Abstract
We produced XX↔XY chimeras by using embryos whose X chromosomes were tagged with EGFP (X*), making the fluorescent green female (XX*) germ cells easily distinguishable from their nonfluorescent male (XY) counterparts. Taking advantage of tagging with EGFP, the XX* “prospermatogonia” were isolated from the testes, and the status of their genomic imprinting was examined. It was shown that these XX cells underwent a paternal imprinting, despite their chromosomal constitution. As previously indicated in sex-reversal XXsxr testes, we also found a few green XX* germ cells developed as “eggs” within the seminiferous tubules of XX*↔XY chimeric testes. These cells were indistinguishable from XX* prospermatogonia at birth but resumed oogenesis in a testicular environment. The biological nature of the “testicular eggs” was examined by recovering the eggs from chimeric testes. The testicular eggs not only formed an egg-specific structure, the zona pellucida, but also were able to fuse with sperm. The collected testicular eggs were indicated to undergo maternal imprinting, despite the testicular environment. The genomic imprinting did not always follow the environmental conditions of where the germ cells resided; rather, it was defined by the sex that was chosen by the germ cells at early embryonic stage.
Keywords: sex differentiation, XX prospermatogonia, EGFP, XX↔XY chimera, genomic imprinting
To reproduce, mammals must develop as either males or females. In general, sex is determined by the sex chromosomal complement at the time of fertilization; i.e., the presence of a Y chromosome confers “maleness.” However, the mammalian gonads are reported to arise as a bipotential primordium with the plasticity to develop into an ovary or a testis (1). In the process of testicular differentiation, Sertoli cells that express Sry are considered to play an important role. For example, if Sry expression were delayed and/or diminished, the resultant animals would show sex reversal (2–4). Conversely, if exogenously integrated Sry is expressed in XX mouse gonads, they develop into male testes (5).
Germ cells also have the plasticity to develop as either oogonia or “prospermatogonia.” If XX primordial germ cells are sequestered in the testicular cord, they are reported to develop as prospermatogonia from their arrested mitosis and prominent nucleoli structures (6–8).
Although mouse primordial germ cells are dimorphic, the fate of “XX prospermatogonia” in the testis after birth is different from that of XY prospermatogonia. All XX prospermatogonia die within the first few days postpartum (dpp), whereas the XY prospermatogonia proliferate and begin spermatogenesis (7, 9, 10). This difference might be due to the absence of Y-linked spermatogenesis genes in XX cells (11, 12). However, it is known that Y chromosomes bearing XXY spermatogonia also disappear from the testis (13, 14). Thus, a precise mechanism of disappearance of XX prospermatogonia before differentiation is yet to be elucidated.
A useful indicator for sex differentiation in germ-line cells could be the establishment of genomic imprinting during gametogenesis (15). After removal of the imprinting during the primordial germ-cell stage, new imprints are imposed in prospermatogonia before they enter meiosis (16, 17). In contrast, nongrowing primary oocytes, such as those in newborn mice, have not established differential methylation in several differentially methylated regions (DMRs). In oocytes, new imprints are imposed later at different stages of oogenesis for different genes, from very early to the antral follicle stage (18, 19).
By tagging the sex chromosomes with a ubiquitously expressed EGFP transgene (20, 21), we can determine the sex of the preimplantation embryos noninvasively (22). Moreover, if the EGFP-tagged (hereafter designated with an *) embryos then are used to make chimeras, we can visualize the contribution of XX* cells by green fluorescence.
XX somatic and germinal lineages undergo random X-chromosome inactivation together; during gastrulation (23), the inactive X chromosome in “XX germ cells” then undergoes reactivation around the time of entry into the genital ridges, whether the embryo is female (24) or male (25). These findings have been confirmed by Tam and colleagues (26, 27) and extended by studies on Xist expression. Therefore, it may not be possible to trace all of the XX* cells in somatic tissue because one of the X chromosomes is silenced by X inactivation (28), but germ-line cells can be traced because X inactivation does not take place in germ-line cells in the genital ridge (26, 27). One of the advantages of using EGFP-tagged cells is the easy identification of cells, even when they are sparsely distributed (29). In the present study, we recovered the XX* germ-line cells from the XX*↔XY chimeric testes to examine the elasticity of germ-cell sex in molecular bases.
Materials and Methods
Animals. The handling and surgical manipulation of all experimental animals were carried out according to the guidelines of the Committee on the Use of Live Animals in Teaching and Research of Osaka University. We produced six mouse lines whose X chromosomes contain a transgene consisting of EGFP expressed from a CAG promoter (combination of a β-actin promoter and a human cytomegalovirus enhancer) (20). For the experiments presented in this report, we used two transgenic lines of X-linked EGFP [B6C3F1 TgN (act EGFP) Osb CX-50 (no. 50) and B6C3F1 TgN (act EGFP) Osb CX-139 (no. 139) (20)]. When we used the former line, we detected the contribution of all cells possessing XX* chromosomes, because the EGFP fluorescence is equally bright in germ-line and somatic cells. With the latter line, the germ cells could be separated by FACS, based on the difference of EGFP fluorescence in germline (bright) and somatic (faint) cells.
Production of XX*↔XY Chimera. Aggregation chimeras were produced as described in ref. 30. Briefly, superovulated (B6C3F1) females (XX) were mated with males whose X chromosome was tagged with EGFP (X*Y). Two- or four-cell-stage embryos were collected and placed in K+-modified simplex optimized medium (31), covered with mineral oil, and incubated overnight at 37°C in 95% air/5% CO2. Male (EGFP-negative) and female (EGFP-positive) embryos were separated at the eight-cell and early morula stage by using a fluorescent microscope (IX-70 with U-MWIBA filter set, Olympus, Melville, NY) micromanipulator. After removing the zona pellucida with acidic Tyrode's solution (Sigma), male and female embryos were paired in aggregation wells and incubated overnight at 37°C in 95% air/5% CO2. XX*↔XY chimeric embryos were then transferred into the uterus of 2.5-days postcoitum (dpc) pseudopregnant recipients.
Preparation of Testicular and Ovarian Germ Cells. Newborn (0-dpp) germ cells were prepared (32). The testes (20–30) of newborn males and XX*↔XY chimera males were incubated in 1 mg/ml collagenase (type I, Sigma) in DMEM buffered with 20 mM Hepes (pH 7.4) at 32°C for 15 min. After pipeting to separate the seminiferous tubules, the tubules were washed in PBS (–) and incubated with 0.25% trypsin in PBS (–) supplemented with 1 mM EDTA at 32°C for 10 min. Single cells were obtained by pipetting, filtering through a nylon mesh, and centrifuging at 700 × g for 5 min at 4°C. Ovarian cells were prepared by incubating ovaries at 37°C for 15 min after mincing them in 1 mM EDTA in PBS (–) followed by pipetting. The freed cells were filtered through nylon mesh, centrifuged, and resuspended in Hepes-buffered saline solution containing 0.1% BSA.
Both male and female cells were sorted by using a FACSVantage cell sorter (Becton Dickinson). “Testicular eggs” were recovered from chimeric male testes at 1–3 weeks of age. After removing the tunica albuginea, the seminiferous tubules were spread out by gently pulling a part of the tubules under a fluorescent microscope. The tubule sections containing testicular eggs were cut with Noyes spring scissors. While holding one end with Dumont no. 5 tweezers, the contents of the tubes were squeezed out by gently pinching and sliding with supplemental tweezers. The testicular eggs were then collected by using a finely drawn pipette.
For further details about experimental materials and methods, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Results
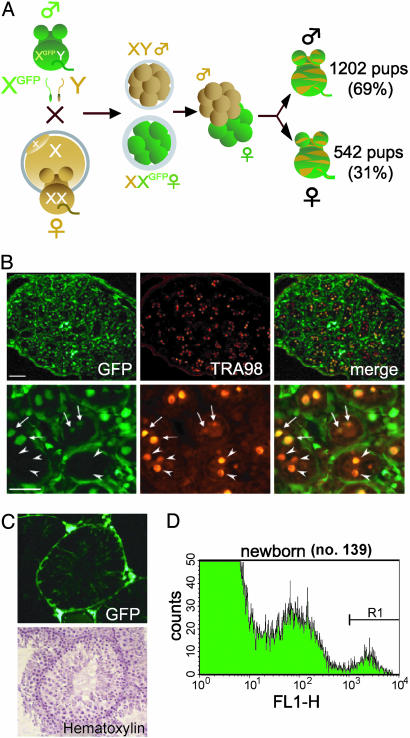
Production of XX*↔XY Chimera by Using EGFP-Tagged X Chromosomes and XX* Derived Cells in Testes. Males with an X-linked EGFP (X*) transgene (20) were bred with wild-type females. Eggs fertilized by X* sperm (female eggs) showed EGFP fluorescence at about the eight-cell stage. After separating male and female embryos based on EGFP fluorescence, we made 4,579 presexed XX*↔XY aggregation chimeric embryos, transplanted them to pseudopregnant females, and obtained 1,744 pups (Fig. 1A). Among the pups, 1,202 were born as males (69%) and 542 as females (31%), defined by their external genital reproductive tract anatomy. Gonadal hermaphroditism was present in 6.1% and 4.8% of grossly phenotypic males and females, respectively.
Fig. 1.
XX*↔XY chimeras containing XX* cells in their testes. (A) Strategy of producing XX*↔XY chimera. Males containing the EGFP transgene on the X chromosome were bred with wild-type females. The male and female embryos were separated, and XX*↔XY chimera embryos were made by aggregation. These embryos were transferred to pseudopregnant females. (B) A testicular section from a newborn XX*↔XY chimera (no. 50). (Upper Left) EGFP-positive XX cells. (Scale bar: 50 μm.) (Upper Center) Immunolabeling (red) for TRA98, a germ cell-specific antigen. (Lower) Higher magnification showing XX* cells (arrows) and XY germ cells (arrowheads) in seminiferous tubules. (Scale bar: 50 μm.) (C) Testicular section from a 5-week-old sexually mature XX*↔XY chimera (no. 50). XX* Sertoli cells are present (Upper); however, XX* spermatogenic cells are absent. (D) Flow cytometric analysis of newborn testicular cells from the no. 139 mouse line. The forward-scatter and side-scatter dot-plot gated fraction was shown to divide into three peaks (negative, medium, and bright) in which the brightest peak consisted of >98% germ cells, proven by TRA98 staining (see Results).
Tagging of the X chromosome by the ubiquitously expressed EGFP transgene allowed us to trace XX* cells residing in testes. Numerous TRA98-positive green (XX*) cells inside the seminiferous tubules were present at birth, indicative of germ-line cells (Fig. 1B). However, at 5 weeks of age, no XX* spermatogenic cells were found in testes observed, despite the presence of XX* Sertoli cells (based on their characteristic shape) inside the tubule and somatic cells in the interstitium in >25 independent observations (Fig. 1C).
Retrieval of XX* Germ Cells from XX*↔XY Chimeric Mice. To clarify the nature of XX* germ cells residing inside the seminiferous tubules in XX*↔XY chimera, we isolated germ cells tagged with X-EGFP by using a FACS. More than 30 chimeric testes were prepared, combined, and subjected to FACS analysis. The experiment was repeated on three different occasions, and the results were similar in all cases. EGFP-positive cells from XX↔XY chimeras using the no. 139 line were quantified as depicted in Fig. 1D. The brightest peak indicated by R1 was corrected as a germ-cell fraction after removing bright somatic cells by gating in a forward-scatter and side-scatter plot. To examine the purity of germ cells in the sorted fraction, some of these cells were attached to glass slides and immunostained with TRA98. Germ cells from newborn testes (and also germ cells collected from newborn ovaries) were successfully purified by this procedure, because 98% of thus prepared EGFP-positive cells were found to be TRA 98-positive (data not shown).
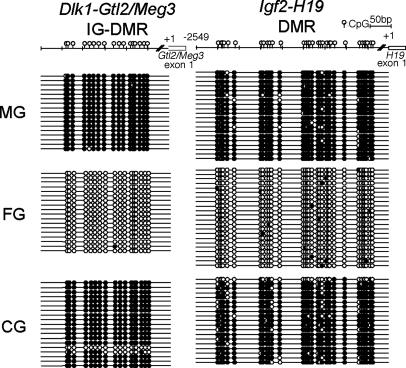
Differentiation of XX* Prospermatogonia in Chimeric Testes. To determine whether XX* germ cells recovered from testes were differentiated as prospermatogonia, the XX* germ cells were released from the seminiferous tubules and sorted by using a FACS and subjected to cell cycle analysis after staining with propidium iodide. The recovered XX* germ cells, confirmed by TRA98 staining, were 2n, as would be found in male germ cells from newborn wild-type mice (data not shown). We then analyzed the status of genomic imprinting in these cells. DMRs of Dlk1-Gtl2/Meg3 and Igf2-H19 were methylated in recovered XX* germ cells in a similar manner to XY germ cells in wild-type males (Fig. 2). The region analyzed for H19 includes 500 base pairs of the imprinting control region, which is shown to be involved in the establishment of imprinting at this locus (17, 33).
Fig. 2.
Characterization of germ cells in testes of newborn XX*↔XY chimera (no. 139). DMR methylation of paternal methylated genes Dlk1-Gtl2/Meg3 and Igf2-H19 in newborn germ cells, analyzed by bisulfite genomic sequencing. Filled ovals indicate methylated CpGs, and open ovals indicate unmethylated CpGs. As expected, DMRs of spermatogonia (MG) showed hypermethylation, and oocytes (FG) showed hypomethylation. XX spermatogonia (CG) showed hypermethylation.
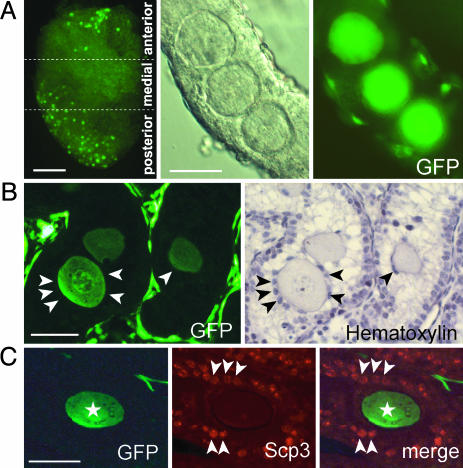
Testicular Eggs Found in 1- to 4-Week-Old Chimeric Testes. We never found large cells in the XX*↔XY chimeric testis at birth. However, starting at 1 week of age, up to 100 large cells could be found inside the seminiferous tubules [19 of 46 (41%), 3 of 23 (13%), and 16 of 74 (22%) chimeric testes were found to possess large cells observed at 1, 2, and 3 weeks after birth, respectively] (Fig. 3A).
Fig. 3.
Testicular eggs in seminiferous tubules. (A)(Left) Large cells in testis indicated as XX* by EGFP fluorescence (no. 139). (Center and Right) Higher magnification of three EGFP-expressing cells in a separated seminiferous tubule. (B) A testicular section from a 7-dpp XX*↔XY chimera. Granulosa-like cells, which surround testicular eggs, are indicated by arrowheads. (C) A testicular section of XX*↔XY chimera at 17 dpp (no. 139). GFP-positive testicular eggs are indicated by asterisks, and GFP-negative, SCP3-positive spermatocytes are marked by arrowheads. (Scale bars: A Left, 500 μm; A Center, B, and C, 50 μm.)
Although somewhat smaller than normal ovarian eggs, these cells reached 50 μm in diameter by 3 weeks, and we called them testicular eggs (Fig. 3). The testicular eggs were found mainly in the anterior (323 eggs) and posterior (142 eggs) parts of the testis, as opposed to the medial area (77 eggs) (Fig. 3A). (Eleven chimeric testes were examined.)
Most of the testicular eggs were surrounded by a few granulosa-like cells, but we never found the follicle-like structures seen inside the seminiferous tubules (Fig. 3B). There were XY (nongreen) spermatogonia near the testicular eggs, indicating the existence of normal Sertoli cells in the vicinity of the testicular eggs (Fig. 3C). It should also be noted that we could not find XY (nongreen) testicular eggs.
In some cases, testes were exposed by operation to allow observation under a dissecting fluorescent microscope. After confirmation of the existence of testicular eggs by surface examination of testes, we replaced the testes into the scrotum and sutured, and the mice were kept until they were 9–20 weeks of age. Sperm from five chimeric testes that possessed testicular eggs were subjected to in vitro fertilization and were found to have normal fertilizing ability (data not shown).
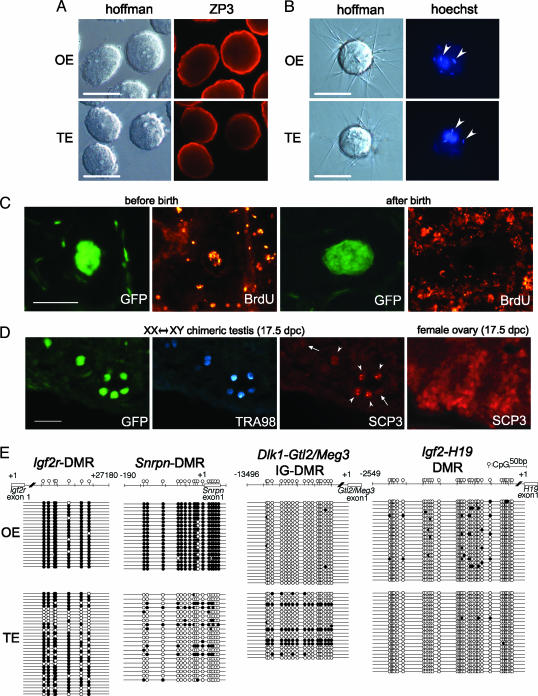
When testicular eggs were immunostained with an anti-oocyte-specific protein (ZP3) that surrounds the oocytes, they were shown to have zona pellucida structures (Fig. 4A). Testicular eggs were inseminated with sperm after removing the zona pellucida. Similar to ovarian eggs, the testicular eggs showed the ability to fuse with sperm at as early as 1 week of age (Fig. 4B).
Fig. 4.
Characterization of testicular eggs in chimeric testes. (A) Immunofluorescence staining of ZP3 in zona pellucida of testicular eggs in 1-week-old chimeric testes and oocytes in “normal” ovaries. (B) Sperm were added to eggs preloaded with Hoechst 33342. Fused sperm (arrowheads) are stained with Hoechst because of dye transfer from the eggs. (C) BrdUrd staining to detect onset of meiosis in testicular eggs. In two Left panels, BrdUrd was injected at 12.5 dpc, and the testicular eggs were recovered at 1 week after birth. In two Right panels, BrdUrd was injected every day from 0 to 5 dpp, and the testicular eggs were recovered at 6 dpp. (D) SCP3-positive cells in 17.5-dpc chimeric testis. EGFP indicates the XX* cells. TRA98 staining (with 7-amino-4-methylcoumarin-3-acetic acid) shows germ cells. SCP3 staining (with tetramethylrhodamine isothiocyanate) shows synaptonemal complexes. Note some XX germ cells (EGFP and TRA98 double positive) also contained SCP3 (arrowheads), whereas others did not (arrows). SCP3 staining in 17.5-dpc ovarian eggs is shown for comparison. (Scale bars: 50 μm.) (E) DMR methylation in maternally methylated (Igf2r and Snrpn) and paternally methylated (Dlk1-Gtl2/Meg3 and Igf2-H19) genes in 3-week-old testicular eggs. Methylation of Igf2r-DMR, but not Snrpn-DMR, was similar in testicular eggs and ovarian eggs. The paternal methylated gene was almost completely nonmethylated in testicular eggs, similar to ovarian eggs.
However, the fertilized eggs derived from germinal vesicle (GV)-stage testicular eggs were not able to initiate development as in the case of normal GV-stage oocytes (data not shown). To detect DNA synthesis just before meiosis, BrdUrd was repeatedly injected into newborn pups from 0 to 5 dpp. However, no BrdUrd incorporation was detected in testicular eggs, although it was shown in other cell types (Fig. 4C). This lack of incorporation presumably occurs because, at birth, the testicular eggs are already 4n. BrdUrd also was injected into recipient mothers at 12.5 dpc, and the pups were analyzed at 1 week of age. Testicular eggs were brightly stained with the anti-BrdUrd antibody; male germ cells were negative because the label was diluted in prospermatogonia-differentiated cells as the result of further divisions between the time of labeling and the time of study (Fig. 4C). These results suggest that the testicular eggs begin meiosis at a similar time as normal eggs. Furthermore, some XX* germ cells were found to express the primary meiotic marker synaptonemal complex protein 3 (SCP3) at 17.5 dpc (Fig. 4D).
We manually collected >1,000 testicular eggs from chimeric testes 3 weeks after birth for an analysis of genomic imprinting. In contrast to XX spermatogonia, testicular eggs did not show paternal imprinting of Dlk-Glt2/Meg3 and Igf2-H19. Instead, Igf2r was heavily methylated, as in normal eggs. Snrpn was found to remain unmethylated (Fig. 4E), whereas it normally becomes methylated in ovarian oocytes, suggesting that the maturation (or development) of testicular eggs is delayed. These testicular eggs remained in the testis until 4 weeks of age and then disappeared. However, the cause of this disappearance is not known.
Discussion
Sex Determination of XX Germ Cells in the Testes. A potential explanation of the formation of testicular eggs is that they are developing in regions where the masculinizing effect of XY somatic cells is being diluted by the existence of XX somatic cells. In general, germ cells are sequestered inside testis cords by 12.5 dpc, and if the sequestration is not completed, the germ cells spontaneously enter meiosis and differentiate into oocytes. Menke et al. (34) reported that Stra8, a premeiotic marker, began to be expressed from 12.5 dpc only in XX germ cells, whereas Adams and McLaren (6) reported prospermatogonia differentiated by 12.5 dpc. Thus, germ cell's sex differentiation in both male and female seems to be started on day 12.5 dpc. Once the meiotic germ cells appear in gonads, it is reported that these cells antagonize mesonephric cell migration and testis cord formation, which leads to a formation of ovotestis (35). However, the testicular eggs were always observed inside the seminiferous tubules (Fig. 3). With consideration to the above reports, we presume the testicular eggs that were always found inside the seminiferous tubules were not derived from the ovotestis area but more likely differentiated in an environment where surrounding somatic tissues are destined to differentiate into testes. It is reported that Sry expression and testicular cord formation emanates from the central region of the gonad (36, 37), whereas the formation of the oogenesis wave starts from the anterior part of the gonads and extends into the posterior region (34). If the differentiation of gonads begins simultaneously as male and female, the anterior part of the gonad is most likely the area where the germ cells differentiate into female. Supporting this assumption, most of the testicular eggs were found in the seminiferous tubules in the anterior and posterior poles of the testis and occasionally around the testicular surface (Fig. 3A). Moreover, the testis cords in which testicular eggs were located prenatally could have been incomplete or in some way abnormal because of the presence of XX somatic cells, with the cords appearing as normal testicular cords at a later stage of development. This environmental condition might have some similarity with the mesonephric rete in the testis of fetal sex-reversed mice. McLaren (38) reported that the existence of the second X chromosome (XX) renders germ cells more susceptible to the meiosis-inducing influence from such an environment. We therefore presume that a cascade of molecular and cellular events leading to oogenesis began in XX germ cells in testes before environmental factors from the testicular cords prohibited meiosis and resulted in testicular eggs.
Genomic Imprinting of XX* Germ Cells in Testis. Many XX* germ cells that were supposed to be prospermatogonia were found in XX*↔XY chimeric testes. However, there was little information about these cells being prospermatogonia. Recently, Durcova-Hills et al. (39) reported using sex-reversal mice in which the imprinted genes Igf2 and H19 were methylated more heavily in embryonic germ-cell lines established with an XY sex chromosome constitution than in those with an XX sex chromosome constitution, irrespective of the phenotypic sex of the genital ridge from which the embryonic germ cells (EGCs) had been derived. They concluded that the aberrant sex-specific methylation of these genes in EGCs is intrinsic and cell-autonomous and is not due to any influence of the genital ridge somatic cells (39). In contrast, the XX* spermatogonia from XX*↔XY chimeric testes clearly showed a male-type methylation pattern in imprinted genes, which was possibly influenced by somatic cells, despite their female set of chromosomes (Fig. 2). One of the reasons for this discrepancy may be the types of cells examined (XX* spermatogonia vs. EGCs). Moreover, Durcova-Hills et al. (39) examined the genomic imprinting of EGCs at 11.5 dpc, which is about the time of the elimination of genomic imprinting. In contrast, we examined the status of genomic imprinting in XX* germ cells at 0 dpp.
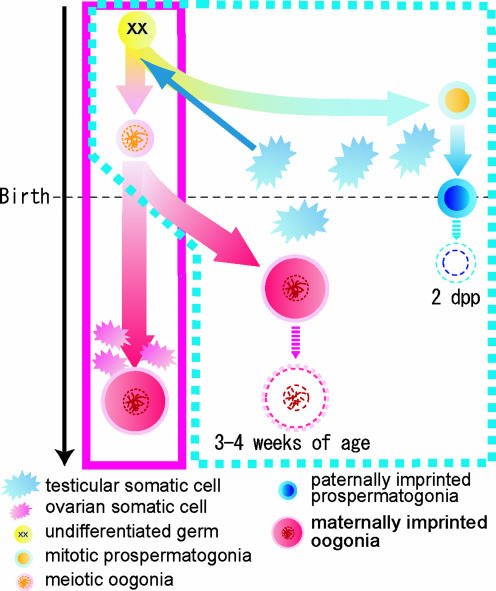
Based on the observation of cell size, a small number of XX germ cells were reported to have developed as testicular eggs in XXsxr sex-reversal mice testes (40). In the present experiment, taking advantage of EGFP tagging, these matured (or grown-up) XX* cells in the testes were recovered and were demonstrated to have zona pellucida and fusing ability with sperm. These characteristics apparently appeared during the growth of “eggs” inside seminiferous tubules after birth. This finding indicates that the testicular environment did not inhibit resuming of oogenesis and subsequent oocyte maturation in seminiferous tubules. Moreover, it should be noted that the testicular eggs must have sequestered inside seminiferous tubules and been exposed to male factors from the beginning of meiosis in the embryonic stage (Fig. 4 C and D) to the methylation-acquiring period after birth (Fig. 4E). The data described in the present study indicate that the sex-specific methylation pattern does not always follow the chromosomal constitution or the environmental conditions where the germ cells reside. Instead, the imprinting pattern seems to be defined by the sex that was chosen by the germ cells at their early stage of development (Fig. 5).
Fig. 5.
XX germ cells are reported to develop as spermatogonia when deposited in a testicular environment (6, 10). However, proof of male type differentiation in molecular bases was not available. As shown in the present study, once XX germ cells were inhibited from entering meiosis, they were demonstrated to acquire paternal imprinting, which indicates the development of XX prospermatogonia. In 1-dpp testes, we found approximately as many spermatogonia surviving as in the 0-dpp testes. However, the XX* spermatogonia were seldom seen at 2 dpp (data not shown). The reason for this disappearance is not known. Occasionally, some XX germ cells initiated meiosis in seminiferous tubules in their embryonic stage and were arrested in 4n stage. Despite continuous exposure to male factors inside seminiferous tubules during the embryonic stage, these cells did not acquire paternal imprinting as XX prospermatogonia. Instead, they resumed meiosis after birth and obtained a maternal imprinting pattern in the testicular environment. Because the maternal imprinting starts after birth in the growing oocytes, the imprinting in testicular eggs may also start after birth, together with their growth in size. Taking these facts together, we postulate that the pattern of genomic imprinting is designated when the germ cells choose the sex to develop at ≈12.5 dpc and that it is not influenced by environmental factors when methylation takes place.
These findings may relate to the symptoms of XX human males [estimated to occur in 1/20,000–1/25,000 births (41)] and Klinefelter syndrome patients [XXY males are estimated to occur in 1/500–1/1,000 births (42)], in which germ cells that contain two X chromosomes are reported to disappear during maturation (7, 13). Because the experimental model that we established allowed us to recover live germ cells, it can be used to investigate more detailed mechanisms of male infertility and sex differentiation in germ cells in general.
Supplementary Material
Acknowledgments
We thank Dr. Yoshitake Nishimune (Osaka University, Osaka) for his generous gift of the TRA98 antibody used in this study. In addition, we thank Dr. Susan S. Suarez and Dr. Stuart B. Moss for critically reading the manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) (15080207) and the 21st Century Center of Excellence Program of MEXT. J.L. is a Fellow of the Japan Society for the Promotion of Science.
Author contributions: M.O. designed research; A.I., T.N., S.K., J.L., and F.I. performed research; S.C. and N.N. contributed new reagents/analytic tools; A.I. and M.O. analyzed data; and A.I. and M.O. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: dpp, days postpartum; dpc, days postcoitum; DMR, differentially methylated region; SCP3, synaptonemal complex protein 3.
References
- 1.Merchant-Larios, H. & Moreno-Mendoza, N. (2001) Arch. Med. Res. 32, 553–558. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht, K. H., Young, M., Washburn, L. L. & Eicher, E. M. (2003) Genetics 164, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, C. H. & Taketo, T. (2001) Genesis 30, 7–11. [DOI] [PubMed] [Google Scholar]
- 4.Lovell-Badge, R. & Robertson, E. (1990) Development (Cambridge, U.K.) 109, 635–646. [DOI] [PubMed] [Google Scholar]
- 5.Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge, R. (1991) Nature 351, 117–121. [DOI] [PubMed] [Google Scholar]
- 6.Adams, I. R. & McLaren, A. (2002) Development (Cambridge, U.K.) 129, 1155–1164. [DOI] [PubMed] [Google Scholar]
- 7.McLaren, A. (1995) Philos. Trans. R. Soc. London B Biol. Sci. 350, 229–233. [DOI] [PubMed] [Google Scholar]
- 8.McLaren, A. & Southee, D. (1997) Dev. Biol. 187, 107–113. [DOI] [PubMed] [Google Scholar]
- 9.Bronson, S. K., Smithies, O. & Mascarello, J. T. (1995) Proc. Natl. Acad. Sci. USA 92, 3120–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer, S. J. & Burgoyne, P. S. (1991) Development (Cambridge, U.K.) 112, 265–268. [DOI] [PubMed] [Google Scholar]
- 11.Burgoyne, P. S., Levy, E. R. & McLaren, A. (1986) Nature 320, 170–172. [DOI] [PubMed] [Google Scholar]
- 12.Levy, E. R. & Burgoyne, P. S. (1986) Cytogenet. Cell Genet. 42, 208–213. [DOI] [PubMed] [Google Scholar]
- 13.Lue, Y., Rao, P. N., Sinha Hikim, A. P., Im, M., Salameh, W. A., Yen, P. H., Wang, C. & Swerdloff, R. S. (2001) Endocrinology 142, 1461–1470. [DOI] [PubMed] [Google Scholar]
- 14.Mroz, K., Carrel, L. & Hunt, P. A. (1999) Dev. Biol. 207, 229–238. [DOI] [PubMed] [Google Scholar]
- 15.Surani, M. A. (2001) Nature 414, 122–128. [DOI] [PubMed] [Google Scholar]
- 16.Davis, T. L., Trasler, J. M., Moss, S. B., Yang, G. J. & Bartolomei, M. S. (1999) Genomics 58, 18–28. [DOI] [PubMed] [Google Scholar]
- 17.Ueda, T., Abe, K., Miura, A., Yuzuriha, M., Zubair, M., Noguchi, M., Niwa, K., Kawase, Y., Kono, T., Matsuda, Y., et al. (2000) Genes Cells 5, 649–659. [DOI] [PubMed] [Google Scholar]
- 18.Obata, Y. & Kono, T. (2002) J. Biol. Chem. 277, 5285–5289. [DOI] [PubMed] [Google Scholar]
- 19.Lucifero, D., Mertineit, C., Clarke, H. J., Bestor, T. H. & Trasler, J. M. (2002) Genomics 79, 530–538. [DOI] [PubMed] [Google Scholar]
- 20.Nakanishi, T., Kuroiwa, A., Yamada, S., Isotani, A., Yamashita, A., Tairaka, A., Hayashi, T., Takagi, T., Ikawa, M., Matsuda, Y. & Okabe, M. (2002) Genomics 80, 564–574. [DOI] [PubMed] [Google Scholar]
- 21.Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. (1997) FEBS Lett. 407, 313–319. [DOI] [PubMed] [Google Scholar]
- 22.Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. (1998) Nat. Genet. 19, 220–222. [DOI] [PubMed] [Google Scholar]
- 23.McMahon, A., Fosten, M. & Monk, M. (1981) J. Embryol. Exp. Morphol. 64, 251–258. [PubMed] [Google Scholar]
- 24.Monk, M. & McLaren, A. (1981) J. Embryol. Exp. Morphol. 63, 75–84. [PubMed] [Google Scholar]
- 25.McLaren, A. & Monk, M. (1981) J. Reprod. Fertil. 63, 533–537. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson, R. V., Zhou, S. X., Wheatley, S. C., Koopman, P. & Tam, P. P. (1998) Dev. Biol. 199, 235–244. [DOI] [PubMed] [Google Scholar]
- 27.Tam, P. P., Zhou, S. X. & Tan, S. S. (1994) Development (Cambridge, U.K.) 120, 2925–2932. [DOI] [PubMed] [Google Scholar]
- 28.Clerc, P. & Avner, P. (2000) Science 290, 1518–1519. [DOI] [PubMed] [Google Scholar]
- 29.Zhou, L., Yoshimura, Y., Huang, Y., Suzuki, R., Yokoyama, M., Okabe, M. & Shimamura, M. (2000) Immunology 101, 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W. & Roder, J. C. (1993) Proc. Natl. Acad. Sci. USA 90, 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho, Y., Wigglesworth, K., Eppig, J. J. & Schultz, R. M. (1995) Mol. Reprod. Dev. 41, 232–238. [DOI] [PubMed] [Google Scholar]
- 32.Ohta, H., Yomogida, K., Dohmae, K. & Nishimune, Y. (2000) Development (Cambridge, U.K.) 127, 2125–2131. [DOI] [PubMed] [Google Scholar]
- 33.Lee, J., Inoue, K., Ono, R., Ogonuki, N., Kohda, T., Kaneko-Ishino, T., Ogura, A. & Ishino, F. (2002) Development (Cambridge, U.K.) 129, 1807–1817. [DOI] [PubMed] [Google Scholar]
- 34.Menke, D. B., Koubova, J. & Page, D. C. (2003) Dev. Biol. 262, 303–312. [DOI] [PubMed] [Google Scholar]
- 35.Yao, H. H., DiNapoli, L. & Capel, B. (2003) Development (Cambridge, U.K.) 130, 5895–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albrecht, K. H. & Eicher, E. M. (2001) Dev. Biol. 240, 92–107. [DOI] [PubMed] [Google Scholar]
- 37.Bullejos, M. & Koopman, P. (2001) Dev. Dyn. 221, 201–205. [DOI] [PubMed] [Google Scholar]
- 38.McLaren, A. (1981) J. Reprod. Fertil. 61, 461–467. [DOI] [PubMed] [Google Scholar]
- 39.Durcova-Hills, G., Burgoyne, P. & McLaren, A. (2004) Dev. Biol. 268, 105–110. [DOI] [PubMed] [Google Scholar]
- 40.McLaren, A. (1980) Nature 283, 688–689. [DOI] [PubMed] [Google Scholar]
- 41.de la Chapelle, A. (1981) Hum. Genet. 58, 105–116. [DOI] [PubMed] [Google Scholar]
- 42.Philip, J., Lundsteen, C., Owen, D. & Hirschhorn, K. (1976) Am. J. Hum. Genet. 28, 404–411. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.