Abstract
Oral squamous cell carcinoma (OSCC) cells are usually resistant to doxorubicin, resulting in limited application of doxorubicin in OSCC treatment. MicroRNA (miR)-221 has been reported to be involved in the development of OSCC; however, it remains unclear if and how miR-221 is implicated in modulating the sensitivity of OSCC cells to doxorubicin. In the present study, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to assess miR-221 expression in OSCC cells in response to doxorubicin treatment. In addition, the SCC4 and SCC9 OSCC cell lines were transfected with anti-miR-221 oligonucleotides and cell viability and apoptosis following doxorubicin treatment were evaluated using an MTT assay and Annexin V-fluorescein isothiocyanate/Hoechst double staining, respectively. The mRNA and protein expression levels of tissue inhibitor of metalloproteinase-3 (TIMP3) in anti-miR-221-transfected cells were assessed using RT-qPCR and western blot analysis, respectively. Furthermore, a luciferase reporter assay was performed to investigate whether TIMP3 may be a direct target gene of miR-221. To explore the roles of TIMP3 in miR-221-mediated cell responses, TIMP3 expression was silenced following transfection with TIMP3-targeting small interfering (si)RNA in cells overexpressing miR-221, and cell viability and apoptosis in response to doxorubicin treatment were measured. The results of the present study demonstrated that miR-221 expression was upregulated in SCC4 and SCC9 cells following treatment with doxorubicin. However, inhibiting the doxorubicin-induced upregulation of miR-221 through transfection with anti-miR-221 oligonucleotides led to an increase in the sensitivity of OSCC cells to doxorubicin. In addition, the results indicated that TIMP3 was a direct target of miR-221 in OSCC cells, as determined by a 3′-untranslated region luciferase reporter assay. Co-transfection of cells with anti-miR-221 oligonucleotides and TIMP3-specific small interfering RNA resulted in reduced sensitivity to doxorubicin compared with the cells transfected with the miR-221 inhibitor alone. In conclusion, these results indicated that OSCC cells are resistant to doxorubicin through upregulation of miR-221, which in turn downregulates TIMP3. Therefore, silencing miR-221 or upregulating TIMP3 may be considered promising therapeutic approaches to enhance the sensitivity of OSCC to doxorubicin.
Keywords: oral squamous cell carcinoma, tissue inhibitor of metalloproteinase-3, microRNA-221, doxorubicin, drug resistance
Introduction
OSCC is the most common type of oral cancer and is the eleventh most common malignancy in humans worldwide (1). Although chemotherapeutic agents have been widely used to treat a broad range of human malignancies, their application is limited in OSCC due to tumor cell resistance. One of these drugs is doxorubicin, whose application in OSCC treatment is limited due to cancer cell resistance. Therefore, an improved understanding regarding the molecular basis of doxorubicin resistance is required, in order to develop strategies to increase the sensitivity of OSCC cells and promote the use of this reagent in the treatment of OSCC.
MicroRNAs (miRNAs/miR) are small noncoding RNAs (22–26 nt) that post-transcriptionally regulate gene expression through base-pair matching with the 3′ untranslated region (3′UTR) of target mRNA transcripts (2). miRNAs are involved in various cellular processes, including cell differentiation, proliferation and apoptosis, and serve critical roles in carcinogenesis (3). miR-221 belongs to the miR-221/222 family, which is located as a cluster in the short arm of the X chromosome (4). miR-221 has been reported to be involved in cancer development and drug resistance (4–6). Wei et al (7) reported that exosomal miR-221/222 mediated tamoxifen resistance in recipient estrogen receptor-positive breast cancer cells. Zhao et al (8) demonstrated that inhibition of miR-21 and miR-221 in tumor-initiating stem-like pancreatic cancer cells reduced chemoresistance against gemcitabine and 5-fluorouracil. Furthermore, inhibition of miR-221 in SNU449 liver cancer cells increased doxorubicin-induced cell apoptosis through upregulating caspase-3 activity (9). Previous studies have indicated that aberrant expression of miR-221 may have important roles in the development of OSCC (5,10). Therefore, the present study aimed to investigate whether miR-221 is involved in the chemoresistance of OSCC to doxorubicin.
Tissue inhibitor of metalloproteinase-3 (TIMP3), which is a member of the TIMP family, acts as an inhibitor of matrix metalloproteinases and is involved in extracellular matrix degradation (11). TIMP3 has been identified as a target of miR-221/222 and is involved in regulating sensitivity to chemotherapeutic agents in numerous types of cancer. Gan et al (12) reported that downregulation of miR-221/222 may enhance the sensitivity of MCF-7 and MDA-MB-231 breast cancer cells to tamoxifen via upregulation of TIMP3. In addition, Garofalo et al (13) demonstrated that, in non-small cell lung cancer (NSCLC) and hepatocarcinoma cells, miR-221/222, by targeting phosphatase and tensin homolog (PTEN) and TIMP3, induced TNF-related apoptosis-inducing ligand (TRAIL) resistance and enhanced cellular migration.
The present study investigated whether the miR-221/TIMP3 axis is involved in regulating the sensitivity of OSCC to doxorubicin. The results demonstrated that inhibition of miR-221 restored sensitivity of the SCC4 and SCC9 OSCC cell lines to doxorubicin via upregulation of TIMP3.
Materials and methods
Cell lines and culture
The SCC4 and SCC9 OSCC cell lines were obtained from the Beijing Institute for Cancer Research (Beijing, China). The cells were cultured in Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Wuhan Boster Biological Technology, Ltd., Wuhan, China) at 37°C in a humidified atmosphere containing 5% CO2. Doxorubicin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in dimethyl sulfoxide (DMSO) at 50 mg/ml and further diluted to various concentrations (0.1, 1.0 and 5.0 µM) in the culture medium. Cells were treated with doxorubicin at the indicated concentrations for 24 h at 37°C and then used for analysis.
Transfection of cells with TIMP3 small interfering (si)RNA and anti-miR-221 oligonucleotides
Cells were plated in 6-well plates at a density of 2×105 cells/well. When cells reached 70% confluence, they were transfected with siRNA oligonucleotides targeting human TIMP3 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) or with a non-targeting control siRNA (Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 50 nM, using Lipofectamine® 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The anti-miR-221 and non-targeting scramble oligonucleotides were obtained from Qiagen, Inc. (Valencia, CA, USA). The miR-221 inhibitor (final concentration, 100 nM) or control oligonucleotides were transfected into the cells using Lipofectamine® 2000 according to the manufacturer's protocol. Total RNA and cell lysates were collected 48 h post-transfection and functional studies were conducted 48 h post-transfection.
MTT assay
Cell viability was measured using an MTT assay (Cell Viability kit 1; Roche Applied Science, Penzberg, Germany) as previously described (14). Briefly, cells were seeded in 96-well plates at 5,000 cells/well in triplicate. After 1 day, cells were administered the treatments or DMSO, as indicated. To measure cell viability, 10 µl MTT solution was added directly to each well, and the plates were incubated for 4 h. Solubilization buffer (100 µl) was then added to each well without removing the medium. The plates were then incubated at 37°C overnight and absorbance was measured at 595 nm using a microplate reader. This experiment was run in triplicate for each sample. Relative cell viability was calculated as the ratio of absorbance at 595 nm of the treatment group to absorbance of the control cells. Results are presented as the mean ± standard deviation of three independent experiments.
Annexin V-fluorescein isothiocyanate (FITC)/Hoechst double staining for apoptosis
Cell apoptosis was detected using Annexin V-FITC apoptosis detection reagent (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) according to the manufacturer's protocol, with modifications. Briefly, cells were treated as indicated and were then harvested by trypsin digestion. After collection, cells were washed twice with PBS and centrifuged at 300 × g for 5 min. Cells were then resuspended in 1X staining buffer and cell density was adjusted to 1×105 cells/100 µl. To 100 µl of cell suspension, 5 µl Annexin V-FITC was added and the cells were incubated at room temperature for 15 min followed by the addition of 400 µl 1X staining buffer. Prior to sample loading, 8 µl Hoechst 33258 was added. Cell apoptosis was then detected using a flow cytometer (LSR II; BD Biosciences, Franklin Lakes, NJ, USA) and data were analyzed using the FlowJo software version 9.0 (FlowJo, LLC, Ashland, OR, USA).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for TIMP3 expression
Total RNA was extracted from the cells using the RNeasy kit (Qiagen, Inc.) according to the manufacturer's protocol. For each sample, 1.5 µg total RNA was reverse transcribed into cDNA using the iScript™ cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's protocol. TIMP3 expression was then detected by RT-qPCR as previously described (15). Briefly, total cDNA was diluted 10-fold and 2 µl diluted cDNA was used as a template for RT-qPCR. RT-qPCR was conducted using a CFX Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.) using the iQ™ SYBR®-Green Supermix (Bio-Rad Laboratories, Inc.) according to the manufacturer's protocol. RT-qPCR cycling conditions were as follows: Initial denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 15 sec and annealing and elongation at 60°C for 30 sec, and 1 cycle at 95°C for 10 sec, at 65°C for 5 sec and at 95°C. GAPDH was used as an internal control. All reactions were performed in triplicate. Fold changes in TIMP3 mRNA expression were calculated using the 2−ΔΔCq method (16). Primer sequences were as follows: GAPDH, forward 5′-CCTGCCGGTGACTAACCCT-3′, reverse 5′-AGGCGCCCAATACGACCAAA-3′; and TIMP3, forward 5′-ACCGAGGCTTCACCAAGATG-3′ and reverse 5′-CCCCGTGTACATCTTGCCAT-3′.
RT-qPCR for miR-221 expression
Total RNA, including miRNAs, was extracted using a miRNeasy mini kit (Qiagen, Inc.) according to the manufacturer's protocol. The miRNAs were converted to cDNA, to which a universal tag was added, using the miScript II RT kit (Qiagen, Inc.) according to the manufacturer's protocol. Quantification of miR-221 expression levels was conducted by RT-qPCR using the miScript SYBR Green PCR kit (Qiagen, Inc.), which contains a primer for the detection of the universal tag on cDNAs of miRNAs. The RT-qPCR assay was performed in triplicate with U6 small nuclear (sn)RNA used as an internal control. The following primers were used: miR-221, 5′-GCAGAGCTACATTGTCTGCT-3′ and U6, 5′-ACGCAAATTCGTGAAGCGTT-3′. The reaction was performed in a final volume of 25 µl, containing 2 µl cDNA, 2.5 µl specific primer, 2.5 µl universal primer, 12.5 µl 2X QuantiTect SYBR-Green PCR Master Mix and 5.5 µl H2O. The PCR cycling conditions were as follows: 95°C for 15 min, followed by 40 cycles at 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec. The expression levels of miR-221 were normalized to U6 snRNA. The fold-change in miR-221 levels was calculated using the 2−ΔΔCq method (16).
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay lysis buffer (1% NP-40, 0.5% Sodium deoxycholate and 0.1% SDS; Wuhan Boster Biological Technology, Ltd.) and protein concentration of whole cell lysates was determined using the Bicinchoninic Acid Protein Assay kit (Beyotime Institute of Biotechnology, Haimen, China). Briefly, 30 µg whole cell lysates were loaded and separated by electrophoresis on 10% SDS-polyacrylamide gels and were then transferred to polyvinylidene fluoride membranes (Beyotime Institute of Biotechnology). After blocking with 5% non-fat milk for 1 h at room temperature, the membranes were incubated with rabbit anti-human TIMP3 (cat no. ab39184; 1:1,000; Abcam, Cambridge, MA, USA) and rabbit anti-human β-actin (cat no. ab8227; 1:2,000; Abcam) overnight at 4°C. The membranes were then washed three times with TBST containing 0.1% Tween-20 and incubated with goat anti-rabbit secondary antibody (cat no. A0208; 1:1,000; Beyotime Institute of Biotechnology) for 1 h at room temperature. Subsequently, membranes were washed a further three times with TBST and were incubated with BeyoECL Plus chemical luminescence solution (Beyotime Institute of Biotechnology). The protein bands were visualized using a ChemiDoc XRS imaging system and were analyzed using Quantity One software version 4.3.0 (Bio-Rad Laboratories, Inc.).
Luciferase reporter assay
The 3′UTR of human TIMP3 mRNA transcripts was obtained from cDNA reverse transcribed from RNA as aforementioned, and amplified by PCR using the following primers: TIMP3, forward 5′-CTCGAGCTGGGCAAAGAAGGGTCTTTCGC-3′ and reverse 5′-GCGGCCGCTTCCAATAGGGAGGAGGCTGGAGGA-3′. The PCR products were cloned downstream of the Renilla luciferase stop codon in a psi-CHECK2 vector (Promega Corporation, Madison, WI, USA) between the XhoI (5′) and NotI (3′) sites. To perform the luciferase assay, SCC9 cells were co-transfected with 1 µg p3′UTR-TIMP3 and 100 nM anti-miR-221 oligonucleotides or 100 nM non-targeting oligonucleotides using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Cells were harvested 24 h post-transfection and were assessed using the Dual Luciferase Reporter Assay (Promega Corporation) according to the manufacturer's protocol, on a Synergy 2 plate reader (BioTek Instruments, Inc., Winooski, VT, USA). The assay was performed in triplicate. Renilla luciferase activity was normalized to firefly luciferase activity for each well to normalize cell number and transfection efficiency.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Student's t-test was used to compare the means of two groups. A one-way analysis of variance followed by Tukey post hoc test was conducted to compare the means of more than two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-221 expression is upregulated in SCC4 and SCC9 cells treated with doxorubicin
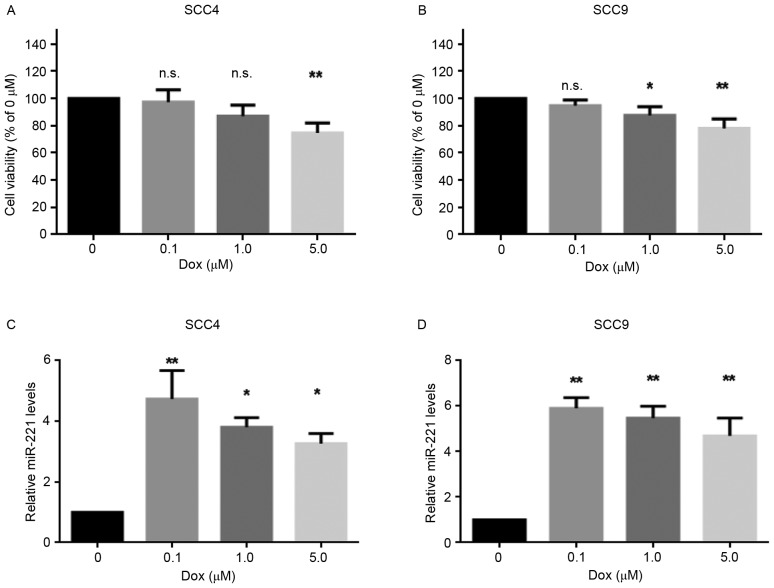
The present study aimed to investigate the effects of doxorubicin on the viability of two OSCC cell lines, SCC4 and SCC9, following treatment with various concentrations (0, 0.1, 1.0 and 5.0 µM) of doxorubicin for 24 h. The results revealed that 0.1 µM doxorubicin was not lethal to the cell lines, whereas 5 µM doxorubicin significantly decreased cell viability (Fig. 1A and B). Furthermore, to determine whether miR-221 was involved in the response of these cells to doxorubicin, the expression levels of miR-221 were detected in SCC4 and SCC9 cells treated with various concentrations of doxorubicin. As presented in Fig. 1C and D, miR-221 expression was upregulated in the cell lines following doxorubicin treatment. In addition, a sublethal dose of doxorubicin (0.1 µM) resulted in the highest upregulation of miR-221 levels. These data indicated that miR-221 expression was induced in response to doxorubicin treatment in SCC4 and SCC9 cells, thus suggesting a potential role for miR-221 in the protection of OSCC cells from doxorubicin.
Figure 1.
miR-221 expression was upregulated in SCC4 and SCC9 cells following doxorubicin treatment. Cells were treated with 0, 0.1, 1.0 and 5.0 µM doxorubicin for 24 h. The cell viability of (A) SCC4 and (B) SCC9 cells was measured by MTT assay. The expression levels of miR-221 were measured by reverse transcription-quantitative polymerase chain reaction in (C) SCC4 and (D) SCC9 cells. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 vs. control (0 µM Dox) group. Dox, doxorubicin; miR, microRNA; n.s., not significant.
Inhibition of miR-221 sensitizes SCC4 and SCC9 cells to doxorubicin
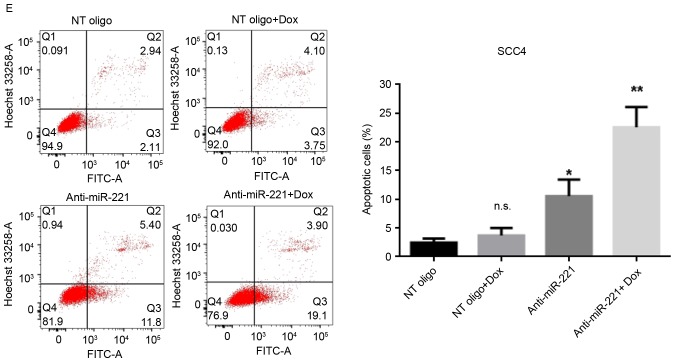
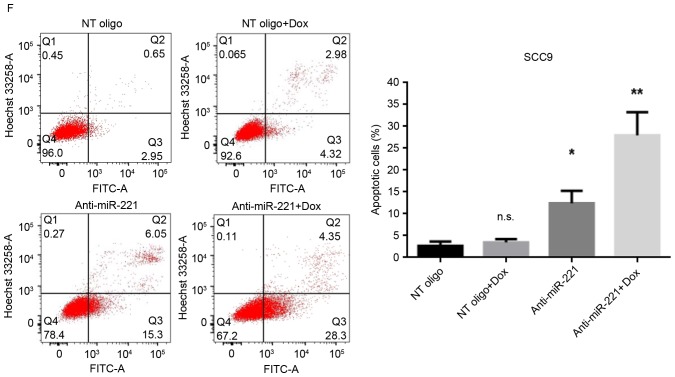
miR-221 has been reported to protect cancer cells from apoptosis in hepatocellular carcinoma (17), astrocytoma (18) and breast cancer (12). Therefore, the present study investigated the roles of upregulated miR-221 in cellular sensitivity to doxorubicin. miR-221 was knocked down in SCC4 and SCC9 cells by transient transfection with anti-miR-221 oligonucleotides; miR-221 knockdown was confirmed using RT-qPCR. The anti-miR-221 oligonucleotides significantly decreased the levels of miR-221 compared with in cells transfected with non-targeting oligonucleotides, with or without doxorubicin treatment (Fig. 2A and B). Subsequently, the effects of silencing miR-221 on cell viability, with or without treatment of doxorubicin, were determined. The results revealed that inhibition of miR-221 alone moderately reduced the viability of SCC4 and SCC9 cells (Fig. 2C and D). However, transfection with miR-221 inhibitory oligonucleotides alongside treatment with doxorubicin significantly inhibited the viability of both cell lines (Fig. 2C and D). The effects of miR-221 inhibition on cell apoptosis, with or without doxorubicin treatment, were then investigated. Without doxorubicin treatment, inhibition of miR-221 in SCC4 and SCC9 cells led to a moderately increased percentage of apoptotic cells compared with the control cells (P<0.05; Fig. 2E and F). However, alongside doxorubicin treatment, transfection of SCC4 and SCC9 cells with anti-miR-221 oligonucleotides further increased the population of apoptotic cells compared with the cells transfected with control oligonucleotides (P<0.01; Fig. 2E and F). These results indicated that knockdown of miR-221 sensitized SCC4 and SCC9 cells to doxorubicin, thus suggesting a protective role for upregulated miR-221 in doxorubicin-mediated cell death.
Figure 2.
Inhibition of miR-221 sensitized SCC4 and SCC9 cells to doxorubicin. Cells were transfected with 100 nM anti-miR-221 or control oligonucleotides for 48 h and were then treated with 0.1 µM doxorubicin or dimethyl sulfoxide for 24 h. The expression levels of miR-221 were measured by reverse transcription-quantitative polymerase chain reaction in (A) SCC4 and (B) SCC9 cells. Cell viability of (C) SCC4 and (D) SCC9 cells was measured by MTT assay. Apoptosis of (E) SCC4 and (F) SCC9 cells was measured by flow cytometry with Annexin V-FITC/Hoechst 33258 double staining. Representative dot plots and quantification of flow cytometry results are presented. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 vs. NT oligo group. Dox, doxorubicin; miR, microRNA; NT, non-targeting; FITC, fluorescein isothiocyanate; n.s., not significant.
TIMP3 is downregulated in SCC4 and SCC9 cells following doxorubicin treatment
TIMP3 has been identified as a direct target of miR-221 in numerous types of cancer, including breast, lung and liver cancers (12,13). To investigate whether TIMP3 is involved in miR-221-mediated resistance to doxorubicin in SCC4 and SCC9 cells, the present study analyzed the protein expression levels of TIMP3 following doxorubicin treatment. The results revealed that the protein expression levels of TIMP3 were decreased following doxorubicin treatment (Fig. 3A) in both cell lines. However, the decrease in TIMP3 protein levels did not occur in a dose-dependent manner, since an increase in the concentration of doxorubicin from 0.1 to 1.0 µM did not further decrease the protein expression levels of TIMP3 (Fig. 3A). To determine whether miR-221 regulated TIMP3 expression in SCC4 and SCC9 cells the mRNA and protein expression levels of TIMP3 in cells transiently transfected with anti-miR-221 or control oligonucleotides were determined. TIMP3 mRNA (Fig. 3B) and protein levels (Fig. 3C) were significantly increased when miR-221 was inhibited in SCC4 and SCC9 cells. Subsequently, the present study confirmed that TIMP3 was a direct target of miR-221 in OSCC using a 3′UTR luciferase reporter assay (Fig. 3D). The reporter assay revealed that transfection of cells with anti-miR-221 oligonucleotides significantly increased the luciferase activity of the TIMP3 3′UTR reporter plasmid in SCC9 cells (Fig. 3E). These data suggested that TIMP3 expression is downregulated through direct regulation by miR-221 in response to doxorubicin treatment in SCC4 and SCC9 cells.
Figure 3.

TIMP3 is downregulated in SCC4 and SCC9 cells in response to doxorubicin treatment. (A) Cells were treated with vehicle, or 0.1 or 1.0 µM of doxorubicin for 24 h. The protein expression levels of TIMP3 in SCC4 and SCC9 cells were measured by western blotting. (B and C) Cells were transfected with 100 nM anti-miR-221 or control oligonucleotides for 48 h. (B) mRNA expression levels of TIMP3 were measured by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard deviation of three independent experiments. **P<0.01 vs. No transfection group. (C) Protein expression levels of TIMP3 were measured by western blotting. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 vs. NT oligo group. (D) Schematic diagram of human TIMP3 3′UTR luciferase constructs with miR-221 binding sites in the TIMP3 3′UTR region. (E) SCC9 cells were co-transfected with the TIMP3 luciferase construct and 100 nM anti-miR-221 or control oligonucleotides. The luciferase assay was performed 48 h post-transfection. Renilla luciferase activity was normalized to firefly luciferase activity. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 vs. NT oligo group. Dox, doxorubicin; miR, microRNA; TIMP3, tissue inhibitor of metalloproteinase-3; NT, non-targeting; UTR, untranslated region; n.s., not significant.
miR-221 protects OSCC cells through downregulating TIMP3 levels
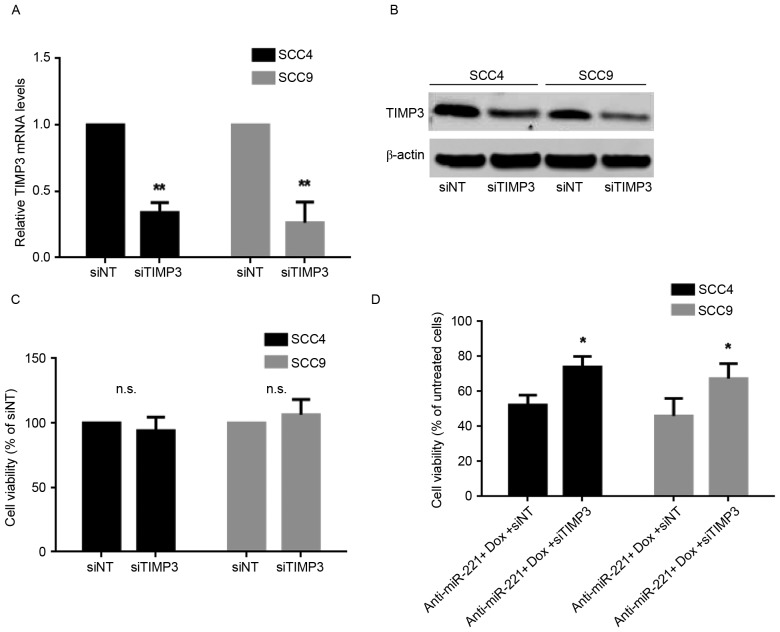
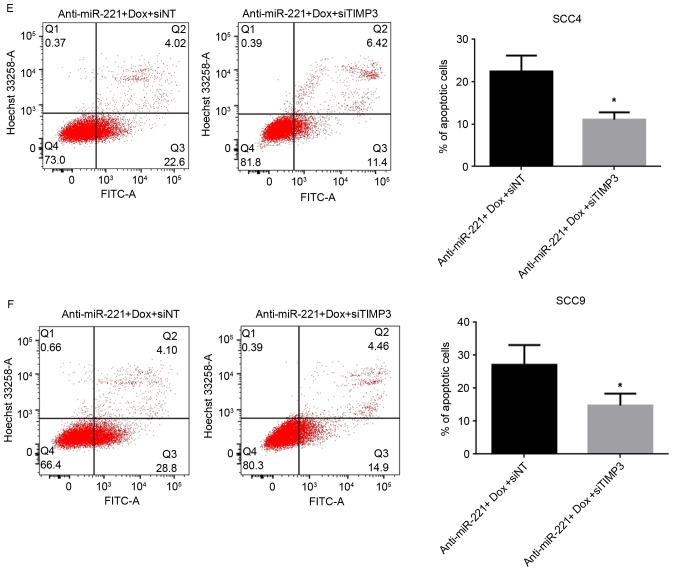
The present study investigated whether miR-221 may protect OSCC cells from doxorubicin-mediated cell death through downregulating TIMP3 levels. TIMP3 was knocked down in SCC4 and SCC9 cells following transfection with TIMP3-specific siRNA; the knockdown was confirmed by RT-qPCR (Fig. 4A) and western blotting (Fig. 4B). Suppression of TIMP3 alone did not lead to significant alterations in cell viability (Fig. 4C). The present study then detected the viability and apoptosis of SCC4 and SCC9 cells transfected with anti-miR-221 oligonucleotides and TIMP3 siRNA, and compared the results to cells transfected with the anti-miR-221 oligonucleotides and non-targeting siRNA under doxorubicin treatment. The cell viability results revealed that co-transfection of cells with anti-miR-221 and TIMP3 siRNA significantly improved the survival rate compared with cells transfected with anti-miR-221 oligonucleotides and control siRNA (P<0.05) under doxorubicin treatment in SCC4 and SCC9 cells (Fig. 4D). Furthermore, the percentage of early apoptotic cells was reduced in SCC4 (P<0.05, Fig. 4E) and SCC9 (P<0.05, Fig. 4F) cells transfected with anti-miR-221 oligonucleotides and TIMP3 siRNA compared with cells transfected with anti-miR-221 and control siRNA under doxorubicin treatment. These results indicated that suppression of TIMP3 partially reversed the anti-miR-221-induced inhibition of cell viability and promotion of apoptosis in OSCC cells, thus suggesting that miR-221 may protect OSCC cells from doxorubicin treatment partially by downregulating TIMP3.
Figure 4.
miR-221 protects OSCC cells through downregulating TIMP3 levels. (A) mRNA expression levels of TIMP3 in cells transfected with TIMP3 siRNA or control oligonucleotides, as detected by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard deviation of three independent experiments. **P<0.01 vs. siNT group. (B) Protein expression levels of TIMP3 in cells transfected with TIMP3 siRNA or control oligonucleotides, as detected by western blotting. (C) Effects of silencing TIMP3 on cell viability, measured using an MTT assay. (D) Viability of SCC4 and SCC9 cells transfected with anti-miR-221 oligonucleotides and TIMP3 siRNA or control oligonucleotides under doxorubicin treatment, measured using an MTT assay Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. anti-miR-221 + Dox + siNT group. Effects of anti-miR-221 and TIMP3 siRNA on (E) SCC4 and (F) SCC9 cell apoptosis under doxorubicin. Representative dot plots and quantification of flow cytometry results are presented. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. anti-miR-221 + Dox + siNT group. Dox, doxorubicin; miR, microRNA; TIMP3, tissue inhibitor of metalloproteinase-3; NT, non-targeting; siRNA, small interfering RNA; FITC, fluorescein isothiocyanate; n.s., not significant.
Discussion
OSCC represents the most common type of oral cancer, which is the sixth most common malignancy in humans (1). Despite advances in therapy, only 40–50% of patients with OSCC are likely to survive for 5 years, which is primarily due to metastasis to distal organs (19,20). Locally advanced tumors and metastatic disease require a comprehensive therapeutic strategy involving chemotherapy; however, the efficacy of chemotherapy is compromised due to acquired and inherent tumor cell resistance to chemotherapeutic reagents. Doxorubicin, which is a drug widely used in cancer treatment, is less often used in OSCC due to the resistance of OSCC cancer cells. Therefore, to improve its use in OSCC a strategy is required to increase the sensitivity of OSCC cells to this reagent.
miR-221 has been reported to be aberrantly expressed in OSCC and promotes the development of OSCC by improving anchorage-independent growth, migration and invasion of cancer cells (5,10). In addition, miR-221 is involved in the resistance of cancer cells to chemotherapeutic reagents (14,15,17). In breast cancer cells, downregulation of miR-221/222 enhances the sensitivity of MCF-7 and MDA-MB-231 to tamoxifen via the upregulation of TIMP3 (12). In NSCLC and hepatocarcinoma cells, miR-221/222 induces TRAIL resistance by targeting PTEN and TIMP3 (13). Furthermore, inhibition of miR-221 in SNU449 liver cancer cells increased doxorubicin-induced cell apoptosis (9). Therefore, the present study aimed to determine whether miR-221 is involved in regulating the sensitivity of OSCC cells to doxorubicin.
The results of the present study demonstrated that miR-221 levels were upregulated in OSCC cells treated with doxorubicin, thus suggesting that miR-221 may serve a protective role in response to doxorubicin. In support of this hypothesis, knockdown of miR-221 in doxorubicin-treated OSCC cells reduced cell viability and induced cell apoptosis, indicating that inhibition of miR-221 may increase the sensitivity of OSCC cells to doxorubicin. miR-221 has also been reported to serve an oncogenic role in OSCC. Gombos et al (21) reported that miR-221 was overexpressed in OSCC samples. Yang et al (10) indicated that miR221 increased growth and anchorageindependent colony formation of OSCC cell lines, and promoted the tumorigenesis of an OSCC cell line in nude mice. Furthermore, He et al (5) demonstrated that inhibition of miR-221 suppressed migration and invasion of UM1 OSCC cells by targeting methyl-CpG-binding domain protein 2. The findings of the present study are consistent with these results, thus indicating that miR-221 functions as an oncogene in OSCC development.
TIMP3 promotes apoptotic cell death in melanoma cells (22). In addition, it has previously been identified as a target of miR-221 in breast cancer, NSCLC and liver cancer (12,13). The present study demonstrated that, unlike miR-221, the protein expression levels of TIMP3 were downregulated in response to doxorubicin treatment. Therefore, downregulation of TIMP3 may be induced by miR-221 and may be involved in induction of resistance to doxorubicin. To test these hypotheses, the present study validated that TIMP3 is a direct target of miR-221 in doxorubicin-treated OSCC cells using a TIMP3 3′UTR luciferase reporter assay. To further demonstrate that TIMP3 contributes to miR-221-induced doxorubicin chemoresistance, TIMP3 siRNA was transfected into OSCC cells to decrease TIMP3 expression under inhibition of miR-221. The results demonstrated that depletion of TIMP3 improved the survival of cells transfected with anti-miR-221 oligonucleotides and treated with doxorubicin. Therefore, it may be concluded that miR-221 induced resistance to doxorubicin in human OSCC cell lines at least in part by downregulating TIMP3. Due to the complexity of the gene networks that are regulated by miR-221, other factors may also contribute to miR-221-induced doxorubicin resistance; therefore, a better understanding of how miR-221 regulates doxorubicin sensitivity is required for designing therapeutic strategies that target miR-221 in OSCC.
In conclusion, the present study is summarized in the diagram presented in Fig. 5. Briefly, miR-221 levels were upregulated in response to doxorubicin treatment in OSCC cell lines, resulting in a decrease in the expression levels of TIMP3. The downregulation of TIMP3 was able to protect OSCC cells from doxorubicin-induced apoptosis, resulting in resistance of OSCC cells to doxorubicin. In concordance with this hypothesis, the present study indicated that inhibiting miR-221, following transfection with inhibitory oligonucleotides, significantly sensitized OSCC cells to doxorubicin. Therefore, inhibition of miR-221 may represent a potential strategy to sensitize OSCC cells to doxorubicin and improve the efficacy of doxorubicin in OSCC.
Figure 5.
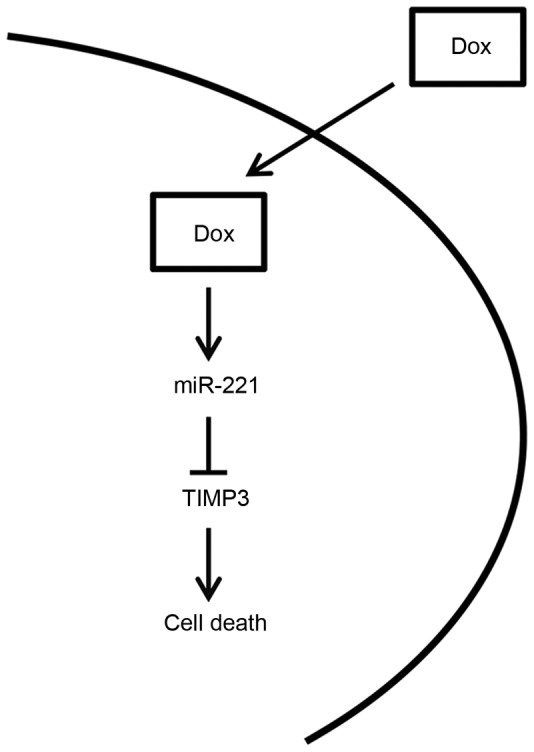
Schematic diagram demonstrating that miR-221 protects OSCC cells from doxorubicin-induced cell death through targeting TIMP3. Dox, doxorubicin; miR, microRNA; TIMP3, tissue inhibitor of metalloproteinase-3.
Glossary
Abbreviations
- OSCC
oral squamous cell carcinoma
- TIMP3
tissue inhibitor of metalloproteinase-3
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. International Agency for Research on Cancer. Lyon, France: 2013. May 19, 2014. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancerbase No. 11 (Internet) Accessed. [Google Scholar]
- 2.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.MacFarlane LA, Murphy PR. MicroRNA: Biogenesis, function and role in cancer. Curr Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garofalo M, Quintavalle C, Romano G, Croce CM, Condorelli G. miR221/222 in cancer: Their role in tumor progression and response to therapy. Curr Mol Med. 2012;12:27–33. doi: 10.2174/156652412798376170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He S, Lai R, Chen D, Yan W, Zhang Z, Liu Z, Ding X, Chen Y. Downregulation of miR-221 inhibits cell migration and invasion through targeting methyl-CpG binding domain protein 2 in human oral squamous cell carcinoma cells. Biomed Res Int. 2015;2015:751672. doi: 10.1155/2015/751672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nassirpour R, Mehta PP, Baxi SM, Yin MJ. miR-221 promotes tumorigenesis in human triple negative breast cancer cells. PLoS One. 2013;8:e62170. doi: 10.1371/journal.pone.0062170. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Wei Y, Lai X, Yu S, Chen S, Ma Y, Zhang Y, Li H, Zhu X, Yao L, Zhang J. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res Treat. 2014;147:423–431. doi: 10.1007/s10549-014-3037-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Zhao L, Ischenko I, Bao Q, Schwarz B, Nieß H, Wang Y, Renner A, Mysliwietz J, Jauch KW. Antisense inhibition of microRNA-21 and microRNA-221 in tumor-initiating stem-like cells modulates tumorigenesis, metastasis, and chemotherapy resistance in pancreatic cancer. Target Oncol. 2015;10:535–548. doi: 10.1007/s11523-015-0360-2. [DOI] [PubMed] [Google Scholar]
- 9.Fornari F, Milazzo M, Galassi M, Callegari E, Veronese A, Miyaaki H, Sabbioni S, Mantovani V, Marasco E, Chieco P, et al. p53/mdm2 feedback loop sustains miR-221 expression and dictates the response to anticancer treatments in hepatocellular carcinoma. Mol Cancer Res. 2014;12:203–216. doi: 10.1158/1541-7786.MCR-13-0312-T. [DOI] [PubMed] [Google Scholar]
- 10.Yang CJ, Shen WG, Liu CJ, Chen YW, Lu HH, Tsai MM, Lin SC. miR-221 and miR-222 expression increased the growth and tumorigenesis of oral carcinoma cells. J Oral Pathol Med. 2011;40:560–566. doi: 10.1111/j.1600-0714.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Munoz W, Khokha R. The role of tissue inhibitors of metalloproteinases in tumorigenesis and metastasis. Crit Rev Clin Lab Sci. 2008;45:291–338. doi: 10.1080/10408360801973244. [DOI] [PubMed] [Google Scholar]
- 12.Gan R, Yang Y, Yang X, Zhao L, Lu J, Meng QH. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther. 2014;21:290–296. doi: 10.1038/cgt.2014.29. [DOI] [PubMed] [Google Scholar]
- 13.Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Starr TK, Scott PM, Marsh BM, Zhao L, Than BL, O'Sullivan MG, Sarver AL, Dupuy AJ, Largaespada DA, Cormier RT. A sleeping beauty transposon-mediated screen identifies murine susceptibility genes for adenomatous polyposis coli (Apc)-dependent intestinal tumorigenesis. Proc Natl Acad Sci USA. 2011;108:5765–5770. doi: 10.1073/pnas.1018012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Than BL, Goos JA, Sarver AL, O'Sullivan MG, Rod A, Starr TK, Fijneman RJ, Meijer GA, Zhao L, Zhang Y, et al. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene. 2014;33:3861–3868. doi: 10.1038/onc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Rong M, Chen G, Dang Y. Increased miR-221 expression in hepatocellular carcinoma tissues and its role in enhancing cell growth and inhibiting apoptosis in vitro. BMC Cancer. 2013;13:21. doi: 10.1186/1471-2407-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu D, Sun YC. Overexpression of miR-221 inhibits proliferation and promotes apoptosis of human astrocytoma cells. Int J Clin Exp Pathol. 2015;8:4851–4856. [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka T, Tanaka M, Tanaka T. Oral carcinogenesis and oral cancer chemoprevention: A review. Pathol Res Int. 2011;2011:431246. doi: 10.4061/2011/431246. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Huang SH, O'Sullivan B. Oral cancer: Current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013;18:e233–e240. doi: 10.4317/medoral.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gombos K, Horváth R, Szele E, Juhász K, Gocze K, Somlai K, Pajkos G, Ember I, Olasz L. miRNA expression profiles of oral squamous cell carcinomas. Anticancer Res. 2013;33:1511–1517. [PubMed] [Google Scholar]
- 22.Das AM, Seynhaeve AL, Rens JA, Vermeulen CE, Koning GA, Eggermont AM, Ten Hagen TL. Differential TIMP3 expression affects tumor progression and angiogenesis in melanomas through regulation of directionally persistent endothelial cell migration. Angiogenesis. 2014;17:163–177. doi: 10.1007/s10456-013-9385-2. [DOI] [PubMed] [Google Scholar]