Abstract
The aim of the present study was to investigate the role of the Toll-like receptor (TLR)4 signaling pathway in cellular response to lipopolysaccharide (LPS) in rat pulmonary artery smooth muscle cells (PASMCs). Chronic obstructive pulmonary disease (COPD) rats were established with passive inhaling cigarette smoke plus injection of LPS. The TLR4 protein in lung tissues was determined with immunohistochemical staining and protein levels of the components of the TLR4 pathway in PASMCs were analyzed with western blotting. The production of interferon (IFN)-γ upon LPS stimulation in PASMCs was measured with ELISA. TLR4 expression in lung tissue from COPD rats was increased obviously compared with that in normal group. LPS enhances TLR4 expression in rat PASMCs and induced production of IFN-γ dramatically. LPS treatment resulted in increased phosphor-interleukin-1 receptor-associated kinase (IRAK), IκB and IκB kinase, as well as the total protein of nuclear factor (NF)-κB p65. TLR4 inhibitor TAK-242, IRAK1/4 inhibitor and NF-κB inhibitor Bay 117082 were capable of suppressing the effects of LPS. TLR4 signaling pathway is functional in PASMCs, and may be involved in the inflammatory response during the pathogenesis of COPD.
Keywords: lipopolysaccharide, Toll-like receptor 4, interferon-γ, nuclear factor-κB, pulmonary arterial smooth muscle cells
Introduction
Chronic obstructive pulmonary disease (COPD) is a devastating lung disease with a basic feature of incomplete reversibility of airflow obstruction, which may be resulted from lung's abnormal inflammatory response to harmful particles or gases (1). Pulmonary tissues of COPD have obvious vascular inflammatory changes and pathological reconstruction in the early days even before hypoxia appears, and pulmonary vascular remodeling of COPD is associated with the role of chronic inflammatory cells and inflammatory mediators. Therefore, chronic inflammation is a central part of the early pulmonary vascular remodeling (2). Pulmonary artery smooth muscle cells (PASMCs) not only are the main effectors involved in pulmonary vascular remodeling, but also can act like immune cells to synthesize and secrete inflammatory mediators to promote the development of pulmonary vascular inflammation, and thus serve key roles during pulmonary vascular remodeling. However, the underlying mechanisms remain largely unclear.
Toll-like receptors (TLRs) are receptors that have the prominent biological function to promote synthesis and release of cytokines to trigger inflammatory response (3). Moreover, it also can promote the maturation of antigen presenting cells to induce the human body's acquired immunity. TLR4 was the first identified mammalian TLR, belonging to cytomembrane type I transmembrane glycoprotein receptor, and can be activated by the lipopolysaccharides (LPS) of pathogenic microorganisms, lipoproteins and peptidoglycans, to promote cells to produce cytokines, chemokines, adhesion molecules and acute phase protein to regulate the inflammatory responses (4). TLR4 is widely expressed in pulmonary macrophages, airway epithelial cells, airway smooth muscle cells and vascular endothelial cells. Previous studies of the authors reported that TLR4 receptor mediates PI3K/NF-κB signaling during airway remodeling in asthma, which is an important signal pathway to regulate the bronchial smooth muscle cells to synthesize and secrete inflammatory factor (5). Some reports have demonstrated an increased expression of TLR4 in lung tissue of COPD patients (6). Yet, the role of TLR4 in synthesis and secretion function of PASMCs is unclear. The present study investigated the TLR4 expression level in PASMCs of COPD rats and its role in PASMCs synthesis and secretion function.
Materials and methods
Materials
LPS was purchased from Sigma-Aldrich (Darmstadt, Germany). The cigarettes were from Liuzhou Cigarette Factory (Liuzhou, China). Gentamicin (cat. no. 15710-064) was from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Rat monoclonal antibody against TLR4 was from Abcam (Cambridge, MA, USA). Horseradish peroxidase-conjugated anti-rat IgG was from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China). The DAB kit was from Sangon Biotech Co., Ltd. (Shanghai, China). TAK-242 was from MedChem Express (Monmouth Junction, NJ, USA). The ELISA kit (cat. no. E-EL-R0009c) was from R&D Systems, Inc. (Minneapolis, MN, USA).
Animal grouping and modeling
The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Guilin Medical University (Guilin, China). A total of 24 specific pathogen-free Wistar rats (12 males and 12 females) weighing 180–220 g were randomly divided into two groups: Control group and COPD group. Rats in the control group were bred normally for 8 weeks and then examined. Rats in the COPD group were administered with 200 µg LPS to the airways on days 1 and 14, and treatment of passive inhaling of cigarette smoke was administered 1 h/day for 8 weeks. Rats in control group were administered with same volume of saline to the airways on day 1 and 14, and were treated in the same apparatus as the COPD group with normal air 1 h/day for 8 weeks.
Lung tissue specimen preparation
Lung tissues were prepared as following: i) Middle lobes of the right lungs of the rats were removed; ii) 10% neutral formalin was injected into the lungs until the middle lobe of the right lung had swelled completely; iii) the hilum of the lung was ligated; iv) the middle lobes were soaked in 10% neutral formalin and fixed for 24 h; v) the specimen was sliced continuously 2–3 mm to the right of the middle lobe; vi) gradient alcohol dehydration, paraffin embedding and slicing were performed according to standard protocol (7); and vii) alterations in the airway and pulmonary alveoli were observed following conventional hematoxylin and eosin staining.
Rat PASMC culture
Rat PASMCs were cultured in medium supplemented with 25% inactivated fetal bovine serum and 100 µg/ml gentamicin, and cells at passages 3–6 were used for experiments. PASMCs were isolated from the 3rd level or lower artery branch of pulmonary lobe segments from Wistar rats.
Immunochemical staining
Paraffin embedded lung tissues were cut into 4 µm thick sections. Floating sections on 0.01 M PBS (Thermo Fisher Scientific, Inc.) were exposed to 0.25% Triton X-100 (J&K Scientific Ltd., Beijing, China) and 3% H2O2, and then blocked in 1% FBS (cat. no. 10099-141; Thermo Fisher Scientific, Inc.). They were then incubated sequentially with rat monoclonal antibody against TLR4 (1:800), horseradish peroxidase-conjugated anti-rat IgG (1:200). Sections were developed with a DAB kit. Mouse PASMCs were seeded in 6-well plates with or without LPS (concentration 1 µg/ml), the cultured cells were washed with PBS and fixed with 4% paraformaldehyde for 30 min. They were then rinsed in PBS and incubated at room temperature with 0.5% Triton X-100 for 15 min, followed by blocking in PBS containing 1% BSA at room temperature for 30 min. The cells were then incubated with mouse monoclonal antibody against TLR4 (cat. no. ab22048; dilution 1:800) at 4°C overnight. The cultured cells were rinsed in PBS and incubated for 1 h with biotinylated anti-mouse IgG (cat. no. zb2305; dilution 1:200; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at room temperature, then developed with a DAB kit.
Western blot analysis
Proteins (15 µg) were separated with SDS-PAGE and then transferred onto polyvinylidene difluoride membrane, which was then blocked in 5% non-fat milk at room temperature for 2 h. The membrane was washed with TBS with 0.1% Tween-20 then incubated with mouse monoclonal antibody against TLR4 (dilution 1:1,000) at 4°C overnight, followed with 3×10 min TBS-T washes. The membrane was then incubated at room temperature with horseradish peroxidase-conjugated anti-rat IgG (cat. no. ZB-2305; dilution 1:5,000) for 2 h. After 3×10 min TBS-T washes, the membrane was developed with ECL Plus western blotting detection reagents (Beyotime Institute of Biotechnology, Haimen, China). The ChemiDoc™ XRS+System (using Image Lab 3.0 software; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to capture and analyze images of the membrane.
ELISA
PASMCs were plated in 6-well plates (5×104 cells/well), and incubated for 24 h in serum-free media, There were four groups analyzed: The control group, LPS (the final concentration is 1 µg/ml) treatment group, TAK-242 (the final concentration is 1 µM/l) treatment group, LPS+TAK-242 treatment group (LPS final concentration was 1 µg/ml, TAK-242 final concentration was 1 µM/l). Each group had four duplicates, and three independent experiments were performed. Cellular supernatants were analyzed by an ELISA kit for mouse IFN-γ.
Statistical analysis
SPSS statistical software (version, 17.0; SPSS, Inc., Chicago, IL, USA) was used to analyze data. A one-way analysis of variance was used for statistical analysis between groups. The SNK (homogeneous variance) or Games-Howell method (inhomogeneous variance) was used to compare two groups with normal distributions. P<0.05 was considered to indicate a statistically significant difference.
Results
Lung tissue pathological changes and enhanced TLR4 expression in COPD rats
Pathological exam revealed that the control group exhibited normal airway structures, intact walls and no inflammatory cell infiltration (Fig. 1A). In contrast, the COPD group had inflammatory cell infiltration in airway walls, the airway epithelium cell proliferation, dramatically thickened PASMCs layer in artery and apparent emphysema lung bullae (Fig. 1B). These pathological changes were consistent with COPD typical features. In particular, the authors observed enhanced TLR4 expression in pulmonary artery smooth muscle layer of COPD rats (Fig. 2).
Figure 1.
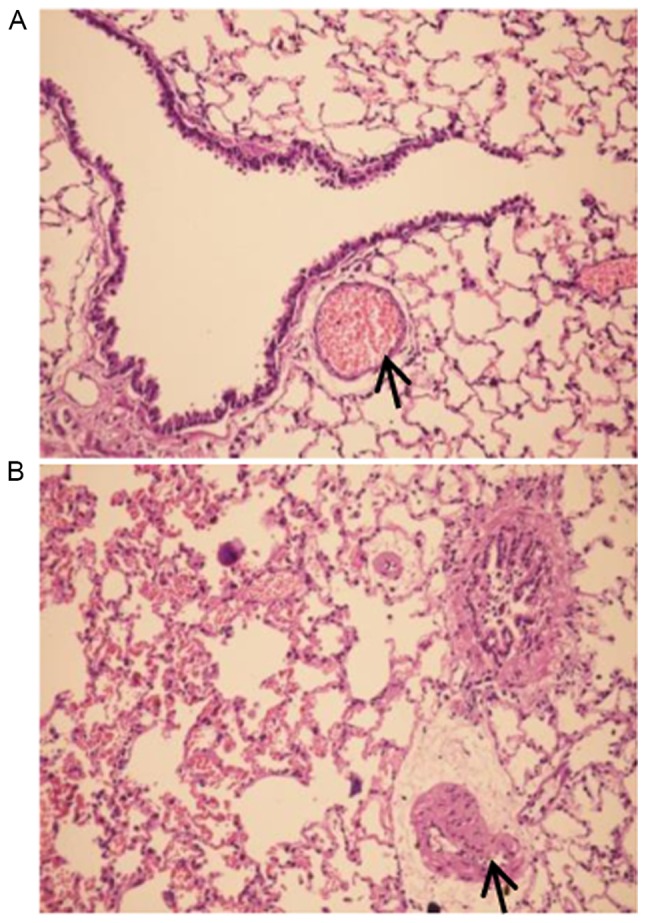
Morphological alteration in lung tissues of COPD rats. The representative pictures of lung tissues from the control group (A, magnification, ×200) and COPD group (B, magnification, ×200) are presented. The pulmonary arteries are marked with arrow. The picture of control group exhibits normal airway structures, intact walls and there is no inflammatory cell infiltration. In contrast, the picture of lung tissue from COPD group present inflammatory cell infiltration in airway walls, a dramatically thickened PASMC layer in the artery and apparent emphysema lung bullae. These pathological changes were consistent with COPD typical features. COPD, chronic obstructive pulmonary disease; PASMC, pulmonary artery smooth muscle cells.
Figure 2.
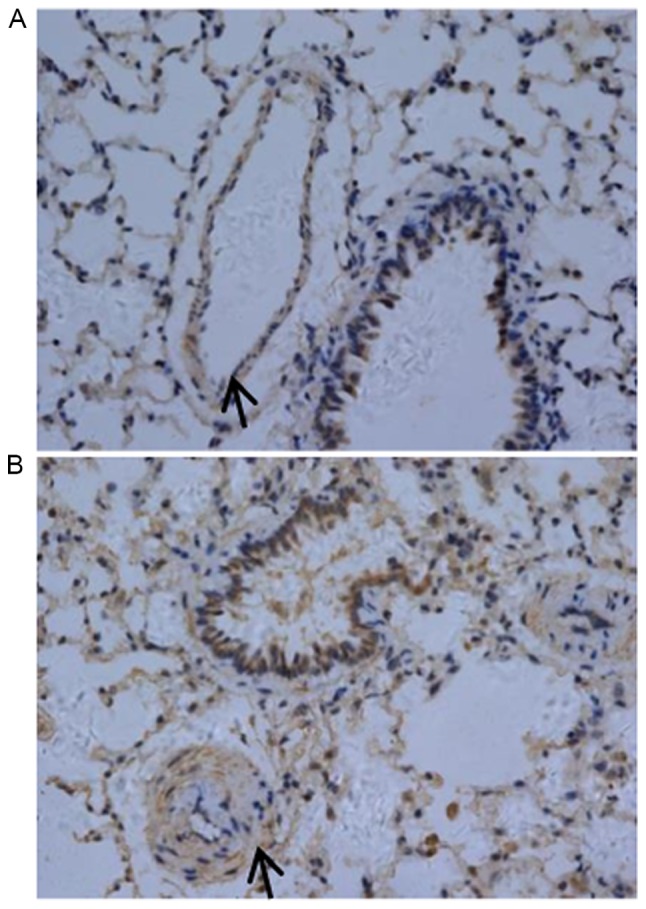
TLR4 expression was increased in lung tissues from COPD rats. There is basal expression of TLR4 in control rat pulmonary artery smooth muscle layer (A, magnification, ×400). However, TLR4 expression was increased in pulmonary artery smooth muscle layer of COPD rats (B, magnification, ×400). The pulmonary arteries are marked with arrow, and brown staining is the positive TLR4 protein. TLR4, Toll-like receptor 4; COPD, chronic obstructive pulmonary disease.
TLR4 expression is enhanced by LPS and inhibited by TAK-242 in PASMCs
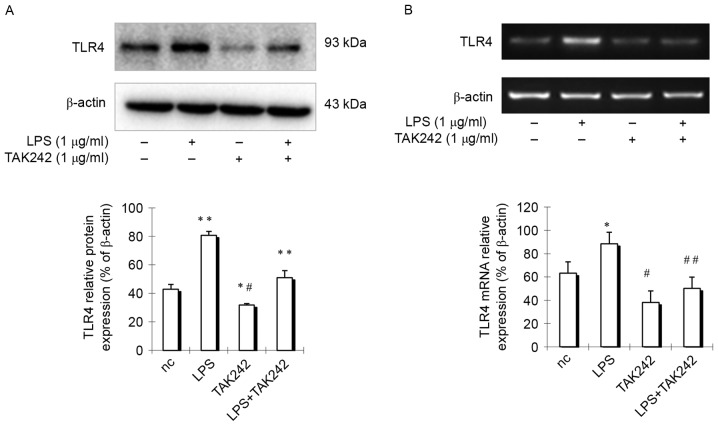
As previous reports demonstrated that human aorta smooth muscle cells express TLR4, which is upregulated by LPS (8), the authors hypothesized that rat pulmonary arterial smooth muscle cells may have similar features. To test the hypothesis, rat PASMCs were isolated and cells were stimulated with LPS for 24 h, then TLR4 expression was detected in PASMCs. Results indicated that TLR4 expression in cells treated with LPS was increased obviously compared with control group cells (Fig. 3), indicating that TLR4 expression is enhanced by LPS treatment. However, if cells were treated with LPS at the presence of TLR4 inhibitor TAK-242, which selectively inhibits TLR4 (9), TLR4 expression was remarkably suppressed (Fig. 3). These findings indicated that there is an active TLR4 signaling pathway in PASMCs.
Figure 3.
TLR4 expression is enhanced by LPS. Pulmonary artery smooth muscle cells were treated with LPS alone or LPS+TAK242. (A) The protein levels of TLR4 were analyzed by western blotting, and (B) the mRNA levels of TLR4 were analyzed by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.001 vs. negative control group. #P<0.01, ##P<0.001 vs. LPS group. TLR4, Toll-like receptor 4; LPS, lipopolysaccharide; nc, negative control.
IFN-γ production is enhanced by LPS and inhibited by TAK-242 in PASMCs
As TLR4 pathway serve a key role in inflammatory response in immune cells. To further examine the function of the TLR4 signaling pathway in PASMCs, cells were treated with LPS at the presence or absence of TAK-242, and the amount of IFN-γ was measured with ELISA. Results demonstrated that IFN-γ expression was increased significantly by LPS stimulation (Fig. 4). Moreover, TLR4 inhibitor TAK-242 antagonized the effect of LPS (Fig. 4). These findings suggested that LPS stimulate PASMCs to produce IFN-γ via the TLR4 signaling pathway.
Figure 4.
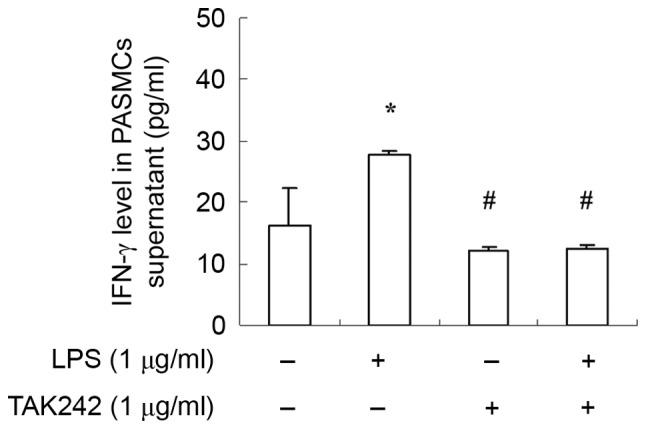
IFN-γ expression is upregulated upon LPS treatment in PASMCs. PASMCs were treated with LPS or LPS+TAK242, and the secreted IFN-γ levels were measured by ELISA assay. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. control group; #P<0.05 vs. LPS group. IFN-γ; interferon-γ; LPS, lipopolysaccharide; PASMCs, pulmonary artery smooth muscle cells.
The cellular response to LPS depends on IRAK and NF-κB in PASMCs
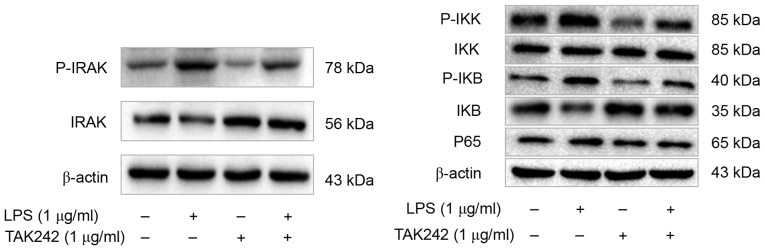
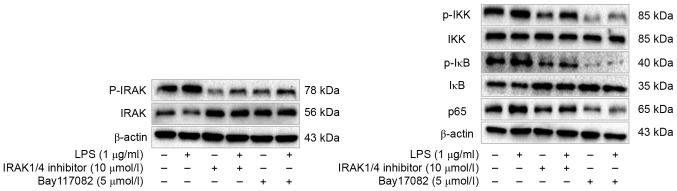
To further elucidate the roles of TLR4 signaling pathway in the cellular response to LPS in PASMCs, the authors analyzed the phosphorylation status or expression of some key components of this pathway, including interleukin-1 receptor-associated kinase (IRAK), IκB kinase (IKK), I-κB and nuclear factor (NF)-κB p65, upon LPS treatment. The authors reported increased amount of phosphorylated IRAK, IKK and I-κB, as well as increased p65 total protein following LPS treatment (Fig. 5). However, this effect of LPS could be inhibited by TAK-242 (Fig. 5). To further verify this observation, cells were firstly treated with IRAK1/4 inhibitor or NF-κB inhibitor Bay117082, and then treated with LPS. Phosphorylation of IRAK, IKK and I-κB was suppressed remarkably, and p65 total protein was decreased dramatically as well (Fig. 6). Moreover, IFN-γ production was also decreased significantly (Fig. 7). These finding suggested that PASMCs' response to LPS stimulation depends on IRAK and NF-κB.
Figure 5.
TLR4 inhibitor TAK-242 suppresses the TLR4/IRAK/NF-ΚB signaling pathway in PASMCs. Western blot analysis was performed to detect the levels of p-IRAK, IRAK, p-IKK, IKK, p-IκB, IκB and NF-κB p65, and the representative blot results are shown. TLR4, Toll-like receptor 4; IRAK, interleukin-1 receptor-associated kinase; NF-κB, nuclear factor-κB; PASMCs, pulmonary artery smooth muscle cells; IKK, IκB kinase; LPS, lipopolysaccharide.
Figure 6.
IRAK1/4 inhibitor and Bay117082 suppress the TLR4/IRAK/NF-ΚB signaling pathway in PASMCs. Western blot analysis was performed to detect the levels of p-IRAK, IRAK, p-IKK, IKK, p-IκB, IκB and NF-κB p65, and the representative blots are shown. IRAK, interleukin-1 receptor-associated kinase; TLR4, Toll-like receptor 4; NF-κB, nuclear factor-κB; PASMCs, pulmonary artery smooth muscle cells; IKK, IκB kinase; LPS, lipopolysaccharide.
Figure 7.
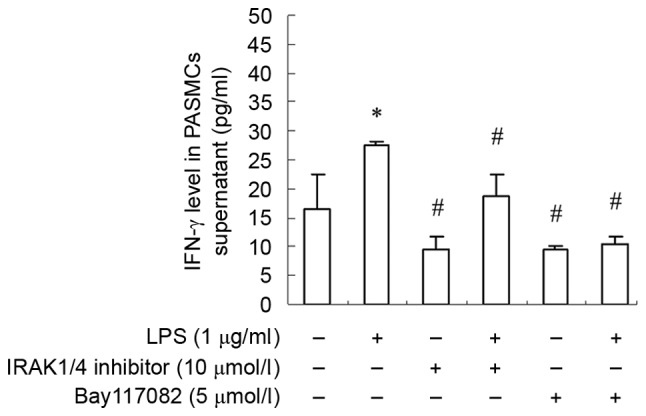
IFN-γ expression is inhibited by IRAK1/4 inhibitor and Bay117082. Data are presented as the mean ± standard error. *P<0.05 vs. control group; #P<0.05 vs. LPS group. IFN-γ, interferon-γ; IRAK, interleukin-1 receptor-associated kinase; PASMCs, pulmonary artery smooth muscle cells; LPS, lipopolysaccharide.
Discussion
COPD is a chronic inflammatory disease, which has high incidence and mortality rate, and has become a significant public health issue. Pulmonary hypertension is closely related to the prognosis of COPD (10). However, the underlying mechanisms remain unclear. In the present study, the authors found PASMCs has a functional TLR4 signaling pathway, which enables cells to respond to LPS and produce cytokine IFN-γ. These findings may provide experimental evidences to understand how pulmonary hypertension occurs in COPD patients.
Vascular inflammation and vascular remodeling are important pathological features of COPD, and PASMCs is the main effector of pulmonary vascular remodeling. Reports have demonstrated that the cellular phenotype transitions of PASMCs caused by the stimulation factors, such as inflammation and trauma, raise inflammatory cell aggregation and inflammatory cascades amplification, and promote the proliferation and migration of PASMCs, differentiation of fibroblasts, and ultimately promote the pulmonary vascular remodeling. This process develops in the early stages of disease, which are the key starting steps prior to cell proliferation and migration (5,11–13). The results indicated that PASMCs themselves are able of producing IFN-γ, indicating PASMCs serve dual roles as both effectors and regulator during pathogenesis of COPD.
TLR4 is the first identified mammalian TLR, which is often expressed in inflammatory cells and can be activated by ligand binding, which eventually activates NF-κB via cellular signaling cascades. NF-κB then translocates to the nucleus to regulate target gene expression. TLR4 in lung tissue may be activated by risk factors such as cigarette smoking, inhaling polluted air, infection of bacteria and virus, which ultimately activates NF-κB and upregulates expression of inflammatory mediators, especially the primary mediator such as tumor necrosis factor (TNF)-α and interleukin (IL)-1, which induces alveolar macrophages to produce a large number of secondary inflammatory cytokines such as IL-6, IL-8, These further induce the neutrophils and CD8 + T lymphocytes to participate in the inflammatory reaction of COPD (14,15). Passive smoking and intratracheal instillation of LPS may cause lung injury similar to chronic obstructive pulmonary disease via the NF-κB signaling pathway, and TLR4 serves an important role in this process (16). A systemic defect in TLR4 signaling increases lipopolysaccharide-induced suppression of IL-2-dependent T-cell proliferation in COPD (3). The TLR4/MyD88 pathway is activated and its downstream inflammatory cytokines such as TNF-α and IL-6 were increased in macrophages from COPD patients (17). The present study indicated that TLR4 signaling in PASMCs depends on IRAK and NF-κB, suggesting potential therapeutic targets for treatment of pulmonary hypertension occurred in COPD patients. To the best of the authors' knowledge, the current study is the first regarding the role of TLR4 signaling in the synthesis and secretion functions of PASMCs. Since TLR4 signaling is able to regulate expression of a variety of inflammatory factors and cytokines in other types of cells, further experiments are needed to investigate whether it is also the case in PASMCs. Moreover, further elucidating the role of TLR4 signaling in proliferation of PASMCs upon LPS stimulation will provide more evidence to understand the mechanisms of COPD and pulmonary hypertension.
In summary, this study revealed the LPS enhances expression of TLR4 and IFN-γ via the TLR4/IRAK/NF-κB signaling pathway in PASMCs, these findings may provide potential therapeutic targets for COPD. However, further experiments are needed to find more details to understand the role of the TLR4 signaling pathway during the pathogenesis of COPD.
Acknowledgements
The current study was supported in part by the Natural Science Foundation of Guangxi (grant nos. 2014GXNSFAA118151 and 2015GXNSFAA139178), the National Natural Science Foundation of China (grant nos. 81360010 and 81560453) and the Medical and Healthy Technology R&D Project from the Guangxi Health and Family Planning Commission (grant no. S2015-34). G.H. was supported by Hundred Talents Program of Guangxi.
References
- 1.Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:995–1013. doi: 10.2147/COPD.S82518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbera JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: Advances in pathophysiology and management. Drugs. 2009;69:1153–1171. doi: 10.2165/00003495-200969090-00002. [DOI] [PubMed] [Google Scholar]
- 3.Knobloch J, Chikosi SJ, Yanik S, Rupp J, Jungck D, Koch A. A systemic defect in Toll-like receptor 4 signaling increases lipopolysaccharide-induced suppression of IL-2-dependent T-cell proliferation in COPD. Am J Physiol Lung Cell Mol Physiol. 2016;310:L24–L39. doi: 10.1152/ajplung.00367.2014. [DOI] [PubMed] [Google Scholar]
- 4.Molteni M, Gemma S, Rossetti C. The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediators Inflamm. 2016;2016:6978936. doi: 10.1155/2016/6978936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi B, Cui J, Ning JN, Wang GS, Qian GS, Lu KZ. Over-expression of PKGIα inhibits hypoxia-induced proliferation, Akt activation, and phenotype modulation of human PASMCs: The role of phenotype modulation of PASMCs in pulmonary vascular remodeling. Gene. 2012;492:354–360. doi: 10.1016/j.gene.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2012;18:1509–1518. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson JG. Immunofluorescence staining. Curr Protoc Cell Biol Chapter. 2001;4 doi: 10.1002/0471143030.cb0403s00. Unit 4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, He Y, Zhang J, Sun S, Sun B. Lipopolysaccharide regulates toll-like receptor 4 expression in human aortic smooth muscle cells. Cell Biol Int. 2007;31:831–835. doi: 10.1016/j.cellbi.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Orr R, Smith LJ, Cuttica MJ. Pulmonary hypertension in advanced chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2012;18:138–143. doi: 10.1097/MCP.0b013e32834f2093. [DOI] [PubMed] [Google Scholar]
- 11.Nazari-Jahantigh M, Wei Y, Schober A. The role of microRNAs in arterial remodelling. Thromb Haemost. 2012;107:611–618. doi: 10.1160/TH11-12-0826. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Ospelt C, Gay S. TLRs and chronic inflammation. Int J Biochem Cell Biol. 2010;42:495–505. doi: 10.1016/j.biocel.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Pace E, Giarratano A, Ferraro M, Bruno A, Siena L, Mangione S, Johnson M, Gjomarkaj M. TLR4 upregulation underpins airway neutrophilia in smokers with chronic obstructive pulmonary disease and acute respiratory failure. Hum Immunol. 2011;72:54–62. doi: 10.1016/j.humimm.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Geraghty P, Dabo AJ, D'Armiento J. TLR4 protein contributes to cigarette smoke-induced matrix metalloproteinase-1 (MMP-1) expression in chronic obstructive pulmonary disease. J Biol Chem. 2011;286:30211–30218. doi: 10.1074/jbc.M111.238824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng Y, Yu CH, Li T, Li W, Cai SX, Li X. Expression and significance of Toll-like receptor-4 in rats lung established by passive smoking or associated with intratracheal instillation of lipopolysaccharide. Zhonghua yi xue za zhi. 2013;93:2230–2234. (In Chinese) [PubMed] [Google Scholar]
- 17.Zeng X, Liu X, Bao H, Zhang Y, Wang X, Shi K, Pang Q. Effects of sulforaphane on Toll-like receptor 4/myeloid differentiation factor 88 pathway of monocyte-derived macrophages from patients with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2014;37:250–254. (In Chinese) [PubMed] [Google Scholar]