Abstract
There is significant need to develop a single-dose rabies vaccine to replace the current multi-dose rabies vaccine regimen and eliminate the requirement for rabies immune globulin in post-exposure settings. To accomplish this goal, rabies virus (RABV)-based vaccines must rapidly activate B cells to secrete antibodies which neutralize pathogenic RABV before it enters the CNS. Increased understanding of how B cells effectively respond to RABV-based vaccines may improve efforts to simplify post-exposure prophylaxis (PEP) regimens. Several studies have successfully employed the TNF family cytokine a proliferation-inducing ligand (APRIL) as a vaccine adjuvant. APRIL binds to the receptors TACI and B cell maturation antigen (BCMA)–expressed by B cells in various stages of maturation–with high affinity. We discovered that RABV-infected primary murine B cells upregulate APRIL ex vivo. Cytokines present at the time of antigen exposure affect the outcome of vaccination by influencing T and B cell activation and GC formation. Therefore, we hypothesized that the presence of APRIL at the time of RABV-based vaccine antigen exposure would support the generation of protective antibodies against RABV glycoprotein (G). In an effort to improve the response to RABV vaccination, we constructed and characterized a live recombinant RABV-based vaccine vector which expresses murine APRIL (rRABV-APRIL). Immunogenicity testing in mice demonstrated that expressing APRIL from the RABV genome does not impact the primary antibody response against RABV G compared to RABV alone. In order to evaluate the necessity of APRIL for the response to rabies vaccination, we compared the responses of APRIL-deficient and wild-type mice to immunization with rRABV. APRIL deficiency does not affect the primary antibody response to vaccination. Furthermore, APRIL expression by the vaccine did not improve the generation of long-lived antibody-secreting plasma cells (PCs) as serum antibody levels were equivalent in response to rRABV-APRIL and the vector eight weeks after immunization. Moreover, APRIL is dispensable for the long-lived antibody-secreting PC response to rRABV vaccination as anti-RABV G IgG levels were similar in APRIL-deficient and wild-type mice six months after vaccination. Mice lacking the APRIL receptor TACI demonstrated primary anti-RABV G antibody responses similar to wild-type mice following immunization with the vaccine vector indicating that this response is independent of TACI-mediated signals. Collectively, our findings demonstrate that APRIL and associated TACI signaling is dispensable for the immune response to RABV-based vaccination.
Keywords: Rabies, Vaccine, APRIL, TACI, Antibody
1. Introduction
Despite known methods of effective RABV PEP, over 55,000 humans are killed by RABV annually; 99% of these deaths occur in resource-poor, canine-rabies endemic countries where control of the RABV reservoir is insufficient or nonexistent and access to medical care is limited (Hampson et al., 2015; WHO Publication, 2010). RABV PEP relies on RABV neutralizing antibodies (RVNAs) to confer protection by preventing the virus from reaching the CNS, causing clinical disease (Li et al., 2011; Schnell et al., 2010). Cell-culture based inactivated RABV vaccines currently used for RABV PEP are safe and effective but they have inherent problems (Shayam et al., 2006); multi-dose vaccination protocols and administration of costly rabies immunoglobulin at the initial clinical intervention are necessary because current vaccines fail to stimulate protective titers of RVNAs following the primary injection (Gacouin et al., 1999; Nagarajan et al., 2014; Wilde et al., 2002). Additionally, poor responders fail to mount protective responses even after repeated booster injections (Cabasso et al., 1974). Generating more immunogenic, protective vaccines against RABV would reduce the costs of prevention and save human lives (McGettigan, 2010). Increased understanding of how B cells effectively respond to RABV-based vaccines will guide the development of more effective, simplified PEP regimens.
In an effort to understand and potentially augment protective B cell responses to RABV-based vaccines, we have evaluated the effects of the TNF family cytokine, APRIL, on the antibody response to RABV vaccination. APRIL is a TNF superfamily cytokine which is expressed by myeloid-derived cells including monocytes, macrophages, and dendritic cells. APRIL, like most TNF superfamily cytokines, forms soluble trimers which can bind to TNF receptors (reviewed in Mackay et al., 2003). APRIL trimers promiscuously bind to the TNF receptors transmembrane activator and calcium-modulator and cyclophilin ligand (CAML)-interactor (TACI), and B cell maturation antigen (BCMA) (Yu et al., 2000). APRIL competes with its sister molecule, B-cell activating factor (BAFF), for receptor binding sites; APRIL and BAFF have diverse interactions, forming functional heterotrimers and regulating signaling through dynamic stoichiometric interactions with each other and receptors (Roschke et al., 2002; Schuepbach-Mallepell et al., 2015).
APRIL plays a role in lymphoid development and activation, influencing humoral immune responses; recombinant APRIL is a costimulator of B and T cells in vitro and leads to increased B cell numbers and T cell activation in vivo (Yu et al., 2000). APRIL transgenic mice exhibit improved T-cell independent (TI) type 2 responses and demonstrate that APRIL boosts antigen-specific antiviral IgM responses to T-cell dependent (TD) antigens (Stein et al., 2002). Live RABV-based vaccine induces neutralizing IgM antibodies that are protective against pathogenic RABV challenge (Dorfmeier et al., 2013a); expression of APRIL in the context of RABV vaccination could improve this early protective response. Importantly, APRIL has also been shown to support antibody-secreting PC survival, suggesting that APRIL in the context of RABV vaccination might improve long-term antibody responses important in sustaining the protective effects of vaccination (Benson et al., 2008; Jourdan et al., 2014).
In this report, we evaluated the hypothesis that APRIL expression in temporospatial association with RABV antigen exposure during vaccination would augment and improve anti-RABV antibody responses. Interestingly, mice immunized with rRABV-mAPRIL demonstrated similar antibody responses to mice immunized with rRABV. In an effort to ascertain the role of APRIL in the response to RABV vaccination, we show that APRIL is dispensable for the anti-RABV G antibody response. Importantly, the antibody response to RABV vaccination does not depend on TACI-mediated signals. Collectively, our work provides new insight into the role of APRIL and TACI signaling in the context of antiviral responses. These results can guide future vaccine development, particularly those which rely on early antibody responses for protection.
2. Materials and Methods
2.1 In vitro splenocyte infection with rRABV and flow cytometry
Spleens were collected from 6–10 week old C57BL/6 mice (The Jackson Laboratory), homogenized, and red blood cells were lysed. Splenocytes were plated at 107 cells/mL, infected with rRABV at a multiplicity of infection (MOI) of 5 and cultured for 48 hours. Splenocytes were harvested, washed with FACS buffer (PBS containing 2% heat-inactivated FBS), blocked with anti-mouse CD16/32 antibody and surface stained with B220 (APC-Cy7, RA3-6B2, BD Biosciences) and CD4 (APC, RM4.5, BD Biosciences). Cells were fixed in 2% paraformaldehyde and stained intracellularly using PermWash buffer (BD Biosciences) for RABV nucleoprotein (N) (FITC, Fujirebio Diagnostics, Inc.) and murine APRIL (PE, A3D8, Biolegend). Cells were resuspended in FACS buffer and analyzed on a BD LSR II flow cytometer. RABV N-positive gates were set so that mock-infected cells analyzed in parallel showed <1% RABV N-positive cells (Lytle et al., 2013; Norton et al., 2014). Data were analyzed using FlowJo (FlowJo, LLC) and Prism 5 (Graphpad).
2.2 Recombinant RABV-based vaccine construction and recovery
rRABV is a molecular clone of the SAD-B19 vaccine strain of RABV (Conzelmann et al., 1990; Schnell et al., 2000). To construct rRABV expressing APRIL, the murine APRIL gene was amplified from pCMV6-Kan/Neo-APRIL (OriGene, MC203367) by RT-PCR with Taq Polymerase (Invitrogen) using forward primer JPMRP-52 (5’- TTT CGT ACG ATT ATG CCA GCC TCA TCT CCA GG -3’) (BsiWI underlined) and reverse primer JPMRP-53 (5’- AAA GCT AGC TCA TAG TTT CAC AAA CCC CAG G -3’) (NheI underlined). Digestion and insertion of this PCR product into the rRABV plasmid using BsiWI and NheI (New England Biolabs) resulted in recombinant RABV plasmid encoding murine APRIL. Infectious virus was recovered, concentrated and purified as described previously (McGettigan et al., 2001; Norton et al., 2014; Schnell et al., 2000) yielding rRABV-mAPRIL.
2.3 One- and multi-step growth curves
Growth kinetics of rRABV and rRABV-mAPRIL were determined as previously described (McGettigan et al., 2003).
2.4 Western blotting
BSR cells were infected with rRABV or rRABV-APRIL at a MOI of 0.1. Cells lysates and 100X concentrated virus-free supernatants were collected and SDS-PAGE separation was performed as previously described (Haley et al., 2017). Membranes were probed for one hour with anti-murine APRIL antibody (PE, A3D8, Biolegend) at a dilution of 1:500 in PBS-0.05% Tween 20 and scanned using a FluorChem M system (ProteinSimple).
2.5 In vitro splenocyte stimulation by rRABV-mAPRIL supernatant and flow cytometry
Splenocytes were collected as described in section 2.1 and cultured in 96-well flat-bottomed plates, 3×105 cells/well, for 72 hours in serial 3-fold dilutions of 100X concentrated supernatant from rRABV or rRABV-mAPRIL infected BSR cells, with a starting dilution of 1:36 in RPMI-based splenocyte medium (Lytle et al., 2013; Norton et al., 2014). Cells were blocked and stained with LIVE/DEAD fixable AQUA (Invitrogen), B220/CD45R (APC-Cy7, clone RA3-6B2, BD Biosciences) and CD4 (PE-CF594, clone GK1.5, BD Biosciences). Samples were fixed and analyzed on a BD LSR Fortessa flow cytometer. Data were analyzed using FlowJo (FlowJo, LLC.) and Prism 5 (Graphpad).
2.6 Evaluation of antibody responses to rRABV-mAPRIL
Groups of female 6–10 week old C57BL/6 mice (The Jackson Laboratory) were immunized intramuscularly (i.m.) with a single dose of 105 FFU of rRABV or rRABV-mAPRIL. On days 3, 5, 7, 10 and 56 post-immunization, blood was collected and serum was isolated for analysis. RABV G-specific IgG and IgM antibodies were determined by ELISA and reported at 1:50 serum dilution as described previously (Cenna et al., 2009, 2008; Dorfmeier et al., 2013b; Dunkel et al., 2015). VNA titers of pooled, heat-inactivated sera were determined using the RFFIT as described previously (Cenna et al., 2009, 2008).
2.7 Evaluation of antibody responses in APRIL-deficient mice
Groups of female 6–10 week old APRIL-deficient mice (B6.Cg-Tnfsf13tm1Pod/J, The Jackson Laboratory) (Xiao et al., 2008) or age-matched female C57BL/6 mice were immunized i.m. with a single dose of 105 FFU of rRABV. On days 3, 5, 7, 10, and 6 months post-immunization, blood was collected and serum was isolated for analysis. RABV G-specific IgG and IgM, and VNA titers were determined as described in section 2.6.
2.8 Evaluation of antibody responses in TACI-deficient mice
Groups of male 6–10 week old TACI-deficient mice (von Bülow et al., 2001) or age-matched male C57BL/6 mice were immunized i.m. with a single dose of 105 FFU of rRABV. On days 7, 10, and 14 post-immunization, blood was collected and serum was isolated for analysis. RABV G-specific IgG antibody titers were determined as described in section 2.6.
3. Results
3.1 B cells upregulate APRIL expression following rRABV
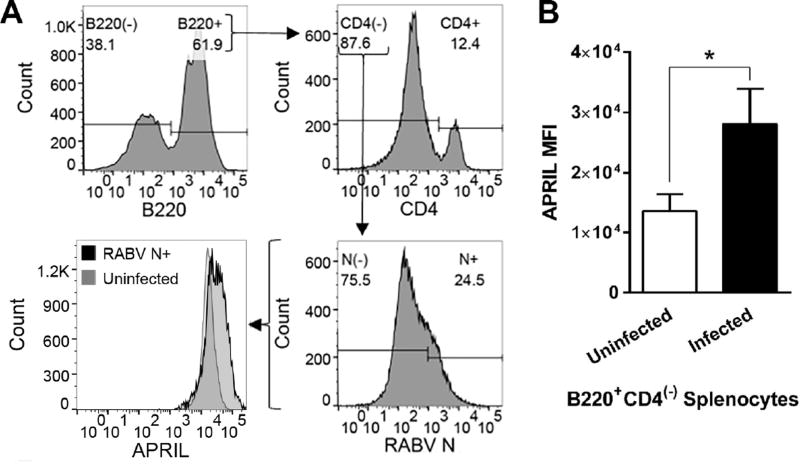
To assess the potential of APRIL as an adjuvant for RABV vaccination, we evaluated the effect of live recombinant RABV-based vaccine (rRABV) treatment on primary murine B cell expression of APRIL ex vivo (Lytle et al., 2013). We isolated splenocytes from C57BL/6 mice, treated these cells with rRABV at a MOI of 5, allowed the cells to propagate in culture for 48 hours and then evaluated the intracellular expression of APRIL and RABV nucleoprotein (N) by flow cytometry (Figure 1A). The results demonstrate that rRABV-infected murine B cells increase APRIL expression by 48 hours post-infection (Figure 1B). The upregulation of APRIL in response to rRABV suggests that APRIL signaling might play a role in effective B cell responses to the vaccine vector. Therefore, we hypothesized that temporospatial APRIL expression in association with RABV antigen exposure would improve the generation of protective antibodies against RABV glycoprotein (G) in response to RABV vaccination.
Figure 1.
3.2 Construction, characterization and functional evaluation of a live, attenuated RABV-based vaccine expressing murine APRIL
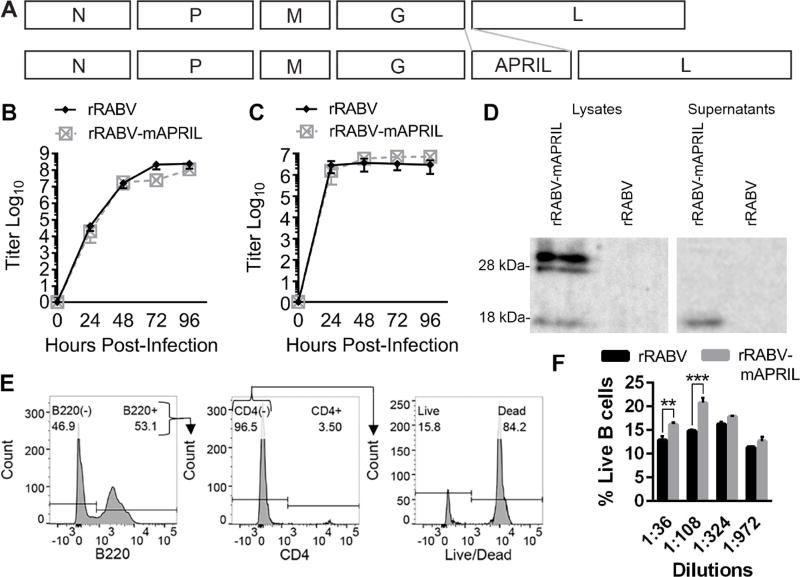
In order to test our hypothesis that the expression of APRIL will enhance RABV vaccine-induced immunity, we constructed and recovered a recombinant, attenuated vaccine strain of RABV expressing murine april, rRABV-mAPRIL (Fig. 2A). The parental vector, rRABV, and rRABV-mAPRIL demonstrated similar growth kinetics (Figs. 2B and 2C), indicating insertion of this gene into the RABV genome does not affect the growth of RABV in vitro.
Figure 2.
APRIL is unique among the TNF cytokines as it is cleaved intracellularly rather than at the cellular membrane. 30 kDa unprocessed APRIL is digested in the golgi by furin convertase yielding soluble 17 kDA monomers (López-Fraga et al., 2001). Western blot analysis of cell lysates from BSR cells infected with rRABV-mAPRIL or rRABV confirmed that rRABV-mAPRIL, but not rRABV, expressed full-length APRIL, which was cleaved into soluble APRIL (Fig. 2D). Western blot analysis of supernatants from rRABV-mAPRIL-infected BSR cells demonstrated the presence of soluble APRIL monomers. The results confirm that rRABV-mAPRIL expresses full-length APRIL which is processed into secreted monomers.
To ensure virally encoded, secreted APRIL was functional, primary murine splenocytes were cultured with serial dilutions of purified and concentrated supernatants from BSR cell cultures infected with rRABV or rRABV-mAPRIL. After 72 hours, splenocytes were harvested, stained with Live/Dead Aqua, α-B220 APC-Cy7, and α-CD4 PE, then analyzed by flow cytometry (Fig. 2E). Exposure of splenocytes to virus-free supernatant from rRABV-mAPRIL-infected cells significantly increased the percentage of live B cells compared to supernatant from rRABV-infected cells (Fig. 2F), indicating that APRIL secreted by rRABV-mAPRIL infected cells is functional.
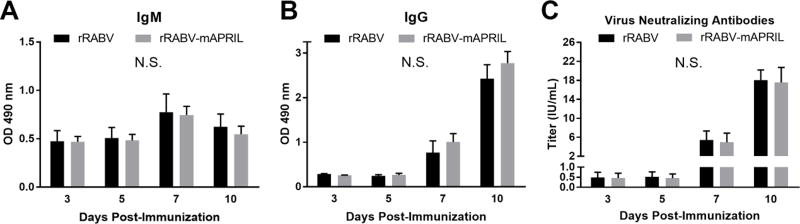
3.3 APRIL expression in association with RABV antigen exposure does not modulate the primary antibody response to RABV vaccination
To evaluate the effect of APRIL expression in association with RABV antigen on the immunogenicity of rabies vaccination, mice were immunized i.m. with 105 FFU rRABV or rRABV-mAPRIL. The kinetics of the primary immune response to vaccination were monitored by serum levels of total anti-RABV G IgM and IgG antibodies at various times post-immunization. rRABV-mAPRIL induced levels of anti-RABV G IgM (Fig. 3A) and IgG (Fig. 3B) comparable to mice immunized with rRABV at all times evaluated. Consistent with the antibody levels determined by ELISA, RVNA titers in mice immunized with rRABV-mAPRIL or rRABV were similar at all times (Fig 3C). RVNAs reached levels indicative of a satisfactory immunization (greater than 0.5 IU/mL) (“CDC - Doctors: Rabies Serology - Rabies,” n.d.) by day 7 post-immunization in response to rRABV and rRABV-mAPRIL.
Figure 3.
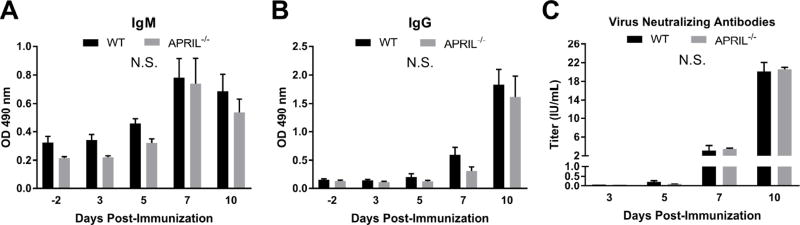
3.4 APRIL is dispensable for the primary antibody response to RABV vaccination
While APRIL expression in the context of rRABV-mAPRIL did not alter the immunogenicity of rRABV, APRIL’s influence on the immune response to RABV vaccination had yet to be evaluated. We employed an APRIL-deficient mouse model to characterize the role of APRIL in the primary anti-RABV G antibody response to vaccination. Mice devoid of APRIL (Xiao et al., 2008) or control (C57BL/6) mice were immunized i.m. with a single dose of 105 FFU rRABV. The kinetics of the primary immune response to vaccination were evaluated by serum levels of total anti-RABV G IgM and IgG antibodies at various times post-immunization. At all times evaluated the anti-RABV G IgM (Fig. 4A) and IgG (Fig. 4B) antibody levels were equivalent between the APRIL-deficient and C57BL/6 mice. RVNA titers were correspondingly similar between C57BL6 and APRIL-deficient mice at all times. These results demonstrate that APRIL is dispensable for the primary antibody response to RABV vaccination.
Figure 4.
3.5 APRIL does not influence long-lived antibody responses to RABV vaccination
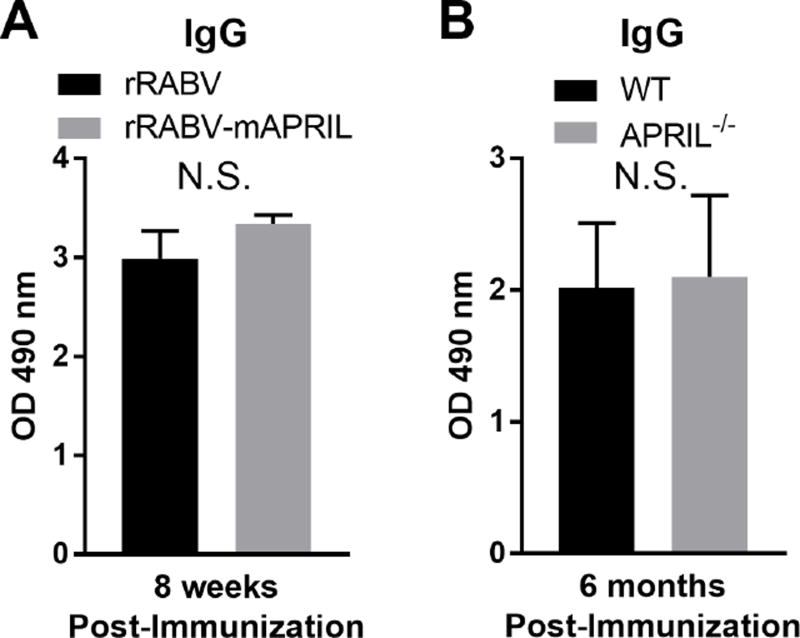
APRIL has been documented to support the survival of antigen-specific PCs (O’Connor et al., 2004). In order to evaluate whether APRIL expression associated with RABV antigen exposure influenced long-lived antibody responses, serum IgG levels in C57BL/6 mice immunized i.m. with 105 FFU rRABV or rRABV-mAPRIL were compared 8 weeks post-immunization. The levels of anti-RABV G IgG at this time were similar between rRABV and rRABV-mAPRIL immunized mice (Fig. 5A).
Figure 5.
In an effort to determine whether APRIL was necessary to support long-lived antibody responses to RABV vaccination, serum IgG levels 6 months after immunization were compared between C57BL/6 mice and APRIL-deficient mice immunized i.m. with 105 FFU rRABV. Sustained antibody responses were similar between APRIL-deficient and control mice indicating that APRIL is dispensable for long-lived anti-RABV antibody responses (Fig. 5B). This is in agreement with previous work which indicates BAFF can signal through BCMA to support PC survival in vivo (Benson et al., 2008). Taken together, neither APRIL expression at the time of antigen exposure nor APRIL deficiency modulates the long-lived antibody-secreting PC response to RABV vaccination.
3.6 The primary antibody response to RABV vaccination is independent of TACI signaling
APRIL binds to TACI and BCMA which are differentially expressed by B cells. Previous studies have demonstrated that BCMA deficiency or transgenic expression does not affect B cell development or antigen-specific B cell responses (Schneider et al., 2001; Xu and Lam, 2001). In contrast, TACI-deficient and soluble TACI transgenic mice have demonstrated significant roles for TACI in the regulation of humoral immune responses (Zhang et al., 2015). In order to confirm that the highest affinity APRIL receptor, TACI, is not required for the primary antibody response to RABV vaccination, TACI-deficient mice were immunized i.m. with a single dose of 105 FFU rRABV. Serum levels of total anti-RABV G IgG antibodies were evaluated at various times post-immunization (Fig. 6). TACI-deficiency did not affect the primary antibody response to RABV vaccination indicating that this response is independent of TACI signaling.
Figure 6.
4. Discussion
Neutralization of APRIL and BAFF by TACI-Fc and BCMA-Fc fusion proteins has been shown to diminish antibody responses against the TD antigens, suggesting that this signaling system might be important for the TD response to rRABV (Haley et al., 2017; Xia et al., 2000; Yu et al., 2000). Increased expression of APRIL in RABV-infected primary murine B cells suggested that APRIL expression in the context of RABV-based vaccines could augment immunogenicity. Indeed, previous HIV vaccine studies have successfully employed APRIL as an adjuvant (Gupta et al., 2015; Kanagavelu et al., 2012; Melchers et al., 2012).
Surprisingly, rRABV-mAPRIL did not elicit more robust anti-RABV antibody responses compared to rRABV. This could be a direct consequence of the systemic immunity needed for effective protection against RABV. APRIL has been a successful adjuvant in vaccines targeting HIV which require strong mucosal immunity since the gut is the major site of HIV replication (Brenchley and Douek, 2008). However, APRIL plays significantly different roles in mucosal and systemic immunity. APRIL secreted by gut endothelium, eosinophils and dendritic cells supports class-switching towards IgA and thereafter maintains IgA+ PCs in the intestinal lamina propria (Barone et al., 2009; Chu et al., 2011; He et al., 2007). While IgA responses are critical for mucosal immunity, the systemic response to RABV required for protection depends on IgG and IgM (Dorfmeier et al., 2013a). Lack of heparin sulfate proteoglycans (HSPGs) in the microenvironment in which APRIL is expressed by rRABV-mAPRIL may also underlie rRABV-mAPRIL’s lack of effect on the anti-RABV antibody response. APRIL cross-linking on HSPGs optimizes APRIL signaling through TACI, the only APRIL receptor involved in early humoral responses (Bossen et al., 2008; Xu and Lam, 2001). If APRIL is unable to cross-link on HSPGs in the context of the response to rRABV then exogenous and endogenous APRIL would likely be irrelevant in the anti-RABV response.
Our results in APRIL-deficient mice confirm that the anti-RABV antibody response to vaccination does not depend on endogenous APRIL. Th1 responses have been associated with strong antibody responses to RABV vaccination (Cenna et al., 2009). Previous findings document intact Th1 immune responses in APRIL-deficient mice (Stein et al., 2002), likely enabling the equivalent responses to RABV vaccination in APRIL-deficient and wild-type mice. It is possible that BAFF outcompetes APRIL for TACI binding sites as BAFF has a higher affinity for TACI than does APRIL (Hymowitz et al., 2005). This scenario is plausible given the intact responses to rRABV immunization in APRIL-deficient mice.
Confirmatory studies in TACI-deficient mice demonstrated comparable antibody responses to control mice against rRABV. Since TACI is the highest affinity APRIL receptor and the only APRIL receptor documented to play a role in early humoral responses, this finding reiterates that APRIL is inconsequential in the anti-RABV response. Our finding agrees with previous reports that TACI-deficient mice have intact in vivo antibody responses to TD antigens (von Bülow et al., 2001; Yan et al., 2001).
Collectively, our results demonstrate that the APRIL:TACI signaling axis is not required for effective RABV vaccination. APRIL expression in temporospatial association with RABV antigen exposure does not improve the anti-RABV antibody response to vaccination. Indeed, we have demonstrated that neither APRIL nor TACI signaling are required for the antibody response to RABV vaccination. Our work provides new insight into the role of APRIL and TACI signaling in the context of antiviral responses. Specifically, the APRIL-TACI signaling axis is unlikely to be successfully manipulated for RABV vaccine development.
Highlights.
Like BAFF, APRIL is a TNF family cytokine that plays critical roles in B cell responses.
Unlike BAFF, little is known about the role of APRIL in vaccine-induced immunity against viral infections.
A recombinant rabies vaccine expressing murine APRIL does not modulate the immunogenicity of the vector.
The APRIL:TACI axis is dispensable for the primary and secondary immune response to a recombinant rabies vaccine.
Acknowledgments
We would like to thank the Flow Cytometry Facility, Kimmel Cancer Center, Thomas Jefferson University for flow cytometry assistance. We would also like to thank Dr. Richard J. Bram, Department of Pediatrics and Adolescent Medicine, College of Medicine, Mayo Clinic for his kind gift of TACI-deficient mice.
Funding: This work was supported by the National Institutes of Health [grant numbers R21AI109135, R56AI123272, and R01AI123272 to JPM].
Glossary
- APRIL
a proliferation inducing ligand
- BAFF
B cell activating factor
- BAFFR
BAFF receptor
- BCMA
B cell maturation antigen
- CAM
calcium-modulator and cyclophilin ligand
- HSPG
heparan sulfate proteoglycans
- G
glycoprotein
- GC
germinal center
- PC
plasma cell
- PEP
post-exposure prophylaxis
- RABV
rabies virus
- rRABV
recombinant SAD-B19 rabies vaccine
- rRABV-mAPRIL
recombinant rabies vaccine expressing murine APRIL
- RVNA
rabies virus neutralizing antibodies
- TACI
T cell activator and CAML inhibitor
- TD
T cell dependent
- TI
T cell independent
- TNF
tumour necrosis family
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barone F, Patel P, Sanderson J, Spencer J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2009;2:495–503. doi: 10.1038/mi.2009.106. [DOI] [PubMed] [Google Scholar]
- Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam K-P, Noelle RJ. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 2008;180:3655–9. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, Scott ML, Maquelin A, Belnoue E, Siegrist C-A, Chevrier S, Acha-Orbea H, Leung H, Mackay F, Tschopp J, Schneider P. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111 doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabasso VJ, Dobkin MB, Roby RE, Hammar AH. Antibody response to a human diploid cell rabies vaccine. 1974;27:553–561. doi: 10.1128/am.27.3.553-561.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC - Doctors: Rabies Serology - Rabies [WWW Document] ndURL http://www.cdc.gov/rabies/specific_groups/doctors/serology.html.
- Cenna J, Hunter M, Tan GS, Papaneri AB, Ribka EP, Schnell MJ, Marx PA, McGettigan JP. Replication-Deficient Rabies Virus–Based Vaccines Are Safe and Immunogenic in Mice and Nonhuman Primates. J. Infect. Dis. 2009;200:1251–1260. doi: 10.1086/605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenna J, Tan GS, Papaneri AB, Dietzschold B, Schnell MJ, McGettigan JP. Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene. Vaccine. 2008;26:6405–14. doi: 10.1016/j.vaccine.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175:485–99. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- Dorfmeier CL, Shen S, Tzvetkov EP, McGettigan JP. Reinvestigating the role of IgM in rabies virus postexposure vaccination. J. Virol. 2013a;87:9217–22. doi: 10.1128/JVI.00995-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP. Investigating the role for IL-21 in rabies virus vaccine-induced immunity. PLoS Negl. Trop. Dis. 2013b;7:e2129. doi: 10.1371/journal.pntd.0002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel A, Shen S, LaBranche CC, Montefiori D, McGettigan JP. A Bivalent, Chimeric Rabies Virus Expressing Simian Immunodeficiency Virus Envelope Induces Multifunctional Antibody Responses. AIDS Res. Hum. Retroviruses. 2015;31:1126–1138. doi: 10.1089/aid.2014.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacouin A, Bourhy H, Renaud JC, Camus C, Suprin E, Thomas R. Human rabies despite postexposure vaccination. Eur. J. Clin. Microbiol. Infect. Dis. 1999;18:233–5. doi: 10.1007/s100960050269. [DOI] [PubMed] [Google Scholar]
- Gupta S, Clark ES, Termini JM, Boucher J, Kanagavelu S, LeBranche CC, Abraham S, Montefiori DC, Khan WN, Stone GW. DNA Vaccine Molecular Adjuvants SP-DBAFF and SP-D-APRIL Enhance Anti-gp120 Immune Response and Increase HIV-1 Neutralizing Antibody Titers. J. Virol. 2015;89:4158–4169. doi: 10.1128/JVI.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley SL, Tzvetkov EP, Meuwissen S, Plummer JR, McGettigan JP. Targeting Vaccine-induced Extrafollicular Pathway of B cell Differentiation Improves Rabies Post-exposure Prophylaxis. J. Virol. JVI.02435-16. 2017 doi: 10.1128/JVI.02435-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin F-X, Metlin A, Miranda ME, Muller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MAN, Zinsstag J, Dushoff J. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal Bacteria Trigger T Cell-Independent Immunoglobulin A2 Class Switching by Inducing Epithelial-Cell Secretion of the Cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Hymowitz SG, Patel DR, Wallweber HJA, Runyon S, Yan M, Yin J, Shriver SK, Gordon NC, Pan B, Skelton NJ, Kelley RF, Starovasnik MA. Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J. Biol. Chem. 2005;280:7218–27. doi: 10.1074/jbc.M411714200. [DOI] [PubMed] [Google Scholar]
- Jourdan M, Cren M, Robert N, Bollore K, Fest T, Duperray C, Guilloton F, Hose D, Tarte K, Klein B. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia. 2014;28:1647–1656. doi: 10.1038/leu.2014.61. [DOI] [PubMed] [Google Scholar]
- Kanagavelu SK, Snarsky V, Termini JM, Gupta S, Barzee S, Wright JA, Khan WN, Kornbluth RS, Stone GW. Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine. Vaccine. 2012;30:691–702. doi: 10.1016/j.vaccine.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Faber M, Dietzschold B, Hooper DC. The role of toll-like receptors in the induction of immune responses during rabies virus infection. Adv. Virus Res. 2011;79:115–26. doi: 10.1016/B978-0-12-387040-7.00007-X. [DOI] [PubMed] [Google Scholar]
- Lopez-Fraga M, Fernandez R, Albar JP, Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–51. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle AG, Norton JE, Dorfmeier CL, Shen S, McGettigan JP. B cell infection and activation by rabies virus-based vaccines. J. Virol. 2013;87:9097–110. doi: 10.1128/JVI.00800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- McGettigan JP. Experimental rabies vaccines for humans. Expert Rev. Vaccines. 2010;9:1177–86. doi: 10.1586/erv.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettigan JP, Naper K, Orenstein J, Koser M, McKenna PM, Schnell MJ. Functional human immunodeficiency virus type 1 (HIV-1) Gag-Pol or HIV-1 Gag-Pol and env expressed from a single rhabdovirus-based vaccine vector genome. J. Virol. 2003;77:10889–99. doi: 10.1128/JVI.77.20.10889-10899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettigan JP, Sarma S, Orenstein JM, Pomerantz RJ, Schnell MJ. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J. Virol. 2001;75:8724–32. doi: 10.1128/JVI.75.18.8724-8732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers M, Bontjer I, Tong T, Chung NPY, Klasse PJ, Eggink D, Montefiori DC, Gentile M, Cerutti A, Olson WC, Berkhout B, Binley JM, Moore JP, Sanders RW. Targeting HIV-1 envelope glycoprotein trimers to B cells by using APRIL improves antibody responses. J. Virol. 2012;86:2488–500. doi: 10.1128/JVI.06259-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan T, Marissen W, Rupprecht C. Monoclonal antibodies for the prevention of rabies: theory and clinical practice. Antib. Technol. J. 2014;4:1. doi: 10.2147/ANTI.S33533. [DOI] [Google Scholar]
- Norton JE, Lytle AG, Shen S, Tzvetkov EP, Dorfmeier CL, McGettigan JP. ICAM-1-Based Rabies Virus Vaccine Shows Increased Infection and Activation of Primary Murine B Cells In Vitro and Enhanced Antibody Titers In-Vivo. PLoS One. 2014;9:e87098. doi: 10.1371/journal.pone.0087098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin L-L, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199:91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschke V, Sosnovtseva S, Ward CD, Hong JS, Smith R, Albert V, Stohl W, Baker KP, Ullrich S, Nardelli B, Hilbert DM, Migone T-S. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 2002;169:4314–21. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, Cachero TG, Finke D, Beermann F, Tschopp J. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J. Exp. Med. 2001;194:1691–7. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Foley HD, Siler CA, McGettigan JP, Dietzschold B, Pomerantz RJ. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3544–9. doi: 10.1073/pnas.050589197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, McGettigan JP, Wirblich C, Papaneri A. The cell biology of rabies virus: using stealth to reach the brain. Nat. Rev. Microbiol. 2010;8:51–61. doi: 10.1038/nrmicro2260. [DOI] [PubMed] [Google Scholar]
- Schuepbach-Mallepell S, Das D, Willen L, Vigolo M, Tardivel A, Lebon L, Kowalczyk-Quintas C, Nys J, Smulski C, Zheng TS, Maskos K, Lammens A, Jiang X, Hess H, Tan S-L, Schneider P. Stoichiometry of Heteromeric BAFF and APRIL Cytokines Dictates Their Receptor Binding and Signaling Properties. J. Biol. Chem. 2015;290:16330–42. doi: 10.1074/jbc.M115.661405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayam C, Duggal AK, Kamble U, Agarwal AK. Post-exposure prophylaxis for rabies 2006 [Google Scholar]
- Stein JV, Lopez-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodriguez D, Gomez-Caro R, De Jong J, Martinez-A C, Medema JP, Hahne M. APRIL modulates B and T cell immunity. J. Clin. Invest. 2002;109:1587–98. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- WHO Publication. Rabies vaccines: WHO position paper--recommendations. Vaccine. 2010;28:7140–2. doi: 10.1016/j.vaccine.2010.08.082. [DOI] [PubMed] [Google Scholar]
- Wilde H, Khawplod P, Hemachudha T, Sitprija V. Postexposure treatment of rabies infection: can it be done without immunoglobulin? Clin. Infect. Dis. 2002;34:477–80. doi: 10.1086/324628. [DOI] [PubMed] [Google Scholar]
- Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, Theill LE, Colombero A, Solovyev I, Lee F, McCabe S, Elliott R, Miner K, Hawkins N, Guo J, Stolina M, Yu G, Wang J, Delaney J, Meng SY, Boyle WJ, Hsu H. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J. Exp. Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Motomura S, Podack ER. APRIL (TNFSF13) regulates collagen-induced arthritis, IL-17 production and Th2 response. Eur. J. Immunol. 2008;38:3450–8. doi: 10.1002/eji.200838640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol. Cell. Biol. 2001;21:4067–74. doi: 10.1128/MCB.21.12.4067-4074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, Tumas D, Grewal IS, Dixit VM. Activation and accumulation of B cells in TACI-deficient mice. Nat. Immunol. 2001;2:638–43. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat. Immunol. 2000;1:252–6. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li J, Zhang Y-M, Zhang X-M, Tao J. Effect of TACI signaling on humoral immunity and autoimmune diseases. J. Immunol. Res. 2015;2015:247426. doi: 10.1155/2015/247426. [DOI] [PMC free article] [PubMed] [Google Scholar]