Abstract
Expression of the IL-2 receptor α chain (CD25) by peripheral CD4 T cells follows cellular activation. However, CD25 expression by CD4 cells is widely used as a marker to identify regulatory T cells (TR), although cells with regulatory properties are also found in the CD4+CD25– subset. By using in vivo functional assays and Foxp3 expression as a faithful marker of TR differentiation, we have evaluated the requirements for CD25 expression by peripheral TR. We first show that in vivo depletion of CD25+ cells prevents the development of spontaneous encephalomyelitis in recombination-activating gene (RAG)-deficient anti-myelin basic protein T cell antigen receptor (TCR) transgenic mice, and allows disease induction in otherwise healthy RAG-competent transgenic mice. Similar treatment in normal thymectomized animals is followed by the fast recovery of a normal number of CD25+ TR. Consistently, Foxp3-expressing TR encompassed in the CD25– cell population convert to CD25+ after homeostatic expansion and are selectable by IL-2 in vitro. Surface expression of CD25 on TR is controlled by the activity of conventional CD4 cells and is fully labile because it can be lost and regained without affecting the functional potential of the cells. These findings reveal that Foxp3-expressing CD25– cells constitute a peripheral reservoir of differentiated TR, recruited to the CD25+ pool upon homeostatic expansion and/or activation. This analysis, together with the notion that physiological commitment of TR takes place exclusively in the thymus should help for the interpretation of experiments assessing peripheral TR differentiation from naive CD4 T cells, defined as CD25–.
Keywords: homeostasis, mice, T lymphocyte
Healthy unmanipulated mice bear a significant number of “naturally” activated B and T lymphocytes, which seem to represent physiological autoreactivity because they are equally represented in “germ-free” and “antigen-free” mice (1). Like other antigen-experienced CD4 cells, naturally activated CD4 cells are encompassed in the CD45RBlow pool (2). As demonstrated in several experimental systems using adoptive transfers, CD4+CD45RBlow cells (from now on denoted CD45RBlow) limit the pathological potential of the complementary CD45RBhigh naive cells (3, 4). A subset of CD45RBlow cells expressing the CD25 marker is highly enriched in regulatory T cells (TR) that limit both protective and pathological immune responses (5). Several reports, however, demonstrate that TR are not exclusively contained within the CD25-expressing subset (6–9). Moreover, surface markers and genes that are highly represented or expressed in the CD25+ cells are also found in the CD45RBlowCD25– subpopulation although at a lower frequency or level, while being absent in the CD45RBhigh subset. This is the case for the surface molecules CD103 (8), glucocorticoid-induced tumor necrosis factor receptor (GITR) (10), cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) (11), Toll-like receptor (TLR)-4, -5, -7, and -8 (12), and the transcription factor Foxp3 (13). Expression of the Foxp3 gene is strictly required for TR development and enough to confer conventional CD4 T cells with regulatory function (13–15). It is to date the only known TR commitment/differentiation factor in mice.
For convenience of experimental design, most current studies use surface expression of CD25 to distinguish “conventional” T cells from TR. This approach seems appropriate, because most in vivo studies successfully associated regulatory activity to this cellular subset, and >95% of the CD25+ cells in a normal mouse express Foxp3, as evaluated in GFP-Foxp3 fusion knock-in mice (16). Clearly, however, the reverse does not apply, because lack of CD25 expression in a cell population cannot be taken for absence of regulatory cells. This reservation is critical, in view of previous claims that regulatory cells can differentiate in the periphery from naive CD4 cells, defined as CD25–.
The CD25 molecule is the α chain of the IL-2 receptor, and its expression results in higher affinity to IL-2 (17). Upon activation, conventional CD4 cells express CD25, while lacking many of the other phenotypic and functional characteristics of TR (18). Similar induction of CD25 expression upon activation may well occur on TR, and it has been proposed that IL-2 promotes acquisition of this marker and functional activation (19). Several groups have reported that CD25+ cells lose CD25 expression upon adoptive transfer in lymphopenic mice, a phenomenon that is less marked if conventional CD4 cells, presumably serving as a source of IL-2, are present and undergoing homeostatic expansion (6, 20). Intriguingly, acquisition of CD25 expression by CD25– cells undergoing homeostatic expansion was also reported, although the nature of the cells contributing to this phenomenon was not assessed (6, 20, 21).
In this study, we investigated the relevance of CD25 surface expression for the definition of TR and, thus, the possibility that they may arise from the naive CD4 pool in the periphery. We show that administration of depleting anti-CD25 mAb in vivo targets both newly activated conventional cells and a limited subset of regulatory T cells. Furthermore, the bulk of Foxp3-expressing T cells encompassed in the CD45RBlowCD25– cell pool convert to a CD25+ phenotype in lymphopenic conditions, and these cells display functional characteristics of TR. Finally, surface expression of CD25 on TR is fully labile because it can be lost and regained without affecting the functional potential of the cells. Taken together, these analyses indicate that a reservoir of TR is contained in the CD45RBlowCD25– population and that such cryptic TR can rapidly be recruited to the CD25+ pool.
Materials and Methods
Mice. BALB/c, C57BL/6, C57BL/6-Thy1.1, Igha, Gpi1a, C57BL/6 RAG2–/–, B10-PL-MBP-TCR-Tg, and B10-PL-RAG1–/–-MBP-TCR-Tg mice were bred and maintained under specific pathogen-free conditions in our animal house. All animals were used between 4 and 10 weeks of age.
Antibodies and Reagents. Allophycocyanin (APC), CyChrome-, and phycoerythrin (PE)-conjugated anti-CD4 mAb (clone RM4-5), CD45RB-PE (clone 16A), and Thy1.2 biotin (CD90.2) were purchased from BD Biosciences. Thy1.1 (CD90.1) biotin and Alexa Fluor 488-CD25 (clone PC61) were home-made. Biotinylated antibodies were revealed with streptavidin-PE or -APC (BD Biosciences). CD25+ cell depletion was performed with 200 μg of anti-CD25 mAb (clone PC61) injected i.p. As a control, mice received the same amount of rat IgG (Sigma–Aldrich). Depletion was evaluated by using the 7D4 anti-CD25 mAb (BD Biosciences). Pertussis toxin from Bordetella pertussis (Sigma–Aldrich) was injected i.v. (200 ng per mouse).
Thymectomy and Disease Evaluation. Four-week-old BALB/c mice were thymectomized (Tx), and absence of noticeable thymic remnants was confirmed at the end of the experiment. Experimental autoimmune encephalomyelitis (EAE) was scored every 3 days as described (22).
Cell Purification and Transfer. Pooled lymph nodes (LNs) stained with a mixture of anti-CD4-PE and CD25-Alexa mAbs, or with anti-CD4-CyChrome, CD25-Alexa, and CD45RB-PE were purified on a MoFlo High Speed Cell Sorter (Cytomation, Fort Collins, CO). Purity was routinely >98% for CD4+CD25+ cells and >99% for the other CD4 subsets. Cells were suspended in PBS and injected in the retroorbital plexus (100 μl per mouse).
Cell Recovery and Flow Cytometric Analysis. Cell suspensions from spleen or mesenteric LNs were prepared, stained, and washed in PBS containing 2% FCS and 0.01% sodium azide. Propidium iodide was added to the final suspension. Analyses were performed inside a live lymphocyte gate on a FACSCalibur (Becton Dickinson) by using cellquest software. Life lymphocyte counts were deduced from the acquisition of a fixed number of 10-μm latex beads (Coulter) mixed with a known volume of unstained cell suspension.
Cell Cultures and Suppression Assays. Cultures were set in RPMI medium 1640 containing 10% FCS, 100 μg/ml penicillin and streptomycin, 50 μM 2-mercaptoethanol (2-ME), 10 mM Hepes, and 1 mM sodium pyruvate (all purchased from Life Technologies, Grand Island, NY). IL-2 production was as follows: 2.5 × 103 CD4+CD25– cells (target) mixed with 5 × 103 irradiated splenocytes and various numbers of the cell populations under test were stimulated with 0.5 μg/ml anti-CD3 mAb (145.2C11; home-made) for 48 h (U-shape 96-well plate, 100 μl final). Fifty microliters of the supernatant was transferred to 103 CTLL-2 cells, and a saturating amount of IL-2 was added to the last 24 h of a 3-day culture (amplification). For pretreatments, the cells (2 × 106 per well, 24-well plate) were stimulated for 6 days with 1 μg/ml soluble anti-CD3 mAb and a saturating amount of IL-2. Alternatively, the cells (106 per well, six-well plate) were sequentially stimulated with 1 μg/ml and 10 μg/ml plate-bound anti-CD3 mAb for 5 and 3 days, respectively. Standard suppression assays are described in ref. 12. All cultures were set in triplicate, and [3H]thymidine [1 μCi per well (1 Ci = 37 GBq); Amersham Pharmacia Biosciences] was added for the last 6 h.
Real-Time PCR. Total RNA was extracted from 104 to 106 cells by using TriPure (Roche Diagnostics), treated with DNaseI and reverse transcribed by using SuperScript II RT and oligo(dT)12–18 primer (all from Life Technologies). PCRs were performed by using the QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA) and the Light Cycler system (Roche), and consisted of 15 min at 95°C and 45 cycles of 15 s at 95°C, 20 s at 61°C (Foxp3), or 55°C [hypoxanthine phosphoribosyltransferase (HPRT)], and 10 s at 72°C. Primer pairs (5′–3′) were: Foxp3, TTCATGCATCAGCTCTCCACT and AAGGTGGTGGGAGGCTGA; HPRT, CCAGCAAGCTTGCAACCTTAACCA and GTAATGATCGTCAACGGGGGAC. The standard curve method was applied for quantification of each amplicon. The normalized values for Foxp3 mRNA were calculated as the quantity of Foxp3 mRNA levels divided by the quantity of HPRT mRNA levels and converted in reference to the CD45RBhighCD25– subset (= 1).
Results
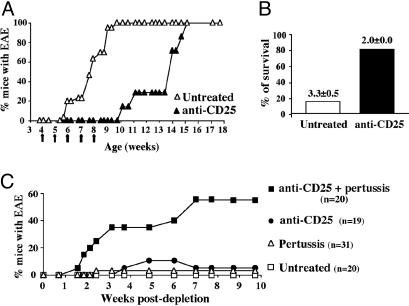
Depleting Anti-CD25 mAb Antibody Targets Both Activated Effector and Regulatory T Cells in Vivo. To establish the contribution of CD25 surface expression to conventional, activated/effector cells and to the TR pool in the same model system, we treated anti-myelin basic protein (MBP) T cell antigen receptor (TCR) transgenic (Tg) mice (23) with a depleting anti-CD25 mAb. Recombination-activating gene (RAG)-deficient monoclonal mice (T/R–) spontaneously develop severe and progressing encephalomyelitis by 2 months of age. In contrast, RAG-competent Tg mice (T/R+) remain healthy, although >90% of the cells are self-reactive, “protected” by TR expressing endogenously encoded TCR (22, 24). Nevertheless, in sick T/R– as in healthy T/R+ mice, ≈5% of all CD4 cells are CD25+ (data not shown). Consistently with the respective phenotype often attributed to activated effector cells and TR (25, 26), the CD4+CD25+ subset detected in T/R– mice expressed higher levels of CD4 and lower levels of CD25 than the CD4+CD25+ T cells observed in T/R+ animals (not shown). Depletion of CD25-expressing cells in T/R– animals prevented EAE when initiated before onset of encephalomyelitis, and interruption of the treatment restored the development of progressing EAE (Fig. 1A). Similar treatment administered to sick animals did not revert the disease process but prevented its progression (Fig. 1B). These findings indicate that newly activated cells express CD25 and that CD25+ cells in this system contain mostly pathological cells. Whether they also encompass a small number of TR, in a too small proportion to ensure tolerance, remains to be assessed.
Fig. 1.
Depletion of both activated and regulatory T cells by anti-CD25 antibody in vivo. T/R– and T/R+ mice were injected with 200 μg of anti-CD25 mAb. (A) One-month-old T/R– animals (n = 7) received five weekly injections and were followed for 4.5 months. The percentage of mice that developed EAE (score ≥2) is represented. (B) Sick T/R– mice (EAE score = 2–3) received a continuous weekly treatment (n = 5). As control, a group of T/R– mice was left untreated (n = 20). Plotted is the percentage of mice alive at 5 months of age and the EAE score of the survivors. Group comparison for the mean EAE score was statistically significant using Student's t test (C) Adult T/R+ mice were treated once with the anti-CD25 mAb (day 0) either alone or together with 200 ng of pertussis toxin (day 0 and 2). Control T/R+ mice were left untreated. EAE level was scored weekly, and plotted is the percentage of mice that developed EAE (score ≥2).
A single injection of the CD25-depleting mAb in healthy T/R+ animals did not lead to significant disease; however, when combined with pertussis administration, ≈55% of the mice developed encephalomyelitis, whereas pertussis alone had no significant effect (Fig. 1C). This result, by revealing that CD25+ cells in the T/R+ animals encompass efficient TR, confirms previous conclusions drawn from adoptive cell transfer experiments (22). More importantly for the present topic, in vivo depletion of all donor T cells by using mAbs to a Thy-1 allotype in T/R– mice, protected by adoptive transfer of normal CD4 cells, was shown to lead to spontaneous severe-progressing EAE (27) whereas we evidence here that depletion of solely CD25+ cells in T/R+ animals does not induce pathology (Fig. 1C). In addition, all T/R+ animals that developed disease upon combined injection of anti-CD25 antibody and pertussis stabilized at a stage of partial hind limbs paralysis (level 2) without progressing to more severe stages of the disease (not shown). Together, these results reveal that administration of CD25-depleting mAb did not lead to total abrogation of regulation in T/R+ mice, confirming that a subset of TR is encompassed in the CD25– cell subset.
The CD25+ TR Pool Is Rapidly Restored in the Periphery After in Vivo Depletion. The results above prompted us to assess the composition of the peripheral CD4 cell pool after anti-CD25 mAb administration in vivo. Because T/R+ animals display a low number of TR, we used normal BALB/c mice to ensure accurate analysis. In a first step, to exclude the potential contribution of newly generated thymic CD4 cells, 4-week-old BALB/c mice were thymectomized and treated with the depleting anti-CD25 mAb 1 month later. Two days after treatment, a maximum of 0.5% of the total CD4 cells in peripheral blood lymphocytes (PBLs), spleen, and LNs stained positive with the 7D4 anti-CD25 mAb, representing <5% of the normal frequency of CD4+CD25+ cells in PBLs (Fig. 2A), spleen and LNs (not shown). In less than a month after depletion, the CD4+CD25+ cell pool progressively recovered normal frequency (Fig. 2 A). Surprisingly, euthymic mice submitted to the same treatment displayed similar kinetics of CD4+CD25+ cell recovery (not shown), indicating that thymic output is not a major factor for peripheral CD25+ T cell homeostasis as shown recently in newborn Tx mice (28). We next assessed whether the repopulating T cells were recently activated or regulatory CD4+ T cells by monitoring their ability to suppress conventional CD4 cell responses to TCR triggering. As shown in Fig. 2B, CD4+CD25+ cells isolated from mice Tx and treated 3 months before with either anti-CD25 mAb or rat IgG were equally efficient in inhibiting the IL-2 production by normal CD25– cells. These results, consistent with a previous report (29), indicate that the peripheral immune system is autonomous to ensure efficient control of TR homeostasis. This control may result from a substantial expansion of the few CD25+ cells that escaped depletion and/or from proficient conversion of other CD4+ T cell subtypes into a CD4+CD25+ TR phenotype.
Fig. 2.
CD4+CD25+ TR recover normal levels after in vivo depletion of Tx mice. Adult-Tx BALB/c(n = 7) mice were injected i.p. with 200 μg of anti-CD25 mAb or rat IgG (controls) twice at 1-week intervals. (A) Peripheral blood lymphocytes were analyzed for the frequency of CD25+ among CD4+ cells at different days after the injection. Shown is the percentage of CD25+ among CD4+ cells in treated animals relative to control animals (n = 7). (B) CD4+CD25+ cells were sort-purified from LNs of either CD25+-depleted (CD25+ Tx-depleted) or from rat IgG-injected (CD25+ Tx control) mice 3 months after depletion and tested for their capacity to suppress IL-2 production by stimulated CD4+CD25– cells. Primary cultures consisted in 2.5 × 103 CD4+CD25– cells isolated from normal mice, stimulated alone or in the presence of different numbers of CD25+ cells isolated from Tx-depleted or Tx control mice. As a negative control, CD25– cells (CD25–) were also tested. Shown is the percentage of inhibition [(cpm in control) – (cpm in experiment)/cpm in control] is plotted versus the ratio of the population tested/CD4+CD25– cell number at the origin of the primary culture.
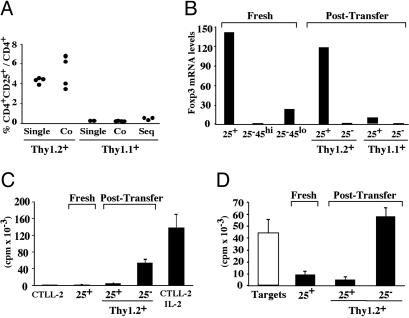
CD25– Foxp3-Expressing Cells Acquire CD25 Expression upon Homeostatic Expansion. To assess the efficiency of CD4+CD25– cells to convert to a CD25+ TR phenotype, we conducted a phenotypic and functional analysis of these cells either Thy1.1+ CD45RBhighCD25– or Thy1.2+ CD45RBlowCD25– 12 days after adoptive transfer into RAG–/– mice. As reported before (6), a significant fraction of the originally CD45RBlowCD25– cells acquired a CD25+ phenotype (≈4.5% in spleen and 15% in LN) whereas the Thy1.1+ cells (originally CD45RBhighCD25–) expressing CD25 were barely detectable (Fig. 3A). More than 80% of the original CD45RBhigh cells became CD45RBlow upon adoptive transfer (not shown); however, sequential transfer of total CD4+CD25– cells (Thy1.2+) in these mice did not increase the frequency of Thy1.1+ cells that converted to a CD25+ phenotype (Fig. 3A). Similar results were obtained in recipients of simultaneous cotransfer, confirming that the two cell subsets are controlled independently for this feature. Finally, Thy1.2+CD45RBlowCD25– cells were mixed with 100-fold fewer Thy1.1+CD4+CD25+ cells before adoptive transfer into RAG–/– animals. This deliberate contamination exceeded that routinely obtained after sort-purification and is similar to that detected in the blood and lymphoid tissues 2 days after administration of depleting anti-CD25 mAb in normal mice. At day 12 posttransfer, Thy1.1+ cells were hardly detectable (average 0.2% and 0.1% of CD4 cells in spleen and mesenteric LN, respectively), whereas the percentage of Thy1.2+ cells expressing the CD25 marker was the same as in single transfer (not shown). It is noteworthy that, inside the CD25+ subset, the contribution of the newly converted Thy1.2+ was therefore >95%. These last results indicate that CD45RBlowCD25– cells undergo efficient conversion to a CD25+ phenotype that largely dominates over the expansion of few contaminating CD4+CD25+ cells. Together these findings demonstrate that acquisition of CD25 surface expression by CD45RBlowCD25– cells in nonimmunized mice is the result of a specific phenotypic modification restricted to a subset of the naturally activated T cell pool.
Fig. 3.
CD45RBlowCD25– cells that acquire in vivo CD25 expression display a regulatory phenotype. RAG2–/– mice received 3 × 105 CD4+CD25– cells either Thy1.2+CD45RBlow or Thy1.1+CD45RBhigh (Single), or an equal number of both cell subsets (Co), and were analyzed 12 days later. For the sequential transfer (Seq), mice that received 3 × 105 Thy1.1+CD45RBhigh cells at day 0 were injected with 3 × 105 Thy1.2+CD4+CD25– cells at day 12 and analyzed at day 24. (A) FACS analysis of splenocytes. Shown is the percentage of CD25+ cells inside a gate either CD4+Thy1.2+ (originally CD25–45RBlo) or CD4+ Thy1.1+ (originally CD25–45RBlo). (B) Foxp3 mRNA levels determined by real-time PCR. (Left) cDNA prepared from freshly isolated (Fresh) CD4+cells, either CD25+ (25+), CD25–CD45RBhigh (25–45hi), or CD25–CD45RBlow (25–45low) served as controls. (Right) cDNA was prepared from CD4+CD25+ or CD4+CD25– cells sort-purified from pooled spleen and LN of either of the single-transfer recipients (Post-Transfer). (C) IL-2 production upon TCR triggering. Thy1.2+CD4+ cells (originally CD25–CD45RBlow) were sort-purified from co-transferred recipients and fractionated as CD25+ or CD25–. Shown is the proliferation of CTLL-2 cells exposed to supernatants of primary cultures that contained 2 × 103 of either cell subset. Control was CD4+CD25+ cells purified from normal C57BL/6 animals. The background (CTLL-2) and the maximum proliferation (CTLL-2 IL-2) are those of CTLL-2 cells maintained in medium either alone or containing saturating amounts of IL-2. (D) Suppression of IL-2 production. As in C except that primary cultures consisted in 2.5 × 103 CD4+CD25– cells isolated from normal mice, stimulated alone (Targets) or in the presence of 1.25 × 103 CD4+ cells (filled bars) either CD25+ or CD25– purified as in C.
We next assessed whether acquisition of CD25 upon homeostatic expansion is a signature of TR by monitoring the level of Foxp3 mRNA in each CD4 subset before and after adoptive transfer (Fig. 3B). Strikingly, the originally CD45RBlowCD25– cells that converted to a CD25+ phenotype displayed levels of Foxp3 mRNA comparable with that of freshly isolated CD25+ cells. In contrast, those that remained CD25– expressed Foxp3 to a lower level than freshly isolated CD45RBlowCD25– cells and rather similar to fresh CD45RBhighCD25– cells. We interpret this result as evidence that CD25– Foxp3-expressing cells inside the CD45RBlow subpopulation selectively express CD25 upon homeostatic expansion. The amount of Foxp3 transcripts in the few CD45RBhighCD25– cells that converted to a CD25+ phenotype remains low, indicating that this cell pool contains recently activated cells. Nevertheless, it is remarkable that the little increase of Foxp3 signal in this cellular subset correlates with a decreased signal (average value 0.2) in the remaining CD25– cells, indicating again that Foxp3-expressing cells convert to a CD25+ phenotype upon homeostatic expansion.
Finally, we tested whether acquisition of CD25 expression by CD45RBlowCD25– cells reveals functional suppressor cells. As references, we used freshly isolated CD4+CD25+ cells. Clearly, the CD25+ but not the CD25– cell subset was unresponsive to TCR stimulation as measured by IL-2 production (Fig. 3C) and suppressed IL-2 production by conventional CD4 cells, to a level comparable with freshly isolated CD4+CD25+ cells (Fig. 3D). We conclude that the CD45RBlowCD25– cells that acquire in vivo CD25 expression display a regulatory phenotype similar to conventional CD4+CD25+ TR.
In Vitro Induction of CD4+CD25– Cells to Suppressor Activity Is Restricted to CD45RBlow Cells. The evidence that CD45RBlowCD25– cells contain Foxp3+ T cells that convert to a CD25+ TR phenotype upon homeostatic expansion prompted us to reevaluate their in vitro suppressor activity. As reported previously (12, 30), freshly isolated CD45RBlowCD25– like CD45RBhighCD25– cells do not show suppressive functions when tested in vitro. However, CD45RBlowCD25– but not CD25–CD45RBhigh cells pretreated with IL-2 and soluble anti-CD3 mAb for 6 days display suppressor function, comparable in efficiency with freshly isolated CD25+ cells (Fig. 4A). Similarly, using a protocol originally described to induce anergic (31) and suppressor (32) cells in vitro, we evidence that CD45RBlowCD25– but not CD25–CD45RBhigh cells pretreated with immobilized anti-CD3 mAb exhibit suppressor activity (Fig. 4B). We propose that these pretreatments selectively expand committed TR and thus mimic in part the homeostatic expansion/activation of the CD25– TR we evidenced above.
Fig. 4.
Activation of CD45RBlowCD25– cells in vitro reveals their regulatory properties. (A) Sorted CD45RBhighCD25– and CD45RBlowCD25– cells (25–45hi and 25–45lo, respectively) maintained for 6 days in culture containing anti-CD3 mAb and IL-2 (IL-2) were washed and cultured with the same number of untreated CD4+CD25– cells, and cultured for another 3 days in the presence of anti-CD3 mAb and antigen-presenting cells. Freshly isolated (Fresh) CD4+CD25+ (25+), CD45RBlowCD25– (25–45RBlo), and CD45RBhighCD25– (25–45hi) cells were used as controls. (B) Sorted CD45RBlowCD25– and CD45RBhighCD25– cells (25–45RBlo and 25–45RBhi, respectively) were stimulated with plate-bound anti-CD3 mAb according to Materials and Methods. The evaluation of their suppressor function was performed by adding them at various ratios to untreated CD4+CD25– cells as in A. Freshly isolated CD4+CD25+ cells (25+fresh) were used as control. The percentage of inhibition of naive T cell proliferation is plotted versus the ratio of the population tested/CD4+CD25– cell number at the origin of the culture, as in Fig. 2B.
CD25+ Regulatory T Cell Expansion and CD25 Surface Expression Are Dissociated. The findings that CD25– TR acquire surface expression of CD25 during homeostatic expansion prompted us to reassess the phenotypic stability of the CD25+ TR isolated from normal mice. As reported before (6, 20, 33), upon adoptive transfer into alymphoid recipients, the vast majority of these cells lose CD25 expression (Fig. 5B). The number of Thy1.2+ cells (originally CD25+) recovered in such transfer experiments is rather low (Fig. 5A), and any proliferative advantage to rare CD25– contaminants in the original preparation may explain this result. However, when the purified Thy1.2+CD4+CD25+ cells were voluntarily contaminated with 1% Thy1.1+CD4+CD25– cells, <10% of the recovered CD4 cells were composed of Thy1.1+ cells, and the frequency of CD25-expressing cells was indistinguishable from that obtained in the pure Thy1.2+ cell transfer group (Fig. 5B). We next confirmed that cotransfer of CD4+CD25– cells at a 1:1 ratio stabilizes to some extent the CD25+ phenotype (33), and further assessed whether the loss of CD25 expression in single transfer is reversible (Fig. 5C). At day 12 posttransfer of Thy1.2+CD25+ cells, a group of mice was analyzed to ensure that ≈80% of the CD4 cells stained negative for CD25 (Fig. 5B), whereas another group received 3 × 105 Thy1.1+CD25– cells. After an additional 12 days, on average, 77% of the Thy1.2+ cells were CD25+. Because the Thy1.2+ cells did not expand significantly after this secondary transfer (Fig. 5A), we favor the interpretation that these cells underwent a reconversion to a CD25+ phenotype. It it noteworthy that the Thy1.2+ cells that were overall CD25– at the time of the second transfer, while converting to a CD25+ phenotype, were also able to control the in vivo expansion of the infused Thy1.1+CD4+CD25– population (Fig. 5A), indicating that loss of CD25 expression does not correlate with loss of function.
Fig. 5.
Functional CD4+CD25+ cells can lose and regain CD25 expression. RAG2–/– mice received CD4 cells either Thy1.2+CD25+ or Thy1.1+CD25– alone (Single), together in same number (Co), at a 100:1 ratio (1%), or sequentially (Seq) and were analyzed 12 days after the last transfer. (A) Efficient control of Thy1.1+ expansion by Thy1.2+ cells. The number of Thy1.1+ (Left) and Thy1.2+ (Right) cells recovered from the spleen is shown for each animal. (B) Loss of CD25 expression upon adoptive transfer. CD4+ cells from mice recipient of 3 × 105 Thy1.2+CD25+ cells transferred alone (Single) or together with 3 × 103 Thy1.1+CD25– cells are analyzed for CD25 and Thy1.1 expression. (C) In cotransfer experiments, CD4+CD25– cells maintain and restore CD25 expression on Thy1.2+ CD4+CD25+ cells. Shown is the CD25 expression on Thy1.2+ cells recovered from mice that received an equal number of Thy1.2+CD4+CD25+ and Thy1.1+CD4+CD25– cells either at the same time (Co) or 12 days apart (Seq).
Discussion
The present work establishes that, upon disruption of homeostasis, CD4 cell subsets contribute to the pool of TR phenotypically defined as CD25+ and Foxp3+ by recruitment from a peripheral reservoir of differentiated CD25– TR. Membrane expression of CD25 appears therefore as a signature of activation equally for conventional T cells and TR. This finding bears several consequences for the potential usage of CD25 as a therapeutic target and for our understanding of TR origin and dynamics.
We show that in vivo depletion of CD25-expressing cells can induce both protection and susceptibility to the very same disease. Depleting anti-CD25 mAbs have already been used to target pathological cells in autoimmune mice and humans. For instance, similarly to what we observed in T/R– animals, clinical trials testing the sustained usage of anti-CD25 mAb in combined therapies for multiple sclerosis gave promising results (34). On the other hand, and similarly to what we report here for the T/R+ animals, a single anti-CD25 mAb administration targets TR and sets a time window where immunization protocols gain in efficiency, an approach explored to improve tumor therapies (35). Short-lasting anti-CD25 mAb administration in mice does not induce or accelerate autoimmune diseases per se unless it is administered early in life or together with self-antigen and strong adjuvants (29, 36). The bulk of our analysis strongly suggests that this temporal limitation and the relative safety of these approaches must rely on the replenishment of the CD25+ TR pool by recruitment of peripheral differentiated CD25– TR. Numerous efforts have been developed worldwide to identify molecular targets that would strictly distinguish all TR from activated cells to improve the efficiency of TR therapeutic depletion protocols. Our results, together with the indication that the number of CD25– cells that can convert to a CD25+ TR phenotype is limited (37), seem to predict that the use of these new tools may lead to an irreversible highly challengeable state of tolerance because it may exhaust the pool of TR.
We established that, upon homeostatic expansion, Foxp3-expressing cells encompassed in the CD45RBlowCD25– subset are the cells contributing to the pool of converted CD25+ TR. We favor the idea that, under similar conditions, the few CD25– TR cells encompassed in the CD45high subpopulation are also those converting to a Foxp3+ CD25+ phenotype. In support of this proposition, it has been shown that Foxp3 expression, although rather low, is detectable in CD45high cells but not in differentiated helper cells (13). Whether acquisition of surface CD25 by Foxp3+ TR is necessary for their regulatory function remains to be formally established. However, we confirmed that CD25 is an activation marker and activated TR are more efficient regulatory cells than untreated cells (12, 19). Our results therefore indicate that, at the steady state, a normal immune system maintains a reservoir of “inactive” TR. Both the frequency of cells converting to a CD25+ phenotype upon homeostatic expansion and their level of Foxp3 mRNA expression indicate that TR contribute to ≈10–20% of the CD45RBlowCD25– cell population, which approximately represents a reservoir of an additional 2 million TR in a normal mouse. This reservoir may serve the purpose of keeping available a large number of inactive TR that could rapidly be recruited to the “active” TR pool upon immune activities. In turn, this subdivision may ensure the robustness of TR-mediated immune regulation while avoiding immunological paralysis by an otherwise excess of regulation. Infections not only trigger immune responses but also often associate with a state of thymic involution and transient lymphopenia, conditions that would favor the recruitment of these cryptic TR.
The original demonstrations that TR are generated intrathymically and selected by recognition of antigens expressed on thymic epithelial cells (TEC) (reviewed in refs. 38 and 39) gained further support from the recent revelation that TR represent a unique differentiative pathway (13–15) that is adopted by CD4 cells with “high avidity” for TEC-antigens (40, 41). The present finding that CD25 expression by TR is fully labile prompted us to propose that, in physiological conditions, peripheral TR and conventional CD4 T cells are strictly compartmentalized according to their previous thymic experience (Fig. 6). Acquisition, maintenance, or loss of CD25 expression by both conventional CD4+ Foxp3– and Foxp3+ TR rely solely on their encounter with activation signals. In uninfected individuals, activation of conventional CD4 cells is limited because their repertoire is biased for low affinity against self-antigens, at least for those encountered in the thymus (41), and does not require exogenous IL-2 (42). The nature of the activating signals for TR remains to be fully established; however, there is little doubt that IL-2 (19), produced mostly by conventional CD4 T cells, and TCR engagement by self-antigen/MHC complexes (16, 40, 41) are crucial. Additional signals are provided by inflammatory cytokines and endogenous ligands binding to the Toll-like receptors expressed by TR (12). Whether the CD25– and CD25+ TR subsets cover different TCR reactivities in normal individuals or whether these cells are highly labile and therefore rapidly switch from an activating to a nonactivating environment remains to be clarified. In addition, activated TR may well limit CD25– TR conversion to a CD25+ phenotype as they do for naive cells, a feature that would explain the remarkable stability of TR CD25+ versus CD25– distribution at the steady state.
Fig. 6.
CD25 is an activation marker of both TR and conventional CD4 cell subsets. In the thymus, conventional CD4 cells undergo positive selection, and these CD4+Foxp3–cells exit the thymus as naive CD45RBhighCD25–. Once in the periphery, if they receive activating signals, they acquire an activated phenotype characterized by a low level of CD45RB and high level of CD25 expression. Exit from the environment where the activation signals are provided associates with the loss of CD25 expression but maintenance of an antigen experienced CD45RBlow phenotype. Thymic TR commitment and differentiation require interaction with activating ligands (gray area), and TR exit the thymus with a CD25+CD45RBlow phenotype. Once in the periphery, Foxp3 expression is maintained. If specific activating signals are absent or below a certain threshold, CD25 expression is lost but CD45RB expression remains low. Reexposure to activation signals reverts this phenotype, and TR may reacquire a CD25 surface expression.
Finally, our findings that surface expression of CD25 is labile in differentiated Foxp3-expressing TR may serve as a word of caution for the interpretation of experiments aiming at the induction of “naive” peripheral CD4+CD25– T cell differentiation to Foxp3+CD25+ TR.
Acknowledgments
We thank members of the Instituto Gulbenkian de Ciência Cell Imaging Unit for cell sorting assistance and antibodies production, and we thank the mouse facility team. We thank Dinis Calado and Shohei Hori for their help in real-time PCR optimization. We thank Jorge Carneiro, Werner Haas, and Antonio Coutinho for valuable suggestions. This work was supported by the Fundação para a Ciência e a Tecnologia (FCT), Portugal, with the coparticipation of the Fundo Europeu de Desenvolvimento Regional (FEDER) through Grants POCTI/MGI/43063/2001 and POCTI/MGI/46477/2002 and fellowships to S.Z., T.L.-C., I.C., and M.R.
Author contributions: S.Z., T.L.-C., I.C., and J.D. designed research; S.Z., T.L.-C., I.C., M.F.M.-F., M.R., and J.D. performed research; S.Z., T.L.-C., I.C., M.F.M.-F., and J.D. analyzed data; and S.Z., T.L.-C., and J.D. wrote the paper.
Abbreviations: TR, regulatory T cells; RAG, recombination-activating gene; TCR, T cell antigen receptor; T/R– and T/R+, anti-myelin basic protein TCR transgenic mice homo- and heterozygous for a null mutation of the RAG-1 gene; Tx, thymectomized; EAE, experimental autoimmune encephalomyelitis; LN, lymph node; PE, phycoerythrin.
References
- 1.Coutinho, A., Kazatchkine, M. D. & Avrameas, S. (1995) Curr. Opin. Immunol. 7, 812–818. [DOI] [PubMed] [Google Scholar]
- 2.Lee, W. T., Yin, X. M. & Vitetta, E. S. (1990) J. Immunol. 144, 3288–3295. [PubMed] [Google Scholar]
- 3.Powrie, F., Leach, M. W., Mauze, S., Caddle, L. B. & Coffman, R. L. (1993) Int. Immunol. 5, 1461–1471. [DOI] [PubMed] [Google Scholar]
- 4.Annacker, O., Burlen-Defranoux, O., Pimenta-Araujo, R., Cumano, A. & Bandeira, A. (2000) J. Immunol. 164, 3573–3580. [DOI] [PubMed] [Google Scholar]
- 5.Mittrucker, H. W. & Kaufmann, S. H. (2004) Eur. J. Immunol. 34, 306–312. [DOI] [PubMed] [Google Scholar]
- 6.Annacker, O., Pimenta-Araujo, R., Burlen-Defranoux, O., Barbosa, T. C., Cumano, A. & Bandeira, A. (2001) J. Immunol. 166, 3008–3018. [DOI] [PubMed] [Google Scholar]
- 7.Curotto de Lafaille, M. A., Muriglan, S., Sunshine, M. J., Lei, Y., Kutchukhidze, N., Furtado, G. C., Wensky, A. K., Olivares-Villagomez, D. & Lafaille, J. J. (2001) J. Exp. Med. 194, 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann, J., Huehn, J., de la Rosa, M., Maszyna, F., Kretschmer, U., Krenn, V., Brunner, M., Scheffold, A. & Hamann, A. (2002) Proc. Natl. Acad. Sci. USA 99, 13031–13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alyanakian, M. A., You, S., Damotte, D., Gouarin, C., Esling, A., Garcia, C., Havouis, S., Chatenoud, L. & Bach, J. F. (2003) Proc. Natl. Acad. Sci. USA 100, 15806–15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3, 135–142. [DOI] [PubMed] [Google Scholar]
- 11.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caramalho, I., Lopes-Carvalho, T., Ostler, D., Zelenay, S., Haury, M. & Demengeot, J. (2003) J. Exp. Med. 197, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057–1061.12522256 [Google Scholar]
- 14.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4, 330–336. [DOI] [PubMed] [Google Scholar]
- 15.Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. (2003) Nat. Immunol. 4, 337–342. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh, C. S., Liang, Y., Tyznik, A. J., Self, S. G., Liggitt, D. & Rudensky, A. Y. (2004) Immunity 21, 267–277. [DOI] [PubMed] [Google Scholar]
- 17.Kono, T., Minami, Y. & Taniguchi, T. (1993) Semin. Immunol. 5, 299–307. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi, S. (2004) Annu. Rev. Immunol. 22, 531–562. [DOI] [PubMed] [Google Scholar]
- 19.Furtado, G. C., Curotto de Lafaille, M. A., Kutchukhidze, N. & Lafaille, J. J. (2002) J. Exp. Med. 196, 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavin, M. A., Clarke, S. R., Negrou, E., Gallegos, A. & Rudensky, A. (2002) Nat. Immunol. 3, 33–41. [DOI] [PubMed] [Google Scholar]
- 21.Almeida, A. R., Legrand, N., Papiernik, M. & Freitas, A. A. (2002) J. Immunol. 169, 4850–4860. [DOI] [PubMed] [Google Scholar]
- 22.Hori, S., Haury, M., Coutinho, A. & Demengeot, J. (2002) Proc. Natl. Acad. Sci. USA 99, 8213–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafaille, J. J., Nagashima, K., Katsuki, M. & Tonegawa, S. (1994) Cell 78, 399–408. [DOI] [PubMed] [Google Scholar]
- 24.Olivares-Villagomez, D., Wang, Y. & Lafaille, J. J. (1998) J. Exp. Med. 188, 1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baecher-Allan, C., Brown, J. A., Freeman, G. J. & Hafler, D. A. (2001) J. Immunol. 167, 1245–1253. [DOI] [PubMed] [Google Scholar]
- 26.Annacker, O., Pimenta-Araujo, R., Burlen-Defranoux, O. & Bandeira, A. (2001) Immunol. Rev. 182, 5–17. [DOI] [PubMed] [Google Scholar]
- 27.Hori, S., Haury, M., Lafaille, J. J., Demengeot, J. & Coutinho, A. (2002) Eur. J. Immunol. 32, 3729–3735. [DOI] [PubMed] [Google Scholar]
- 28.Dujardin, H. C., Burlen-Defranoux, O., Boucontet, L., Vieira, P., Cumano, A. & Bandeira, A. (2004) Proc. Natl. Acad. Sci. USA 101, 14473–14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurie, K. L., Van Driel, I. R. & Gleeson, P. A. (2002) Immunol. Cell Biol. 80, 567–573. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi, T., Kuniyasu, Y., Toda, M., Sakaguchi, N., Itoh, M., Iwata, M., Shimizu, J. & Sakaguchi, S. (1998) Int. Immunol. 10, 1969–1980. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins, M. K., Chen, C. A., Jung, G., Mueller, D. L. & Schwartz, R. H. (1990) J. Immunol. 144, 16–22. [PubMed] [Google Scholar]
- 32.Lombardi, G., Sidhu, S., Batchelor, R. & Lechler, R. (1994) Science 264, 1587–1589. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura, E., Sakihama, T., Setoguchi, R., Tanaka, K. & Sakaguchi, S. (2004) Int. Immunol. 16, 1189–1201. [DOI] [PubMed] [Google Scholar]
- 34.Bielekova, B., Richert, N., Howard, T., Blevins, G., Markovic-Plese, S., McCartin, J., Wurfel, J., Ohayon, J., Waldmann, T. A., McFarland, H. F. & Martin, R. (2004) Proc. Natl. Acad. Sci. USA 101, 8705–8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu, J., Yamazaki, S. & Sakaguchi, S. (1999) J. Immunol. 163, 5211–5218. [PubMed] [Google Scholar]
- 36.McHugh, R. S. & Shevach, E. M. (2002) J. Immunol. 168, 5979–5983. [DOI] [PubMed] [Google Scholar]
- 37.Curotto de Lafaille, M. A., Lino, A. C., Kutchukhidze, N. & Lafaille, J. J. (2004) J. Immunol. 173, 7259–7268. [DOI] [PubMed] [Google Scholar]
- 38.Le Douarin, N., Corbel, C., Bandeira, A., Thomas-Vaslin, V., Modigliani, Y., Coutinho, A. & Salaun, J. (1996) Immunol. Rev. 149, 35–53. [DOI] [PubMed] [Google Scholar]
- 39.Modigliani, Y., Bandeira, A. & Coutinho, A. (1996) Immunol. Rev. 149, 155–120. [DOI] [PubMed] [Google Scholar]
- 40.Bensinger, S. J., Bandeira, A., Jordan, M. S., Caton, A. J. & Laufer, T. M. (2001) J. Exp. Med. 194, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A. & Caton, A. J. (2001) Nat. Immunol. 2, 301–306. [DOI] [PubMed] [Google Scholar]
- 42.Sadlack, B., Lohler, J., Schorle, H., Klebb, G., Haber, H., Sickel, E., Noelle, R. J. & Horak, I. (1995) Eur. J. Immunol. 25, 3053–3059. [DOI] [PubMed] [Google Scholar]