This case-control study assesses the association of cytotoxic, anti-inflammatory, and immunomodulating agents with the risk for developing myeloid neoplasms among patients with autoimmune disease.
Key Points
Question
What is the risk for developing a therapy-related myeloid neoplasm after treatment of autoimmune disease?
Findings
In this multicenter, case-control study of 40 011 patients with primary autoimmune disease, 86 had a proven therapy-related myeloid neoplasm (acute myeloid leukemia or myelodysplastic syndrome). Detailed medical record review for all systemic exposures to cytotoxic and immunomodulating agents revealed only azathioprine sodium use more frequently in these cases.
Meaning
Azathioprine was the only agent associated with a significant risk for myeloid neoplasm.
Abstract
Importance
Therapy-related myeloid neoplasms are a potentially life-threatening consequence of treatment for autoimmune disease (AID) and an emerging clinical phenomenon.
Objective
To query the association of cytotoxic, anti-inflammatory, and immunomodulating agents to treat patients with AID with the risk for developing myeloid neoplasm.
Design, Setting, and Participants
This retrospective case-control study and medical record review included 40 011 patients with an International Classification of Diseases, Ninth Revision, coded diagnosis of primary AID who were seen at 2 centers from January 1, 2004, to December 31, 2014; of these, 311 patients had a concomitant coded diagnosis of myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). Eighty-six cases met strict inclusion criteria. A case-control match was performed at a 2:1 ratio.
Main Outcomes and Measures
Odds ratio (OR) assessment for AID-directed therapies.
Results
Among the 86 patients who met inclusion criteria (49 men [57%]; 37 women [43%]; mean [SD] age, 72.3 [15.6] years), 55 (64.0%) had MDS, 21 (24.4%) had de novo AML, and 10 (11.6%) had AML and a history of MDS. Rheumatoid arthritis (23 [26.7%]), psoriasis (18 [20.9%]), and systemic lupus erythematosus (12 [14.0%]) were the most common autoimmune profiles. Median time from onset of AID to diagnosis of myeloid neoplasm was 8 (interquartile range, 4-15) years. A total of 57 of 86 cases (66.3%) received a cytotoxic or an immunomodulating agent. In the comparison group of 172 controls (98 men [57.0%]; 74 women [43.0%]; mean [SD] age, 72.7 [13.8] years), 105 (61.0%) received either agent (P = .50). Azathioprine sodium use was observed more frequently in cases (odds ratio [OR], 7.05; 95% CI, 2.35- 21.13; P < .001). Notable but insignificant case cohort use among cytotoxic agents was found for exposure to cyclophosphamide (OR, 3.58; 95% CI, 0.91-14.11) followed by mitoxantrone hydrochloride (OR, 2.73; 95% CI, 0.23-33.0). Methotrexate sodium (OR, 0.60; 95% CI, 0.29-1.22), mercaptopurine (OR, 0.62; 95% CI, 0.15-2.53), and mycophenolate mofetil hydrochloride (OR, 0.66; 95% CI, 0.21-2.03) had favorable ORs that were not statistically significant. No significant association between a specific length of time of exposure to an agent and the drug’s category was observed.
Conclusions and Relevance
In a large population with primary AID, azathioprine exposure was associated with a 7-fold risk for myeloid neoplasm. The control and case cohorts had similar systemic exposures by agent category. No association was found for anti–tumor necrosis factor agents. Finally, no timeline was found for the association of drug exposure with the incidence in development of myeloid neoplasm.
Introduction
Therapy-related secondary myeloid neoplasms constitute a spectrum of diseases, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), which are serious consequences resulting from the transformation of a myeloid clone induced by cytotoxic therapy or ionizing radiation exposure. From 10% to 20% of patients diagnosed with myeloid neoplasms have a history of exposure to cytotoxic therapy and/or radiotherapy for treatment of an unrelated, primary malignant or benign disease, such as an autoimmune disease (AID). The basis of radiotherapy- or chemotherapy-related hematologic malignant disease is proposed to be a loss of DNA repair mechanisms and resulting genomic instability. The most common therapeutic agents implicated are alkylating compounds, antimetabolites, and topoisomerase inhibitors. Medical literature has highlighted therapy-related myeloid neoplasms after treatment of several nonmalignant diseases, especially inflammatory bowel disease and rheumatoid arthritis. Further confounding this risk for myeloid neoplasms is the nature of AID itself, which is characterized by an incessant, chronically activated inflammatory cascade that has been associated with an increased risk for myeloid malignant neoplasms alone.
A recent epidemiologic study by Wilson et al highlights a statistically significant but small increased risk odds ratio (OR) of 1.5 for MDS occurring with any AID compared with patients with no AID, especially in those with AID for 10 years or longer, with an adjusted OR of 2.1 for MDS. Anderson et al used the US Surveillance Epidemiology and End Results Medicare database and concluded that the existence of an autoimmune condition was associated with an increased risk for myeloid neoplasms. The addition of therapeutic agents to the treatment of AID theoretically amplifies the opportunity for a myeloid malignant neoplasm to develop in genetically susceptible individuals.
Previous studies identified a possible association between AID and myeloid neoplasms but have not systematically assessed or addressed the potential influence of therapies used in AID on the incidence and risk for myeloid neoplasms. Thus, the true influence and effect of treatment for AID in the development of myeloid neoplasms beyond the higher risk of AID itself is uncertain. Other questions arise, such as the risk associated with extended duration of drug exposure or the type of agents used and the background of AID. In particular, tumor necrosis factor (TNF) inhibitors used to treat autoimmune conditions have a documented risk for lymphoma, but the risk for secondary myeloid neoplasms is undefined. Autoimmune disease affects a large group of patients, and disease-modifying agents are increasingly prescribed for AID, especially the TNF inhibitors and/or antagonists. Characterization of risk can guide drug selection, affect safety assessments, and potentially permit individualized patient drug selection and monitoring during treatment. To our knowledge, no large studies have comprehensively evaluated a population with primary AID who developed myeloid neoplasms in association with a detailed therapeutic intervention for the underlying AID.
Methods
Study Aims
This study aimed to characterize the association of immunomodulating, cytotoxic, and other systemic agents for the treatment of AID with the risk for therapy-related MDS or AML. Secondary aims were to characterize the latency period from onset and treatment of AID to development of myeloid neoplasms. This protocol was approved by the institutional review board of the Mayo Clinic, which did not require informed consent for this retrospective review. Data were deidentified according to Health Insurance Portability and Accountability Act guidelines.
Study Design
All patients with an International Classification of Diseases, Ninth Revision (ICD-9)–coded diagnosis of AID seen at the Mayo Clinic campuses in Phoenix, Arizona, and Jacksonville, Florida, from January 1, 2004, to December 31, 2014, underwent assessment for a concurrent ICD-9 code for MDS or AML. The ICD-9 AID search terms included rheumatoid arthritis, primary biliary cirrhosis, autoimmune hepatitis, inflammatory bowel disease, Crohn disease, ulcerative colitis, Behçet disease, systemic lupus erythematosus (SLE), scleroderma, systemic sclerosis, dermatomyositis, polymyositis, polymyalgia rheumatica, Sjögren syndrome, pemphigus vulgaris, psoriasis, psoriatic arthritis, inflammatory arthritis, granulomatosis with polyangiitis, myasthenia gravis, polyarteritis nodosa, mixed connective tissue disease, CREST (calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome, juvenile rheumatoid arthritis, multiple sclerosis, and transverse myelitis. From this population of patients with concomitant coexisting diagnosis of MDS and/or AML and any AID, patient medical records were individually assessed. We confirmed 86 cases meeting strict inclusion criteria. Specifically, the case population required positive documentation of at least 1 of the 27 AID diagnoses (as above) with subsequent development of MDS or AML more than 1 month after an established diagnosis of AID. This criterion was used to ensure that no potential cases were missed and to reduce potential overlap with a diagnosis of a paraneoplastic phenomenon occurring in the acute care setting. We collected patient demographics, date of AID diagnosis, drug exposure with a detailed timeline (when available), and myeloid neoplasm disease characteristics, including cytogenetics, and outcomes. The classification of the myeloid neoplasms followed the 2008 World Health Organization classification of myeloid malignant neoplasms.
Exclusion Criteria
Patients with prior malignant disease or myeloproliferative diseases (including chronic myelomonocytic leukemia) were excluded, as were patients who received a chronologic diagnosis of myeloid neoplasm preceding the diagnosis of AID. Last, cases with an inadequate or an incomplete medical record for review or an inability to validate the above diagnoses were excluded from this study.
Cytogenetic Analysis
We performed Giemsa-banded karyotyping on bone marrow samples using conventional methods. When available, at least 20 metaphases were analyzed. Karyotypes of Giemsa-banded chromosomes were described according to the 2009 International System of Human Cytogenetic Nomenclature. Cytogenetic abnormalities associated with the individual myeloid neoplasms were subclassified following the guidelines of the 2008 World Health Organization classification of myeloid malignant neoplasms.
Controls and Matching
Cases were matched to controls (2 controls per case) by age, sex, and autoimmune-specific diagnosis from the primary population with AID. We used the Greedy algorithm, which minimized the weighted sum of the absolute differences in the matching variables between each case and all remaining possible controls.
Statistical Analysis
Cases were compared with controls using the χ2 test for frequency data. Likewise, exposure outcomes between cases and controls were compared using χ2 tests for trend. Unadjusted ORs with 95% CIs were constructed using conditional logistic regression for each agent and medication categories overall. A multivariate model was constructed in which agents with P < .20 were included. Use of a particular agent or category was considered if a patient had any documented exposure to the drug of interest. Multiple drug exposures were considered separate agents even if overlapping in exposure timeline. Patients with unknown exposure times were excluded from any analysis involving length of exposure outcomes. The study had 80% power (α = .05, 2-tailed) to detect a 10% or greater difference in drug agent exposure between cases and controls. Overall survival for cases was calculated as the time from AML or MDS diagnosis until the date of death or last follow-up. Surviving patients were considered censored at the time of the last follow-up. The distribution of survival time was estimated using the Kaplan-Meier method and compared between disease groups by log-rank test. We used SAS software (version 9.4; SAS Institute) for analysis.
Results
Patient Populations and Demographics
We identified a total of 40 011 patients with any of the 27 AID codes in the 10 years from 2004 to 2014. Of these, 311 patients carried a concurrent ICD-9 code for AID and MDS or AML. After detailed medical record validation and application of inclusion and exclusion criteria as described, 86 cases (49 men [57.0%]; 37 women [43.0%]; mean [SD] age, 72.3 [15.6] years) met strict eligibility criteria (Figure). Detailed medical records from the final control population of 172 patients (98 men [57.0%]; 74 women [43.0%]; mean [SD] age, 72.7 [13.8] years) matched 2:1 for 86 cases were reviewed.
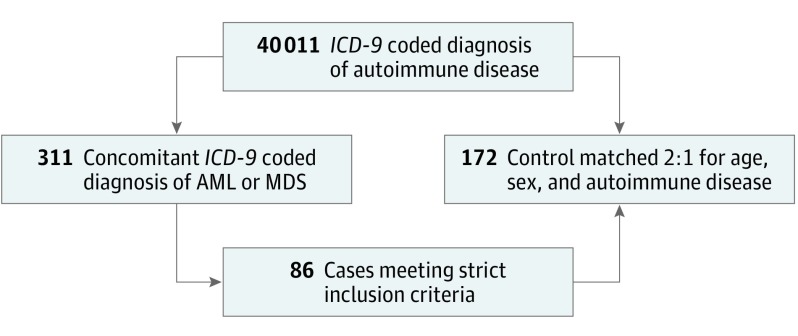
Figure. Flowchart Algorithm for Selection of Case and Control Populations.
Among 311 potential cases with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), we excluded 225 for the following reasons: 93 (41.3%) had no confirmed diagnosis of MDS or AML; 50 (22.2%) had no validated diagnosis of autoimmune disease (AID); 43 (19.1%) had an alternate hematologic diagnosis (ie, acute lymphocytic leukemia, essential thrombocytopenia, multiple primary neoplasms, non–Hodgkin lymphoma, monoclonal disorder); 15 (6.7%) had inadequate medical record information for review; 10 (4.4%) had MDS before development of AID; 7 (3.1%) had a history of solid malignant tumor; and 7 (3.1%) had paraneoplastic disorder. Paraneoplastic criteria were met if the diagnosis of MDS presented in the same 30-day period of autoimmune phenomenon. Among the total of 236 medical records reviewed for potential controls, 64 were excluded for the following reasons: 48 (75.0%) had no validated diagnosis of AID; 12 (18.8%) had inadequate information in the medical record for review; and 4 (6.3%) had a history of solid malignant tumor. ICD-9 indicates International Classification of Diseases, Ninth Revision.
Case cohort demographics (Table 1) highlight myeloid disease specifics of the 86 patients, including 55 (64.0%) with MDS, 21 with (24.4%) AML, and 10 with (11.6%) AML and a history of MDS. Per the Surveillance Epidemiology and End Results data, during the same 10-year period in a comparable population of 40 000, the anticipated incidence of myeloid disease was 60 to 80 cases of MDS and 44 to 60 cases of AML, which is not significantly different from our case population. Rheumatoid arthritis (23 [26.7%]), psoriasis (18 [20.9%]), and SLE (12 [14.0%]) were the most common autoimmune profiles, followed by the inflammatory bowel diseases Crohn disease (8 [9.3%]) and ulcerative colitis (6 [7.0%]). The median (interquartile range [IQR]) time of onset, from diagnosis of AID to diagnosis of myeloid neoplasm, was 8 (4-15) years (Table 1). With a median follow-up time of 21.6 (IQR, 8.6-61.3) months, 45 deaths occurred in the case cohort. Patients with AML and a history of MDS had the shortest median survival of 11.2 (95% CI, 8.2-63.2) months, followed by cases with de novo AML at 14 (95% CI, 1.6 to not reached) months and MDS at 86.8 (95% CI, 38.2-156.1) months (P = .004).
Table 1. Study Cohort Demographic Characteristics.
| Characteristic | No. (%) of Participants | |
|---|---|---|
| Cases (n = 86) |
Controls (n = 172) |
|
| Age, mean (SD), y | 72.3 (15.6) | 72.7 (13.8) |
| Sex, No. (%) | ||
| Male | 49 (57.0) | 98 (57.0) |
| Female | 37 (43.0) | 74 (43.0) |
| AID diagnosis (ICD-9 code) | ||
| Rheumatoid arthritis (714) | 23 (26.7) | 55 (32.0) |
| Psoriasis (696.1) | 18 (20.9) | 37 (21.5) |
| SLE (710) | 12 (14.0) | 23 (13.4) |
| Crohn disease (555.9) | 8 (9.3) | 15 (8.7) |
| Ulcerative colitis (556.9) | 6 (7.0) | 13 (7.6) |
| Multiple sclerosis (340) | 4 (4.7) | 8 (4.7) |
| Granulomatosis with polyangiitis (446.4) | 4 (4.7) | 8 (4.7) |
| Autoimmune hepatitis (571.42) | 1 (1.2) | 2 (1.2) |
| Psoriatic arthropathy (696) | 3 (3.5) | 4 (2.3) |
| Systemic sclerosis (710.1) | 2 (2.3) | 1 (0.6) |
| Dermatomyositis (710.3) | 1 (1.2) | 2 (1.2) |
| Other | 4 (4.7) | 4 (2.3) |
| MDS/AML diagnosis | ||
| MDS | 55 (64.0) | NA |
| AML | 21 (24.4) | NA |
| AML with history of MDS | 10 (11.6) | NA |
| Time from AID diagnosis, ya | ||
| 0-5 | 23 (26.7) | 29 (16.9) |
| 6-10 | 16 (18.6) | 10 (5.8) |
| 11-20 | 14 (16.3) | 25 (14.5) |
| ≥21 | 16 (18.6) | 19 (11.0) |
| Unknown | 17 (19.8) | 89 (51.7) |
| Median (IQR)b | 8.0 (4-15) | NA |
Abbreviations: AID, autoimmune disease; AML, acute myeloid leukemia; ICD-9, International Classification of Diseases, Ninth Revision; IQR, interquartile range; MDS, myelodysplastic syndrome; NA, not applicable; SLE, systemic lupus erythematosus.
Measured as time to last follow-up or death for the control cohort.
Includes 69 cases.
Cytogenetics
Cytogenetic findings of the 86 myeloid neoplasms (55 MDS, 21 de novo AML, and 10 AML with an antecedent MDS) are subclassified based on the guidelines of the 2008 World Health Organization classification of myeloid malignant neoplasms. Among the MDS group, 23 patients had a normal karyotype; 3, −7/deletion (del)(7q); 4, −5/del(5q); 1, −13/del(13q); 1, del(11q); 1, del(12p)/t(12p); 3, +8; 6, del(20q); 2, balanced chromosomal translocations; 4, complex abnormalities defined as 3 or more abnormalities; 5, noncomplex unclassifiable abnormalities; and 2, unknown cytogenetic results. Among the AML group, 10 patients had a normal karyotype; 4, recurrent cytogenetic abnormalities associated with AML, including 1 with t(8;21)(q22;q22), 1 with inversion(16)(p13.1q22), 1 with t(6;9)(p23;q34), and 1 with t(9;22)(q34;q11.2); 4, −7/del(7q); 1, −5/del(5q); 1, −7/del(7q); 1, −5/del(5q); 1, +8; 1, del(20q); 6, complex abnormalities; 1, noncomplex unclassifiable abnormalities; and 1, unknown cytogenetic results.
Systemic Treatment Exposure
Case and control populations who were treated with systemic therapies had similar exposures by agent category (Table 2), including cytotoxic, immunomodulation, nonsteroidal anti-inflammatory agents, anti-inflammatory agents, and corticosteroids. Fifty-seven of 86 cases (66.3%) received a cytotoxic or an immunomodulating agent. In comparison, 105 of 172 controls (61.0%) received either agent (P = .50). For most agents, we found a similar exposure for systemic agents in the case and control groups, including the anti-TNF drug class. Azathioprine sodium was the only agent for which statistically significant exposure differences were observed, and exposure was more frequent in the case cohort than in controls (OR, 7.05; 95% CI, 2.35- 21.13; P < .001). Cyclophosphamide (OR, 3.58; 95% CI, 0.91-14.11) was the second most common, although not reaching statistical significance (Table 2). In a multivariate model, azathioprine remained significant (OR, 6.92; 95% CI, 2.21-21.66) when controlling for cyclophosphamide, methotrexate sodium, and leflunomide use.
Table 2. Systemic Treatments and ORs.
| Treatment Type | No. (%) of Participants | Unadjusted OR (95% CI)a | P Valueb | |
|---|---|---|---|---|
| Cases (n = 86) |
Controls (n = 172) |
|||
| Cytotoxic | ||||
| Overall | 41 (47.7) | 77 (44.8) | 1.15 (0.65-2.04) | .63 |
| Azathioprine sodium | 19 (22.1) | 11 (6.4) | 7.05 (2.35-21.13) | <.001 |
| Cyclophosphamide | 10 (11.6) | 11 (6.4) | 3.58 (0.91-14.11) | .07 |
| Methotrexate sodium | 17 (19.8) | 46 (26.7) | 0.60 (0.29-1.22) | .16 |
| Mitoxantrone | 2 (2.3) | 2 (1.2) | 2.73 (0.23-33.0) | .43 |
| Mercaptopurine | 3 (3.5) | 9 (5.2) | 0.62 (0.15-2.53) | .51 |
| Mycophenolate mofetil hydrochloride | 5 (5.8) | 14 (8.1) | 0.66 (0.21-2.03) | .47 |
| Immunomodulating | ||||
| Overall | 29 (33.7) | 65 (37.8) | 0.78 (0.41-1.48) | .45 |
| Abatacept | 1 (1.2) | 0 | NA | .38 |
| TNF inhibitors | 13 (15.1) | 33 (19.2) | 0.71 (0.33-1.53) | .17 |
| Belimumab | 1 (1.2) | 0 | NA | .79 |
| Glatiramer acetate | 1 (1.2) | 1 (0.6) | NA | NA |
| Cyclosporine | 1 (1.2) | 0 | NA | NA |
| Lenalidomide | 0 | 1 (0.6) | NA | NA |
| Leflunomide | 3 (3.5) | 14 (8.1) | 0.41 (0.11-1.46) | NA |
| Hydroxychloroquine sulfate | 20 (23.3) | 38 (22.1) | 1.12 (0.50-2.48) | NA |
| Ustekinumab | 0 | 1 (0.6) | NA | NA |
| Corticosteroid | 35 (40.7) | 64 (37.2) | 1.22 (0.66-2.26) | .53 |
| Anti-inflammatory | ||||
| Overall | 5 (5.8) | 8 (4.7) | 1.13 (0.40-4.10) | .68 |
| Mesalamine | 5 (5.8) | 8 (4.7) | 1.13 (0.40-4.10) | .68 |
| Sulfasalazine | 2 (2.3) | 7 (4.1) | 0.57 (0.12-2.75) | .49 |
| NSAID | 7 (8.1) | 15 (8.7) | 0.92 (0.33-2.54) | .86 |
Abbreviations: NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug; OR, odds ratio.
Calculated according to individual exposures compared with controls. Conditional logistic regression was used owing to matching.
Calculated using the χ2 test.
Azathioprine Exposure in Cases and Control
Variable exposure to azathioprine occurred in 19 cases (Table 3) and 11 controls (Table 4). The most common disease treated with azathioprine was SLE in 7 of 19 cases. All cases with SLE were treated with azathioprine in combination with additional agents. A total of 6 of 19 cases (31.6%) were exposed to azathioprine as monotherapy, compared with 2 of 11 (18.2%) controls. Median duration of azathioprine exposure was 23.5 (IQR, 12-36) months in cases and 36 (IQR, 24-45) months in controls. In cases and controls, several patients had unknown documented exposure time to azathioprine. The median latency period (time from AID diagnosis to myeloid neoplasm diagnosis) in azathioprine-exposed cases was 8 (IQR, 3-25) years. A similar timeline was seen in nonazathioprine-exposed cases, with a median of 8 (IQR, 4-14.5) years. No significant cytogenetic pattern was identified.
Table 3. Details of All 19 Cases With Exposure to Azathioprine.
| Patient No./AID/Year | Azathioprine Monotherapya | Additional Therapies | Duration of Azathioprine Exposure | MDS Diagnosis | AML Diagnosis | CG Finding | MN Treatment | Last Follow-up or DOD | Outcome at Last Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1/RA/2009 | Yes | None | 23 mo | 2012 | NA | 46XX, −20q | Observation | 2014 | Stable |
| 2/SLE/1995 | No | Methotrexate sodium, cyclophosphamideb | 3 y | NA | 2008 | 46XY | BSC | 2009 | Deceased |
| 3/SLE/2009 | No | Hydroxychloroquine sulfate, anti-TNF | 11 mo | 2010 | 2011 | 46XX | Inductionc | 2011 | Deceased |
| 4/SLE/1980 | No | Hydroxychloroquine | 1 y | NA | 2011 | 46XX | Inductionc | 2012 | Deceased |
| 5/Crohn disease/2007 | No | Mesalamine | 1 dose | 2010 | NA | 46XY | BSC | 2014 | Stable |
| 6/SLE/2007 | No | Mycophenolate mofetil hydrochloride, methotrexate, hydroxychloroquine | 1 y | 2012 | NA | 46XX | Observation | 2014 | Stable |
| 7/Crohn disease/2000 | Yes | None | 7 y | 2008 | NA | 46XX, −7q | HSCT | 2012 | Relapse, deceased |
| 8/SLE/1987 | No | Mycophenolate mofetil | 2 y | NA | 2012 | 46XX | Inductionc | 2012 | Deceased |
| 9/AIH/2002 | Yes | None | 11 y | NA | 2013 | Complex | Azacitadine | 2013 | Deceased |
| 10/SLE/1978 | No | Hydroxychloroquine, cyclophosphamide, mycophenolate mofetil | 22 y | 2012 | NA | 46XX, −20q | Observation | 2014 | Stable |
| 11/UC/1980s | No | Sulfasalazine | Unknown | NA | 2013 | 46XY, −20q | HSCT | 2014 | Remission |
| 12/GP/2006 | No | Cyclophosphamideb | 19 mo | NA | 2007 | 46XY, −5q | Unknown | 2007 | Unknown |
| 13/SLE/1991 | No | Cyclophosphamide, methotrexate, hydroxychloroquine | Unknown | 2006 | NA | 46XY, +8 | BSC | 2008 | Deceased |
| 14/PBC/1996 | Yes | None | Unknown | 2010 | NA | Complex | Azacitadine | 2011 | Deceased |
| 15/PAN/2002 | Yes | None | 3 y | 2010 | NA | 46XY | Unknown | 2010 | Unknown |
| 16/Behçet syndrome/1980s | No | Cyclosporine, cyclophosphamide | Unknown | 2006 | NA | Unbalanced t(1;7) | BSC | 2008 | Deceased |
| 17/GP/2004 | No | Cyclophosphamide | 2 y | 2008 | 2013 | Complex | Decitabine | 2013 | Unknown |
| 18/GP/2005 | No | Methotrexate, cyclophosphamideb | 1 y | 2008 | NA | 46XX, +8, +9 |
None | 2009 | Unknown |
| 19/PMR/2010 | Yes | None | Unknown | 2011 | NA | 46XY, +13 | BSC | 2011 | Deceased |
Abbreviations: AID, autoimmune disease; AIH, autoimmune hepatitis; BSC, best supportive care; CG, cytogenetics; DOD, date of death; GP, granulomatosis with polyangiitis (formerly Wegener granulomatosis); HSCT, allogeneic hematopoietic stem cell transplant; MN, myeloid neoplasm; PAN, polyarteritis nodosa; PBC, primary biliary cirrhosis; PMR, polymyalgia rheumatica; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TNF, tumor necrosis factor; UC, ulcerative colitis.
Indicates no other systemic cytotoxic or immunomodulating exposures historically or in combination with azathioprine. Azathioprine was given as azathioprine sodium.
Combination regimen during same time period, otherwise sequential therapies.
Indicates standard 7 + 3 induction chemotherapy.
Table 4. Details of All 11 Control Participants With Exposure to Azathioprine.
| Participant No./AID/Year | Azathioprine Monotherapya | Additional Therapies | Duration of Azathioprine Exposure | Last Follow-up or DOD | Outcome at Last Follow-up |
|---|---|---|---|---|---|
| 1/GP/2000 | No | Cyclophosphamide | 8 y | 2010 | Alive |
| 2/RA/1990 | No | Methotrexate sodium, anti-TNF | Unknown | 2006 | Alive |
| 3/RA/1974 | No | Gold, hydroxychloroquine sulfate | 3 y | 2015 | Alive |
| 4/GP/2005 | No | Cyclophosphamide | Unknown | 2014 | Alive |
| 5/Psoriasis/2011 | No | Methotrexate | 2 mo | 2015 | Alive |
| 6/GP/2013 | No | Cyclophosphamide | Unknown | 2014 | Alive |
| 7/SLE/1994 | No | Mycophenolate mofetil hydrochloride, cyclophosphamide,b mesalamine | 3 y | 2004 | Alive |
| 8/Crohn disease/1997 | Yes | None | Unknown | 2014 | Alive |
| 9/AIH/2011 | Yes | None | 4 y | 2015 | Alive |
| 10/SLE/1978 | No | Hydroxychloroquine | Unknown | 2015 | Alive |
| 11/RA/2006 | No | Methotrexate, anti-TNF, leflunomide, rituximab | 1 y | 2009 | Alive |
Abbreviations: AID, autoimmune disease; AIH, autoimmune hepatitis; DOD, date of death; GP, granulomatosis with polyangiitis (formerly Wegener granulomatosis); RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TNF, tumor necrosis factor.
Indicates no other systemic cytotoxic or immunomodulating exposures historically or in combination with azathioprine. Azathioprine was given as azathioprine sodium.
Combination regimen during same time period, otherwise sequential therapies.
Exposure Time and Onset of MDS and AML
We analyzed exposure time in association with MDS or AML onset (eTable 1 in the Supplement). No significant association for duration of agent exposure by drug category was found. Exposure time for immunomodulating agents was similar between the cases and controls treated with systemic agents. Of note, a large portion of the control cohort was exposed to a systemic agent for several years without development of myeloid neoplasm. When we compared the cumulative exposures of cases and controls, both groups had similar exposure times to several systemic therapies in total (eTable 1 in the Supplement).
Autoimmune Therapies and Disease Association With MDS and AML
We found a slightly higher, but not statistically significant, incidence of AML vs MDS for azathioprine and hydroxychloroquine exposure. However, the opposite was seen for methotrexate, anti-TNF treatment, corticosteroid, and mesalamine exposures, with a slightly higher incidence of MDS vs AML (eTable 2 in the Supplement).
AID vs Hematologic Disease
Notable but not statistically significant trends were as follows: rheumatoid arthritis accounted for nearly one-third of the total population with MDS, and psoriasis followed by SLE represented the highest AID association for de novo AML (eTable 3 in the Supplement).
Discussion
The clinical consequence of medications used to treat AID and their risk for development of secondary malignant neoplasms is increasingly recognized. However, little is known regarding the risk and incidence of myeloid neoplasms in association with treatments used for AID. The pathogenesis of myeloid neoplasm in AID may be related to inflammatory cytokines chronically activating the myeloid hematopoetic progenitor compartment. The cytotoxic and immunomodulating therapies used to treat AID can be perpetual offenders as well, and in other disease entities these drugs can clearly contribute to the development of secondary myeloid neoplasms. Although studies have examined the risk for solid tumors and lymphomas, until now, to our knowledge, the risk and influence of drugs used in AID and their association with myeloid neoplasms has not been characterized in a comprehensive study of a population with primary AID that has validated diagnoses and a detailed therapeutic timeline of exposures compared with a control cohort. Furthermore, although several recent studies looked at the concurrent incidence of myeloid neoplasms or assessed a specific drug in a specific subtype of AID (ie, TNF antagonists in spondyloarthritis), to our knowledge no large-scale comprehensive study of a population with primary AID (ie, 27 disease entities) and the description of the development of myeloid neoplasms has been undertaken. Our data also linked total agent exposure with the onset of myeloid neoplasms after the diagnosis of AID. We chose this time criterion because the AID diagnosis must exist first and be under treatment to assess the potential influence of therapeutic interventions on the incidence of myeloid neoplasms. The time frame from the onset of AID to the development of myeloid neoplasms in our study (median, 8 years) was similar to that in the study by Kwong, which evaluated azathioprine treatment in a cohort with autoimmune disease; however, that study also included solid organ transplant recipients. Thus, our study is representative of a real-life population that first develops AID and subsequently myeloid neoplasms.
In our population with primary AID, only azathioprine exposure was associated with a statistically significant 7-fold risk for myeloid neoplasms (OR, 7.05; 95% CI, 2.35- 21.13; P < .001). This risk was also observed in multivariate analysis and was identical to the risk in a prospective cohort of patients undergoing thiopurine treatment in inflammatory bowel disease. Our study demonstrated notable, but not statistically significant, risks among the case cohort with exposure to cyclophosphamide (OR, 3.58; 95% CI, 0.91-14.11), followed by mitoxantrone (OR, 2.73; 95% CI, 0.23-33.00). Methotrexate, mercaptopurine, and mycophenolate mofetil had favorable ORs, but these were not statistically significant, possibly owing to an underpowered sample size, even in such a large study overall.
The data presented herein are supported by other studies in selected populations with AID, such as inflammatory bowel disease and rheumatoid arthritis only, in which azathioprine exposure was shown to be a risk factor for development of myeloid neoplastic disease. However, none of the previous studies examined and compared the variety of medications used in a large population with AID spectrum at this scale and with the development of myeloid neoplasms.
Among the proposed mechanisms of leukemic transformation in patients with AID are the preexisting dysfunctional immune response, chronic inflammation, and amplification of this risk by immunosuppressive and cytotoxic therapies used to treat the disease. The leukemogenic mechanism of antimetabolites, including azathioprine, is thought to occur by nonrepaired, DNA double-strand breaks that form highly mutagenic DNA bases. A genetic predisposition or inflammatory-mediated propensity for inefficient repair of drug-induced genetic damage may explain the exposure time to onset and susceptibility to the development of myeloid malignant neoplasms, as suggested in previous studies. In line with a repair defect, the exposure time from commencement of cytotoxic therapy to development of myeloid neoplasms occurred within 5 years for more than half the case cohort, much shorter than in the control population, in which approximately 35% had exposure of 5 years or less (eTable 1 in the Supplement). Alternatively, preexisting age-related, hematopoietic clones with p53 mutation have been identified in a high frequency among healthy elderly individuals who are naïve to chemotherapy. Therefore, these preexisting clones may be selectively promoted after treatment exposure, thus contributing to the development of therapy-related AML.
Elucidating and molecularly characterizing these mechanisms may provide clues to secondary myeloid pathogenesis, serve as potential biomarkers, and influence therapeutic decisions in the future. Theoretically, we speculate these leukemias may respond favorably to DNA and cell cycle checkpoint inhibitors, given that these drugs exploit underlying repair defects in AML cells. As a consequence, an area of future research should be exploration of underlying DNA damage repair defects, specifically mutations in such genes, that might enable transformation under (cytotoxic) drug treatment for AID.
In our study, the most common immunomodulating categories of exposures in both cohorts permitting OR assessment (Table 2) included hydroxychloroquine sulfate (OR, 1.12; 95% CI, 0.50-2.48), TNF inhibitors (OR, 0.71; 95% CI, 0.33-1.53), and leflunomide (OR, 0.41; 95% CI, 0.11-1.46). Hydroxychloroquine exposure was equal among both cohorts at approximately 23% and thus carried no association with development of therapy-related myeloid neoplasms. Leflunomide and TNF inhibitors had nonsignificant favorable OR exposure to outcome.
This study is, to our knowledge, the first to show that TNF inhibitors do not seem to have a higher risk of contributing to myeloid neoplasm formation in patients with AID. In fact, several smaller studies used anti-TNF agents to treat MDS, which showed a favorable result overall. This observation is very important because of concerns of TNF inhibitors and secondary malignant neoplasms; however, our data need further confirmation from other data sets.
Limitations
Limitations of our study are associated with its retrospective nature, possible underrepresentation of certain autoimmune populations, underpowered comparisons for individual rare diseases, lack of control for disease severity, and inability to calculate cumulative drug exposure. Potential cases of myeloid neoplasms may have been missed because our institution is a referral center. In addition, AID drug exposure data and a detailed timeline were not available in all cases. Small sample size (particularly for individual drug classification) also limits our interpretation, because many of these comparisons are underpowered, resulting in nonsignificant ORs and associated wide CIs. Nevertheless, to our knowledge this study is one of the largest, most comprehensive AID case-control studies performed among more than 40 000 patients with AID in a single case cohort analyzing a detailed drug exposure timeline with detailed therapeutic exposure in regards to the incidence of myeloid neoplasm.
Conclusions
Physicians who prescribe cytotoxic and immunomodulating therapies are mindful of risk-benefit evaluation, but obstacles to identifying patients at highest risk exist, including a variable latency period from the drug exposure to myeloid disease diagnosis, concomitant multiple-drug exposure, and scarcity of studies in the literature. Future mechanistic and genomic profiling studies may help to identify patients at risk. Results of the present study are expected to contribute to knowledge of therapy-related myeloid malignant neoplasms in patients treated for AID, suggesting that individualized drug selection and monitoring during treatment could become possible, especially for patients requiring treatment with azathioprine and cytotoxic agents. Tumor necrosis factor inhibitors did not show an increased incidence of myeloid neoplasms, which is an important observation. Although our results are intriguing, they should at this stage not change or replace the clinical judgments, monitoring, and current standard treatments or interventions in AID.
eTable 1. Exposure Time to Cytotoxic and Immunomodulating Agents
eTable 2. Systemic Autoimmune Therapies vs Hematologic Disease
eTable 3. Autoimmune Disease vs Hematologic Disease
References
- 1.Zhang L, Wang SA. A focused review of hematopoietic neoplasms occurring in the therapy-related setting. Int J Clin Exp Pathol. 2014;7(7):3512-3523. [PMC free article] [PubMed] [Google Scholar]
- 2.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5(12):943-955. [DOI] [PubMed] [Google Scholar]
- 3.Churpek JE, Larson RA. The evolving challenge of therapy-related myeloid neoplasms. Best Pract Res Clin Haematol. 2013;26(4):309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ornstein MC, Mukherjee S, Mohan S, et al. . Predictive factors for latency period and a prognostic model for survival in patients with therapy-related acute myeloid leukemia. Am J Hematol. 2014;89(2):168-173. [DOI] [PubMed] [Google Scholar]
- 5.Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol. 2007;137(6):513-529. [DOI] [PubMed] [Google Scholar]
- 6.Kwong YL, Au WY, Liang RH. Acute myeloid leukemia after azathioprine treatment for autoimmune diseases: association with −7/7q−. Cancer Genet Cytogenet. 1998;104(2):94-97. [DOI] [PubMed] [Google Scholar]
- 7.Lopez A, Mounier M, Bouvier AM, et al. ; CESAME Study Group . Increased risk of acute myeloid leukemias and myelodysplastic syndromes in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12(8):1324-1329. [DOI] [PubMed] [Google Scholar]
- 8.Ramadan SM, Fouad TM, Summa V, Hasan SKh, Lo-Coco F. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica. 2012;97(6):805-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong YL. Azathioprine: association with therapy-related myelodysplastic syndrome and acute myeloid leukemia. J Rheumatol. 2010;37(3):485-490. [DOI] [PubMed] [Google Scholar]
- 10.Bernatsky S, Clarke AE, Suissa S. Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis. Arch Intern Med. 2008;168(4):378-381. [DOI] [PubMed] [Google Scholar]
- 11.Jones M, Symmons D, Finn J, Wolfe F. Does exposure to immunosuppressive therapy increase the 10 year malignancy and mortality risks in rheumatoid arthritis? a matched cohort study. Br J Rheumatol. 1996;35(8):738-745. [DOI] [PubMed] [Google Scholar]
- 12.Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson AB, Neogi T, Prout M, Jick S. Relative risk of myelodysplastic syndromes in patients with autoimmune disorders in the General Practice Research Database. Cancer Epidemiol. 2014;38(5):544-549. [DOI] [PubMed] [Google Scholar]
- 14.Kristinsson SY, Björkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-α inhibitor therapy. Eur J Gastroenterol Hepatol. 2011;23(12):1150-1156. [DOI] [PubMed] [Google Scholar]
- 16.Mariette X, Tubach F, Bagheri H, et al. . Lymphoma in patients treated with anti-TNF. Ann Rheum Dis. 2010;69(2):400-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geborek P, Bladström A, Turesson C, et al. . Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis. 2005;64(5):699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swerdlow SH; International Agency for Research on Cancer . World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 19.Shaffer LG, Slovac ML, Campbell LJ. An International System for Human Cytogenetic Nomenclature: Recommendations of the International Standing Committee on Human Cytogenetic Nomenclature. Basel, Switzerland: Karger AG; 2009. [Google Scholar]
- 20.Faries D, Leon AC, Haro JM, Obenchain RL. Analysis of Observational Health Care Data Using SAS. Cary, NC: SAS Institute Inc; 2010. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 22.Braun T, Fenaux P. Myelodysplastic syndromes (MDS) and autoimmune disorders (AD). Best Pract Res Clin Haematol. 2013;26(4):327-336. [DOI] [PubMed] [Google Scholar]
- 23.Komrokji RS, Kulasekararaj A, Al Ali NH, et al. . Autoimmune diseases and myelodysplastic syndromes. Am J Hematol. 2016;91(5):E280-E283. [DOI] [PubMed] [Google Scholar]
- 24.Mekinian A, Grignano E, Braun T, et al. . Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia. Rheumatology (Oxford). 2016;55(2):291-300. [DOI] [PubMed] [Google Scholar]
- 25.Hellgren K, Dreyer L, Arkema EV, et al. ; ARTIS Study Group, For the DANBIO Study Group . Cancer risk in patients with spondyloarthritis treated with TNF inhibitors. Ann Rheum Dis. 2016;76(1):105-111. [DOI] [PubMed] [Google Scholar]
- 26.Sill H, Olipitz W, Zebisch A, Schulz E, Wölfler A. Therapy-related myeloid neoplasms. Br J Pharmacol. 2011;162(4):792-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong TN, Ramsingh G, Young AL, et al. . The role of TP53 mutations in the origin and evolution of therapy-related AML. Nature. 2015;518(7540)552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhuri L, Vincelette ND, Koh BD, et al. . CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo. Haematologica. 2014;99(4):688-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibes R, Bogenberger JM, Chaudhuri L, et al. . RNAi screening of the kinome with cytarabine in leukemias. Blood. 2012;119(12):2863-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott BL, Ramakrishnan A, Storer B, et al. . Prolonged responses in patients with MDS and CMML treated with azacitidine and etanercept. Br J Haematol. 2010;148(6):944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott BL, Ramakrishnan A, Fosdal M, et al. . Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149(5):706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeg HJ, Jiang PY, Holmberg LA, Scott B, Petersdorf EW, Appelbaum FR. Hematologic responses of patients with MDS to antithymocyte globulin plus etanercept correlate with improved flow scores of marrow cells. Leuk Res. 2004;28(11):1177-1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Exposure Time to Cytotoxic and Immunomodulating Agents
eTable 2. Systemic Autoimmune Therapies vs Hematologic Disease
eTable 3. Autoimmune Disease vs Hematologic Disease