Abstract
Previous studies have confirmed that exposure to particulate matter with a diameter of ≤2.5 µm (PM2.5) is associated with inflammation. PM2.5 decreases cardiac cell viability and increases apoptosis through overproduction of reactive oxygen species (ROS). In the present study, the role of PM2.5 in ECs was investigated in vitro. Human umbilical vein endothelial cells and human microvascular endothelial cells (ECs) were incubated with PM2.5 (100–800 µg/ml) to investigate the effects of PM2.5 on EC viability, migration, tube formation and intracellular levels of ROS. Cell viability and cell apoptosis were determined by MTT assay and flow cytometry analysis. Cell migration was assessed using a Boyden chamber assay, and tube formation was determined by matrigel assay. Tumor necrosis factor-α and interleukin-8 levels were measured by ELISA, and ROS levels were assessed with 2′,7′-dichlorofluorescin diacetate. The results indicated that PM2.5 decreases EC viability and increases EC apoptosis in a concentration-dependent manner. PM2.5 also decreased EC tube formation in a dose-dependent manner. The results also demonstrated that PM2.5 suppresses adhesion to EC extracellular matrix proteins. Furthermore, PM2.5 exposure significantly induced ROS generation, indicative of oxidative stress. Finally, it was demonstrated that PM2.5 decreased angiogenesis in vivo. These results suggested that repeated exposure to PM2.5 induces vascular inflammation.
Keywords: particulate matter, human umbilical vein endothelial cells, human microvascular endothelial cells, migration, angiogenesis
Introduction
Epidemiological studies have demonstrated that particulate matter (PM) is a serious environmental contaminant and is responsible for multiple human diseases, including cardiopulmonary diseases and cancers (1–5). Toxicological studies have revealed that PM with a diameter of ≤2.5 µm (PM2.5) has a higher toxicity than larger particles (5,6). Long-term contact with high doses of PM2.5 enhances cardiovascular disease mortality rates (7). However, the underlying mechanisms of PM2.5 have yet to be elucidated.
Endothelial cell (EC) dysfunction is necessary for a number of diseases, including cardiovascular diseases (8,9). There have not been many studies on the effects of PM2.5 on ECs, however, previous studies have demonstrated that PM2.5 induces oxidative stress in human umbilical vein endothelial cells (HUVECs) (10) and endothelial progenitor cells (11). However, the effects of PM2.5 on ECs and its mechanisms remain to be elucidated.
PM2.5 can be quickly released via the respiratory tract and then affect the organs and blood vessels. PM2.5 also can be phagocytosed by macrophages, releasing a number of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α (12). PM2.5 exposure is capable of inducing inflammation, which is regarded as the major mechanism of PM2.5-mediated toxicity (13–15).
It has also been demonstrated that oxidative stress is a key target of PM2.5-mediated cytotoxicity, however, these studies mainly focused on human lung epithelial cells (16,17). The present study focused on ECs exposed to high doses of PM2.5 and evaluated the effect on cell viability, migration and tube formation. The current understanding of whether PM2.5 exposure accelerates cell damage via ROS-mediated inflammation is limited and warrants more investigation. The present study, therefore aimed to determine the potential mechanisms underlying PM2.5-induced vascular toxicity.
To the best of our knowledge, the data in the present study has demonstrated for the first time that PM2.5 effectively suppressed migration, tube formation and adhesion to extracellular matrix (ECM) proteins in ECs. The present study also demonstrated the mechanisms by which PM2.5 induced vascular toxicity in ECs, at least in part, via a ROS-dependent signaling pathway. These findings may provide a strategy for the prevention and treatment of PM2.5-induced vascular inflammation in the clinical setting.
Materials and methods
Materials and reagents
PM2.5 was purchased from the National Institute of Standards and Technology (NIST; Gaithersburg, MD, USA), 2′,7′-dichlorofluorescin diacetate (DCFH-DA) and fetal bovine serum (FBS) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), MTT reagents were obtained from Dojindo Molecular Technologies, Inc. (Shanghai, China) and the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Cell culture
HUVECs and HMEC-1 human microvascular endothelial cells were obtained from AllCells, LLC (H-001F; Shanghai, China). Cells were cultured in RPMI 1640 medium with 20% FBS, 60 µg/ml of endothelial cell growth supplement (BD Biosciences, San Jose, CA, USA) and 100 U/ml of penicillin with 100 µg/ml of streptomycin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified atmosphere with 5% CO2 at 37°C.
PM2.5 was resuspended in PBS and the solutions were stored at −20°C until use. ECs were treated with various concentrations of PM2.5 to select the optimal concentration and time.
Detection of cell viability
Cell viability was determined by MTT assay. The ECs were cultured in 96-well plates (1.0×104 cells/well) in 100 µl medium for 24 h. ECs were then exposed to 0–800 µg/ml PM2.5 for 0–24 h. Following exposure, 20 µl of MTT was added to the wells for 1 h at 37°C. Cells were then treated with 100 µl of dimethyl sulfoxide. The absorbance was measured at 570 nm using a microplate reader. The viability of the treated cells was calculated as a percentage of the untreated control group, which was regarded as 100%.
Detection of cell apoptosis
ECs were cultured in 6-well plates (2×105 cells/well) for 24 h. ECs were then exposed to 0–800 µg/ml PM2.5 for 24 h. The ECs were harvested and resuspended in PBS. EC apoptosis was evaluated by flow cytometry analysis using an Annexin V-FITC/PI kit according to the manufacturer's protocols. The number of apoptotic cells was calculated by FlowJo (version 9.6.2; FlowJo LLC, Ashland, OR, USA).
Detection of cell migration
EC migration assays were performed using a modified Boyden chamber (8 µm pore size; BD Biosciences, Oxford, UK). ECs (5×104 cells/well) were treated with 0–800 µg/ml PM2.5 in RPMI 1640 medium for 6 h. RPMI 1640 medium supplemented with 500 µl 10 ng/ml vascular endothelial growth factor (VEGF; 293-VE-050; R&D Systems, Inc., Minneapolis, MN, USA) was added into the bottom chambers and the PM2.5-treated ECs were added to the upper chambers for 8 h. The migrating cells were stained with 1% calcein-AM and quantified by counting under a fluorescence microscope. Experiments were repeated at least three times, and the migrated cells were counted in five random fields of each filter.
Detection of tube formation
The 96-well plates were pre-coated with Matrigel for 2 h. ECs were exposed to 0–800 µg/ml PM2.5 in RPMI 1640 medium for 6 h. Then 2×104 ECs/well were incubated in RPMI 1640 medium with 10 ng/ml VEGF for 24 h at 37°C. Quantification of tube formation was performed by using IncuCyte angiogenesis version 2.0 image analysis (Essen Bioscience, Ann Arbor, MI, USA).
Detection of ECM cell adhesion
Cell adhesion of ECs was evaluated by an Extracellular Matrix Cell Adhesion Array kit (ECM545; Chemicon; EMD Millipore, Billerica, MA, USA). The assay was carried out according to the manufacturer's instructions. The wells were pre-coated with different human ECM proteins (collagen I, collagen II, collagen IV, fibronectin, laminin, tenascin and vitronectin) and a BSA-pre-coated well as control. The ECs were treated with 0–800 µg/ml PM2.5 for 6 h. Following washing with PBS, attached cells were stained with CyQuant GR® Dye (ECM545; Chemicon; EMD Millipore) according to the manufacturer's protocol and the cell-bound stain was then extracted in extraction buffer (0.05% trypsin in Hanks Balanced Salt Solution containing 25 mM HEPES). The absorbance of the stain was determined at 450 nm. The change in absorbance was presented as the fold of untreated control.
ROS detection
ECs (2×105 cells/well) were cultured in 35 mm dishes and exposed to PM2.5 (0–800 µg/ml) for 24 h, when the cells were harvested for intracellular ROS detection using DCFH-DA. First, ECs were washed with PBS and resuspended in RPMI 1640 medium containing 10 µM of DCFH-DA at 37°C for 40 min in the dark. Then the ECs were washed with PBS and ROS were detected using a confocal fluorescence microscope and analyzed by flow cytometry.
ELISA
ECs (2×105 cells/well) were cultured in 35 mm dishes and exposed to PM2.5 (0–800 µg/ml) for 24 h. The ECs were centrifuged at 10,000 × g for 10 min at 4°C, and TNF-α (RAB1089) and interleukin (IL)-8 (RAB0595) levels were determined by ELISA (Sigma-Aldrich; Merck KGaA) according to the manufacturer's instructions.
In vivo matrigel plug assay
All animal care and experimental procedures complied with the guidelines of the Animal Experimentation Ethics Committee of Tongji University. Male C57BL/6 mice (24–25 g, 6 weeks old, n=15) were supplied and maintained by Tongji University Laboratory Animal Service Center at 23±2°C, 55±5% humidity on a 12 h light/dark cycle with food and water supplied ad libitum. Matrigel (500 µl) with heparin (10 U/ml), VEGF 100 ng/ml and PM2.5 (0–800 µg/ml) were mixed and injected into the right flanks of mice. Negative controls were obtained by injecting mice with Matrigel and twice-distilled water. The mice were sacrificed by cervical dislocation after 7 days, and the Matrigel plugs were removed and photographed. The hemoglobin content of the Matrigel plugs was calculated using a Drabkin's reagent kit (5252; Sigma-Aldrich; Merck KGaA).
Statistical analysis
All data are presented as mean ± standard deviation obtained from at least three experiments. Statistical analysis of two samples was performed with Student's t-test analysis. Statistical analyses among multiple groups were performed using one-way analysis of variance followed by Bonferroni's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
PM2.5 decreases HUVEC and HMEC-1 viability
The cytotoxicity of PM2.5 was determined by MTT assay in HUVECs and HMEC-1 after 24 h. PM2.5 at 200–800 µg/ml significantly reduced EC viability compared with the 0 µg/ml PM2.5 control group (Fig. 1). As demonstrated in Fig. 1, PM2.5 significantly inhibited the viability of HUVECs and HMEC-1 in a dose-dependent manner following 24 h treatment. The concentrations producing 50% growth inhibition of PM2.5 on HUVEC and HMEC-1 were ~200 µg/ml and 300 µg/ml, respectively.
Figure 1.
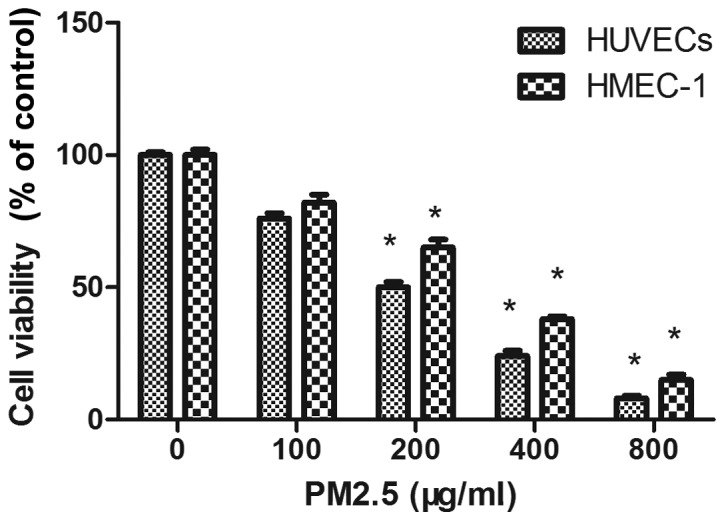
Effect of PM2.5 on HUVEC and HMEC-1 viability. Cells were treated with 0–800 µg/ml PM2.5 for 24 h, then cell viability was determined by MTT assay. *P<0.05 vs. control group (0 µg/ml PM2.5). PM2.5, particulate matter with a diameter of ≤2.5 µm; HUVEC, human umbilical vein endothelial cell; HMEC, human microvascular endothelial cell.
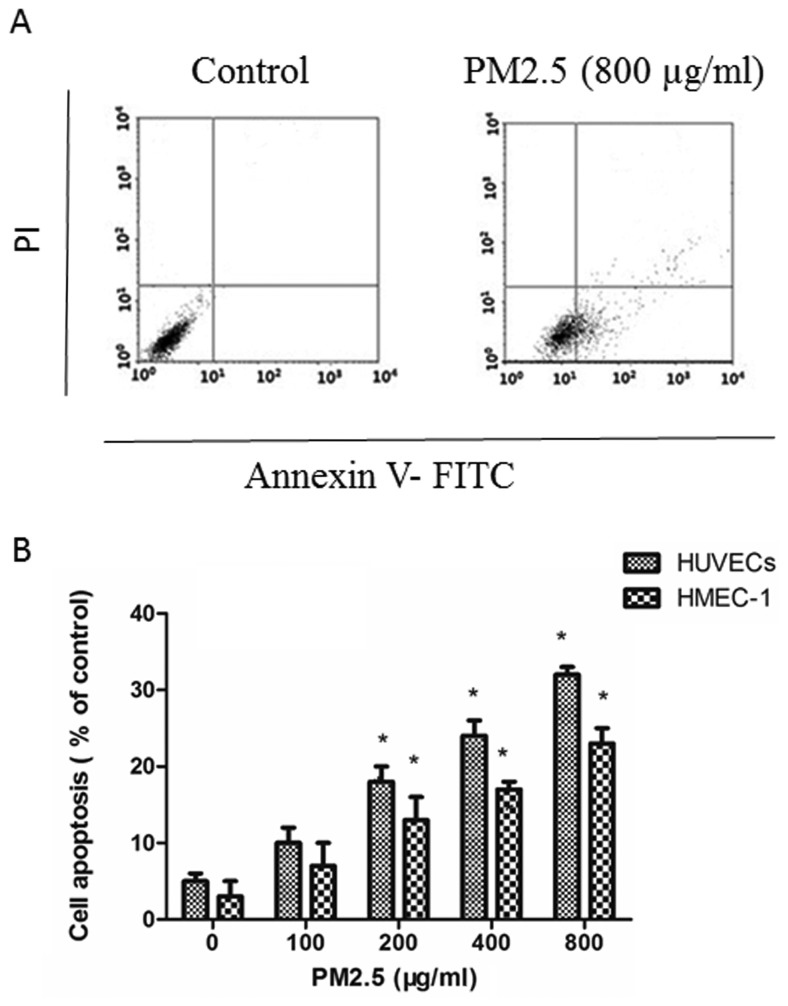
PM2.5 induces HUVECs and HMEC-1 apoptosis
Annexin V-FITC/PI assays demonstrated that PM2.5 induced apoptosis in ECs in a dose-dependent manner: PM2.5 at 200–800 µg/ml significantly increased the proportion of apoptotic ECs after 24 h compared with the 0 µg/ml PM2.5 control group (Fig. 2).
Figure 2.
Effect of PM2.5 on HUVEC and HMEC-1 apoptosis. (A) Cells were treated with 0–800 µg/ml PM2.5 for 24 h, then cell apoptosis was assessed by Annexin V-fluorescein isothiocyanate/propidium iodide assay. (B) Quantification of cell apoptosis. *P<0.05 vs. control group (0 µg/ml PM2.5). PM2.5, particulate matter with a diameter of ≤2.5 µm; HUVEC human umbilical vein endothelial cell; HMEC, human microvascular endothelial cell.
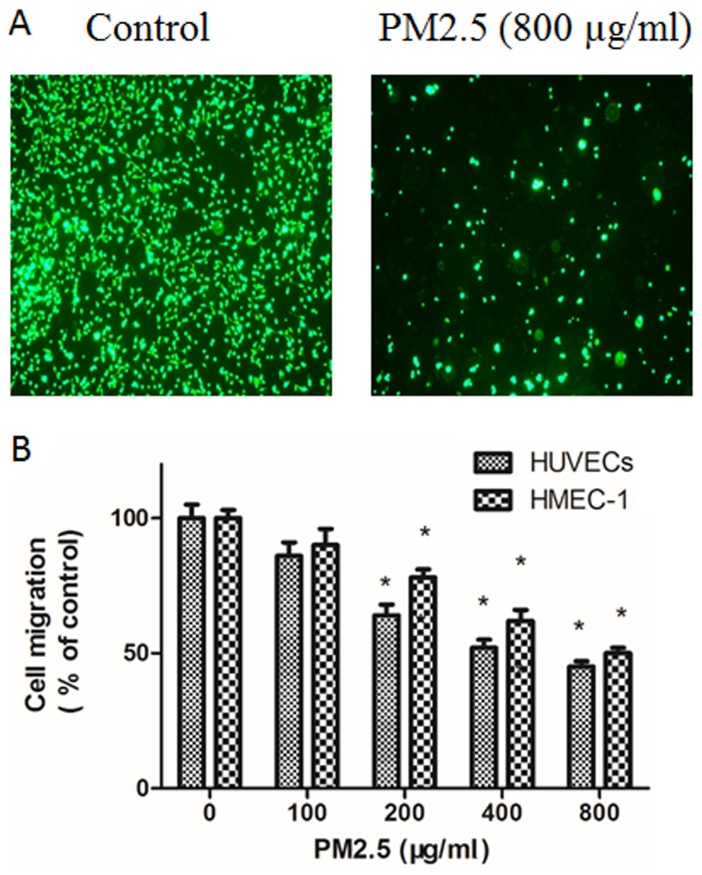
PM2.5 decreases HUVECs and HMEC-1 migration
The effect of PM2.5 on the migration of ECs was explored using Transwell migration assays. Compared with basal medium, RPMI 1640 medium with VEGF triggered EC migration; however, this effect was dose-dependently inhibited by PM2.5 treatment (Fig. 3). In the Boyden chamber assay, 200–800 µg/ml PM2.5 treatment significantly inhibited EC migration (Fig. 3).
Figure 3.
Effect of PM2.5 on HUVEC and HMEC-1 migration. (A) Cells were treated with 0–800 µg/ml PM2.5 for 24 h, then cell migration was determined by Boyden chamber assay Transwell assay. (B) Quantitative analysis of cell migration. *P<0.05 vs. control group (0 µg/ml PM2.5). PM2.5, particulate matter with a diameter of ≤2.5 µm; HUVEC, human umbilical vein endothelial cell; HMEC, human microvascular endothelial cell.
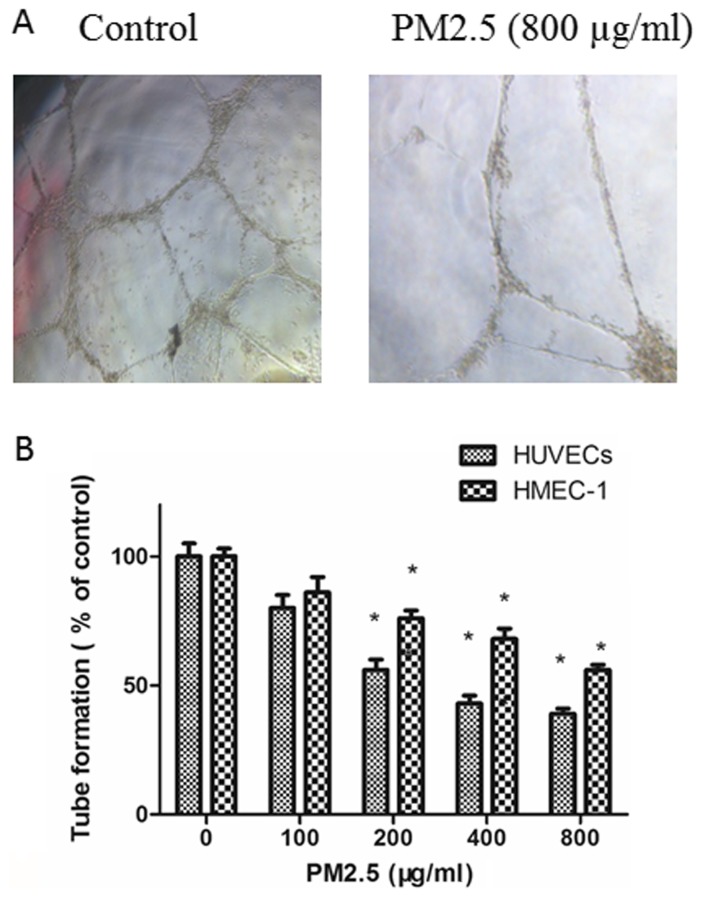
PM2.5 decreases HUVEC and HMEC-1 tube formation
The effects of PM2.5 on the angiogenesis of ECs were explored with a tube formation assay. ECs in RPMI 1640 medium containing 10 ng/ml VEGF were planted on Matrigel and the amount of tube formation was measured. As demonstrated in Fig. 4, compared with untreated ECs, tube formation was inhibited in ECs by treatment with PM2.5 in in a dose-dependent manner.
Figure 4.
Effect of PM2.5 on HUVEC and HMEC-1 tube formation. (A) Cells were treated with 0–800 µg/ml PM2.5 for 24 h, then tube formation was determined by Matrigel tube formation assay. (B) Quantitative analysis of tube formation. *P<0.05 vs. control group (0 µg/ml PM2.5). PM2.5, particulate matter with a diameter of ≤2.5 µm; HUVEC, human umbilical vein endothelial cell; HMEC, human microvascular endothelial cell.
PM2.5 decreases EC adhesion to ECM proteins
EC adhesion to various ECM proteins (collagen I, collagen II, collagen IV, fibronectin, laminin, tenascin and vitronectin) was measured in cells were treated with 0 or 800 µg/ml PM2.5. The decreased adhesions were observed in the two cell lines (P<0.05). Collagen IV, tenascin and vitronectin adhesion was significantly reduced in PM2.5-treated HUVECs and HMEC-1s, compared with control (P<0.05; Fig. 5).
Figure 5.
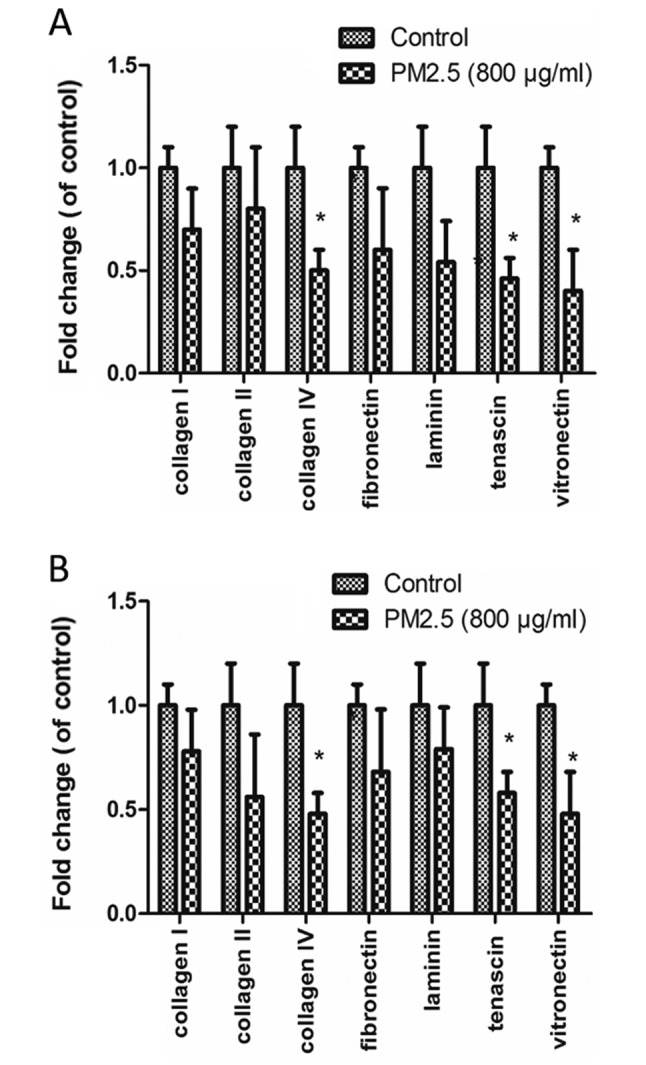
Effect of PM2.5 on cell adhesion of (A) HUVEC and (B) HMEC-1 to ECM proteins. Cells were treated with 0–800 µg/ml PM2.5 for 24 h, then the absorbance of ECM proteins was determined. *P<0.05 vs. control group. PM2.5, particulate matter with a diameter of ≤2.5 µm; HUVEC, human umbilical vein endothelial cell; HMEC, human microvascular endothelial cell; ECM, extracellular matrix; Group 1, control.
PM2.5 increases intracellular HUVEC and HMEC-1 ROS generation
DCFH-DA staining demonstrated that PM2.5 treatment increased ROS accumulation in a dose-dependent manner; flow cytometry demonstrated that the mean fluorescence intensity of ECs incubated with PM2.5 at 200, 400 and 800 µg/ml for 24 h was significantly increased compared with the 0 µg/ml PM2.5 control group (P<0.05; Fig. 6).
Figure 6.
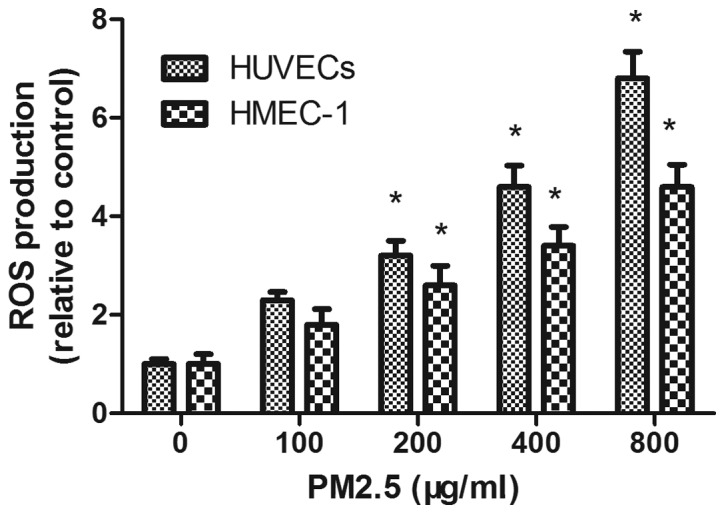
Effect of PM2.5 on HUVEC- and HMEC-1-induced ROS production. Cells were treated with 0–800 µg/ml PM2.5 for 24 h, then ROS production was determined by 2′,7′-dichlorofluorescin diacetate assay. *P<0.05 vs. control group (0 µg/ml PM2.5). PM2.5, particulate matter with a diameter of ≤2.5 µm; HUVEC, human umbilical vein endothelial cell; HMEC, human microvascular endothelial cell; ROS, reactive oxygen species.
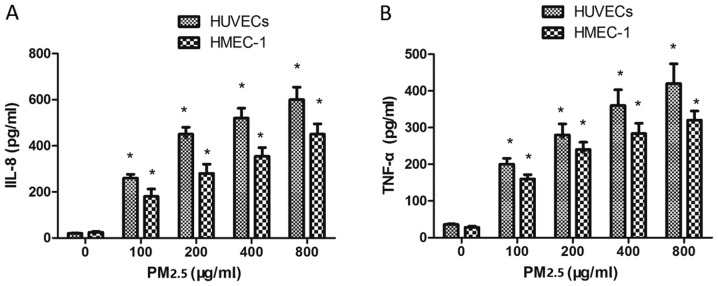
Effect of PM2.5 on IL-8 and TNF-α expression in HUVECs and HMEC-1
To investigate whether PM2.5 treatment affected IL-8 and TNF-α expression, ECs were incubated with PM2.5 for 24 h. PM2.5 (100–800 µg/ml) induced IL-8 (Fig. 7A) and TNF-α (Fig. 7B) expression in ECs compared with the 0 µg/ml PM2.5 control group, indicating that PM2.5 induced vascular inflammation via IL-8 and TNF-α overexpression.
Figure 7.
Effect of PM2.5 on HUVEC- and HMEC-1-induced IL-8 and TNF-α expression. Cells were treated with 0–800 µg/ml PM2.5 for 24 h, then (A) IL-8 and (B) TNF-α expression was determined by ELISA. *P<0.05 vs. control group (0 µg/ml PM2.5). PM2.5, particulate matter with a diameter of ≤2.5 µm; HUVEC human umbilical vein endothelial cell; HMEC, human microvascular endothelial cell; IL, interleukin; TNF, tumor necrosis factor.
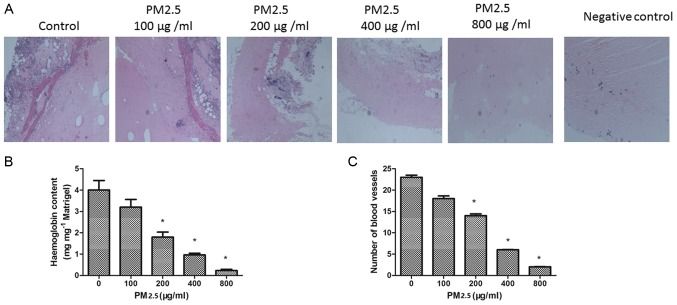
PM2.5 inhibits angiogenesis in vivo
To confirm the effect of PM2.5 on angiogenesis in vivo, a Matrigel plug assay was performed. The plugs containing VEGF demonstrated greater levels of angiogenesis compared with the negative control diluent plugs. In the presence of PM2.5 treatment, the number of blood vessels in the plugs was decreased. The hemoglobin concentration was quantified in the plugs; the hemoglobin content was significantly decreased in the 200–800 µg/ml PM2.5 treated groups compared with the 0 µg/ml PM2.5 group (P<0.05; Fig. 8B). This result is consistent with the inhibition of angiogenesis by PM2.5 in vivo (Fig. 8C).
Figure 8.
(A) Effect of PM2.5 on VEGF-induced angiogenesis in vivo. (B) Hemoglobin content and (C) angiogenesis of Matrigel plugs was determined. Magnification, ×10. *P<0.05 vs. control group (0 µg/ml PM2.5). PM2.5, particulate matter with a diameter of ≤2.5 µm; VEGF, vascular endothelial growth factor.
Discussion
PM2.5 has previously been demonstrated to exercise adverse effects on ECs (18–20); however, its effect on angiogenesis in ECs remains unclear. In the present study, the vascular inflammation activities of PM2.5 were demonstrated for the first time in HUVEC and HMEC-1. Although the molecular mechanisms of PM2.5 in ECs have not been verified, the data from the present study demonstrated that high doses of PM2.5 decreased EC viability, migration and tube formation. High doses of PM2.5 also increased ROS production and IL-8 and TNF-α expression. Based on the results of the ECs lines, high doses (>200 µg/ml) of PM2.5 are able to significantly inhibit angiogenesis. Therefore, it is possible that high-doses of PM2.5 can induce vascular inflammation.
Angiogenesis requires a number of steps, including cell proliferation, migration, tube formation and remodeling (21). The results of the present study demonstrated that the inhibitory effect of PM2.5 on viability were stronger in HUVECs than HMEC-1s. PM2.5 treatment may also inhibit migration and tube formation in the two EC lines, implying that PM2.5 can inhibit angiogenesis. The endothelial recovery involved endothelial proliferation and migration to the injury site; the findings of the present study add a previously unrecognized role of PM2.5 exposure in the regulation of ECs biological function following vascular injury.
To further investigate mechanisms through which PM2.5 induces inhibitory effects on migration of ECs, the effects of PM2.5 on the ECs ability to adhere to ECM proteins were investigated. PM2.5 decreased the ability to adhere to collagen type IV, tenascin and vitronectin. Collagen IV serves an important role in cell adhesion and motility (22) and tenascin has an important role in promoting cell survival and migration (23,24). Vitronectin is a high molecular weight glycoprotein known to promote cell adhesion and affect cell migration (25). Therefore, treatment with PM2.5 resulted in decreased adhesion of endothelial cells to these matrix proteins. It is suggested that the consequent effect was to disrupt endothelial cell-cell junctions leading to an increase in vascular permeability to the environment affected by local injury to blood vessels.
The effect of PM2.5 on ROS production in ECs was also evaluated. ROS have key roles in EC apoptosis. The data from the present study suggested that PM2.5-induced ECs apoptosis may act through the ROS overproduction. PM2.5 also caused the release of the pro-inflammatory cytokines IL-8 and TNF-α. A previous study demonstrated that intracellular ROS contributed to the release of pro-inflammatory cytokines (26). In the present study, TNF-α and IL-8 were secreted from the ECs following 24 h exposure to PM2.5, which may have induced endothelial permeability. Promotion of inflammation is considered a key step in the adverse health effects associated with PM2.5 exposure.
The effects of PM2.5 were studied using an in vivo Matrigel plug model. PM2.5 decreased the hemoglobin content in plugs in comparison with the 0 µg/ml VEGF group (Fig. 8B). The data are consistent with the inhibition of angiogenesis by PM2.5 in vitro.
In conclusion, the anti-angiogenic effects by high-dose PM2.5 exposure on ECs may be exerted through increased ROS production and IL-8 and TNF-α expression, leading to reduced EC proliferation and migration. These findings suggest that high-dose PM2.5 exposure may be an important mediator of vascular inflammation in ECs.
References
- 1.Aaron CP, Chervona Y, Kawut SM, Roux AV Diez, Shen M, Bluemke DA, Van Hee VC, Kaufman JD, Barr RG. Particulate matter exposure and cardiopulmonary differences in the multi-ethnic study of atherosclerosis. Environ Health Perspect. 2016;124:1166–1173. doi: 10.1289/ehp.1409451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurston G, Lippmann M. Ambient particulate matter air pollution and cardiopulmonary diseases. Semin Respir Crit Care Med. 2015;36:422–432. doi: 10.1055/s-0035-1549455. [DOI] [PubMed] [Google Scholar]
- 3.Amatullah H, North ML, Akhtar US, Rastogi N, Urch B, Silverman FS, Chow CW, Evans GJ, Scott JA. Comparative cardiopulmonary effects of size-fractionated airborne particulate matter. Inhal Toxicol. 2012;24:161–171. doi: 10.3109/08958378.2011.650235. [DOI] [PubMed] [Google Scholar]
- 4.Cui P, Huang Y, Han J, Song F, Chen K. Ambient particulate matter and lung cancer incidence and mortality: A meta-analysis of prospective studies. Eur J Public Health. 2015;25:324–329. doi: 10.1093/eurpub/cku145. [DOI] [PubMed] [Google Scholar]
- 5.Fu J, Jiang D, Lin G, Liu K, Wang Q. An ecological analysis of PM2.5 concentrations and lung cancer mortality rates in China. BMJ Open. 2015;5:e009452. doi: 10.1136/bmjopen-2015-009452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue H, Yun Y, Gao R, Li G, Sang N. Winter polycyclic aromatic hydrocarbon-bound particulate matter from peri-urban North China promotes lung cancer cell metastasis. Environ Sci Technol. 2015;49:14484–14493. doi: 10.1021/es506280c. [DOI] [PubMed] [Google Scholar]
- 7.Hu H, Dailey AB, Kan H, Xu X. The effect of atmospheric particulate matter on survival of breast cancer among US females. Breast Cancer Res Treat. 2013;139:217–226. doi: 10.1007/s10549-013-2527-9. [DOI] [PubMed] [Google Scholar]
- 8.Lin CP, Lin FY, Huang PH, Chen YL, Chen WC, Chen HY, Huang YC, Liao WL, Huang HC, Liu PL, et al. Endothelial progenitor cell dysfunction in cardiovascular diseases: Role of reactive oxygen species and inflammation. Biomed Res Int. 2013;2013:845037. doi: 10.1155/2013/845037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang GZ, Wang ZJ, Bai F, Qin XJ, Cao J, Lv JY, Zhang MS. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules. 2015;20:6626–6639. doi: 10.3390/molecules20046626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Y, Xie X, Jia F, He J, Li Z, Fu M, Hao H, Liu Y, Liu JZ, Cowan PJ, et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell Physiol Biochem. 2015;35:353–363. doi: 10.1159/000369701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong GQ, Zhang ZH, Zhao Y, Liu JJ, Han JB. Traffic-related PM2.5 induces cytosolic [Ca2+] increase regulated by Orai1, alters the CaN-NFAT signaling pathway, and affects IL-2 and TNF-α cytoplasmic levels in Jurkat T-cells. Arch Environ Contam Toxicol. 2015;68:31–37. doi: 10.1007/s00244-014-0077-8. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Kou X, Xie L, Cheng F, Geng H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ Sci Pollut Res Int. 2015;22:20167–20176. doi: 10.1007/s11356-015-5222-z. [DOI] [PubMed] [Google Scholar]
- 14.Ostro B, Malig B, Broadwin R, Basu R, Gold EB, Bromberger JT, Derby C, Feinstein S, Greendale GA, Jackson EA, et al. Chronic PM2.5 exposure and inflammation: Determining sensitive subgroups in mid-life women. Environ Res. 2014;132:168–175. doi: 10.1016/j.envres.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potera C. Toxicity beyond the lung: Connecting PM2.5, inflammation, and diabetes. Environ Health Perspect. 2014;122:A29. doi: 10.1289/ehp.122-A29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Lin Z, Huang H, He H, Yang L, Chen T, Yang T, Ren N, Jiang Y, Xu W, et al. AMPK is required for PM2.5-induced autophagy in human lung epithelial A549 cells. Int J Clin Exp Med. 2015;8:58–72. [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, Chen D, Ding W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro. 2013;27:1762–1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Wang FF, Geng CM, Hao WD, Zhao YD, Li Q, Wang HM, Qian Y. The cellular toxicity of PM2.5 emitted from coal combustion in human umbilical vein endothelial cells. Biomed Environ Sci. 2016;29:107–116. doi: 10.3967/bes2016.012. [DOI] [PubMed] [Google Scholar]
- 19.Montiel-Dávalos A, Alfaro-Moreno E, López-Marure R. PM2.5 and PM10 induce the expression of adhesion molecules and the adhesion of monocytic cells to human umbilical vein endothelial cells. Inhal Toxicol. 2007;19:91–98. doi: 10.1080/08958370701495212. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 20.Rui W, Guan L, Zhang F, Zhang W, Ding W. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J Appl Toxicol. 2016;36:48–59. doi: 10.1002/jat.3143. [DOI] [PubMed] [Google Scholar]
- 21.Abdelrahim M, Konduri S, Basha R, Philip PA, Baker CH. Angiogenesis: An update and potential drug approaches (Review) Int J Oncol. 2010;36:5–18. [PubMed] [Google Scholar]
- 22.Favreau AJ, Vary CP, Brooks PC, Sathyanarayana P. Cryptic collagen IV promotes cell migration and adhesion in myeloid leukemia. Cancer Med. 2014;3:265–272. doi: 10.1002/cam4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hessel M, Steendijk P, den Adel B, Schutte C, van der Laarse A. Pressure overload-induced right ventricular failure is associated with re-expression of myocardial tenascin-C and elevated plasma tenascin-C levels. Cell Physiol Biochem. 2009;24:201–210. doi: 10.1159/000233246. [DOI] [PubMed] [Google Scholar]
- 24.Ide M, Saito K, Tsutsumi S, Tsuboi K, Yamaguchi S, Asao T, Kuwano H, Nakajima T. Over-expression of 14-3-3σ in budding colorectal cancer cells modulates cell migration in the presence of tenascin-C. Oncol Rep. 2007;18:1451–1456. [PubMed] [Google Scholar]
- 25.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: Vitronectin provides the key. J Cell Biol. 2007;177:927–939. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu G, Huang Q, Zheng W, Huang Y, Hua J, Yang S, Zhuang J, Wang J, Chang J, Xu J, Ye J. LPS upregulated VEGFR-3 expression promote migration and invasion in colorectal cancer via a mechanism of increased NF-κB binding to the promoter of VEGFR-3. Cell Physiol Biochem. 2016;39:1665–1678. doi: 10.1159/000447868. [DOI] [PubMed] [Google Scholar]