Abstract
A previous study demonstrated that particulate matter (≤2.5 µm in diameter; PM2.5) may promote atherosclerosis. However, the underlying mechanisms of PM2.5 in human microvascular endothelial cells (HMEC-1) remain to be elucidated. It has been reported that inflammation and oxidative stress can be reduced by curcumin, and in the present study, the aim was to investigate the protective effects of curcumin on PM2.5-induced oxidative stress and inflammatory response in HMEC-1. HMEC-1 were stimulated with curcumin and PM2.5. The HMEC-1 viability and apoptosis were detected by MTT and annexin V-fluorescein isothiocyanate/propidium iodide assays. The levels of oxidized low-density lipoprotein (oxLDL), tumor necrosis factor (TNF)-α and interleukin (IL)-8 were detected by ELISA. The intracellular reactive oxygen species formation in HMEC-1 was detected using flow cytometry and 2′,7′-dichlorofluorescin diacetate. Nuclear factor (NF)-κB, caspase 3 activity and adhesion molecule expression were also investigated. The results suggested that curcumin reduced PM2.5 (300 µg/ml)-induced cell apoptosis and intracellular caspase 3 activity in HMEC-1. ELISA analysis demonstrated that curcumin reduced PM2.5-induced oxLDL, TNF-α and IL-8 levels. Curcumin induced NF-κB, cell adhesion molecule 1 and vascular cell adhesion protein 1 expression. Thus, curcumin treatment may reduce PM2.5-induced oxidative stress and inflammation in HMEC-1. In summary, it was indicated that the effects of PM2.5 are associated with oxLDL via the NF-κB signaling pathway, thereby inducing PM2.5 mediated oxidative and inflammatory responses. The results also suggested that curcumin may be able to reduce the oxidative and inflammatory effects of PM2.5 in HMEC-1.
Keywords: curcumin, PM2.5, oxidized low-density lipoprotein, human microvascular endothelial cells, reactive oxygen species, inflammation
Introduction
Curcumin is regarded as a direct and an indirect antioxidant agent through its reduction of reactive oxygen species (ROS) (1,2) and enhancement of the expression of cytoprotective enzymes, including glutathione-S-transferase (3,4). It has been reported that curcumin induces antioxidant response mechanisms by regulating transcription factors including the nuclear factor (NF)-κB pathway (5–7). The nuclear factor (NF)-κB pathway modulates DNA repair enzymes and the expression of anti-inflammatory response proteins. These proteins increased the ability of the cell to repair oxidative damage. Curcumin is therefore able to induce a protective effect and activate the NF-κB pathway in cells following exposure to oxidative and inflammatory influences.
There is a connection between the exposure to PM2.5 and cardiopulmonary diseases (8–10). Between 2014 and 2016, the concentration of PM2.5 has risen to >600 µg/m3 in Shanghai and Beijing, particularly in winter (11–15). There is growing concern about protecting people from PM2.5 injury. Although a number of findings have produced experimental data confirming that PM2.5 injures the cardiovascular system and other organs (16,17), knowledge of the biological mechanisms remains limited.
The vascular endothelial cells (ECs) are the primary organ system exposed to PM2.5, and have therefore been inferred to be susceptible. In previous studies, HUVECs were treated with PM2.5 to detect the effects on ECs viability and apoptosis (18,19). These studies demonstrated that PM2.5 induced HUVECs apoptosis via ROS expression. ECs dysfunction is regarded as an important marker in the development of atherosclerosis (AS) (20). A previous study also demonstrated that air pollution enhanced the atherosclerosis pathogenesis via abnormal oxidized lipid accumulation in vessels in vivo (21). Oxidized low-density lipoprotein (oxLDL), a key risk factor for atherosclerosis, has several functions during AS development, including inducing EC apoptosis (22,23). Ox-LDL induces EC apoptosis by eliciting ROS expression. A previous study demonstrated that PM2.5 (200 µg/ml) induces apoptosis in HUVECs via the activation of ROS and that a direct cytotoxic effect was observed in HUVECs (24). Therefore, new strategies are required to counter PM2.5-induced injury. The strategies may enhance the antioxidant potential of EC and in this context, curcumin has a number of biological effects including direct and indirect antioxidant response in animal and cell models.
A number of previous studies have demonstrated that PM2.5 and oxLDL induce the inflammatory response (18,25). The EC adhesion molecules vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) are the mark of the inflammatory response. A previous study demonstrated that oxLDL increased the surface expression of ICAM-1 in HUVECs (26). The present study investigated whether curcumin could regulate PM2.5-treated HMEC-1 adhesion molecule expression.
The purpose of the present study was to investigate the protective effects of curcumin on PM2.5-induced HMEC-1 cytotoxicity. It demonstrated, for the first time to the best of the authors' knowledge, that curcumin reduced PM2.5-induced oxLDL-mediated vascular inflammation in HMEC-1. In addition, curcumin countered PM2.5-enhanced ROS, ICAM-1 and VCAM-1 expression levels, which serve a key role in the inflammatory process via the expression of NF-κB. An improved understanding of the multiple functions of curcumin against PM2.5-induced vascular inflammation may inform the prevention and treatment of PM2.5-induced injury.
Materials and methods
Materials
Curcumin [1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; high purity ≥98.5%] was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). PM2.5 (National Institute of Standards and Technology Boulder Laboratories, Boulder, CO, USA) was suspended in PBS for 24 h and centrifuged at 17,000 × g and 4°C for 10 min.
HMEC-1 culture
Human microvascular endothelial cells (HMEC-1) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 U/ml penicillin with 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere under 5% CO2 at 37°C.
Cell viability assay
MTT assay was used to evaluate cell viability. In the MTT assay, HMEC-1 (1×104 cells/ml) were plated in 96-well plates and treated with curcumin (0–50 µM) or PM2.5 (0–600 µg/ml) for 24 h. Then, 20 µl MTT was added to each well, and the cells were incubated at 37°C for 4 h. The supernatant was removed and 150 µl dimethyl sulfoxide was added to each well for 10 min in darkness. The optical density was calculated at 490 nm with a microplate reader.
Analysis of apoptotic cells
Apoptotic cells were determined by flow cytometry using annexin V-fluorescein isothiocyanate/propidium iodide staining following the manufacturer's protocols.
Measurement of intracellular oxidative stress
To determine whether PM2.5 affected the ROS levels of HMEC-1, a 2′,7′-dichlorofluorescin diacetate (DCFDA) kit (Sigma-Aldrich; Merck KGaA) was used. Curcumin/PM2.5 treated HMEC-1 (2×105 cells/well) were added to DCFDA (20 µM) for 20 min, washed with PBS and then the ROS expression was analyzed by flow cytometry All experiments were repeated three times.
The malondialdehyde (MDA) level and superoxide dismutase (SOD) activity of HMEC-1 were detected by a Lipid Peroxidation (MDA) assay kit (Sigma-Aldrich; Merck KGaA) and SOD assay kit (Sigma-Aldrich; Merck KGaA), respectively.
Measurement of cytokine levels
The plasma oxLDL was measured using an ELISA kit (cat. no. ABIN366743; Cusabio Biotech Co., Ltd., Wuhan, China) as a marker of oxidative stress. Levels of tumor necrosis factor (TNF)-α (cat. no. DTA00C; R&D Systems, Inc., Minneapolis, MN, USA), interleukin (IL)-8 (cat. no. D8000C; R&D Systems, Inc.), ICAM-1 (cat. no. ELHS-ICAM1-1; RayBiotech, Inc., Norcross, GA, USA) and VCAM-1 (cat. no. ab100661; Abcam, Cambridge, UK) were also measured by ELISA kits, according to the manufacturer's protocols.
NF-κB p65 transcription factor assay
To determine NF-κB activity, the nuclear fractions of curcumin/PM2.5 treated HMEC-1 were isolated and measured by NF-κB p65 Transcription Factor Assay kit (Abcam). The assay was conducted according to the manufacturer's protocols.
Measurement of caspase 3 activity
The activity of caspase 3 was measured using a Caspase Activity Detection kit (Sigma-Aldrich; Merck KGaA) was detected according to the manufacturer's protocols. The cells were lysed using the colorimetric buffers included in the Caspase Activity Detection kit. Proteins were centrifuged at 12,000 × g for 10 min at 4°C and measured by the BCA Protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Subsequently, 100 µg protein was incubated with 5 µl caspase 3 substrate (Ac-DEVD-pNA) in a 96-well plate. The activity of caspase 3 was determined using a spectrophotometer at 405 nm.
Statistical analysis
The experimental results are expressed as the mean ± standard error and are accompanied by a number of observations. For analysis of the results, one-way analysis of variance was used with the post-hoc Bonferroni's test for multiple comparisons using SigmaStat software, version 3.5 (Systat Software, Inc., San Jose, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Curcumin reduces PM2.5-induced HMEC-1 apoptosis
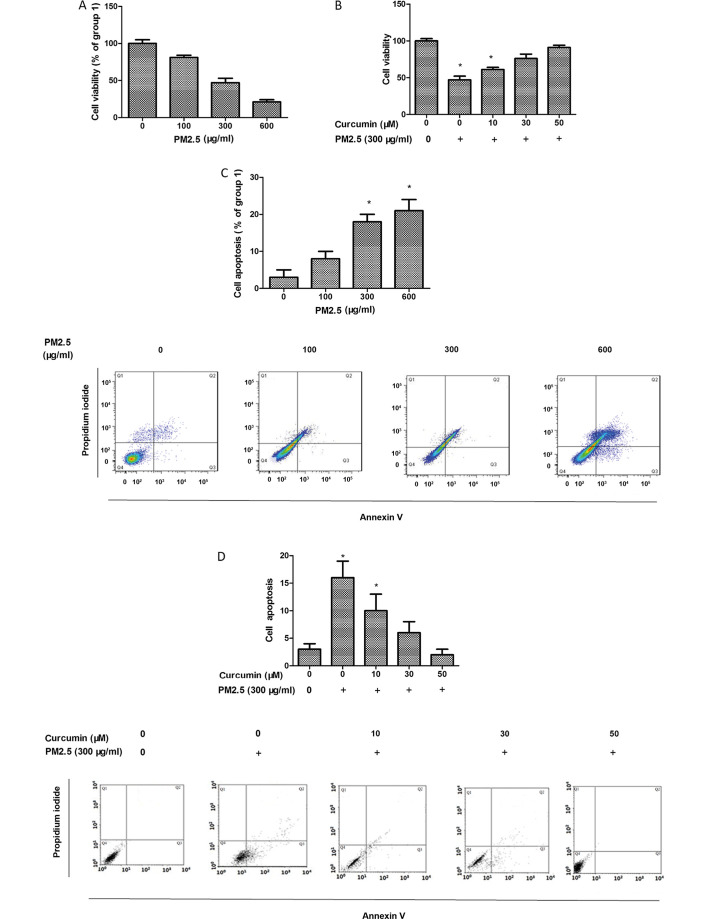
HMEC-1 viability was assessed using the MTT assay and the data demonstrated that PM2.5 reduced HMEC-1 viability (Fig. 1A). HMEC-1 viability remained unchanged following 5–50 µM curcumin treatment for 24 h (data not shown). Therefore, 50 µM curcumin was used for subsequent experiments. In the cells exposed to PM2.5, curcumin pretreatment increased HMEC-1 viability (Fig. 1B).
Figure 1.
Effect of curcumin on PM2.5-induced proliferation and apoptosis of HMEC-1. (A) Effect of PM2.5 on HMEC-1 proliferation by MTT. (B) Effect of curcumin against PM2.5-induced proliferation by MTT. (C) Apoptosis of HMEC-1 following PM2.5 treatment assessed using annexin V-fluorescein isothiocyanate/propidium iodide assay. (D) Apoptosis of HMEC-1 pretreated with curcumin following PM2.5 treatment assessed using annexin V-fluorescein isothiocyanate/propidium iodide assay. Cells in Q2 and Q3 were regarded as apoptotic cells. *P<0.05 vs. control (n=3 independent experiments). PM2.5, particulate matter ≤2.5 µm diameter; HMEC-1, human microvascular endothelial cells.
HMEC-1 apoptosis was detected using flow cytometry. As presented in Fig. 1C, PM2.5 enhanced HMEC-1 apoptosis. In addition, the protective effect of curcumin against PM2.5-induced HMEC-1 injury was also assessed. Curcumin eliminated PM2.5-induced HMEC-1 apoptosis (Fig. 1D).
Curcumin reduces PM2.5-induced oxidative stress in HMEC-1
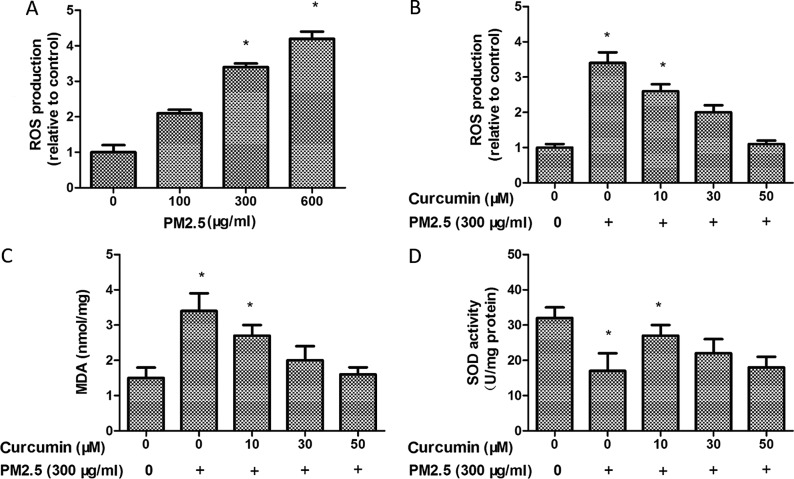
The key hypotheses concerning the effects of PM2.5 is the ability to enhance oxidative stress. The ROS production was observed using H2DCF-DA by flow cytometry. It was observed that PM2.5 treatment induced the production of ROS in HMEC-1 (Fig. 2A). However, ROS production was decreased following curcumin (50 µM) pretreatment for 24 h prior to PM2.5 exposure (Fig. 2B).
Figure 2.
Effect of curcumin on PM2.5-induced oxidative stress in HMEC-1. (A) Intracellular ROS production in HMEC-1 with PM2.5 treatment. (B) Effect of curcumin against PM2.5-induced ROS levels. (C) Effect of curcumin against PM2.5-induced MDA levels. (D) Effect of curcumin against PM2.5-induced SOD levels. *P<0.05 vs. control (n=3 independent experiments). PM2.5, particulate matter ≤2.5 µm diameter; HMEC-1, human microvascular endothelial cells; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase.
The MDA level was markedly increased in the PM2.5 group (Fig. 2C). By contrast, SOD activity decreased in the PM2.5 group compared with the control group (Fig. 2D). PM2.5 significantly decreased the MDA level (Fig. 2C) and enhanced SOD activity (Fig. 2D) in the curcumin pretreated HMEC-1 group compared with the PM2.5 group. Therefore, curcumin may block PM2.5-induced oxidative stress in HMEC-1.
Curcumin reduces PM2.5-induced oxLDL expression in HMEC-1
Fig. 3 demonstrates that plasma oxLDL level was overexpressed in the PM2.5 group compared with the control group. The data suggested that PM2.5 induces oxidative stress via the oxLDL level. Treatment with curcumin (50 µM) decreased the expression of oxLDL in HMEC-1 exposed to PM2.5 (Fig. 3).
Figure 3.
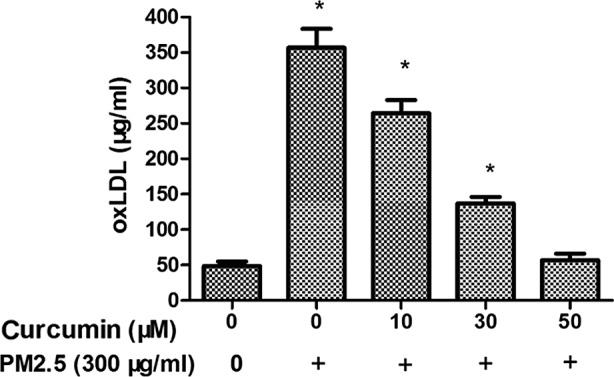
Effect of curcumin on PM2.5-induced oxLDL expression in HMEC-1. The intracellular oxLDL production in HMEC-1 with treatment as indicated. *P<0.05 vs. control (n=3 independent experiments). PM2.5, particulate matter ≤2.5 µm diameter; oxLDL, oxidized low-density lipoprotein; HMEC-1, human microvascular endothelial cells.
Curcumin reduces PM2.5-induced inflammatory responses in HMEC-1
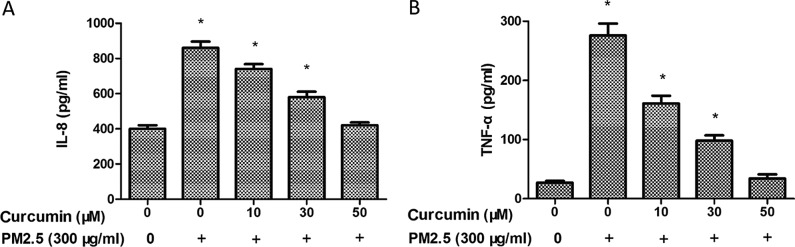
To further investigate the inflammatory effects of PM2.5 in HMEC-1, the expression of inflammatory chemokines was measured by ELISA. As demonstrated in Fig. 4, PM2.5 exposure induced IL-8 (Fig. 4A) and TNF-α (Fig. 4B) levels in the PM2.5 groups. A significant decrease of the expression of IL-8 (Fig. 4A) and TNF-α was identified in HMEC-1 pretreated with curcumin prior to PM2.5 exposure (Fig. 4B).
Figure 4.
Effect of curcumin on PM2.5-induced the expression of (A) IL-8 and (B) TNF-α in HMEC-1. ELISA analysis for IL-8 and TNF-α in HMEC-1 following treatment with curcumin and PM2.5. *P<0.05 vs. control (n=3 independent experiments). PM2.5, particulate matter ≤2.5 µm diameter; IL-8, interleukin-8; TNF-α, tumor necrosis factor-α; HMEC-1, human microvascular endothelial cells.
Curcumin reduces PM2.5-induced caspase 3 activity in HMEC-1
The caspase family of cysteine proteases have a key role in cell death, and caspase 3 is an important member of the caspase family. Therefore, the caspase 3 activity in PM2.5-treated HMEC-1 was investigated. As demonstrated in Fig. 5, the activity of caspase 3 in the PM2.5 group was increased. Furthermore, the activity of caspase 3 was suppressed in HMEC-1 pretreated with curcumin prior to PM2.5 exposure.
Figure 5.
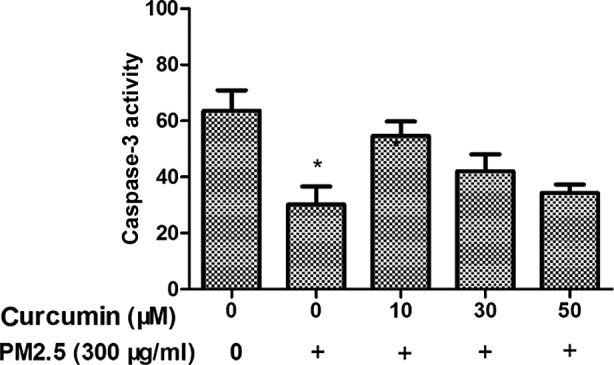
Effect of curcumin on PM2.5-induced expression of caspase 3 in HMEC-1. *P<0.05 vs. control (n=3 independent experiments). PM2.5, particulate matter ≤2.5 µm diameter; HMEC-1, human microvascular endothelial cells.
Curcumin reduces PM2.5-induced NF-κB signaling in HMEC-1
To investigate whether NF-κB is regulated by PM2.5 treatment, phosphorylated (p)NF-κB expression was examined. As presented in Fig. 6, PM2.5 induced intracellular pNF-κB levels in HMEC-1 (P<0.05). Fig. 6 demonstrates the effects of pretreatment with curcumin on PM2.5-induced pNF-κB upregulation in HMEC-1.
Figure 6.
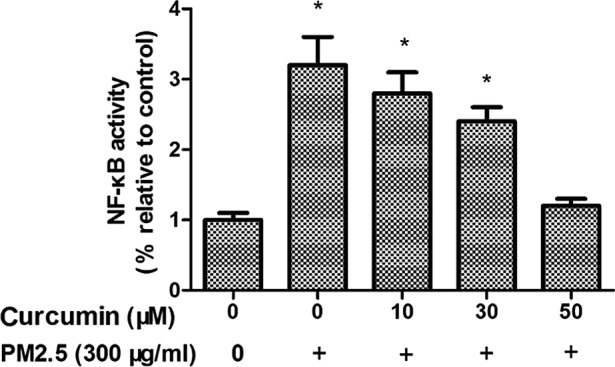
Effect of curcumin on PM2.5-induced activity of NF-κB in HMEC-1. *P<0.05 vs. control (n=3 independent experiments). PM2.5, particulate matter ≤2.5 µm diameter; NF-κB, Nuclear factor κ B; HMEC-1, human microvascular endothelial cells.
Curcumin attenuates PM2.5 induced adhesion molecules expression in HMEC-1
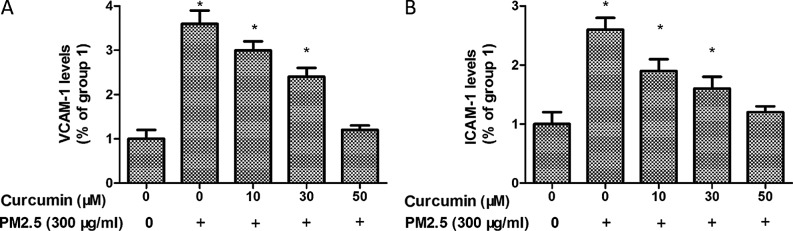
To further analyze the potential inflammatory effect of PM2.5 in HMEC-1 cells, ICAM-1 and VCAM-1 expression were identified. As demonstrated in Fig. 7, the levels of ICAM-1 and VCAM-1 were significantly upregulated by PM2.5 treatment.
Figure 7.
Curcumin suppresses PM2.5-induced expression of (A) VCAM-1 and (B) ICAM-1 in HMEC-1. *P<0.05 vs. control (n=3 independent experiments). PM2.5, particulate matter ≤2.5 µm diameter; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; HMEC-1, human microvascular endothelial cells.
In addition, pretreated with curcumin induced a significant reduction of ICAM-1 and VCAM-1 levels in PM2.5 exposed HMEC-1 (Fig. 7).
Discussion
Vascular endothelial injury leads to vascular disease in patients. Previous studies have demonstrated that PM2.5 induces oxidative stress (27–30). Notably, epidemiological and clinical studies have indicated that air pollution is associated with the development of vascular disease. However, the exact cellular mechanisms of PM2.5-induced vascular endothelial injury remain to be elucidated. There are no studies, to the best of the authors' knowledge, concerning the potential protective effects of curcumin on PM2.5-induced vascular endothelial injury. The implication of the present study is that PM2.5 markedly induced oxidative stress and inflammation in HMEC-1. Curcumin reversed the effects of PM2.5 by decreasing the expression of ROS and inhibiting inflammation via the NF-κB pathway.
Increasing evidence has suggested that PM2.5 induces cells apoptosis (31–33), however the precise molecular mechanisms of PM2.5-induced apoptosis remain unclear, although it is known that PM2.5 decreases viability of HUVECs in vitro (14). The present study documented that PM2.5 could promote HMEC-1 apoptosis by activity the caspase 3.
Certain previous studies have suggested that lipid levels are associated with PM2.5 (34–36), although this remains to be confirmed. A previous study indicated that exposure to air pollution aggravated the oxLDL levels in vivo (21). OxLDL is well known to trigger the ECs to induce adhesion molecules and chemotactic cytokines expression, attracting monocytes to the vascular wall for an inflammatory response. ECs secrete inflammatory cytokines that promote the proliferation and migration of smooth-muscle cells, resulting in atherosclerotic lesion formation. It was identified that PM2.5 exposure induced an increase in oxLDL levels in the present study and it has been previously reported that PM2.5 exposure is a potential pathogenic mediator for atherogenesis in vivo (37). Therefore, oxLDL levels were associated with PM2.5 exposure. Further studies are required to explain the effects of PM2.5 exposure on lipid metabolism and its role.
Activation of IL-8 and TNF-α has been associated with vascular inflammation. The data from the present study demonstrated that PM2.5 induced IL-8 and TNF-α release in HMEC-1. ROS overexpression is a mediator of numerous biological processes, including cell inflammation. ROS overproduction has additionally been regarded as a potential mediator of vascular diseases. The present study suggested that PM2.5 induced vascular inflammation via ROS activation. It also identified that PM2.5 increased the expression of ICAM-1 and VCAM-1, which mediate vascular inflammation. The results of the present study suggested that PM2.5 exhibits a number of effects on vascular inflammation. In addition, it confirmed that curcumin reversed PM2.5-induced oxidative stress in HMEC-1 through the inhibition of ROS. Curcumin decreased oxLDL, IL-8, TNF-α, ICAM-1 and VCAM-1 expression and increased cell viability in the context of PM2.5 treatment. These results suggested that curcumin protected HMEC-1 from PM2.5-induced injury at least in part by decreasing the activation of inflammation.
A previous study reported that the activation of the NF-κB pathway was involved in the apoptosis process (38). Additional studies have also demonstrated that the NF-κB pathway participates in PM2.5-induced oxidative stress (29,39,40). The present study demonstrated that PM2.5 significantly increased the NF-κB activity in HMEC-1. Therefore, the downregulation of NF-κB may be required for the protective effects of curcumin against PM2.5-induced injury in HMEC-1.
In conclusion, the key results of the present study suggested that curcumin reduces PM2.5-induced injury in HMEC-1 and curcumin is able to reverse PM2.5-induced oxidant activity via decreased ROS, oxLDL, ICAM-1 and VCAM-1 levels in HMEC-1. The present study demonstrated that curcumin inhibited PM2.5-induced vascular inflammation in HMEC-1. These results suggested that curcumin may be able to be used as a potential agent for the prevention of vascular inflammatory processes. However, further in vivo investigations are required to confirm the results of the present study.
Acknowledgements
The present study was supported by grants from the Natural Science Foundation of China (grant nos. 81401870 and 81400927), the Shanghai Municipal Science and Technology Commission (grant no. 13ZR1459000) and Major Science and Technology Program for Water Pollution Control and Treatment, China (grant no. 2014ZX07405002D).
References
- 1.Lee YJ, Kim NY, Suh YA, Lee C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol. 2011;15:1–7. doi: 10.4196/kjpp.2011.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng A, Li H, Wang X, Feng Z, Xu J, Cao K, Zhou B, Wu J, Liu J. Anticancer effect of a curcumin derivative B63: ROS production and mitochondrial dysfunction. Curr Cancer Drug Targets. 2014;14:156–166. doi: 10.2174/1568009613666131126115444. [DOI] [PubMed] [Google Scholar]
- 3.Garige M, Walters E. Curcumin inhibits development and cell adhesion in Dictyostelium discoideum: Implications for YakA signaling and GST enzyme function. Biochem Biophys Res Commun. 2015;467:275–281. doi: 10.1016/j.bbrc.2015.09.175. [DOI] [PubMed] [Google Scholar]
- 4.Tapia E, Soto V, Ortiz-Vega KM, Zarco-Márquez G, Molina-Jijón E, Cristóbal-García M, Santamaría J, García-Niño WR, Correa F, Zazueta C, Pedraza-Chaverri Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid Med Cell Longev. 2012;2012:269039. doi: 10.1155/2012/269039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao F, Liu T, Xu Y, Xu D, Feng S. Curcumin inhibits cell proliferation and promotes apoptosis in human osteoclastoma cell through MMP-9, NF-κB and JNK signaling pathways. Int J Clin Exp Pathol. 2015;8:6037–6045. [PMC free article] [PubMed] [Google Scholar]
- 6.Katsori AM, Palagani A, Bougarne N, Hadjipavlou-Litina D, Haegeman G, Vanden Berghe W. Inhibition of the NF-κB signaling pathway by a novel heterocyclic curcumin analogue. Molecules. 2015;20:863–878. doi: 10.3390/molecules20010863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royt M, Mukherjee S, Sarkar R, Biswas J. Curcumin sensitizes chemotherapeutic drugs via modulation of PKC, telomerase, NF-kappaB and HDAC in breast cancer. Ther Deliv. 2011;2:1275–1293. doi: 10.4155/tde.11.97. [DOI] [PubMed] [Google Scholar]
- 8.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, Zanobetti A. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern united states. Environ Health Perspect. 2016;124:23–29. doi: 10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buczyńska AJ, Krata A, Van Grieken R, Brown A, Polezer G, De Wael K, Potgieter-Vermaak S. Composition of PM2.5 and PM1 on high and low pollution event days and its relation to indoor air quality in a home for the elderly. Sci Total Environ. 2014;490:134–143. doi: 10.1016/j.scitotenv.2014.04.102. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed MO, Song WW, Ma WL, Li WL, Li YF, Khan AU, Ibrahim MA, Maarouf OA, Ahmed AA, Ambuchi JJ. Potential toxicological and cardiopulmonary effects of PM2.5 exposure and related mortality: Findings of recent studies published during 2003–2013. Biomed Environ Sci. 2016;29:66–79. doi: 10.3967/bes2016.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Yang W, Han B, Zhang W, Chen M, Bai Z. Gravimetric analysis for PM2.5 mass concentration based on year-round monitoring at an urban site in Beijing. J Environ Sci (China) 2016;40:154–160. doi: 10.1016/j.jes.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Fang X, Li R, Xu Q, Bottai M, Fang F, Cao Y. A two-stage method to estimate the contribution of road traffic to PM2.5 concentrations in Beijing, China. Int J Environ Res Public Health. 2016;13 doi: 10.3390/ijerph13010124. pii: E124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han L, Zhou W, Li W. Fine particulate (PM2.5) dynamics during rapid urbanization in Beijing, 1973–2013. Sci Rep. 2016;6:23604. doi: 10.1038/srep23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan L, Xiu G, Feng L, Cheng N, Wang C. The mercury species and their association with carbonaceous compositions, bromine and iodine in PM2.5 in Shanghai. Chemosphere. 2016;146:263–271. doi: 10.1016/j.chemosphere.2015.11.058. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Qiao T, Huang Z, Zhu M, Xu W, Xiu G, Tao J, Lee S. Comparison of ionic and carbonaceous compositions of PM2.5 in 2009 and 2012 in Shanghai, China. Sci Total Environ. 2015;536:695–703. doi: 10.1016/j.scitotenv.2015.07.100. [DOI] [PubMed] [Google Scholar]
- 16.Haikerwal A, Akram M, Del Monaco A, Smith K, Sim MR, Meyer M, Tonkin AM, Abramson MJ, Dennekamp M. Impact of fine particulate Matter (PM2.5) exposure during wildfires on cardiovascular health outcomes. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001653. pii: e001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu J, Liberda EN, Qu S, Guo X, Li X, Zhang J, Meng J, Yan B, Li N, Zhong M, et al. The role of metal components in the cardiovascular effects of PM2.5. PLoS One. 2013;8:e83782. doi: 10.1371/journal.pone.0083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montiel-Dávalos A, Alfaro-Moreno E, López-Marure R. PM2.5 and PM10 induce the expression of adhesion molecules and the adhesion of monocytic cells to human umbilical vein endothelial cells. Inhal Toxicol. 2007;19:S91–S98. doi: 10.1080/08958370701495212. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 19.Yang GZ, Wang ZJ, Bai F, Qin XJ, Cao J, Lv JY, Zhang MS. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules. 2015;20:6626–6639. doi: 10.3390/molecules20046626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soares SR, Carvalho-Oliveira R, Ramos-Sanchez E, Catanozi S, da Silva LF, Mauad T, Gidlund M, Goto H, Garcia ML. Air pollution and antibodies against modified lipoproteins are associated with atherosclerosis and vascular remodeling in hyperlipemic mice. Atherosclerosis. 2009;207:368–373. doi: 10.1016/j.atherosclerosis.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 22.Takenaka T, Takahashi K, Kobayashi T, Oshima E, Iwasaki S, Suzuki H. Oxidized low density lipoprotein (Ox-LDL) as a marker of atherosclerosis in hemodialysis (HD) patients. Clin Nephrol. 2002;58:33–37. doi: 10.5414/CNP58033. [DOI] [PubMed] [Google Scholar]
- 23.Wang GF, Shi CG, Sun MZ, Wang L, Wu SX, Wang HF, Xu ZQ, Chen DM. Tetramethylpyrazine attenuates atherosclerosis development and protects endothelial cells from ox-LDL. Cardiovasc Drugs Ther. 2013;27:199–210. doi: 10.1007/s10557-013-6440-6. [DOI] [PubMed] [Google Scholar]
- 24.Bo L, Jiang S, Xie Y, Kan H, Song W, Zhao J. Effect of vitamin e and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS One. 2016;11:e0152216. doi: 10.1371/journal.pone.0152216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Kou X, Xie L, Cheng F, Geng H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ Sci Pollut Res Int. 2015;22:20167–20176. doi: 10.1007/s11356-015-5222-z. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HP, Zheng FL, Zhao JH, Guo DX, Chen XL. Genistein inhibits ox-LDL-induced VCAM-1, ICAM-1 and MCP-1 expression of HUVECs through heme oxygenase-1. Arch Med Res. 2013;44:13–20. doi: 10.1016/j.arcmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Deng X, Rui W, Zhang F, Ding W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol Toxicol. 2013;29:143–157. doi: 10.1007/s10565-013-9242-5. [DOI] [PubMed] [Google Scholar]
- 28.Su R, Jin X, Zhang W, Li Z, Liu X, Ren J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere. 2017;167:444–453. doi: 10.1016/j.chemosphere.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Chen M, Zhong M, Hu Z, Qiu L, Rajagopalan S, Fossett NG, Chen LC, Ying Z. Exposure to concentrated ambient PM2.5 shortens lifespan and induces inflammation-associated signaling and oxidative stress in drosophila. Toxicol Sci. 2017;156:199–207. doi: 10.1093/toxsci/kfw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong Z, Guo Z, Zhang R, Xu J, Dong W, Zhuang G, Deng C. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med. 2016;239:117–125. doi: 10.1620/tjem.239.117. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Deng Z, Feng Y, Liao F, Zhou F, Feng S, Wang X. PM2.5 induced apoptosis in endothelial cell through the activation of the p53-bax-caspase pathway. Chemosphere. 2017;177:135–143. doi: 10.1016/j.chemosphere.2017.02.144. [DOI] [PubMed] [Google Scholar]
- 32.Deng X, Zhang F, Wang L, Rui W, Long F, Zhao Y, Chen D, Ding W. Airborne fine particulate matter induces multiple cell death pathways in human lung epithelial cells. Apoptosis. 2014;19:1099–1112. doi: 10.1007/s10495-014-0980-5. [DOI] [PubMed] [Google Scholar]
- 33.Soberanes S, Urich D, Baker CM, Burgess Z, Chiarella SE, Bell EL, Ghio AJ, De Vizcaya-Ruiz A, Liu J, Ridge KM, et al. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem. 2009;284:2176–2186. doi: 10.1074/jbc.M808844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice MB, Cavallari J, Fang S, Christiani D. Acute decrease in HDL cholesterol associated with exposure to welding fumes. J Occup Environ Med. 2011;53:17–21. doi: 10.1097/JOM.0b013e3182028d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan B, Li J, Guo J, Ma P, Wu Z, Ling Z, Guo H, Hiroshi Y, Yanagi U, Yang X, et al. The toxic effects of indoor atmospheric fine particulate matter collected from allergic and non-allergic families in Wuhan on mouse peritoneal macrophages. J Appl Toxicol. 2016;36:596–608. doi: 10.1002/jat.3217. [DOI] [PubMed] [Google Scholar]
- 36.Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J, Kupper L, Williams R, Neas L, Cascio W, et al. Coarse particulate matter (PM2.5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Perspect. 2007;115:709–714. doi: 10.1289/ehp.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brucker N, Moro AM, Charão MF, Durgante J, Freitas F, Baierle M, Nascimento S, Gauer B, Bulcão RP, Bubols GB, et al. Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers. Sci Total Environ. 2013;463–464:884–493. doi: 10.1016/j.scitotenv.2013.06.098. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Wang Y, Li H, Shen G, Hu S. Ox-LDL influences peripheral Th17/Treg balance by modulating Treg apoptosis and Th17 proliferation in atherosclerotic cerebral infarction. Cell Physiol Biochem. 2014;33:1849–1862. doi: 10.1159/000362963. [DOI] [PubMed] [Google Scholar]
- 39.Kafoury RM, Madden MC. Diesel exhaust particles induce the over expression of tumor necrosis factor-alpha (TNF-alpha) gene in alveolar macrophages and failed to induce apoptosis through activation of nuclear factor-kappaB (NF-kappaB) Int J Environ Res Public Health. 2005;2:107–13. doi: 10.3390/ijerph2005010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu LZ, Sun H, Chen JH. Histone deacetylases 3 deletion restrains PM2.5-induced mice lung injury by regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed Pharmacother. 2017;85:756–762. doi: 10.1016/j.biopha.2016.11.094. [DOI] [PubMed] [Google Scholar]