Abstract
Engraftment and homing of mesenchymal stem cells (MSCs) are modulated by priming factors including the bioactive lipid sphingosine-1-phosphate (S1P), by stimulating CXCR4 receptor signaling cascades. However, limited in vivo efficacy and the remaining priming molecules prior to administration of MSCs can provoke concerns regarding the efficiency and safety of MSC priming. Here, we showed that valproic acid (VPA), a histone deacetylase inhibitor, enforced the priming effect of S1P at a low dosage for human umbilical cord-derived MSCs (UC-MSCs). A DNA-methylation inhibitor, 5-azacytidine (5-Aza), and VPA increased the expression of CXCR4 in UC-MSCs. In particular, UC-MSCs primed with a suboptimal dose (50 nM) of S1P in combination with 0.5 mM VPA (VPA+S1P priming), but not 1 µM 5-Aza, significantly improved the migration activity in response to stromal cell-derived factor 1 (SDF-1) concomitant with the activation of both MAPKp42/44 and AKT signaling cascades. Both epigenetic regulatory compounds had little influence on cell surface marker phenotypes and the multi-potency of UC-MSCs. In contrast, VPA+S1P priming of UC-MSCs potentiated the proliferation, colony forming unit-fibroblast, and anti-inflammatory activities, which were severely inhibited in the case of 5-Aza treatment. Accordingly, the VPA+S1P-primed UC-MSCs exhibited upregulation of a subset of genes related to stem cell migration and anti-inflammation response. Thus, the present study demonstrated that VPA enables MSC priming with S1P at a low dosage by enhancing their migration and other therapeutic beneficial activities. This priming strategy for MSCs may provide a more efficient and safe application of MSCs for treating a variety of intractable disorders.
Keywords: sphingosine-1-phosphate, valproic acid, mesenchymal stem cell, priming
Introduction
Mesenchymal stem cells (MSCs) are multipotent progenitor cells which are isolated from bone marrow (BM), adipose, dental pulp, umbilical cord (UC), and UC blood (UCB) (1,2). MSC administration provides a therapeutic benefit by their recruitment to damaged organs where they are actively involved in tissue repair (3–8). The beneficial effect is also attributed to paracrine factors (9), such as cytoprotective effects modulated by cytokines (10) and pro-angiogenic and pro-arteriogenic effects (11). However, current MSC therapy has limited therapeutic effectiveness, particularly due to poor in vivo engraftment and survival of the transplanted cells (12). Most (≥99%) intravenously injected MSCs adhere to the lungs, and a mere 2–3% of them are released into the circulation (13). Thus, understanding the precise mechanism underlying the migration and engraftment of MSCs during tissue repair is crucial.
Some chemokines [stromal cell-derived factor 1 (SDF-1)] and growth factors [e.g., vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) or hepatocyte growth factor (HGF)] play a crucial role in the mobilization and engraftment of adult MSCs (14–19). Among them, SDF-1 and its receptor, the CXCR4, is a pivotal factor in the homing/engraftment of stem cells. Indeed, hematopoietic stem/progenitor cells (HSPCs) engraft in the BM by following an SDF-1 gradient that is upregulated in the BM after conditioning for transplantation (e.g., total body irradiation and myeloablative chemotherapy) (14,18). The sensitivity/responsiveness of HSPCs to a SDF-1 gradient is positively affected ('primed') by a subset of molecules enriched in the damaged tissues including bioactive lipids [e.g., sphingosine-1-phosphate (S1P) (20) and ceramide-1-phosphate (C1P) (21,22), neutrophil-derived cationic peptide cathelicidin (LL-37), β2-defensin, and soluble membrane attack complex (sMAC) C5b-9 (21)].
We recently demonstrated that this priming phenomenon observed in the process of HSPCs occurs similarly in MSCs primed with S1P and C1P bioactive lipids and a cationic peptide, LL-37 (3,23). In particular, the primed MSCs exhibit cell migration, colony-forming activity, and anti-inflammatory capacity in the cell culture condition, which promote their therapeutic benefits to treat pulmonary arterial hypertension (PAH). However, the primed MSCs still exhibit limited in vivo engraftment into damaged tissues (3,23). More importantly, even an extensive washing step prior to MSC administration fails to completely remove the remaining priming factors which can provoke an adverse inflammatory response (3). Thus, development of priming strategies at a low dosage is needed. The expression of CXCR4, a main target for stem cell priming, is upregulated by the DNA-demethylating agent, 5-azacytidine (5-Aza) (24), and histone deacetylase inhibitor, valproic acid (VPA) (25). In the present study, we investigated the role of these epigenetic regulatory modulators in improving MSC priming strategies for accelerating their therapeutic application.
Materials and methods
Culturing human umbilical cord-derived MSCs
Human UC was obtained from healthy normal full-term newborns after obtaining written informed parental consent in accordance with the guidelines approved by the Ethics Committee on the Use of Human Subjects at Asan Medical Center. Informed consent was obtained from all pregnant mothers before UC collection. UC-derived MSCs (UC-MSCs) used in the present study were grown in low-glucose Dulbecco's modified Eagle's medium (DMEM) (HyClone, Pittsburgh, PA, USA) supplemented with 2 mM L-glutamine, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.3), minimum essential medium (MEM) nonessential amino acid solution, penicillin/streptomycin (Corning Cellgro; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 1 mg/ml ascorbic acid (Sigma-Aldrich), 10% heat-inactivated fetal bovine serum (FBS) (HyClone), 5 ng/ml human epidermal growth factor (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/ml basic fibroblast growth factor, and 50 mg/ml long-R3 insulin-like growth-factor 1 (ProSpec, Rehovot, Israel) in a humidified atmosphere with 5% CO2 at 37°C. UC-MSCs expanded less than five passages were used to ensure their multipotency. The expression of surface proteins was analyzed as described previously (3).
Cell migration assay
The 8 µm polycarbonate membranes were coated with 50 µl 1.0% gelatin (Sigma-Aldrich) for 1 h. UC-MSCs were primed with S1P (50 nM; Cayman Chemical, Ann Arbor, MI, USA), VPA (0.5 mM), or 5-Aza (1 µM) (both from Sigma-Aldrich) alone or in combination with 50 nM S1P for 1 day. The UC-MSCs detached with TrypLE solution (Thermo Fisher Scientific, Pittsburgh PA, USA) were washed and resuspended in DMEM containing 0.5% bovine serum albumin (BSA), and seeded at a density of 3×104 cells/well into the upper chambers of Transwell inserts (Corning Costar, Pittsburgh, PA, USA). The lower chambers were filled with 150 ng/ml SDF-1 (R&D Systems, Minneapolis, MN, USA) in 0.5% BSA DMEM. After 1 day, the inserts were removed from the Transwell plates. Cells remaining in the upper chambers were scraped off using cotton wool, and cells that had transmigrated were fixed with 4% paraformaldehyde (PFA) solution in phosphate-buffered saline (PBS) and stained with 0.5% crystal violet (Sigma-Aldrich). Stained cells on the lower side of the membranes were quantified by digital image analysis using the Image Pro 5.0 software (Media-Cybernetics, Rockville, MD, USA).
Cell proliferation and colony forming unit-fibroblast (CFU-F) assay
Cell proliferation after priming with the indicated compounds for 24 h was determined by MTT assay (Sigma-Aldrich) according to the manufacturer's protocol. Reduction of MTT reagent was performed for 4 h and quantified by measuring the absorbance at 570 nm on a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). For the CFU-F assay, UC-MSCs were treated with the indicated priming factors for 24 h, after which cells were re-plated at a clonal density (600 cells/well) in 6-well culture plates, and further maintained in culture media for 14 days. The established colonies were washed twice with PBS, fixed, and stained with 0.5% crystal violet (Sigma-Aldrich).
In vitro differentiation assay
In vitro differentiation into chondrogenic, osteogenic or adipogenic lineages was performed as described previously (26). Briefly, UC-MSCs treated with the indicating priming factors were cultured in StemPro chondrogenesis (Invitrogen, Carlsbad, CA, USA), osteogenic (DMEM supplemented with 5% FBS, 50 µM L-ascorbate-2-phosphate, 0.1 µM dexamethasone, and 10 mM glycerophosphate), or adipogenic (DMEM supplemented with 5% FBS, 1 µM dexamethasone, 10 µM insulin, 200 µM indomethacin, and 0.5 mM isobutylmethylxanthine) differentiation medium. The chondrogenic differentiation was examined by Alcian Blue staining (Sigma-Aldrich) and osteogenic differentiation was noted by positive staining with Alizarin Red staining (Sigma-Aldrich), which is specific for calcium. Adipogenic differentiation characterized with intracellular lipid accumulation was visualized by Oil Red O staining (Sigma-Aldrich).
Anti-inflammatory assay of MSCs
The in vitro anti-inflammatory potency of MSCs was examined as described previously (3,23). Briefly, MH-S, a murine alveolar macrophage cell line, was maintained in DMEM-high-glucose supplemented with 10% heat-inactivated FBS and penicillin/streptomycin. For the inflammatory assay, 1×105 MH-S cells were seeded in 12-well culture plates, followed by stimulation with 0.1 µg/ml lipopolysaccharide (LPS) (Sigma-Aldrich) in the absence or the presence of the conditioned medium (CM), which were collected from IMR90 cells or UC-MSCs with or without priming. After 5 h, medium conditioned by the MH-S macrophages was collected and clarified by centrifugation at 500 × g for 10 min. A total of 50 µl of MH-S medium was used for murine tumor necrosis factor-α (TNF-α) enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher Scientific).
Western blot analysis
UC-MSCs primed with 50 nM S1P and 0.5 mM VPA were starved for 12 h in DMEM containing 0.5% BSA at 37°C, stimulated with 150 ng/ml SDF-1 for 5, 10, 20 or 30 min, and the phosphorylation of mitogen-activated protein kinases (MAPK), phospho-MAPKp42/44 (#4376) or total MAPKp42/44 (#9102) and phospho-AKT (#9271) or AKT (Ser473; #9272) (all from Cell Signaling Technology, Danvers, MA, USA) was analyzed by western blot analysis. Cell extracts were prepared using cell lysis buffer (0.5% NP-40, 0.5% Triton X-100, 20 mM HEPES, 1.5 mM MgCl2, 2 mM DTT, 2 mM EDTA, 150 mM NaCl) supplemented with protease and phosphatase inhibitor cocktails (Roche Life Science, Basel, Switzerland). Protein lysate was quantitated using BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) and 30 µg cell extracts were separated by 10% SDS-PAGE and transferred onto nitrocellulose blotting membranes (GE Healthcare, München, Germany). All primary antibodies were diluted 1:1,000 and the peroxidase conjugated anti-rabbit secondary antibody (#111-035-045) was diluted 1:2,000 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA).
RT-qPCR
For gene expression analysis, total RNA was prepared and reverse-transcribed from the indicated cells using the RNeasy Mini kit (Qiagen, Hilden, Germany) and TaqMan Reverse Transcription Reagents (Applied Biosystems, Carlsbad, CA, USA), respectively. The indicated transcripts were quantified by qPCR as previously described (4,27). Primers were designated as follows: CXCR4 forward, 5′-ACTACACCGAGGAAATGGGCT-3′ and reverse, 5′-CCCACAATGCCAGTTAAGAAGA-3′; MMP12 forward, 5′-GATCCAAAGGCCGTAATGTTCC-3′ and reverse, 5′-TGAATGCCACGTATGTCATCAG-3′; PDGFB forward, 5′-GGCAACACTGCTGTCCACAT-3′ and reverse, 5′-GTCCCACACCCACCTGGAA-3′; PDGFRB forward, 5′-GATGCCGAGGAACTATTCATCT-3′ and reverse, 5′-TTTCTTCTCGTGCAGTGTCAC-3′; cMET forward, 5′-AGCGTCAACAGAGGGACCT-3′ and reverse, 5′-GCAGTGAACCTCCGACTGTATG-3′; VEGFB forward, 5′-TCAGGGATAGCCCAGTCAATACA-3′ and reverse, 5′-GCCACAGAAGGCTGTCTCCTT-3′; VEGFC forward, 5′-AGTTCCACCACCAAACATGCA-3′ and reverse, 5′-CACTATATGAAAATCCTGGCTCACA-3′; ANGPT1 forward, 5′-TGCTCACGTGGCTCGACTATA-3′ and reverse, 5′-AGCACAGCAAGCTCAGCAGTTT-3′; ANGPT2 forward, 5′-GGTTTGATGCATGTGGTCCTT-3′ and reverse, 5′-AATGCCGTTGAACTTATTTGTGTTC-3′; IDO1 forward, 5′-TCCGTGAGTTTGTCCTTTCAAA-3′ and reverse, 5′-CAGGGAGACCAGAGCTTTCACA-3′; IDO2 forward, 5′-GATTGATGCTCACCAGCTTCAAG-3′ and reverse, 5′-GCTCCCGGTGACCCTTCAG-3′; LIF forward, 5′-GAAAGCTTTGGTAGGTTCTTCGTT-3′ and reverse, 5′-TGCAGGTCCAGCCATCAGA-3′; GAPDH forward, 5′-TGAATGCCACGTATGTCATCAG-3′ and reverse, 5′-TGTTGCTGTAGCCAAATTCGTT-3′.
Statistical analysis
Data were analyzed using a non-parametric Mann-Whitney test or a one-way ANOVA with the Bonferroni post-hoc test to detect statistically significant differences. We used GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA, USA) to perform all analyses, and statistical significance was defined as p<0.05, p<0.01 or p<0.001.
Results
Effect of 5-Aza on S1P-primed human UC-MSCs
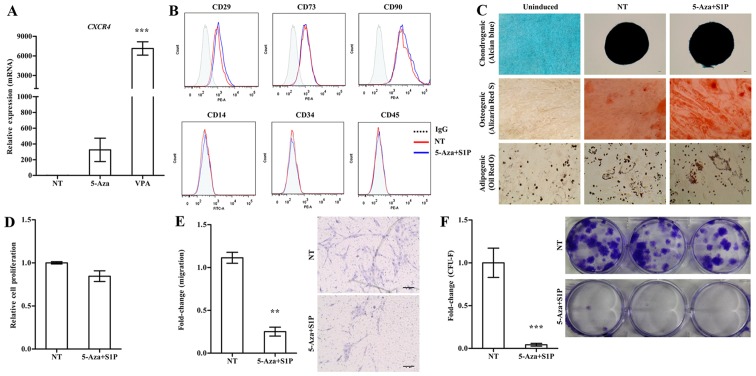
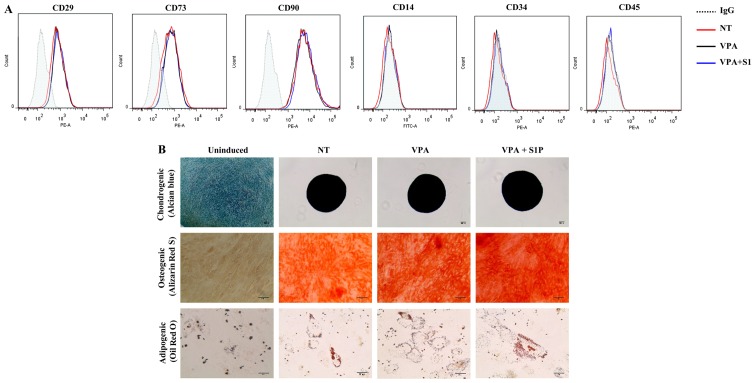
Treatment with 5-Aza and VPA at a low concentration (1 µM and 0.5 mM, respectively) markedly increased the expression of CXCR4 in the UC-MSCs (Fig. 1A). We next examined the effect of these epigenetic regulators on the basic features of UC-MSCs. Both 5-Aza and VPA, independently of the priming with 50 nM S1P, had less influence on the expression of surface marker proteins which were positive for CD29, CD73 and CD90 but negative for CD34 and CD45 (Figs. 1B and 2A). Furthermore, S1P priming together with 5-Aza (5-Aza+S1P) or VPA (VPA+S1P) had little effect on the multi-lineage differentiation capacity based on an in vitro differentiation assay into the chondrogenic, osteogenic and adipogenic lineages which were estimated by an increased level of glycosaminoglycans (Alcian blue), mineral deposition (Alizarin Red S), and lipid accumulation (Oil Red O staining), respectively (Figs. 1C and 2B).
Figure 1.
Adverse effect of 5-azacytidine (5-Aza) on umbilical cord-derived mesenchymal stem cells (UC-MSCs) primed with sphingosine-1-phosphate (S1P). (A) RT-qPCR analysis of CXCR4 in human UC-MSCs treated with 1 µM 5-Aza or 0.5 mM valproic acid (VPA) for 24 h. The relative expression level of the indicated genes is represented as the fold-change compared to the value of non-treated MSCs (NT) and are shown as means ± SEM (n=4), ***p<0.001, one-way ANOVA test. (B) Flow cytometric analysis of the expression of the indicated MSC surface (CD29, CD73 and CD90) and hematopoietic lineage (CD14, CD34 and CD45) proteins in UC-MSCs primed with 1 µM 5-Aza together with 50 nM S1P for 24 h. (C) Representative images of the chondrogenic, osteogenic or adipogenic differentiation assay using UC-MSCs primed with 5-Aza+S1P. The chondrogenesis, osteogenesis and adipogenesis were determined using Alcian Blue, Alizarin Red S and Oil Red O staining, respectively. (D) Cell proliferation (n=3), (E) chemotaxis to stromal derived factor-1 (SDF-1) (n=6), and (F) colony-forming unit-fibroblast (CFU-F) assay (n≥6) of UC-MSCs primed with 5-Aza+S1P for 24 h. All data are presented as the mean ± SEM. **p<0.01 and ***p<0.001, compared to non-primed cells (non-parametric Mann-Whitney test). Representative images of the migrated cells (E) and stained colonies (F) are shown in the right panels.
Figure 2.
The effect of valproic acid (VPA) + sphingosine-1-phosphate (S1P) priming on the basal features of umbilical cord-derived mesenchymal stem cells (UC-MSCs). (A) Flow cytometric analysis of surface antigens of the indicated MSC surface (CD29, CD73 and CD90) and hematopoietic (CD14, CD34 and CD45) lineage proteins in UC-MSCs primed with 0.5 mM VPA alone (VPA) or in combination with 50 nM S1P (VPA+S1P) for 24 h. (B) Representative images of the chondrogenic, osteogenic and adipogenic differentiation assays in which each lineage differentiation was determined using Alcian Blue (magnification, ×100; scale bar, 100 µm), Alizarin Red S (magnification, ×200; scale bar, 100 µm), and Oil Red O staining (magnification, ×200; scale bar, 50 µm), respectively.
Contradictory to the upregulation of CXCR4 (Fig. 1A), UC-MSCs primed with 5-Aza+S1P had decreased migration activity in response to SDF-1 in a Transwell migration assay; with ~30% less migration activity than the unprimed cells (Fig. 1E). In addition, 5-Aza+S1P priming severely impaired the capacity of clonogenic CFU-F, which represents the number of self-renewing clonogenic progenitor cells (Fig. 1F). The cellular proliferation of MSCs was only slightly changed by the priming with 5-Aza+S1P (Fig. 1D). Therefore, these results demonstrate that treatment with 5-Aza provides an adverse outcome for the priming of UC-MSCs with S1P.
VPA enforces the priming of UC-MSCs with a suboptimal dose of S1P
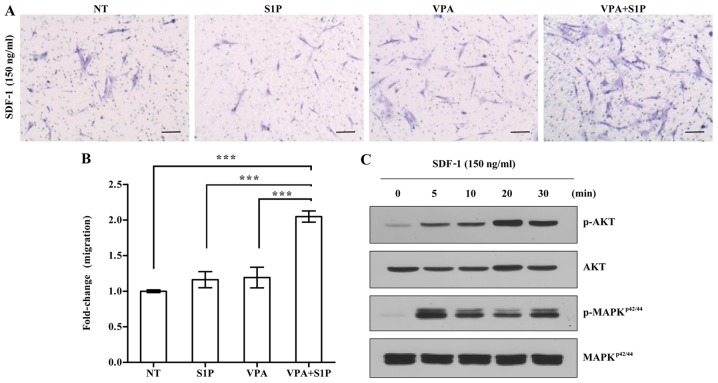
Next, we explored the effect of VPA on the priming of UC-MSCs using a low dose of S1P. To address this issue, we employed 50 nM S1P, which is 4-fold less than the optimal dose (200 nM) used for priming of both adipose- and UCB-derived MSCs (3). In agreement, the priming with a suboptimal dosage of S1P alone had little effect on the responsiveness of UC-MSCs to the SDF-1 chemokine (Fig. 3A). In contrast, the priming of UC-MSCs with VPA+S1P, but not VPA alone, significantly increased the chemotactic activity to SDF-1 by 3-fold when compared with that of the unprimed cells (Fig. 3A and B). This enforced response to SDF-1 by VPA+S1P priming completely differed from the case of 5-Aza+S1P priming. We next explored the status of the signaling pathways implicated in the migration of HSPCs (20,21,28,29). In line with the results of the chemotaxis assay, UC-MSCs exposed to VPA+S1P had increased levels of phosphorylated MAPKp42/44 and AKT proteins, indicating activation of their signaling pathways (Fig. 3C). These results indicate that VPA, and not 5-Aza, promote the priming of UC-MSCs at the suboptimal dose of S1P by activating the MAPKp42/44 and AKT signaling pathways.
Figure 3.
Enhanced responsiveness to stromal derived factor-1 (SDF-1) in umbilical cord-derived mesenchymal stem cells (UC-MSCs) primed with valproic acid (VPA)+sphingosine-1-phosphate (S1P). (A and B) Chemotaxis assays for 150 ng/ml SDF-1 of MSCs exposed to 0.5 mM VPA alone (VPA) or in combination with 50 nM S1P (VPA+S1P) for 24 h. (A) Representative images of the Transwell insets from the migration assay. (B) The relative amount of migration was quantified as the fold-change in the number of transmigrated cells and is shown as means ± SEM (n=6). **p<0.01 and ***p<0.001 compared to nonprime cells (NT) (one-way ANOVA with Bonferroni post-test). (C) Western blot analysis of phosphorylated (p-) or total AKT and MAPKp42/44. MSCs primed with VPA+S1P were treated with 150 ng/ml SDF-1 for the indicated time after serum-starvation for 12 h.
Enhanced therapeutic capacities of UC-MSCs primed with VPA+S1P
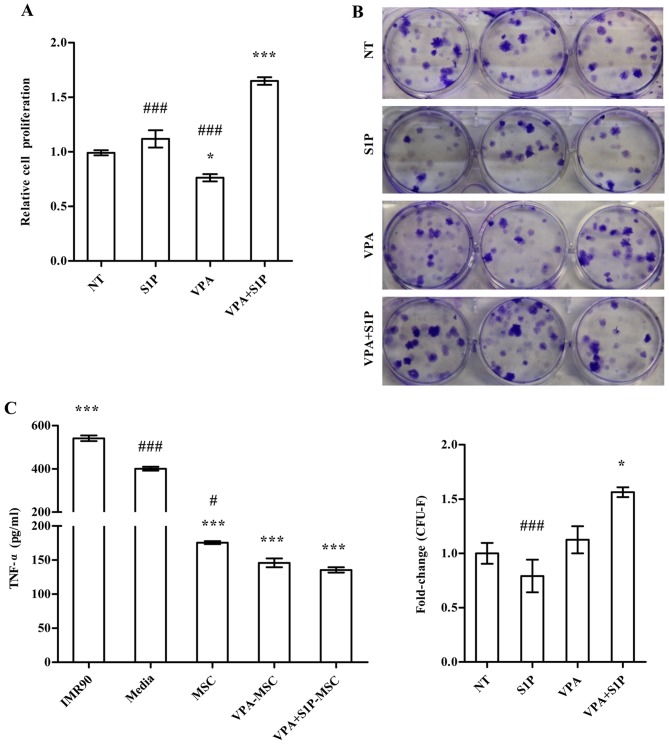
We further examined the effect of VPA+S1P priming on other cellular activities which are related to the therapeutic effect of MSCs. Unlike 5-Aza+S1P (Fig. 1), UC-MSCs primed with VPA+S1P had enhanced capacity for both cell proliferation (Fig. 4A) and clonogenic CFU-F abilities (Fig. 4B). These beneficial effects were not observed in the UCB-MSCs primed by VPA or S1P alone (Fig. 4A and B). Since MSCs exert anti-inflammatory and immunomodulatory properties (30–32), we next explored whether VPA+S1P priming can influence the anti-inflammatory effect of UC-MSCs. To address this issue, we prepared the CM collected from UC-MSCs unprimed, primed by VPA or S1P alone, and primed by VPA+S1P and examined whether CM could suppress the secretion of TNF-α from MH-S cells, an alveolar macrophage cell line, by stimulation with LPS (3). CM from UC-MSCs was effective to reduce the secretion of TNF-α from LPS-stimulated MH-S cells (Fig. 4C). In particular, CM derived from the VPA+S1P-primed UC-MSCs further inhibited TNF-α secretion to a greater extent than UC-MSCs unprimed or primed with VPA or S1P alone, although a significant difference was observed only between naive and VPA+S1P-primed cells. When we used CM from IMR90, a type of human lung fibroblast as a control, a further increase in TNF-α secretion was observed in our in vitro anti-inflammation assays (Fig. 4C).
Figure 4.
Enhanced therapeutic potency of umbilical cord-derived mesenchymal stem cells (UC-MSCs) primed with valproic acid (VPA)+sphingosine-1-phosphate (S1P). (A and B) Cell proliferation analysis [(A) n=4)] and colony-forming unit-fibroblast (CFU-F) assay [(B) n=6)] of UC-MSCs primed with VPA (0.5 mM) or S1P (50 nM) alone as well as VPA+S1P for 24 h. For the CFU-F assay, 600 cells were seeded into 6-well culture plates and cultured for 14 days, and the number of colonies was quantified. Representative stained colonies of adherent cells are shown in the right panel. (C) Quantification of TNF-α protein (n=4) secreted from a murine alveolar macrophage cell line stimulated with LPS for 5 h in the absence or presence of conditioned medium (CM) harvested from the indicated cells. All data are presented as means ± SEM, *p<0.05, **p<0.01 and ***p<0.001 compared with non-treated (NT) umbilical cord blood (UCB)-MSCs; #p<0.05 and ###p<0.001 compared with VPA+S1P primed cells, one-way ANOVA test ANOVA with Bonferroni post-test.
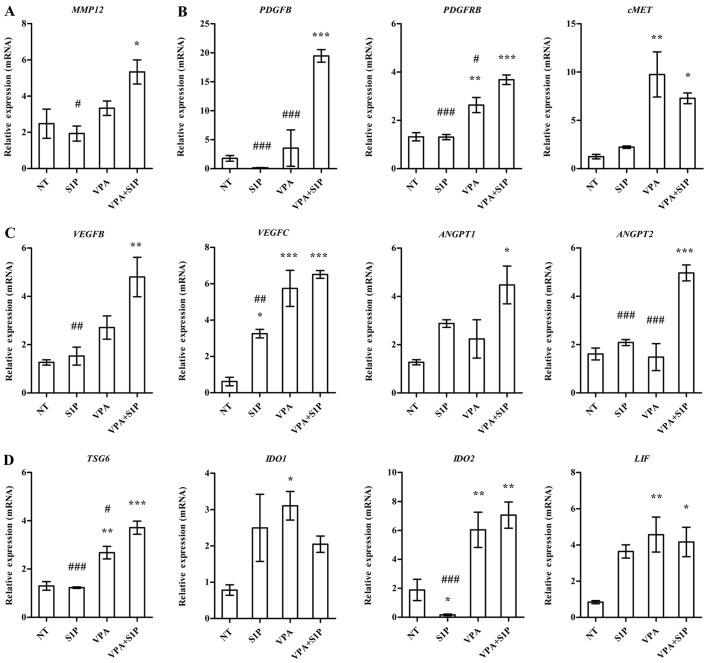
To obtain a mechanistic insight into the effect of VPA+S1P priming, we examined the expression of growth factors, pro-inflammation cytokines and anti-inflammatory factors secreted by MSCs (3,23). In line with the aforementioned data, UC-MSCs primed with VPA+S1P had significantly upregulated expression of a subset of growth factors and their receptors (PDGFB, PDGFRB and cMET) (Fig. 5B), pro-angiogenesis factors (VEGFB, VEGFC, ANGPT1 and ANGPT2) (Fig. 5C), anti-inflammatory factors (LIF, TSG6 and IDO2) (Fig. 5D) and a stem cell migration-related protein (MMP12) (Fig. 5A). Most of the aforementioned genes affected by VPA+S1P priming were downregulated by 5-Aza+S1P priming (data not shown) and little change was noted following priming with VPA or S1P alone (Fig. 5). Collectively, these results indicate that priming of MSCs with a minimal dose of VPA and S1P can effectively promote the migration, proliferation, self-renewal and anti-inflammatory capacity of MSCs, which are crucial for their therapeutic potency.
Figure 5.
Valproic acid (VPA)+sphingosine-1-phosphate (S1P) priming upregulates genes related to therapeutic outcomes of mesenchymal stem cells (MSCs). (A-D) The expression level of (A) MMP-12, (B) growth factors, (C) angiogenesis- and (D) anti-inflammation-related genes in umbilical cord-derived MSCs (UC-MSCs) primed with 0.5 mM VPA or 50 nM S1P alone as well as in combination with VPA and S1P (VPA+S1P) for 24 h. The expression level was calculated as the ratio of the value of the primed UC-MSCs to unprimed ones (NT). Data are shown as means ± SEM (n≥4); *p<0.05, **p<0.01 and ***p<0.001 compared to NT group; #p<0.05, ##p<0.01 and ###p<0.001 compared to VPA+S1P group (one-way ANOVA with Bonferroni post-test).
Discussion
Our present study highlights that the priming of UC-MSCs combined with VPA and S1P enhanced migration, self-renewal, and anti-inflammatory potency of MSCs using a minimal dosage of these compounds. Priming molecules, such as bioactive lipids, are enriched in damaged tissue and can function as strong chemo-attractants for HSPCs and MSCs. However, the priming of MSCs with a high dose of these molecules could pose a risk of stimulating the inappropriate inflammatory response when these factors left remaining inside the primed cells are released after MSC therapy. Thus, the present study provides crucial experimental clues for the optimization of priming of MSCs with a minimal amount of priming factors by enforcing CXCR4 signaling, which can assist in developing more practical and safe protocols for clinical applications.
Previously, we reported that bioactive lipids (S1P and C1P) or cationic peptide (LL-37) stimulate the migratory potential of several tissue (UCB, adipose and BM)-derived MSCs (3,23). However, compared with HSPCs, the priming of MSCs still exhibits a limited effect, particularly in vivo engraftment of infused stem cells. Unlike HSPCs, only a small proportion of MSCs express functionally active CXCR4 receptor on their surfaces, and this expression diminishes with passage (33). CXCR4 is a key to mediating specific migration of these cells (14) and its upregulation could overcome the limited in vivo engraftment ability of primed MSCs (33). By employing epigenetic regulatory compounds, we found that a DNA-demethylation agent (5-Aza) and a histone deacetylase inhibitor (VPA) significantly increased the expression of CXCR4 in UC-MSCs (Fig. 1A). Importantly, the priming combined with VPA and S1P increased the responsiveness to SDF-1, and the CXCR4 signaling cascade was activated by a suboptimal dose (Fig. 3). Unexpectedly, the treatment of 5-Aza severely impaired the chemotactic activity to SDF-1 (Fig. 1E) and the clonogenic ability of CFU-F (Fig. 1F), although the influence on cellular proliferation was less (Fig. 1D). These results indicate that the expression level of CXCR4 alone may not be sufficient to enhance the effects of MSC priming; thus further in-depth investigation of the molecular nature of MSC priming is required.
Mechanistically, priming molecules such as bioactive lipids (S1P) or cationic peptide (LL-37) enhance the mobilization or engraftment of adult SCs including HSPCs and MSCs by the incorporation of CXCR4 into lipid rafts which is better connected with downstream signaling proteins, and in turn, enables the primed cells to better respond to the SDF-1α gradient (34). Of importance, the priming of MSCs also potentiates several important cellular properties of MSCs, such as self-renewal, anti-inflammation and pro-angiogenesis which are pivotal to the beneficial outcomes of MSC therapy (3,23). In line with previous studies, we confirmed that the optimized priming strategies combined with VPA and S1P with a suboptimal dose also enhanced these beneficial outcomes of UC-MSCs in in vitro cell culture assays (Fig. 4), paralleled with upregulation of several trophic, immunosuppressive, anti-inflammatory and proangiogenic factors (Fig. 5). Thus, the in vivo efficacy of UC-MSCs primed with VPA+S1P should be further examined.
The priming factors for HSPCs and MSCs are generally enriched in damaged tissues as part of the repair process; thus, they are reported to increase tissue inflammation and remodeling (21,28,29). Thus, priming of stem cells with a high concentration of these factors abnormally increases their content inside the cells. In our previous study of UCB-MSCs primed with S1P, cells under normal culture contained a low level of S1P; however, the priming of S1P increased the S1P content significantly (3). In particular, extensive washing of cells with normal saline solution before administration of S1P-primed MSCs cannot completely remove the remaining S1P. Thus, the optimization of priming strategies with a lower dosage could be crucial to prevent the possibility of residual priming factors provoking an inappropriate inflammatory response and vascular remodeling. The present study provides a strong candidate to ensure the safe application of the MSC priming method. Notably, a high dose of VPA (~10 mM) was found to decrease the proliferation potential and multi-lineage differentiation capability of human MSCs by activating the transcription of p21CIP1/WAF1, which eventually arrested the cell cycle at the G2⁄M phase (35). In addition, aberrant epigenetic regulation can lead to tumorigenesis and premature stem cell aging (36). Thus, to translate the MSC priming to human clinical trials, it will be important to optimize types or combinations of priming factors and also to carefully consider their safety. To address issues of safety, long-term monitoring of the hypothetical risks for tumor development from primed MSCs should be thoroughly examined.
In conclusion, VPA as a combination factor ensures the priming effect of S1P with a suboptimal dose, which can provide the optimized and safe application of MSC therapy in the clinic. Our findings suggest that combination strategies for MSC priming would overcome the poor in vivo engraftment of the infused MSCs, and finally improve the therapeutic potency of MSCs.
Acknowledgments
The present study was co-supported by the Global High-Tech Biomedicine Technology Development Program of the National Research Foundation (NRF) and Korea Health Industry Development Institute (KHIDI) (MSIP&MOHW) (no. 2015M3D6A1065364), by Basic Science Research Program through the NRF (no. 2015R1A2A1A15054754), and by the Korea Korean Health Technology R&D Project, Ministry of Health & Welfare of the Republic of Korea (no. HI14C3339).
Glossary
Abbreviations
- 5-Aza
5-azacytidine
- BM
bone-marrow
- C1P
ceramide-1-phosphate
- CD
cluster of differentiation
- CFU-F
colony-forming unit-fibroblast
- CM
conditioned medium
- HGF
hepatocyte growth factor
- HSPC
hematopoietic stem/progenitor cell
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MSCs
mesenchymal stem cells
- PAH
pulmonary artery hypertension
- PDGF
platelet-derived growth factor
- S1P
sphingosine-1-phosphate
- SDF-1
stromal cell-derived factor 1
- sMAC
soluble membrane attack complex
- TNF-α
tumor necrosis factor-α
- UC-MSCs
umbilical cord-derived MSCs
- UCB
umbilical cord blood
- VPA
valproic acid
- VEGF
vascular endothelial growth factor
References
- 1.Knaän-Shanzer S. Concise review: The immune status of mesenchymal stem cells and its relevance for therapeutic application. Stem Cells. 2014;32:603–608. doi: 10.1002/stem.1568. [DOI] [PubMed] [Google Scholar]
- 2.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang H, Kim K-H, Lim J, Kim YS, Heo J, Choi J, Jeong J, Kim Y, Kim SW, Oh YM, et al. The therapeutic effects of human mesenchymal stem cells primed with sphingosine-1 phosphate on pulmonary artery hypertension. Stem Cells Dev. 2015;24:1658–1671. doi: 10.1089/scd.2014.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin HJ, Lee HJ, Heo J, Lim J, Kim M, Kim MK, Nam HY, Hong GH, Cho YS, Choi SJ, et al. Senescence associated MCP-1 secretion is dependent on a decline in BMI1 in human mesenchymal stromal cells. Antioxid Redox Signal. 2015;24:471–485. doi: 10.1089/ars.2015.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res Ther. 2014;16:R79. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song M, Lim J, Yu HY, Park J, Chun JY, Jeong J, Heo J, Kang H, Kim Y, Cho YM, et al. Mesenchymal stem cell therapyalleviates interstitial cystitis by activating Wnt signaling pathway. Stem Cells Dev. 2015;24:1648–1657. doi: 10.1089/scd.2014.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim A, Yu HY, Heo J, Song M, Shin JH, Lim J, Yoon SJ, Kim Y, Lee S, Kim SW, et al. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci Rep. 2016;6:30881. doi: 10.1038/srep30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song M, Heo J, Chun JY, Bae HS, Kang JW, Kang H, Cho YM, Kim SW, Shin DM, Choo MS. The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem Cells Dev. 2014;23:654–663. doi: 10.1089/scd.2013.0277. [DOI] [PubMed] [Google Scholar]
- 10.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 12.Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: Systemic or local administration? Neurosci Lett. 2006;398:129–134. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 13.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 15.Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha→ hypoxia response element→ VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000;60:6248–6252. [PubMed] [Google Scholar]
- 16.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corpechot C, Barbu V, Wendum D, Chignard N, Housset C, Poupon R, Rosmorduc O. Hepatocyte growth factor and c-Met inhibition by hepatic cell hypoxia: A potential mechanism for liver regeneration failure in experimental cirrhosis. Am J Pathol. 2002;160:613–620. doi: 10.1016/S0002-9440(10)64881-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucia M, Zhang YP, Reca R, Wysoczynski M, Machalinski B, Majka M, Ildstad ST, Ratajczak J, Shields CB, Ratajczak MZ. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006;20:18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Guo W, Xiong M, Han H, Chen J, Mao D, Tang B, Yu H, Zeng Y. Effect of SDF-1/CXCR4 axis on the migration of transplanted bone mesenchymal stem cells mobilized by erythropoietin toward lesion sites following spinal cord injury. Int J Mol Med. 2015;36:1205–1214. doi: 10.3892/ijmm.2015.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: A novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106–116. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C, Schneider G, Abdel-Latif A, Mierzejewska K, Sunkara M, Borkowska S, Ratajczak J, Morris AJ, Kucia M, Ratajczak MZ. Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells–implications for tissue regeneration. Stem Cells. 2013;31:500–510. doi: 10.1002/stem.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim J, Kim Y, Heo J, Kim KH, Lee S, Lee SW, Kim K, Kim IG, Shin DM. Priming with ceramide-1 phosphate promotes the therapeutic effect of mesenchymal stem/stromal cells on pulmonary artery hypertension. Biochem Biophys Res Commun. 2016;473:35–41. doi: 10.1016/j.bbrc.2016.03.046. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Kim H-S, Roh K-H, Lee BC, Shin TH, Yoo JM, Kim YL, Yu KR, Kang KS, Seo KW. DNA methyltransferase inhibition accelerates the immunomodulation and migration of human mesenchymal stem cells. Sci Rep. 2015;5:8020. doi: 10.1038/srep08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Cui X, Wu Z, Jia L, Yu Y, Zhou Q, Hu X, Xu W, Luo D, Liu J, et al. Transplantation of bone marrow mesenchymal stem cells pretreated with valproic acid in rats with an acute spinal cord injury. Biosci Trends. 2014;8:111–119. doi: 10.5582/bst.8.111. [DOI] [PubMed] [Google Scholar]
- 26.Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han DW, Park SB, Choi SW, Kang SK, Schöler H, et al. CD49f enhances multi-potency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells. 2012;30:876–887. doi: 10.1002/stem.1052. [DOI] [PubMed] [Google Scholar]
- 27.Heo J, Lim J, Lee S, Jeong J, Kang H, Kim Y, Kang JW, Yu HY, Jeong EM, Kim K, et al. Sirt1 regulates DNA methylation and differentiation potential of embryonic stem cells by antagonizing Dnmt3l. Cell Rep. 2017;18:1930–1945. doi: 10.1016/j.celrep.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 28.Wu W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, Ratajczak J, Laughlin MJ, Ratajczak MZ. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2012;26:736–745. doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8:54–68. doi: 10.15283/ijsc.2015.8.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Han Y, Zhuang Y, Fu J, Liu H, Shi Q, Ju X. Overexpression of COX-2 but not indoleamine 2,3-dioxygenase-1 enhances the immunosuppressive ability of human umbilical cord-derived mesenchymal stem cells. Int J Mol Med. 2015;35:1309–1316. doi: 10.3892/ijmm.2015.2137. [DOI] [PubMed] [Google Scholar]
- 32.Yang W, Yang Y, Yang JY, Liang M, Song J. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway. Int J Mol Med. 2016;37:1075–1082. doi: 10.3892/ijmm.2016.2498. [DOI] [PubMed] [Google Scholar]
- 33.Jones GN, Moschidou D, Lay K, Abdulrazzak H, Vanleene M, Shefelbine SJ, Polak J, de Coppi P, Fisk NM, Guillot PV. Upregulating CXCR4 in human fetal mesenchymal stem cells enhances engraftment and bone mechanics in a mouse model of osteogenesis imperfecta. Stem Cells Transl Med. 2012;1:70–78. doi: 10.5966/sctm.2011-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Park JR, Seo MS, Roh KH, Park SB, Hwang JW, Sun B, Seo K, Lee YS, Kang SK, et al. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009;42:711–720. doi: 10.1111/j.1365-2184.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner W, Weidner CI, Lin Q. Do age-associated DNA methylation changes increase the risk of malignant transformation? BioEssays. 2015;37:20–24. doi: 10.1002/bies.201400063. [DOI] [PubMed] [Google Scholar]