Abstract
Colorectal carcinoma (CRC) is one of the most common types of malignancy worldwide. Recently, neoadjuvant chemotherapy has become an important treatment strategy for CRC. However, treatment frequently fails due to the development of chemoresistance, which is a major obstacle for positive prognosis. However, the underlying mechanisms of chemoresistance remain unclear. The present study assessed the functions of nucleus accumbens-associated protein 1 (NAC1), an important transcriptional regulator, in CRC progression. Reverse transcription-quantitative polymerase chain reaction, western blot analysis and immunohistochemistry were performed to detect the expression levels of NAC1. It was identified that NAC1 was significantly overexpressed in CRC compared with non-tumorous tissues, indicating an oncogenic role. Following this, gain and loss of function analyses were performed in vitro to further investigate the function of NAC1. Cell viability and caspase-3/7 activity assays were used to assess chemotherapy-induced apoptosis. These results indicated that overexpression of NAC1 in CRC cells increased resistance to chemotherapy and inhibited apoptosis. Additionally, RNA interference-mediated knockdown of NAC1 restored the chemosensitivity of CRC cells. Furthermore, mechanistic investigation revealed that NAC1 increased drug resistance via inducing homeobox A9 (HOXA9) expression, and that knockdown of HOXA9 abrogated NAC1-induced drug resistance. In conclusion, the results of the present study demonstrated that NAC1 may be a critical factor in the development of chemoresistance, offering a potential novel target for the treatment of CRC.
Keywords: nucleus accumbens-associated protein 1, homeobox A9, drug resistance, apoptosis, colorectal carcinoma
Introduction
Colorectal carcinoma (CRC) is one of the most common types of malignancy, comprising ~10% of all primary cancers (1,2). In the last decade, advances have been made in the use of neoadjuvant chemotherapy in combination with surgery, increasing the long-term survival rate of patients with CRC (3,4). Notably, 5-fluorouracil (5-FU) and oxaliplatin are commonly used as first-line anticancer drugs (5). However, patients who respond poorly to these drugs may suffer metastasis and relapse. Furthermore, drug-resistance often emerges following clinical treatment; this is a primary obstacle limiting positive outcomes (6,7). Thus, investigation into the molecular mechanisms underlying chemotherapy resistance is required for the development of novel clinical treatments for CRC.
Nucleus accumbens-associated protein 1 (NAC1) is a member of the broad-complex, tramtrack and bric a brac/pox virus and zinc finger protein family, which is involved in numerous cellular processes including proliferation, apoptosis and transcriptional regulation (8–10). NAC1 contributes to cancer development and progression via activating key proteins and interacting with signaling pathways. Previous studies have demonstrated that NAC1 serves a key role in carcinogenesis, including ovarian, breast and cervical cancers (11). It was revealed that NAC1 expression levels were significantly increased in recurrent post-treatment ovarian tumors, compared with pretreated tumors (12,13). Furthermore, downregulated expression levels of NAC1 maintained cell survival, prevented cell senescence and activated autophagy via high mobility group box 1 protein (8,14). Additionally, NAC1 was demonstrated to predominantly localize to the nucleus, directly bind to nanog homeobox and interact with importin α3/4 to regulate the pluripotency network (15). Furthermore, NAC1 was identified to regulate forkhead box Q1 (FOXQ1) expression levels to mediate cell motility and invasion in cancer cells (16). A previous study indicated that NAC1 was additionally detected in the cytosol, particularly during mitosis, suggesting that NAC1 serves various functions in tumor initiation, development and progression (17).
Homeobox (HOX) genes, comprising 39 genes organized in four clusters, encode a class of transcription factors which control self-renewal and differentiation of cells (18). Previous studies have identified that HOX family genes are involved in cancer development and therapy (19–21). NIH 3T3 fibroblast clones bearing the activated HOX-2.4 gene produced fibrosarcomas in nude mice, indicating its oncogenic potential (22). HOX protein HOXA9 is typically expressed during the development of the reproductive tract, and is overexpressed in ovarian cancer, acute leukemia and breast cancer (23). Furthermore, HOXA9 expression levels in epithelial ovarian cancers cells have been demonstrated to induce cancer-associated fibroblasts and promote a microenvironment permissive for tumor growth (24). However, the potential roles of HOXA9 in drug resistance remain to be fully elucidated.
In the present study, it was demonstrated that NAC1 was overexpressed in colorectal carcinoma tissues, and that increased expression levels of NAC1 contributed to chemotherapy resistance. These data suggested that overexpression of NAC1 in HCT8 and SW480 cells conferred drug resistance and reduced caspase-3/7 activity to inhibit cell apoptosis. Opposite effects were observed when small interfering (si)RNA was used to knock down NAC1 expression in HCT116 and SW620 cells. Further investigation indicated that NAC1 promoted tumor progression viamediating HOXA9 expression. These findings identified a potential novel target for the treatment of CRC.
Materials and methods
Patients and tissue samples
The present study was approved by the Research Ethics Committee of Hangzhou First People's Hospital (Hangzhou, China). A total of 94 CRC and paired non-tumorous tissues were collected from patients following surgery at the Department of Gastrointestinal Surgery, Hangzhou First People's Hospital. Written informed consent was obtained from all patients. Tumor and paired non-tumorous tissues were stored at −80°C.
Cell lines
HCT8, HCT116, SW480 and SW620 human CRC cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The HEK293T human embryogenic kidney cell line was obtained from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin.
NAC1 overexpression and siRNA transfection
For ectopic expression of NAC1, NAC1 cDNA was cloned into the vector pLKO.1 (catalog no. 10878; Addgene, Inc., Cambridge, MA, USA). Lentivirals were constructed in HEK293T cells and cell lines were infected to establish NAC1 stable overexpression cells as described previously (25). The siRNAs targeting NAC1 (UGA UGU ACA CGU UGG UGC CUG UCA CCA and GAG GAA GAA CUC GGU GCC CUU CUC CAU) and HOXA9 (ACU ACU ACG UGG ACU CGU UC and AAU CAA CAA AGA CCG AGC AAA) were purchased from Genepharm Biotech (Taipei, Taiwan). A scrambled sequence was used as a negative control. Cell transfection was performed using Lipofectamine® 3000 according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissue samples and cells was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). First-strand cDNA was synthesized using the PrimeScript cDNA Synthesis kit (Takara Biotechnology, Co., Ltd., Dalian, China), according to the manufacturer's protocol. qPCR was performed using the SYBR® -Green Master mix kit (Takara Biotechnology Co., Ltd., Dalian, China) and the Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Cycling conditions were as follows: An initial predenaturation step at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 30 sec and annealing at 60°C for 30 sec. All reactions were performed in triplicate. The primers used for detecting NAC1 were as follows: Forward, 5′-AAGCTGAGGATCTGCTGGAA-3′ and reverse, 5′-CCAGACACTGCAGATGGAGA-3′. The primers used for GAPDH were as follows: Forward, 5′-ACCACAGTCCATGCCATCA-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. The expression levels of NAC1 were normalized to that of GAPDH in each sample using the 2−∆∆Cq method (26).
Western blot analysis
Total proteins from tissues and cells were extracted using radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Beyotime Institute of Biotechnology, Haimen, China). For western blot analysis, 60 µg of total protein lysates underwent 10% SDS-PAGE and were subsequently transferred onto polyvinylidene difluoridemembranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA) as described previously (27). The protein was detected using an Enhanced Chemiluminescence substrate (Beijing Transgen Co., Ltd., Beijing, China). Rabbit anti-NAC1 (catalog no. ab29047) and rabbit anti-HOXA9 (catalog no. ab83480) primary antibodies were purchased from Abcam (Cambridge, UK). A mouse anti-β-actin (sc-47778) primary antibody was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and served as an internal control. Membranes were probed with primary antibodies diluted 1:1,000 at 4°C overnight, followed by a horse radish peroxidase-conjugated goat-anti-rabbit or anti-mouse secondary antibody (catalog no. ab6789; Abcam, Cambridge, UK) diluted 1:5,000 for 1 h at room temperature.
Immunohistochemical staining
CRC and paired non-tumorous tissues were fixed in formalin, embedded in paraffin and sectioned into 4-µm thick slices. Following deparaffinization and rehydration, antigen retrieval was performed using boiling 0.01 M citrate buffer (Boster Systems, Inc., Pleasanton, CA, USA) for 30 min and endogenous peroxidases were blocked with 3% H2O2. Sections were washed three times with PBS and goat serum (Boster Systems, Inc.) was used to block nonspecific binding for 30 min. Tissues were subsequently incubated with an anti-NAC1 antibody (1:200 dilution; catalog no. ab29047; Abcam) at 4°C overnight followed by incubation with a peroxidase-labeled goat anti-rabbit secondary antibody (catalog no. BA1055; Boster Systems, Inc.) for 1 h at 37°C. 3′3-Diaminobenzidine (Boster Systems, Inc.) was used to visualize tissue antigens. Following this, the sections were counterstained with hematoxylin and dehydrated and observed under a microscope.
Caspase-3/7 activity and cell viability assay
The indicated cells were treated with 5-FU (50 ng/ml) oroxaliplatin (10 µM) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and cultured for 12 h at 37°C. To measure capase-3/7 activity of cells following treatment with a Caspase-Glo® 3/7 assay kit (Promega Corporation, Madison, WI, USA) was used according to the manufacturer's protocol. Cell viability was evaluated using the Cell Counting kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). The absorbance was measured at a wavelength of 450 nm using a microplate reader. Experiments were repeated at least three times.
Luciferase reporter assay
HCT116 and SW620 cells were transfected with NAC1 or control siRNAs. The cells were subsequently transfected with a pGL3 control vector or the pGL3-HOXA9 promoter vector and Renilla. A luciferase activity assay was performed using the Dual-Luciferase® Reporter assay system (Promega Corporation) according to the manufacturer's protocol. Firefly luciferase activity was normalized to Renilla activity.
Statistical analysis
SPSS software version 21.0 (IBM SPSS, Armonk, NY, USA) was used for statistical analysis. Data are presented as the mean ± standard error of at least three experiments. Data were analyzed by an unpaired Student's t-test for comparison between two groups, or a one-way analysis of variance followed by Student-Newman-Keuls post hoc test for comparison between multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of NAC1 are significantly elevated in CRC tissue
To investigate the involvement of NAC1 in the progression of CRC, the mRNA expression levels of NAC1 in 30 CRC and adjacent non-tumorous tissues were analyzed by RT-qPCR. The results indicated that compared with non-tumorous tissue, NAC1 expression levels were significantly upregulated in CRC tissue (Fig. 1A; P=0.0008). Additionally, a GSE6988 dataset generated from the Gene Expression Omnibus database consisting of 28 healthy and 49 CRC tissues was investigated, and the mRNA expression levels of NAC1 were significantly increased in CRC tissues (Fig. 1B; P=0.004). Furthermore, western blot analysis and immunohistochemistry revealed that the protein expression levels of NAC1 were elevated in CRC tissue (Fig. 1C and D). The results indicated that the expression levels of NAC1 were increased in tumor compared with non-tumor tissues, implicating an oncogenic function for NAC1 in CRC.
Figure 1.
NAC1 is upregulated in colorectal carcinoma tissues. (A) Relative mRNA expression levels of NAC1 in 30 paired samples of CRC tissue and adjacent non-tumorous tissue were measured by reverse transcription-quantitative polymerase chain reaction analysis. (B) The expression levels of NAC1 in GSE6988 datasets. (C) The protein expression levels of NAC1 were measured by western blot analysis in 10 paired CRC tissues and adjacent non-tumorous tissues. (D) Immunohistochemical staining demonstrating upregulation of NAC1 in CRC tissues. Scale bar, 100 µm. CRC, colorectal carcinoma; T, colorectal carcinoma tissue; N, non-tumorous tissue; NAC1, nucleus accumbens-associated protein 1.
NAC1 confers resistance of CRC cells to chemotherapy in vitro
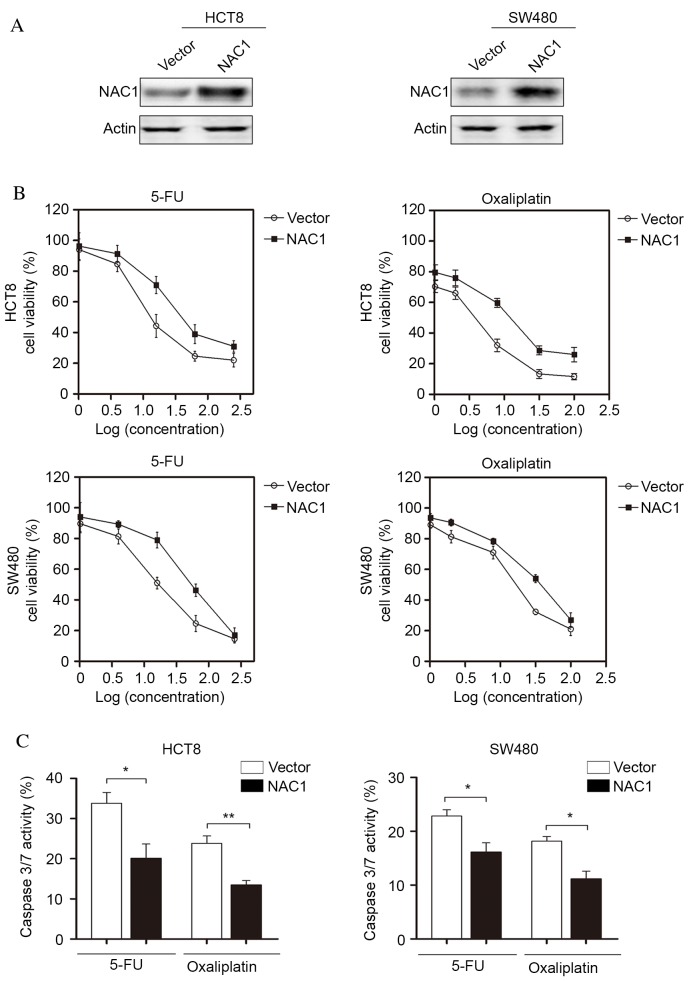
Chemoresistance is a major challenge for CRC treatment; therefore, the present study investigated the potential function of NAC1 in CRC cells following chemotherapy. NAC1 was stably expressed in HCT8 and SW480 cell lines and western blot analysis was used to confirm the overexpression of NAC1 (Fig. 2A). The cells were subsequently treated with 5-FU and oxaliplatin at a range of doses. The concentrations of 5-FU were as follows: 1, 4, 16, 64 and 256 ng/ml, and the concentrations of oxaliplatin were 1, 2, 8, 32 and 100 µM. The results indicated that overexpression of NAC1 in HCT8 and SW480 cells significantly increased the resistance of cells to 5-FU and oxaliplatin-induced cell death (Fig. 2B). In addition, caspase-3/7 activity was significantly decreased following overexpression of NAC1. This suggested a low level of apoptosis, and was consistent with the cell viability assay (Fig. 2C). Taken together, these data suggested that NAC1 increased the resistance of CRC cells to cytotoxic drugs.
Figure 2.
Overexpression of NAC1 increases colon cancer cell resistance to chemotherapy in vitro. (A) Representative western blot images of protein expression levels of NAC1 in HCT8 and SW480 cells following ectopic expression of NAC1. (B) HCT8 and SW480 cells overexpressing NAC1 were treated with 5-FU and oxaliplatin at a range of concentrations and cell viability was analyzed using the Cell Counting kit-8. (C) Caspase-3/7 activity of HCT8 and SW480 cells following NAC1 overexpression was assessed. Data are presented as the mean ± standard error (n=3). *P<0.05; **P<0.01. NAC1, nucleus accumbens-associated protein 1; 5-FU, 5-fluorouracil.
Knocking down the expression of NAC1 restores the chemosensitivity of CRC cells
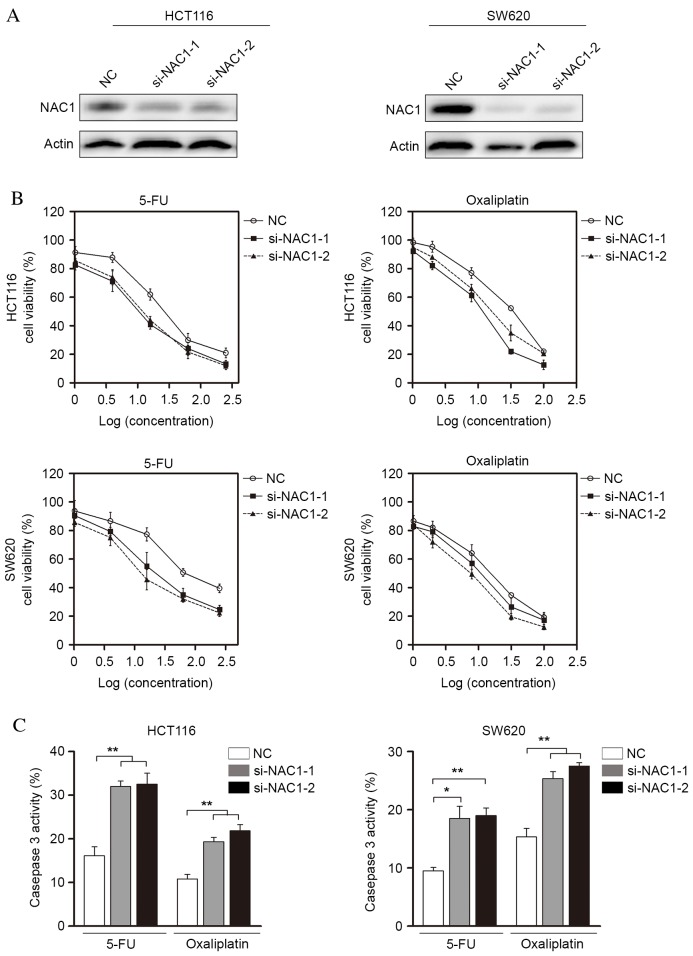
To further characterize the role of NAC1 in the regulation of CRC cell death, the present study transfected target-specific siRNA against NAC1 into HCT116 and SW620 cells. NAC1 siRNA led to a significant decrease of protein expression levels in the two cell lines (Fig. 3A). These NAC1-knockdown cells became sensitive to apoptosis induced by 5-FU and oxaliplatin (Fig. 3B), suggesting an anti-apoptotic role for NAC1 in CRC cells. In addition, the cytotoxic agent-induced activation of caspase-3/7 significantly increased following NAC1 knockdown (Fig. 3C). Collectively, these data indicated that knockdown of NAC1 expression resulted in restored sensitivity to 5-FU and oxaliplatin in CRC cells.
Figure 3.
Inhibition of NAC1 expression increases CRC cell sensitivity to chemotherapy in vitro. (A) Representative western blot images of NAC1 protein expression levels in HCT116 and SW620 cells transfected with control or NAC1 siRNA. β-actin served as an internal control. (B) HCT116 and SW620 cells with knockdown of NAC1 were treated with 5-FU oroxaliplatin and cell viability was measured using the Cell Counting kit-8. (C) Caspase-3/7 activity in HCT116 and SW620 cells following inhibition of NAC1 expression was assessed. Data are presented as the mean ± standard error (n=3).*P<0.05;**P<0.01. NAC1, nucleus accumbens-associated protein 1; siRNA, small interfering RNA; 5-FU, 5-fluorouracil; NC, negative control.
NAC1 promotes chemoresistance of CRC cells via increased HOXA9 expression
The present study investigated the potential mechanism underlying NAC1-mediated chemoresistance in CRC cells. NAC1 may transcriptionally regulate the gene expression of a variety of pluripotency factors and modulate stem cell properties. The HOX gene family was selected for investigation due to its involvement in mediating cell stemness. It was revealed that HOXA9 promoter activity was significantly decreased following knockdown of NAC1 (Fig. 4A). These findings indicated that HOXA9 may be a downstream regulator of NAC1. Following this, western blot analysis revealed that knockdown of NAC1 suppressed HOXA9 expression (Fig. 4B). Furthermore, overexpression of NAC1 was demonstrated to induce HOXA9 expression, which was subsequently silenced by siRNA (Fig. 4C). The caspase-3/7 activity of CRC cells overexpressing NAC1 was determined, following silencing of HOXA9 expression (Fig. 4D). The results revealed that the NAC1-induced reduction in cell apoptosis was reversed by silencing HOXA9 expression, suggesting that HOXA9 is a downstream regulator of NAC1-mediated CRC cell resistance to anticancer drugs.
Figure 4.
HOXA9 is an NAC1 downstream gene involved in NAC1-mediated chemoresistance. (A) Luciferase activity of the HOXA9 promoter was measured in HCT116 and SW620 cells, following transfection with control siRNA or siNAC1. Representative western blot images and analysis of HCT116 and SW620 cells demonstrating (B) an reduction in HOXA9 protein expression levels, following NAC1 knockdown, and (C) an increase in HOXA9 protein expression levels, following ectopic NAC1 expression. (D) Caspase-3/7 activity in HCT8 and SW480 cells, following overexpression of NAC1 and knockdown HOXA9 expression, was assessed. Data are presented as the mean ± standard error (n=3). *P<0.05; **P<0.01; ***P<0.001. HOXA9, homeobox A9; NAC1, nucleus accumbens-associated protein 1; si, small interfering; NC, negative control.
Discussion
CRC is one of the most common types of malignancy worldwide. Despite recent developments in therapeutic strategies, finding an effective treatment approach remains challenging. For example, 5-FU, the widely-used first-line anticancer drug, has an efficacy of only ~20% when administered as a single drug treatment. Numerous patients with CRC develop drug resistance and this may lead to cancer progression, including metastasis and angiogenesis (28). The present study investigated whether NAC1-mediated upregulation of HOXA9 significantly contributes to drug resistance in CRC.
Emerging evidence has indicated that NAC1 has multifunctional roles in tumorigenesis, including increasing cell survival and proliferation, enhancing cell motility, and regulating cellular senescence and autophagy. Although NAC1 overexpression has frequently been observed in a wide variety of cancers, it has not been studied in detail in CRC. The present study investigated the clinical significance and biological function of NAC1 in CRC. It was demonstrated that NAC1 was frequently upregulated in CRC tissues, and that NAC1 may mediate CRC chemoresistance. Furthermore, NAC1 siRNA was revealed to increase apoptosis in response to anticancer drug-induced tumor cell apoptosis, indicating that inhibition of NAC1 may be a novel strategy for CRC treatment.
Cancer cell responses to chemotherapy have a variety of underlying mechanisms, ranging from activation of survival signaling pathways to inhibition of cell death, and from increasing cell membrane transportation to initiating cell senescence (29–31). However, the underlying molecular mechanisms of NAC1-mediated chemoresistance remain unclear. Recently, NAC1-mediated downregulation of various downstream genes was investigated in the SKOV3 ovarian cancer cell line. The FOXQ1 gene was identified as a regulator of NAC1-mediated cell motility (16). The present study examined the potential involvement of the HOX gene family in tumor chemoresistance, due to its roles in regulating cell transcription, morphogenesis and differentiation (32). Luciferase activity experiments were performed to assess HOX gene transcriptional regulation by knocking down NAC1 expression levels. HOXA9 promoter activity was suppressed when NAC1 expression was inhibited. Additionally, the present study further revealed that the protein expression levels of HOXA9 decreased following NAC1 knockdown. Ectopic expression of NAC1 was revealed to induce HOXA9 expression. Furthermore, inhibition of NAC1 or HOXA9 increased the drug sensitivity of CRC cells. A previous study demonstrated that the homeobox genes HOXA9 and A10 were upregulated in chemoresistant glioblastoma cells, and increased levels of HOXA9 were associated with a reduced survival rate (33). HOXA9 has additionally been revealed to mediate resistance to temozolomide treatment in gliomas, via upregulation of B-cell lymphoma 2 (34). Similarly, HOXA9 overexpression was inversely correlated with drug resistance, relapse and overall survival rate in acute leukemia (35). In addition, HOXA9 may induce transforming growth factor-β2, consequently modulating tumor stroma and promoting tumor growth and metastasis. Furthermore, cancer-associated fibroblasts that may be induced by HOXA9 have been revealed to mediate chemoresistance in cancer cells (36). Therefore, the present study demonstrated that HOXA9 may be important to maintain NAC1-induced chemoresistance.
In conclusion, the present study demonstrated the upregulation of NAC1 in CRC samples, and that overexpression of NAC1 in CRC cells induced resistance to anticancer drugs. Furthermore, knockdown of NAC1 restored the chemosensitivity of CRC cells, indicating an oncogenic role. The underlying molecular mechanisms of NAC1 may be mediated via the upregulation of HOXA9. This indicates that NAC1 may serve as a potential target for the treatment of CRC.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 3.Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: A pilot trial. J Clin Oncol. 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–190. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 5.Cecchin E, D'Andrea M, Lonardi S, Zanusso C, Pella N, Errante D, De Mattia E, Polesel J, Innocenti F, Toffoli G. A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenomics J. 2013;13:403–409. doi: 10.1038/tpj.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong M, Zheng W, Lu X, Ao L, Li X, Guan Q, Cai H, Li M, Yan H, Guo Y, et al. Identifying clinically relevant drug resistance genes in drug-induced resistant cancer cell lines and post-chemotherapy tissues. Oncotarget. 2015;6:41216–41227. doi: 10.18632/oncotarget.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, Patel R, Liu D, Qin ZH, Shih IM, Yang JM. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31:1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsunoda K, Oikawa H, Tada H, Tatemichi Y, Muraoka S, Miura S, Shibazaki M, Maeda F, Takahashi K, Akasaka T, et al. Nucleus accumbens-associated 1 contributes to cortactin deacetylation and augments the migration of melanoma cells. J Invest Dermatol. 2011;131:1710–1719. doi: 10.1038/jid.2011.110. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Cheng Y, Ren X, Hori T, Huber-Keener KJ, Zhang L, Yap KL, Liu D, Shantz L, Qin ZH, et al. Dysfunction of nucleus accumbens-1 activates cellular senescence and inhibits tumor cell proliferation and oncogenesis. Cancer Res. 2012;72:4262–4275. doi: 10.1158/0008-5472.CAN-12-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinawath N, Vasoontara C, Yap KL, Thiaville MM, Nakayama K, Wang TL, Shih IM. NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene. 2009;28:1941–1948. doi: 10.1038/onc.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama K, Nakayama N, Davidson B, Sheu JJ, Jinawath N, Santillan A, Salani R, Bristow RE, Morin PJ, Kurman RJ, et al. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci USA. 2006;103:18739–18744. doi: 10.1073/pnas.0604083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson B, Berner A, Trope' CG, Wang TL, Shih IeM. Expression and clinical role of the bric-a-brac tramtrack broad complex/poxvirus and zinc protein NAC-1 in ovarian carcinoma effusions. Hum Pathol. 2007;38:1030–1036. doi: 10.1016/j.humpath.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Yang JW, Ren X, Yang JM. NAC1 and HMGB1 enter a partnership for manipulating autophagy. Autophagy. 2011;7:1557–1558. doi: 10.4161/auto.7.12.17910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazaki K, Nakayama N, Nariai Y, Nakayama K, Miyazaki K, Maruyama R, Kato H, Kosugi S, Urano T, Sakashita G. Nuclear localization signal in a cancer-related transcriptional regulator protein NAC1. Carcinogenesis. 2012;33:1854–1862. doi: 10.1093/carcin/bgs193. [DOI] [PubMed] [Google Scholar]
- 16.Gao M, Wu RC, Herlinger AL, Yap K, Kim JW, Wang TL, Shih IeM. Identification of the NAC1-regulated genes in ovarian cancer. Am J Pathol. 2014;184:133–140. doi: 10.1016/j.ajpath.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu PH, Hung SH, Ren T, Shih IeM, Tseng Y. Cell cycle-dependent alteration in NAC1 nuclear body dynamics and morphology. Phys Biol. 2011;8:015005. doi: 10.1088/1478-3975/8/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larochelle A, Choi U, Shou Y, Naumann N, Loktionova NA, Clevenger JR, Krouse A, Metzger M, Donahue RE, Kang E, et al. In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene. J Clin Invest. 2009;119:1952–1963. doi: 10.1172/JCI37506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida T, Takahashi F, Chiba N, Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco Md, Wijendran V, Shioda T, et al. HOXB9, a gene overexpressed in breast cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad Sci USA. 2010;107:1100–1105. doi: 10.1073/pnas.0912710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Iwasaki M, Ficara F, Lin C, Matheny C, Wong SH, Smith KS, Cleary ML. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell. 2010;17:597–608. doi: 10.1016/j.ccr.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aberdam D, Negreanu V, Sachs L, Blatt C. The oncogenic potential of an activated Hox-2.4 homeobox gene in mouse fibroblasts. Mol Cell Biol. 1991;11:554–557. doi: 10.1128/MCB.11.1.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 24.Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrarese R, Harsh GR, VI, Yadav AK, Bug E, Maticzka D, Reichardt W, Dombrowski SM, Miller TE, Masilamani AP, Dai F, et al. Lineage-specific splicing of a brain-enriched alternative exon promotes glioblastoma progression. J Clin Invest. 2014;124:2861–2876. doi: 10.1172/JCI68836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Zhan S, Tan W, Cheng R, Gong H, Zhu Q. P300 binds to and acetylates MTA2 to promote colorectal cancer cells growth. Biochem Biophys Res Commun. 2014;444:387–390. doi: 10.1016/j.bbrc.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 28.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medina-Ramirez CM, Goswami S, Smirnova T, Bamira D, Benson B, Ferrick N, Segall J, Pollard JW, Kitsis RN. Apoptosis inhibitor ARC promotes breast tumorigenesis, metastasis, and chemoresistance. Cancer Res. 2011;71:7705–7715. doi: 10.1158/0008-5472.CAN-11-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang WJ, Song MJ, Park EY, Lee JJ, Park JH, Park K, Park JH, Kim HP. Transcription factors Sp1 and Sp3 regulate expression of human ABCG2 gene and chemoresistance phenotype. Mol Cells. 2013;36:368–375. doi: 10.1007/s10059-013-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breau MA, Wilkinson DG, Xu Q. A Hox gene controls lateral line cell migration by regulating chemokine receptor expression downstream of Wnt signaling. Proc Natl Acad Sci USA. 2013;110:16892–16897. doi: 10.1073/pnas.1306282110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaspar N, Marshall L, Perryman L, Bax DA, Little SE, Viana-Pereira M, Sharp SY, Vassal G, Pearson AD, Reis RM, et al. MGMT-independent temozolomide resistance in pediatric glioblastoma cells associated with a PI3-kinase-mediated HOX/stem cell gene signature. Cancer Res. 2010;70:9243–9252. doi: 10.1158/0008-5472.CAN-10-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brumatti G, Salmanidis M, Kok CH, Bilardi RA, Sandow JJ, Silke N, Mason K, Visser J, Jabbour AM, Glaser SP, et al. HoxA9 regulated Bcl-2 expression mediates survival of myeloid progenitors and the severity of HoxA9-dependent leukemia. Oncotarget. 2013;4:1933–1947. doi: 10.18632/oncotarget.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alharbi RA, Pettengell R, Pandha HS, Morgan R. The role of HOX genes in normal hematopoiesis and acute leukemia. Leukemia. 2013;27:1000–1008. doi: 10.1038/leu.2012.356. [DOI] [PubMed] [Google Scholar]
- 36.Johansson AC, Ansell A, Jerhammar F, Lindh MB, Grénman R, Munck-Wikland E, Östman A, Roberg K. Cancer-associated fibroblasts induce matrix metalloproteinase-mediated cetuximab resistance in head and neck squamous cell carcinoma cells. Mol Cancer Res. 2012;10:1158–1168. doi: 10.1158/1541-7786.MCR-12-0030. [DOI] [PubMed] [Google Scholar]