Abstract
Endothelial progenitor cells (EPCs) are an independent factor predicting cardiovascular events. Visfatin plays an important role in the pathogenesis of various metabolic disorders. In this study, we examined the effects of visfatin on the apoptosis of EPCs and the mechanisms underlying these effects. Cultured EPCs pre-treated with various concentrations of visfatin, FK866 (visfatin inhibitor) and BAY11-7085 [referred to as BAY11; nuclear factor-κB (NF-κB) inhibitor] were used to investigate the association between visfatin and EPC apoptosis. Following treatment with visfatin for 48 h, the EPCs exhibited a dose-dependent increase in apoptosis and an upregulated expression of Bax, caspase-3 and NF-κB at both the mRNA and protein level, and a decreased protein expression of Bcl-2. Compared with the untreated control group, the increase in EPC apoptosis, as well as in Bax and caspase-3 expression was significant following treatment with 150 ng/ml visfatin, which also induced a dose-dependent and significant increase in the protein expression of interleukin-6 (IL-6) and intercellular adhesion molecule-1 (ICAM-1). All the visfatin-induced effects were suppressed by pre-treatment with FK866. Pre-incubation of the EPCs with BAY11 for 1 h followed by treatment with visfatin (150 ng/ml) for 48 h also abolished visfatin-induced apoptosis; it also abolished the promoting effects of visfatin on the expression of caspase-3, Bax, ICAM-1 and IL-6, and its suppressive effects on the protein expression of Bcl-2. On the whole, our data indicate that visfatin induces EPC apoptosis by increasing the expression of pro-inflammatory mediators partly through the regulation of NF-κB.
Keywords: visfatin, endothelial progenitor cells, apoptosis, inflammation
Introduction
Visfatin, also known as pre-B-cell colony-enhancing factor and nicotinamide phosphoribosyltransferase (Nampt), was originally cloned in 1994 and is a multifaceted molecule (1). As a nicotinamide adenine dinucleotide (NAD) biosynthetic enzyme, visfatin determines the activity of NAD-consuming enzymes, such as sirtuins and influences a variety of metabolic responses, prompting increasing attention in the literature.
Visfatin plays an important role in regulating insulin secretion, as it functions as an immunomodulatory cytokine and is involved in the inflammatory responses (2). Visfatin has also been implicated in the pathogenesis of various metabolic disorders, with increased plasma concentrations of visfatin reported in overweight/obese subjects, as well as in pateints with type 2 diabetes mellitus, metabolic syndrome, atherosclerosis and cardiovascular diseases (3–7). In atherosclerosis, visfatin has been implicated causatively by inducing oxidative stress and inflammation in endothelial cells, which subsequently damages vascular endothelial function (8). Visfatin also participates in the inflammatory process in rat spleen by modulating macrophages and inflammatory cytokines (9). In addition, treatment with visfatin in vitro has been shown to promote the proliferation of vascular smooth muscle cells (10) and to increase the endothelial expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and E-selectin, all of which are considered as biomarkers of endothelial dysfunction (11). Thus, these cell types are implicated as both the targets and cellular sources of visfatin. Finally, a pathological role for visfatin was also reported by a study showing that the administration of visfatin impairs microvascular endothelium-dependent relaxation through a mechanism involving NADPH oxidase stimulation (12). Despite these in vitro and in vivo data, the mechanisms underlying the function of visfatin remain controversial.
Endothelial progenitor cells (EPCs) are progenitor cells that can migrate to the peripheral circulation to differentiate into mature endothelial cells. In the case of vessel impairment or tissue ischemia, EPCs mobilize to peripheral blood circulation, specifically home in to the damaged or ischemic tissue, and then differentiate further into mature endothelial cells (13). Thus, the restoration and regeneration of endothelial cells depends not only on the proliferation and migration of mature endothelial cells, but also on the circulating EPC levels. Indeed, the decrease in the number of circulating EPCs has been used to independently predict atherosclerotic progression and poor prognosis in patients with coronary heart disease (14,15). It is also known that apoptosis plays a crucial role in the loss of EPCs, with data suggesting several factors that may be associated with traditional and the disease-related risk of eliciting apoptosis in EPCs, including C-reactive protein, oxidized low-density lipoprotein (LDL) and interferon-α (16–18).
Emerging evidence has established a potential link between adipokines and vascular dysfunction, while our previous studies have correlated EPC dysfunction in obese rats with serum visfatin levels (19), with higher levels of serum visfatin and a lower level of circulating EPCs in the obese population (7). However, the specific mechanisms remain unclear, and few studies have specifically investigated visfatin and EPCs, and the related mechanisms of action. In the present study, we investigated whether visfatin plays a role in promoting EPCs apoptosis and aimed to elucidate the mechanisms underlying such effects.
Materials and methods
Isolation and culture of EPCs
This study was approved by the Ethics Committee of Hebei General Hospital. Informed consent was obtained from all subjects. Umbilical vein blood mononuclear cells (MNCs) of healthy pregnant woman donors were isolated by Ficoll density gradient centrifugation (Dakewe, Shenzhen, China). After washing with phosphate-buffered saline (PBS), the MNCs were resuspended in EGM-2MV medium (Lonza, Walkersville, MD, USA), supplemented with 20% fetal bovine serum, 1% Pen/Strep, 0.04% hydrocortisone, 0.1% heparin and 0.1% ascorbic acid in the presence of insulin-like growth factor-1 (IGF-1; 50 ng/ml), epidermal growth factor (EGF; 10 ng/ml), fibroblast growth factor (FGF; 50 ng/ml), vascular endothelial growth factor (VEGF; 50 ng/ml) and fibronectin (10 µg/ml). Six-well tissue culture plates pre-coated with fibronectin (Solarbio, Beijing, China) and the cells were seeded at a density of 2×106 cells/ml and cultured in a 5% CO2 incubator at 37°C. After 48 h of culture, non-adherent cells were removed by washing with PBS, and EGM-2MV medium was added to each well. The medium was changed every 2 days until treatment. The cells were observed daily under an inverted microscope (TS100F; Nikon, Tokyo, Japan).
Immunophenotyping of EPCs
Immunophenotyping of cultured cells was detected by flow cytometry. Briefly, the EPCs were washed twice with PBS and incubated with EPC-specific markers for 30 min at 4°C in the dark. The following FITC- or PE-conjugated monoclonal antibodies (mAbs) were used as EPCs markers: anti-human CD34 (555748) and anti-human CD309 (554680), also termed kinase insert domain receptor (KDR) (both from Becton-Dickinson, Franklin Lakes, NJ, USA). Isotype-matched irrelevant mAbs were used as negative controls. The cells were electronically gated according to their light scattering properties to exclude cell debris. CD309+CD34+ double-positive cells were considered as EPCs. Data were detected on a FACSCanto flow cytometer (Becton-Dickinson) and analysed using SPSS 13.0 software (IBM, Armonk, NY, USA).
Uptake of Dil-acetylated low-density lipoprotein and staining for Ulex europaeus lectin
The EPCs were marked by cellular uptake of DiI-labeled acetylated LDL (DiI-ac-LDL; Molecular Probes, Eugene, OR, USA) and binding of FITC-conjugated lectin from Ulex europaeus agglutinin (FITC-Lectin-UEA-1; Sigma-Aldrich, St. Louis, MO, USA). After 10 days in culture, the attached cells were incubated with Dil-ac-LDL (2.5 µg/ml) in EGM-2MV medium for 3 h at 37°C. The cells were then fixed with 2% paraformaldehyde for 10 min and incubated for 1 h with FITC-Lectin-UEA-1 (10 µg/ml). The cells were then observed under an Olympus Fluoview FV1000 laser scanning confocal microscope (OLFV-34CMXE; Olympus, Tokyo, Japan). Orange cells positive for both DiI-ac-LDL and FITC-Lectin-UEA-1 were identified as EPCs.
Treatment of EPCs with visfatin
After being cultured for 7 days, the adherent EPCs were serum-starved for 24 h, and then incubated with human recombinant visfatin (Peprotech, Rocky Hill, NJ, USA) at various concentrations (0, 50, 100 and 150 ng/ml) for 48 h. In some experiments, the EPCs were pretreated with visfatin inhibitor (FK866, 10 µM; Sigma-Aldrich) or nuclear factor-κB (NF-κB) inhibitor [BAY11-7085 (referred to as BAY11), 5 µM; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA] 1 h prior to incubation with visfatin at various concentrations for 48 h.
Flow cytometry
The apoptosis of the EPCs was evaluated by flow cytometry. The EPCs were fluorescently labeled by the addition of 500 µl of binding buffer, 5 µl of Annexin V-FITC and 5 µl of propidium iodide (Miltenyi Biotec, Bergisch Gladbach, Germany). Following incubation in the dark at room temperature for 15 min, the cells were subjected to flow cytometric analysis. A minimum of 10,000 cells in the gated region was analyzed using a BD FACSCalibur Flow Cytometer (Becton-Dickinson). The esults were presented by the percentage of total cells.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the EPCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA was reverse-transcribed into cDNA according to the instructions of the Easy Script First Strand cDNA Synthesis Super Mix kit (TransGen Biotech, Beijing, China). Specific primers designed for the amplification of caspase-3, Bax, Bcl-2, NF-κB and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were verified by NCBI Blast; primer sequences are listed in Table I. Reactions were carried out on an ABI PRISM 7300 PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR-Green I GoTaq® qPCR Master Mix (Promega, Madison, WI, USA). PCR reactions were performed in a total of 25 µl as follows: an initial cycle at 95°C for 5 min, followed by 40 cycles of 95°C for 15 sec, 58°C for 20 sec and 72°C for 30 sec. The gene expression was analyzed in duplicate and normalized against GAPDH. The results of each gene are expressed as relative expression using the ΔCt method.
Table I.
Sequences of primers used for RT-qPCR.
| Genes | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| GADPH | TGAACGGGAAGCTCACTGG | GCTTCACCACCTTCTTGATGTC |
| Caspase-3 | TGGACTGTGGCATTGAGAC | AATAACCAGGTGCTGTGGAGT |
| Bax | GGCTGGACATTGGACTTCCT | CCACAAAGATGGTCACGGT |
| Bcl-2 | GTGGATGACTGAGTACCTGAAC | CGCATCTCGGACCTGTG |
| NF-κB | CAAGGCAGCAAATAGACGAG | TGTTGAGAGTTAGCAGTGAGGC |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NF-κB, nuclear factor-κB.
Western blot analysis
The cells were washed twice with ice-cold PBS and cellular proteins from EPCs under various treatments were prepared with lysis buffer [1% NP-40, 150 mM NaCl, 50 mM Tris (pH 8.0), 0.1% aprotinin, 0.1% leupeptin, 0.035% pepstain A and 100 µg/ml PMSF] supplemented with protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany) and solubilized for 30 min at 4°C. The samples were centrifuged at 11,600 × g for 30 min at 4°C. Protein concentrations were determined via Nanojob (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein samples were then denatured in glyceraldehyde 3-phosphate dehydrogenase (SDS) sample buffer (125 mM Tris-HCl, pH 6.8, 50% glycerol, 2% SDS, 5% β-mercaptoethanol and 0.01% bromophenol blue) for 10 min at 100°C. Equal amounts of proteins were separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Subsequently, 5% non-fat milk in Tris-buffered saline was used for blocking for 4 h at room temperature. After blocking, the membranes were incubated overnight at 4°C with appropriately diluted rabbit anti-human primary antibodies to caspase-3 (sc-2048; Santa Cruz Biotechnology, Inc.), Bax (50599-2-Ig; Proteintech, Chicago, IL, USA), Bcl-2 (2870; Cell Signaling Technology, Danvers, MA, USA), NF-κB (#21013; Signalway Antibody, Baltimore, MD, USA), ICAM-1 (ab53013) and interleukin (IL)-6 (ab32530) (both from Abcam, Cambridge, MA, USA), or mouse anti-human β-actin (66009-1-Ig; Proteintech), followed by incubation with the relevant secondary antibodies [caspase-3, Bax, Bcl-2, NF-κB, ICAM, IL-6: anti-rabbit IgG (L3012-1); β-actin: anti-mouse IgG (L3032-2); all from Signalway Antibody] for 2 h at room temperature. The immunoreactive proteins were visualized by the enhanced chemiluminescence (ECL) detection system. β-actin served as an internal control protein.
Statistical analysis
All experiments were repeated at least 3 times. Data are presented as the means ± SD and were analyzed using the SPSS statistical package (SPSS 13.0; IBM). Data were analyzed by homogeneity testing for variance. Statistical significance was determined by ANOVA (post-hoc used Student-Newman-Keuls test) for comparison of normally distributed data with homogeneous variance relevant groups. P-values <0.05 were considered to indicate statistically significant differences.
Results
Culture of EPCs
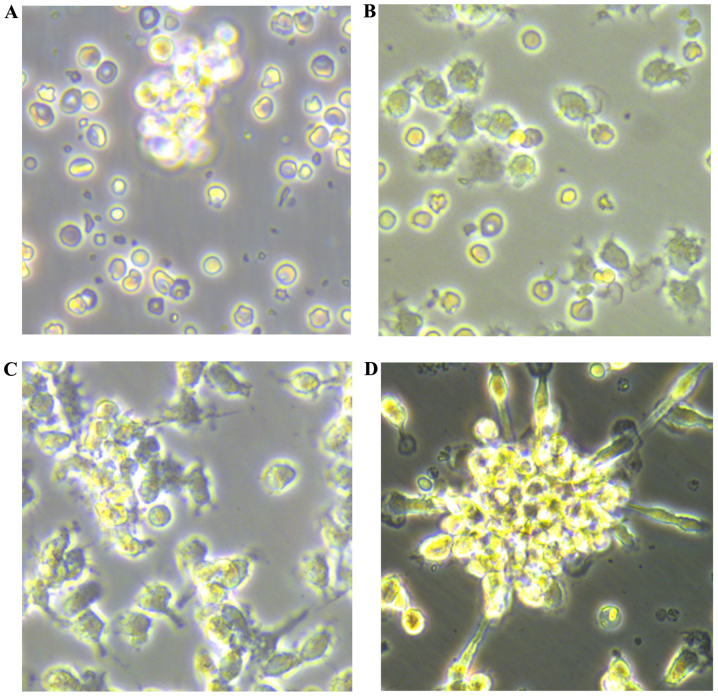
Newly isolated umbilical vein blood MNCs were round in shape when suspended in medium (Fig. 1A). After 48 h of plating, the cells were partly attached (Fig. 1B), with the adherent cells gradually elongating into a spindle shape (Fig. 1C). At 7–10 days after plating, the adherent cells grew as colonies. A colony of EPCs was defined as a central core of round cells with elongated sprouting cells at the periphery (Fig. 1D). The cells were round, triangle, oval or irregular. After 10 days, the cultured EPCs were linearly grown.
Figure 1.
Culture of umbilical vein blood mononuclear cells (MNCs). Umbilical vein blood MNCs were isolated and cultured from healthy pregnant woman. Morphology of MNCs under an inverted microscope. Isolated MNCs: (A) newly isolated, after culture for (B) 48 h (×400 magnification), (C) 7 days (×400 magnification), (D) 10 days (×400 magnification).
Immunophenotyping of EPCs
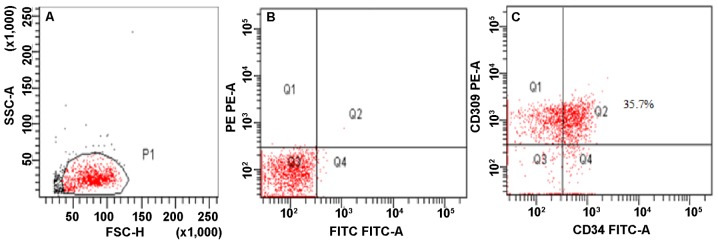
By flow cytometry, CD309+CD34+ double-positive cells were determined as EPCs (Fig. 2).
Figure 2.
Immunophenotyping of cultured cells was detected by flow cytometry. (A) Gate for MNCs. (B) Negative controls of isotype-matched irrelevant mAbs. (C) CD309+CD34+ double-positive cells were determined as EPCs.
Uptake of Dil-ac-LDL and staining for FITC-Lectin-UEA-1
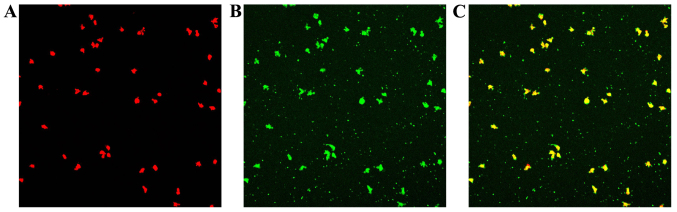
Following the uptake of Dil-ac-LDL and the binding of FITC-Lectin-UEA-1, double-stained EPCs were observed under a laser scanning confocal microscope (Fig. 3).
Figure 3.
Endothelial progenitor cells (EPCs) were characterized by the cellular uptake of DiI-labeled acetylated low-density lipoprotein (LDL) and binding of fluorescein isothiocyanate-conjugated lectin from Ulex europaeus agglutinin. (A) DIL-Ac-LDL uptaken by EPCs (red); (B) FITC-Lectin-UEA-1 combined by EPCs (green); (C) EPCs stained with DIL-Ac-LDL and FITC-Lectin-UEA-1 (orange).
Visfatin induces the apoptosis of EPCs
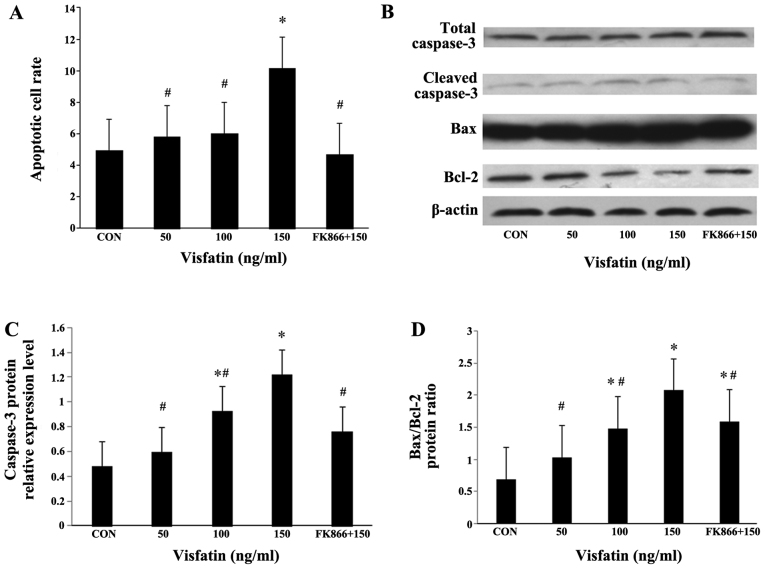
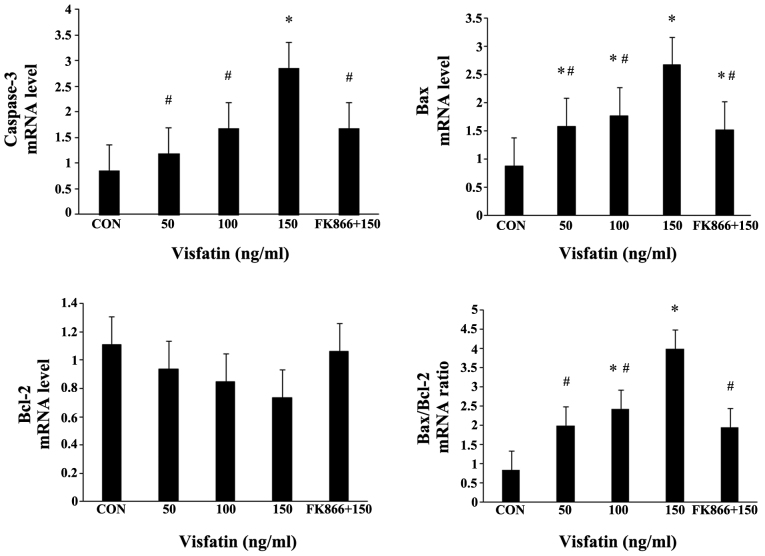
Recombinant human visfatin induced a dose-dependent and significant increase in EPC apoptosis, and this effect was completely abrogated by pre-treatment with FK866 (a visfatin inhibitor) (Fig. 4A). As expected, visfatin significantly increased the expression levels of cleaved caspase-3 in a dose-dependent manner at both the mRNA and protein level, and pre-treatment with FK866 markedly suppressed the increased expression of cleaved caspase-3 (Fig. 4B and C, and Fig. 5). The Bax/Bcl-2 expression ratio is critical for the induction of apoptosis; thus, we examined the expression of Bax and Bcl-2 in EPCs by both RT-qPCR and western blot analysis. Following treatment with visfatin, Bax expression was significantly upregulated at both the mRNA and protein level; however, the protein expression of Bcl-2 was decreased in a dose-dependent manner (P>0.05); these effects were all abolished by pre-treatment with FK866 (Figs. 4 and 5). The mRNA expression of Bcl-2 in the EPCs exhibited no statistically significant change, although there was a decreased trend following treatment with visfatin. As a result, the ratio of Bax/Bcl-2 expression was markedly augmented in the EPCs (Figs. 4 and 5).
Figure 4.
Effect of visfatin on endothelial progenitor cell (EPC) apoptosis. (A) EPCs were treated with visfatin (0-150 ng/ml) for 48 h in the presence or absence of visfatin inhibitor (FK866, 10 µM). Apoptotic cells were determined by flow cytometry. The apoptotic EPCs are presented by the percentage of total cells. Dose-dependent induction of apoptosis in EPCs by visfatin in the presence or absence of FK866. (B-D) Effects of visfatin on the expression of apoptosis-related proteins (caspase-3, Bax and Bcl-2), as determined by western blot analysis. Data are presented as fold change relative to β-actin. *P<0.05 vs. CON (no treatment); #P<0.05 vs. 150 ng/ml visfatin. Values represent the means ± standard deviation.
Figure 5.
mRNA expression levels of caspase-3, Bax, Bcl-2 and Bax/Bcl-2 ratio in endothelial progenitor cells (EPCs) treated with visfatin in the presence or absence of FK866. RT-qPCR was performed to determine the mRNA expression levels. *P<0.05 vs. CON (no treatment); #P<0.05 vs. 150 ng/ml visfatin. Values represent the means ± standard deviation.
Pro-inflammatory mediators are involved in the apoptosis of EPCs induced by visfatin
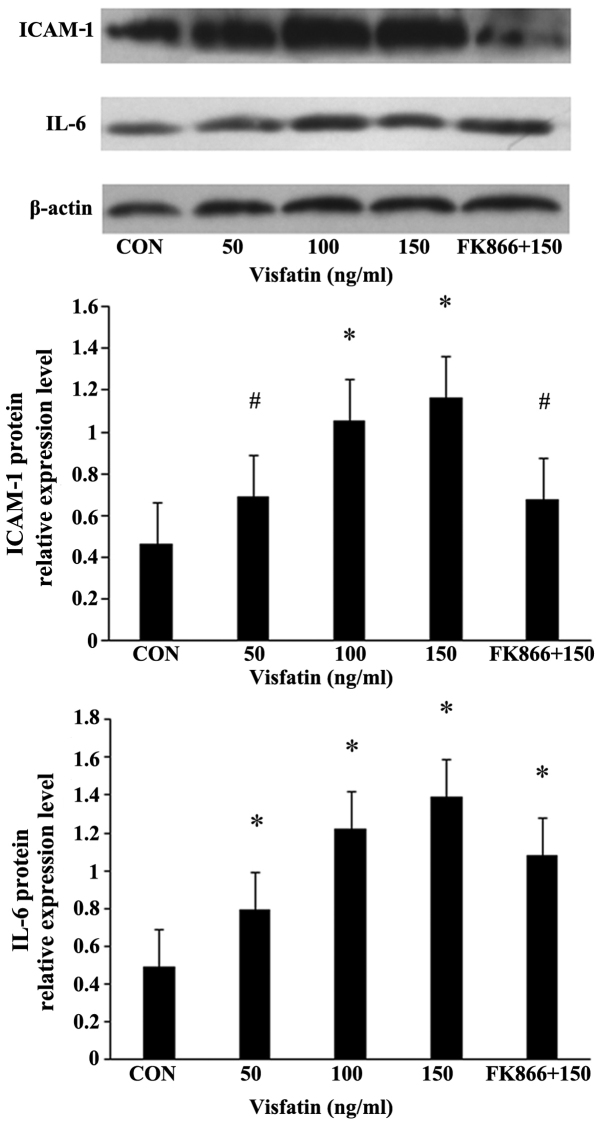
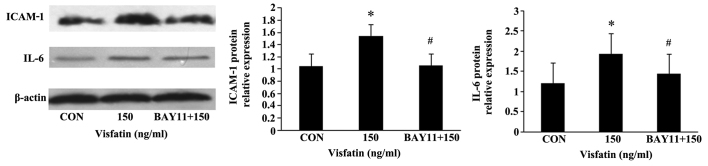
Treatment of the EPCs with various concentrations of visfatin resulted in a dose-dependent and significant increase in the protein expression of ICAM-1 and IL-6, as determined by western blot analysis (Fig. 6). FK866 suppressed these upregulatory effects, indicating the involvement of pro-inflammatory mediators in the apoptosis of EPCs induced by visfatin.
Figure 6.
Visfatin-induced intercellular adhesion molecule-1 (ICAM-1) and interleukin-6 (IL-6) production in endothelial progenitor cells (EPCs). EPCs were treated with visfatin (0-150 ng/ml) for 48 h in the presence or absence of visfatin inhibitor (FK866, 10 µM). The protein expression levels of ICAM-1 and IL-6 were measured by western blot analysis. *P<0.05 vs. CON (no treatment); #P<0.05 vs. 150 ng/ml visfatin. Values represent the means ± standard deviation.
NF-κB mediates visfatin-induced EPC apoptosis
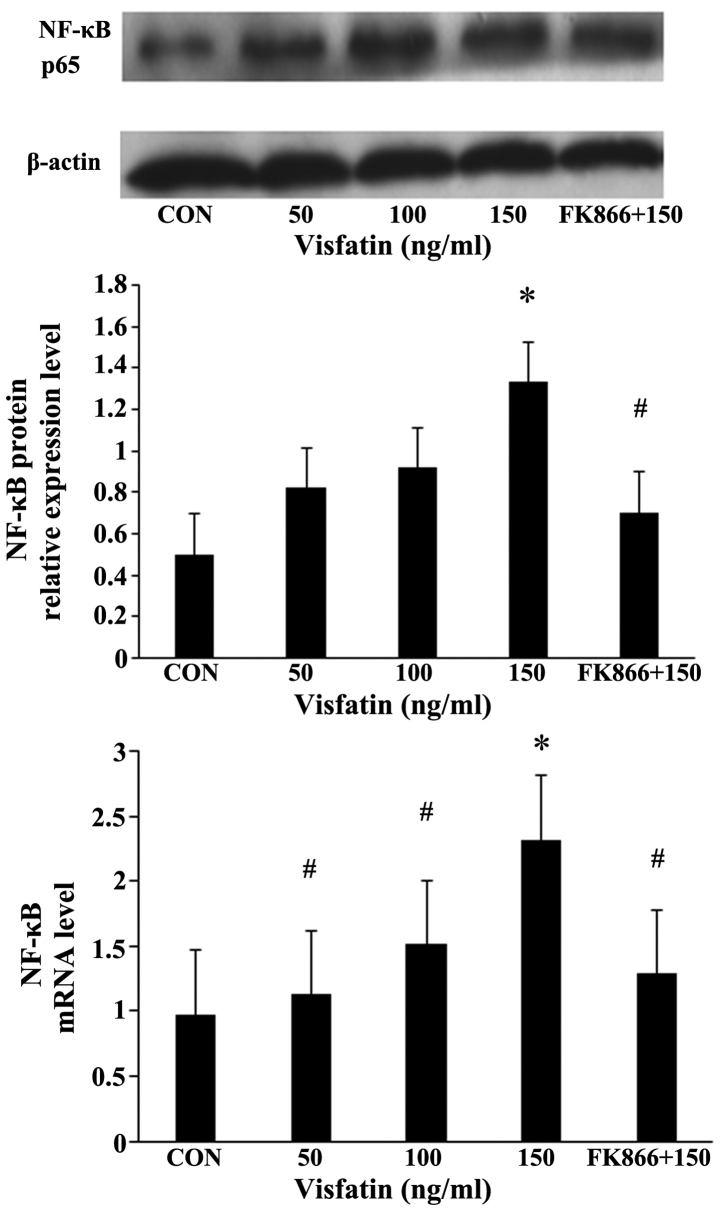
Compared with the EPCs not incubated with visfatin, the expression of NF-κB in the EPCs following treatment with various concentrations of visfatin increased in a significant and dose-dependent manner at both the mRNA and protein level, an effect that was inhibited by pre-treatment with FK866 (Fig. 7).
Figure 7.
Nuclear factor-κB (NF-κB) mediates visfatin-induced endothelial progenitor cell (EPC) apoptosis. Activation of NF-κB p65 in visfatin-treated EPCs. The expression of NF-κB was determined by RT-qPCR and western blot analysis. *P<0.05 vs. CON (no treatment); #P<0.05 vs. 150 ng/ml visfatin. Values represent the means ± standard deviation.
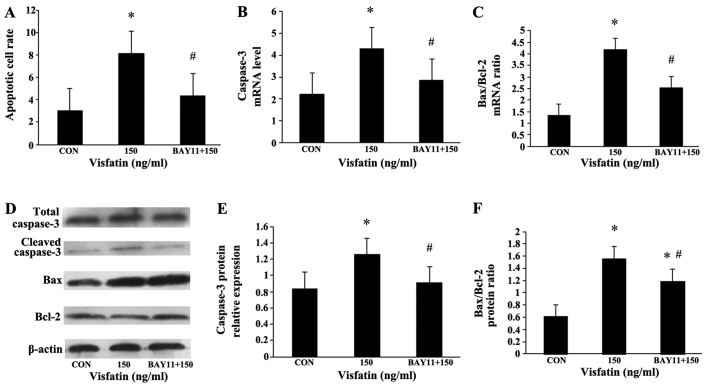
The EPCs were additionally pre-incubated with NF-κB inhibitor (BAY11-7085, 5 µM) for 1 h, and then treated with visfatin (150 ng/ml) for 48 h. Compared with the visfatin-alone-treated group, BAY11 significantly diminished the increase in EPC apoptosis induced by visfatin. In addition, the increased expression of caspase-3 and Bax, and the decreased expression of Bcl-2 were all abolished in the BAY11-treated cells (Fig. 8), suggesting that NF-κB may mediate visfatin-induced EPC apoptosis. Moreover, BAY11 also abolished the promoting effects of visfatin on the protein expression of ICAM-1 and IL-6 in the EPCs (Fig. 9), indicating that visfatin promotes EPC apoptosis by regulating ICAM-1 and IL-6 expression through NF-κB.
Figure 8.
Inhibition of endothelial progenitor cell (EPC) apoptosis and apoptosis-related proteins by BAY11-7085. EPCs were pre-incubated with BAY11 (5 µM) for 1 h, and then incubated with 150 ng/ml visfatin for 48 h. (A) Apoptotic cells were determined by flow cytometry. (B and C) mRNA expression levels of caspase-3 and Bax/Bcl-2 ratio in EPCs treated with visfatin in the presence or absence of BAY11. RT-qPCR was performed to determine the mRNA expression levels. (D-F) Effects of visfatin on the expression of apoptosis-related proteins, as determined by western blot analysis. Data are presented as a fold-change relative to β-actin. *P<0.05 vs. CON (no treatment); #P<0.05 vs. 150 ng/ml visfatin. Values represent the means ± standard deviation.
Figure 9.
Downregulation of intercellular adhesion molecule-1 (ICAM-1) and interleukin-6 (IL-6) protein expression in endothelial progenitor cells (EPCs) pre-treated with BAY11-7085, as determined by western blot analysis. *P<0.05 vs. CON (no treatment); #P<0.05 vs. 150 ng/ml visfatin. Values represent the means ± standard deviation.
Discussion
In the present study, we provide evidence that visfatin promotes the apoptosis of EPCs, as determined by flow cytometry, and that the pro-apototic effects were inhibited by pre-treatment with FK866, a visfatin inhibitor.
The number of EPCs in the blood circulation are regarded as an indicator of endothelial function and cardiovascular disease prognosis (20). Following vessel impairment and tissue ischemia, EPCs can originate from the bone marrow and home into ischemic locations where they participate in the re-endothelialization of impaired blood vessels (13). Therefore, EPCs play an important role in maintaining the completeness of endothelial structure and the normal function of the vascular endothelium. Several chronic factors have been implicated in mediating the apoptosis and dysfunction of EPCs, such as gluco-lipotoxicity, hyperglycemia, hyperlipidemia and oxidative stress (21). Apoptosis is a multi-step process and an increasing number of genes have been identified to be involved in the control or execution of apoptosis (22). There are two major pathways through which apoptosis occurs; the extrinsic pathway and the intrinsic pathway (23). Although these two pathways may act independently of each other, they converge at the level of caspase-3. As the most important member of the caspase-family, caspase-3 is responsible for many biochemical mechanisms driving apoptosis that lead to the cleavage of nuclear and cytosolic material, chromatin condensation, fragmentation of DNA, and disassembly into membrane-enclosed vesicles (24). Apoptosis is also regulated by members of the Bcl-2 family of genes (25), with the effect of the anti-apoptotic gene, Bcl-2, counteracted by the pro-apoptotic gene, Bax. Indeed, the balance between these opposing genes may be the key determinant of cell survival by regulating the apoptotic process, whereby an increase in the Bax/Bcl-2 ratio leads to the activation of caspase-3, and thus determines a cell's susceptibility to undergo apoptosis (25,26). Therefore, we detected the expression of apoptosis-related proteins in this study.
In this study, following treatment with visfatin, we detected decreased levels of the anti-apoptotic gene, Bcl-2, and increased expression levels of the pro-apoptotic gene, caspase-3, as well as an increased Bax/Bcl-2 ratio; all these effects were inhibited by FK866. This finding suggests that visfatin regulates the expression of Bax/Bcl-2/caspase-3 in the process of EPC death, which may explain, at least in part, the decreased number of EPCs.
Due to its role in inflammation, visfatin has been implicated in the pathogenesis of various metabolic disorders, such as metabolic syndrome, type 2 diabetes mellitus and obesity (6,7,27). Our previous studies also revealed that visfatin treatment impaired the migration and adhesion capacity of EPCs (19). A possible mechanism through which visfatin exerts these effects lies in the inflammation induced by visfatin, which may upregulate a series of inflammatory factors, leading to the apoptosis of EPCs and resulted in the reduction of their number and function (19). Inflammation in EPCs has also been linked to pro-inflammatory adhesion molecules, which can be inducers of leukocyte recruitment (28). Vascular inflammation is a common feature of vascular damage in a number of diseases and is a complex process that is initiated by activation of the immune system, leading to the increased expression of pro-inflammatory cytokines (29,30). Previous studies have shown that IL-6 is a potent pro-inflammatory cytokine involved in a number of pathological processes of vascular inflammation and injury, such as proliferative diabetic retinopathy and atherosclerosis, while IL-6 blockade can result in the prevention or therapeutic suppression of disease development (31–34). ICAM-1 is linked to the development of atherosclerosis and is well characterized for its roles in cell adhesion and actin polymerization processes (35,36). In addition, both IL-6 and ICAM-1 can be stimulated by the activation of the NF-κB signaling pathway (37,38).
In this study, we found that visfatin enhanced the protein expression of ICAM-1 and IL-6 in the EPCs, and that this effect was inhibited by pre-treatment with FK866 or BAY11-7085. These results suggest that in cultured EPCs, the synthesis of IL-6 and ICAM-1 is modulated by visfatin, and our data in agreement with those of a previous study, showing that visfatin enhances the expression of adhesion molecules (ICAM-1 and VCAM-1), as well as that of other inflammatory mediators through NF-κB activation in human endothelial cells (38). We also found that treatment with visfatin increased the expression of NF-κB in the EPCs in a dose-dependent manner at both the mRNA and protein level. Given the regulatory effects of visfatin on Bcl-2/Bax/caspase-3, the present data showing that pre-treatment with the inhibitors, FK866 or BAY11-7085, effectively suppressed visfatin-induced apoptosis, as well as ICAM-1 and IL-6 protein expression, support the notion that NF-κB in part mediates visfatin-induced apoptosis. Taken together, the present data suggest that visfatin is an influential upstream factor inducing inflammation and the apoptosis of EPCs, and that its activity results in decreased quantities of EPCs via the upregulation of NF-κB. These findings are in line with those of previous studies demonstrating the involvement of NF-κB in endothelial cell dysfunction and impaired angiogenesis (38,39).
It has previously been reported that FK866 attenuates inflammatory responses and cellular apoptosis following organ injury, ultimately leading to improved cell survival by inhibiting NF-κB activation (40). As FK866 is a specific inhibitor of visfatin, it is reasonable to speculate that visfatin induces apoptosis, at least in part, through NF-κB activation.
However, different cells show different responses following visfatin stimulation. Patel et al (41) demonstrated that the exogenous expression of visfatin in PC3 prostate cancer cells increased tumor cell proliferation and the expression of matrix metalloproteinase (MMP)-2/9 at the molecular level. Yang et al (42) generated stable cell lines from fibrosarcoma HT1080 and 293 cells which overexpress human visfatin protein and stable cell lines with visfatin knockdown using siRNA, and found that the stable visfatin-overexpressing cells were substantially more resistant to apoptosis induced by methylmethane sulfonate (MMS) than the control. FK866 also activates the apoptotic cascade in cancer cells with the release of cytochrome c and the activation of caspase-3 (43,44). In addition, differences in metabolism have been noted between normal cells and cancer cells (45). The different metabolic pathways that cancer cells utilize to survive and proliferate under stress environments (46) may be explained by the discrepancy between normal cells and cancer cells in response to visfatin and FK866.
Furthermore, the effect of visfatin seems also to rely on dosage and the state of cellular activation. On the one hand, circulating visfatin induces the cellular expression of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-8, IL-6 and MMP-9 by activating the p38-MAPK signaling pathway (47,48), in accordance with our data on EPCs, whereby IL-6 was prominently upregulated by visfatin. On the other hand, other studies have suggested that higher concentrations of visfatin result in the upregulation of anti-inflammatory mediators, such as IL-10 and IL-1 receptor antagonists (9,11). It is known that visfatin has dual functions in that it acts extracellularly as a pro-inflammatory cytokine and intracellularly as an enzyme catalyzing the rate-limiting step of the NAD salvage pathway from nicotinamide. Specifically, Rongvaux et al (49) demonstrated that visfatin, as an enzyme catalyzing the condensation of nicotinamide with phosphoribosyl pyro-phosphate, participated in cellular resistance to genotoxic or oxidative stress, and it conferred to cells of the immune system the ability to survive during stressful situations. In addition, visfatin enables proliferating human endothelial cells to resist the oxidative stress indcued by high glucose, and to productively utilize excess glucose to support replicative longevity and angiogenic activity via SIRT1 (50), while it also induces angiogenesis via the MAPK and PI3K/Akt signaling pathways (51). The reason for such discrepancies in the published findings surrounding visfatin is unclear. The doses and species used or exposure times are different among the studies and may have an effect that generates the different results.
To date, the mechanisms of action of visfatin as a cytokine remain unclear and numerous questions remain to be answered. However, our data indicate that visfatin induces EPC apoptosis by increasing the expression of pro-inflammatory mediators partly through the regulation of NF-κB. To fully understand the physiological machanism of visfatin and given the clear importance of this molecule to several physiological and pathological processes, further research is required.
Acknowledgments
This study was supported by grants from the Hebei Natural Science Foundation of China (no. H2014307035). We are indebted to Chao Wang, Hanying Xing, Zhihua Wang and Jingci Yang for assistance and technical advice.
Abbreviations
- EPCs
endothelial progenitor cells
- NF-κB
nuclear factor-κB
- ICAM-1
intercellular adhesion molecule-1
- IL-6
interleukin-6
- Nampt
nicotinamide phosphoribosyltransferase
- NAD
nicotinamide adenine dinucleotide
- VCAM-1
vascular cell adhesion molecule-1
- MNCs
mononuclear cells
- KDR
kinase insert domain receptor
- DiI-acLDL
DiI-labeled acetylated LDL
- FITC-Lectin-UEA-1
fluorescein isothiocyanate-conjugated lectin from Ulex europaeus agglutinin
- PVDF
polyvinylidene fluoride
- ECL
enhanced chemiluminescence
References
- 1.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/MCB.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garten A, Petzold S, Schuster S, Körner A, Kratzsch J, Kiess W. Nampt and its potential role in inflammation and type 2 diabetes. Handb Exp Pharmacol. 2011;203:147–164. doi: 10.1007/978-3-642-17214-4_7. [DOI] [PubMed] [Google Scholar]
- 3.Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou A, Tsanikidis H, Vitta I, Karkos C, Karayannacos PE, Gerasimidis T, Liapis CD. Visfatin (nampt) and ghrelin as novel markers of carotid atherosclerosis in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2010;118:75–80. doi: 10.1055/s-0029-1237360. [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G, Iorio M, Napoli N, Cotesta D, Zinnamosca L, Marinelli C, Petramala L, Minisola S, D'Erasmo E, Letizia C. Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J Endocrinol Invest. 2011;34:e12–e15. doi: 10.1007/BF03346703. [DOI] [PubMed] [Google Scholar]
- 5.Unlütürk U, Harmanci A, Yildiz BO, Bayraktar M. Dynamics of Nampt/visfatin and high molecular weight adiponectin in response to oral glucose load in obese and lean women. Clin Endocrinol (Oxf) 2010;72:469–474. doi: 10.1111/j.1365-2265.2009.03674.x. [DOI] [PubMed] [Google Scholar]
- 6.de Luis DA, Aller R, Gonzalez Sagrado M, Conde R, Izaola O, de la Fuente B. Serum visfatin levels and metabolic syndrome criteria in obese female subjects. Diabetes Metab Res Rev. 2013;29:576–581. doi: 10.1002/dmrr.2430. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Sun L, Gao H, Ren L, Liu N, Song G. Visfatin and oxidative stress influence endothelial progenitor cells in obese populations. Endocr Res. 2015;40:83–87. doi: 10.3109/07435800.2014.952016. [DOI] [PubMed] [Google Scholar]
- 8.Boini KM, Zhang C, Xia M, Han WQ, Brimson C, Poklis JL, Li PL. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim Biophys Acta. 2010;1801:1294–1304. doi: 10.1016/j.bbalip.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao K, Zou WH, Yang Z, Rehman Zia ur, Ansari AR, Yuan HR, Zhou Y, Cui L, Peng KM, Song H. The role of visfatin on the regulation of inflammation and apoptosis in the spleen of LPS-treated rats. Cell Tissue Res. 2015;359:605–618. doi: 10.1007/s00441-014-1997-3. [DOI] [PubMed] [Google Scholar]
- 10.van der Veer E, Nong Z, O'Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 11.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 12.Vallejo S, Romacho T, Angulo J, Villalobos LA, Cercas E, Leivas A, Bermejo E, Carraro R, Sánchez-Ferrer CF, Peiró C. Visfatin impairs endothelium-dependent relaxation in rat and human mesenteric microvessels through nicotinamide phosphoribosyltransferase activity. PLoS One. 2011;6:e27299. doi: 10.1371/journal.pone.0027299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 15.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 16.Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, McCune WJ, Kaplan MJ. Interferon-alpha promotes abnormal vasculogenesis in lupus: A potential pathway for premature atherosclerosis. Blood. 2007;110:2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26:2476–2482. doi: 10.1161/01.ATV.0000242794.65541.02. [DOI] [PubMed] [Google Scholar]
- 18.Tie G, Yan J, Yang Y, Park BD, Messina JA, Raffai RL, Nowicki PT, Messina LM. Oxidized low-density lipoprotein induces apoptosis in endothelial progenitor cells by inactivating the phosphoinositide 3-kinase/Akt pathway. J Vasc Res. 2010;47:519–530. doi: 10.1159/000313879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Chen S, Song G, Ren L, Wei L, Liu N, Zhang D, Lv X. Effect of visfatin on the function of endothelial progenitor cells in high-fat-fed obese rats and investigation of its mechanism of action. Int J Mol Med. 2012;30:622–628. doi: 10.3892/ijmm.2012.1032. [DOI] [PubMed] [Google Scholar]
- 20.Bakogiannis C, Tousoulis D, Androulakis E, Briasoulis A, Papageorgiou N, Vogiatzi G, Kampoli AM, Charakida M, Siasos G, Latsios G, et al. Circulating endothelial progenitor cells as biomarkers for prediction of cardiovascular outcomes. Curr Med Chem. 2012;19:2597–2604. doi: 10.2174/092986712800492995. [DOI] [PubMed] [Google Scholar]
- 21.Peng J, Liu B, Ma QL, Luo XJ. Dysfunctional endothelial progenitor cells in cardiovascular diseases: Role of NADPH oxidase. J Cardiovasc Pharmacol. 2015;65:80–87. doi: 10.1097/FJC.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 22.Joseph B, Marchetti P, Formstecher P, Kroemer G, Lewensohn R, Zhivotovsky B. Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment. Oncogene. 2002;21:65–77. doi: 10.1038/sj.onc.1205018. [DOI] [PubMed] [Google Scholar]
- 23.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 24.Salvesen GS, Dixit VM. Caspases: Intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/S0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 25.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59(Suppl 7):1693s–1700s. [PubMed] [Google Scholar]
- 26.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 27.Laudes M, Oberhauser F, Schulte DM, Freude S, Bilkovski R, Mauer J, Rappl G, Abken H, Hahn M, Schulz O, et al. Visfatin/PBEF/Nampt and resistin expressions in circulating blood monocytes are differentially related to obesity and type 2 diabetes in humans. Horm Metab Res. 2010;42:268–273. doi: 10.1055/s-0029-1243638. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Ingram DA, Murphy MP, Saadatzadeh MR, Mead LE, Prater DN, Rehman J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009;296:H1675–H1682. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30:65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- 30.Satofuka S, Ichihara A, Nagai N, Yamashiro K, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, et al. Suppression of ocular inflammation in endotoxin-induced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci. 2006;47:2686–2692. doi: 10.1167/iovs.05-1458. [DOI] [PubMed] [Google Scholar]
- 31.Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales' disease. Retina. 2008;28:817–824. doi: 10.1097/IAE.0b013e31816576d5. [DOI] [PubMed] [Google Scholar]
- 32.Tang JN, Shen DL, Liu CL, Wang XF, Zhang L, Xuan XX, Cui LL, Zhang JY. Plasma levels of C1q/TNF-related protein 1 and interleukin 6 in patients with acute coronary syndrome or stable angina pectoris. Am J Med Sci. 2015;349:130–136. doi: 10.1097/MAJ.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 33.Kitaba S, Murota H, Terao M, Azukizawa H, Terabe F, Shima Y, Fujimoto M, Tanaka T, Naka T, Kishimoto T, et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am J Pathol. 2012;180:165–176. doi: 10.1016/j.ajpath.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Okiyama N, Sugihara T, Iwakura Y, Yokozeki H, Miyasaka N, Kohsaka H. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A. Arthritis Rheum. 2009;60:2505–2512. doi: 10.1002/art.24689. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa K, Matsumoto M, Sasaki T, Hashimoto H, Kuwabara K, Ohtsuki T, Hori M. Involvement of ICAM-1 in the progression of atherosclerosis in APOE-knockout mice. Atherosclerosis. 2002;160:305–310. doi: 10.1016/S0021-9150(01)00587-1. [DOI] [PubMed] [Google Scholar]
- 36.Bielinski SJ, Pankow JS, Li N, Hsu FC, Adar SD, Jenny NS, Bowden DW, Wasserman BA, Arnett D. ICAM1 and VCAM1 polymorphisms, coronary artery calcium, and circulating levels of soluble ICAM-1: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;201:339–344. doi: 10.1016/j.atherosclerosis.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH, Koo TH, Jang HO, Yun I, Kim KW, Kwon YG, et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim Biophys Acta. 2008;1783:886–895. doi: 10.1016/j.bbamcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Wan R, Liu Y, Li L, Zhu C, Jin L, Li S. Urocortin increased endothelial ICAM1 by cPLA2-dependent NF-kappaB and PKA pathways in HUVECs. J Mol Endocrinol. 2013;52:43–53. doi: 10.1530/JME-13-0182. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda A, Yang WL, Jacob A, Aziz M, Matsuo S, Matsutani T, Uchida E, Wang P. FK866, a visfatin inhibitor, protects against acute lung injury after intestinal ischemia-reperfusion in mice via NF-κB pathway. Ann Surg. 2014;259:1007–1017. doi: 10.1097/SLA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 41.Patel ST, Mistry T, Brown JE, Digby JE, Adya R, Desai KM, Randeva HS. A novel role for the adipokine visfatin/pre-B cell colony-enhancing factor 1 in prostate carcinogenesis. Peptides. 2010;31:51–57. doi: 10.1016/j.peptides.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 44.Wosikowski K, Mattern K, Schemainda I, Hasmann M, Rattel B, Löser R. WK175, a novel antitumor agent, decreases the intracellular nicotinamide adenine dinucleotide concentration and induces the apoptotic cascade in human leukemia cells. Cancer Res. 2002;62:1057–1062. [PubMed] [Google Scholar]
- 45.Crabtree HG. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23:536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Tan BK, Chen J, Digby JE, Keay SD, Kennedy CR, Randeva HS. Increased visfatin messenger ribonucleic acid and protein levels in adipose tissue and adipocytes in women with polycystic ovary syndrome: Parallel increase in plasma visfatin. J Clin Endocrinol Metab. 2006;91:5022–5028. doi: 10.1210/jc.2006-0936. [DOI] [PubMed] [Google Scholar]
- 48.Kocelak P, Olszanecka-Glinianowicz M, Owczarek A, Bożentowicz-Wikarek M, Brzozowska A, Mossakowska M, Zdrojewski T, Grodzicki T, Więcek A, Chudek J. Plasma visfatin/nicotinamide phosphoribosyltransferase levels in hypertensive elderly - results from the PolSenior substudy. J Am Soc Hypertens. 2015;9:1–8. doi: 10.1016/j.jash.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Rongvaux A, Galli M, Denanglaire S, Van Gool F, Drèze PL, Szpirer C, Bureau F, Andris F, Leo O. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 50.Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell. 2009;8:100–112. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- 51.Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and I3K/Akt signalling pathways: Novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78:356–365. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]