different bioeffects (partial tissue disruption or complete disintegration) were observed for these 2 cases. In-line imaging with the use of more sophisticated imaging sequences, higher frequency probes yielding better spatial resolution (e.g., small convex arrays) and synchronization of imaging with BH pulses may help resolve this ambiguity.
CONCLUSIONS
The acoustic parameter space of boiling histotripsy within the frequency range of 1–1.9 MHz relevant to deep abdominal-tissue ablation was systematically investigated using high-speed filming of BH exposures in transparent gel phantoms and B-mode imaging in ex vivo tissue samples. It was concluded that the frequency range of 1.2–1.5 MHz may be optimal for deep tissue ablation, with lower frequencies being prone to prefocal cavitation and higher frequencies producing substantially smaller lesions and resulting in lower ablation rates. The increase in focal pressure amplitudes allowed the use of shorter pulses at higher pulse repetition frequencies to accelerate treatments. A fixed number of pulses delivered per focal spot yielded lesions of the same dimensions regardless of the pulse duration, provided that each pulse induced boiling. The use of the pressure amplitudes that are too high (in situ peak negative pressures of 11–14.5 MPa for the corresponding frequencies of 1–1.5 MHz) resulted in intense prefocal cavitation and formation of disrupted tissue areas, as opposed to liquefied cavities. These data will be used in the optimization of large animal in vivo studies of BH ablation of deep abdominal targets.
Supplementary Material
Acknowledgments
This work was sponsored in part by National Institute of Health, Bethesda, MD, USA (Grants K01 EB015745, P01 DK043881, K01 DK104854 and R01 EB 7643), RFBR 16-02-00653 and NSBRI through NASA NCC 9-58.
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ultrasmedbio.2017.04.030.
References
- Canney MS, Khokhlova VA, Bessonova OV, Bailey MR, Crum LA. Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol. 2010;36:250–267. doi: 10.1016/j.ultrasmedbio.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duck F. Physical properties of tissue: A comprehensive reference book. London: Academic Press; 1990. [Google Scholar]
- Hamilton M, Blackstock D. Nonlinear acoustics. London: Academic Press; 1998. p. 106. [Google Scholar]
- Khokhlova TD, Canney MS, Khokhlova VA, Sapozhnikov OA, Crum LA, Bailey MR. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am. 2011;130:3498–3510. doi: 10.1121/1.3626152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova VA, Fowlkes JB, Roberts WW, Schade GR, Xu Z, Khokhlova TD, Hall TL, Maxwell AD, Wang YN, Cain CA. Histotripsy methods in mechanical disintegration of tissue: Towards clinical applications. Int J Hyperthermia. 2015;31:145–162. doi: 10.3109/02656736.2015.1007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Maxwell AD, Hall TL, Xu Z, Lin KW, Cain CA. Rapid prototyping fabrication of focused ultrasound transducers. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:1559–1574. doi: 10.1109/TUFFC.2014.3070. [DOI] [PubMed] [Google Scholar]
- Lafon C, Zderic V, Noble ML, Yuen JC, Kaczkowski PJ, Sapozhnikov OA, Chavrier F, Crum LA, Vaezy S. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound Med Biol. 2005;31:1383–1389. doi: 10.1016/j.ultrasmedbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Li T, Chen H, Khokhlova T, Wang YN, Kreider W, He X, Hwang JH. Passive cavitation detection during pulsed HIFU exposures of ex vivo tissues and in vivo mouse pancreatic tumors. Ultrasound Med Biol. 2014;40:1523–1534. doi: 10.1016/j.ultrasmedbio.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Kreider W, Yuldashev PR, Khokhlova TD, Schade GR, Sapozhnikov OA, Bailey MR, Khokhlova VA. Development of high-output therapy systems for transcutaneous application of boiling histotripsy; 14th International Symposium on Therapeutic Ultrasound; USA: Las Vegas. Apr 2–5, 2014. [Google Scholar]
- Parker KJ. The thermal pulse decay technique for measuring ultrasonic absorption coefficients. J Acoust Soc Am. 1983;74:1356–1361. [Google Scholar]
- Rosnitskiy PB, Yuldashev PV, Khokhlova VA. Effect of the angular aperture of medical ultrasound transducers on the parameters of nonlinear ultrasound field with shocks at the focus. Acoust Phys. 2015;61:301–307. [Google Scholar]
- Rosnitskiy PB, Yuldashev PV, Sapozhnikov OA, Maxwell AD, Kreider W, Bailey MR, Khokhlova VA. Design of HIFU transducers for generating specified nonlinear ultrasound fields. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64:374–390. doi: 10.1109/TUFFC.2016.2619913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JC, Sapozhnikov OA, Khokhlova VA, Wang YN, Crum LA, Bailey MR. Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound. Phys Med Biol. 2012;57:8061–8078. doi: 10.1088/0031-9155/57/23/8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Xu Z, Hall T, Fowlkes J, Roberts W, Cain C. Active focal zone sharpening for high-precision treatment using histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:305–315. doi: 10.1109/TUFFC.2011.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YN, Khokhlova T, Bailey M, Hwang JH, Khokhlova V. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound Med Biol. 2013;39:424–438. doi: 10.1016/j.ultrasmedbio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Xie FL, Jin CB, Su HB, Gao GW. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med Biol. 2004;30:245–260. doi: 10.1016/j.ultrasmedbio.2003.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


