Abstract
Saponins, which are glycosylated, represent a diverse group of biologically functional products in plants. In the present study, we investigated the effects of soyasaponin Ag, a secondary metabolite extracted from soybean, on α-melanocyte-stimulating hormone (α-MSH)-induced melanin synthesis in B16F10 mouse melanoma cells and the underlying molecular mechanisms. To elucidate the mechanisms through which soyasaponin Ag inhibits melanin synthesis, we performed cellular tyrosinase activity assays and analyzed the expression of the melanogenesis-related genes, tyrosinase, tyrosinase-related protein (TRP)-1 and TRP-2. We demonstrated that soyasaponin Ag inhibited α-MSH-induced melanin synthesis in melanoma cells. Of note, soyasaponin Ag had no inhibitory effect on intracellular tyrosinase activity. However, soyasaponin Ag inhibited TRP-2 expression in a dose-dependent manner. Therefore, the depigmenting effect of soyasaponin Ag may be due to the inhibition of tyrosinase expression or the enhancement of tyrosinase degradation. Moreover, soyasaponin Ag did not exert any toxic on B16F10 mouse melanoma cells, suggesting that soyasaponin is a safe component for use in skin care cosmetic formulations that are used for skin whitening.
Keywords: soyasaponin Ag, B16F10 melanoma cells, melanogenesis, tyrosinase, tyrosinase-related protein 2
Introduction
Melanin determines the color of human skin, hair and eyes as a final product of melanogenesis (1,2), and plays a protective role against sun exposure-associated ultraviolet radiation (3). This pigment is synthesized by a complex pathway in highly specialized cells of the epidermis known as melanocytes (4). Melanocytes are normally positioned at the basal layer of the epidermal part of the skin (5). Specifically, melanin is produced in cellular sub-compartments denominated as melanosomes and is transported to other cells of the epidermis, including keratinocytes and Langerhans cells (5). Importantly, the overproduction or abnormal distribution of melanin can lead to skin disorders and cosmetic complications, such as melisma and freckles (5). Melanin is synthesized by the three enzymes, tyrosinase, tyrosinase-related protein (TRP)-1 and DOPAchrome tautomerase (DCT) also known as TRP-2 (6). UV radiation-sensitized human epidermal keratinocytes produce factors which stimulate melanogenesis, including α-melanocyte-stimulating hormone (α-MSH) (7). Secreted α-MSH/ACTH binds to the melanocortin 1 receptor (MC1R) on melanocytes to activate the cAMP/PKA/CREB pathway, which subsequently increases the expression levels of tyrosinase, TRP-1 and TRP-2 (7,8). Tyrosinase converts L-tyrosine into L-3,4-dihydroxyphenylalanine (L-DOPA); L-DOPA can then be converted into DOPAquinone, which spontaneously undergoes cyclization and further rearrangement, yielding l-DOPAchrome (9). DCT/TRP-2 catalyzes the conversion of DOPAchrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA). TRP-1 is important for tyrosinase stabilization and trafficking, thus ultimately facilitating tyrosinase activity (10). Two types of melanin are produced in mammalian cells, the reddish-yellow-colored pheomelanin and the dark-brown-colored eumelanin. Tyrosinase is required for the synthesis of pheomelanin and eumelanin, whereas TRP-1 and TRP-2 are required for the synthesis of only eumelanin (11,12). Therefore, the downregulation of tyrosinase and related protein expression and/or activity have been proposed to be involved in the reduction of melanin production (13).
Soyasaponins are bioactive secondary plant metabolites in soybeans (Glycine max) and other legumes, such as lentils (Lens culinaris) and green peas (Pisum sativum L. cv. Azad) (14–16). Soyasaponins are oleanene-type triterpenoid saponins with polar sugar chains and a non-polar pentacyclic ring structure (17). These metabolites are generally divided into four main groups on the basis of their aglycone (soyasapogenol) structure, referred to as groups A, B, E and DDMP (2,3-dihydro-2,5-dihydroxy-6-methyl-4-pyrone) (14). Group A soyasaponins contain bidesmosides with two sugar chains at the C-3 and C-22 position of soyasapogenol A. Previously, eight isomers of group A saponins, named Aa, Ab, Ac, Ad, Ae, Af, Ag and Ah, according to their elution order in reversed-phase high-performance liquid chromatography (HPLC), were isolated from soybeans and characterized in the study by Shiraiwa et al (15).
Soyasaponins have been shown to have a number of biological functions, including anti-obesity, anti-inflammatory and anti-carcinogenic effects, as well as hepatoprotective effects (14,18,19). However, to the best of our knowledge, no study to date has investigated the putative anti-melanogenic effects of soyasaponins. Therefore, in the present study, we investigated the anti-melanogenic effects of soyasaponin Ag on α-MSH-induced melanin synthesis in B16F10 melanoma cells.
Materials and methods
Chemicals and antibodies
α-MSH, 3-(4,5,-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), L-DOPA and arbutin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies to tyrosinase (sc-73244), TRP-1 (sc-58438), TRP-2 (sc-74439) and microphthalmia-associated transcription factor (MITF; sc-52938) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Isolation of soyasaponin
Soybean saponins Ag were purified by the method previously described in the study by Shiraiwa et al (15) with some modifications. The seed hypocotyls were extracted in a 10-fold volume (v/v) of 80% (v/v) aqueous methanol for 24 h at room temperature. The extracts were concentrated in vacuo to evaporate the solvents. The dried extracts were suspended in a mixture of 2-butanol, water and acetic acid (final concentration, 0.1%; equal to the 10-fold volume used for extraction) and followed by overnight incubation at room temperature. Following incubation, the upper (butanol) phase was collected, evaporated and dried under a vacuum. To prepare crude saponin solutions, the dried powders were dissolved in a small amount of preparative HPLC solvent [34% (v/v) acetonitrile, 6% (v/v) 2-propanol, 0.1% (v/v) acetic acid and 59.9% (v/v) water]. The crude saponin solutions were then filtered through 0.45-mm membrane filters and were analyzed repeatedly on a preparative reverse-phase HPLC system. The purity of saponin Ag measured by liquid chromatography-photodiode array-tandem mass spectrometry (LC-PDA-MS/MS) analysis was >95%, based on the total peak area monitored at 205 nm.
LC-PDA-MS/MS analysis
Analysis of the saponin components was carried out by LC-PDA-MS/MS with an analytical reverse-phase column (Develosil C30-UG-5, 4.6×150 mm; Nomura Chemical Co., Ltd., Aichi, Japan) at 40°C. Solvent A (94% acetonitrile, 6% 2-propanol and 0.1% formic acid) and solvent B (94% water, 6% 2-propanol, and 0.1% formic acid) were used. Gradient elution from 10.64% (v/v) solvent A (10% acetonitrile) to 53.19% (v/v) solvent A (50% acetonitrile) was performed for 40 min (gradient increased by 1% acetonitrile per minute) at a flow rate of 0.15 ml/min (Prominence UFLP system; Shimadzu Corp., Kyoto, Japan). Then, the column was washed by increasing the concentration of solvent A to 100% (v/v) for 5 min; after which, the eluates composition returned to the initial state of 10.64% (v/v) solvent A for 15 min. The eluate from the column was monitored at 205 and 292 nm using a PDA detector, and MS and MS/MS data of the eluates were acquired with a tandem mass spectrometer (LTQ Orbitrap XL; Thermo Fisher Scientific, Inc., Waltham MA, USA) in positive-ion mode using electrospray ionization [ESI(+)]. Saponin contents were calculated from a standard curve on the basis of the peak area of purified saponin Ag monitored at 205 nm.
Cell culture
B16F10 cells, established to produce melanin and respond to α-MSH (20) were purchased from the American Type Culture Collection (ATCC; CRL-6475). Typically, the B16F10 cells were cultured in α-modified Eagle's minimal essential medium containing 10% fetal bovine serum (HyClone, Logan, UT, USA) and 1% penicillin-streptomycin (10,000 unit/10,000 µg/ml) (Gibco, Grand Island, NY, USA) in a humidified atmosphere containing 5% CO2 in air at 37°C.
Cell viability assay
Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich). The B16F10 cells were cultured in 96-well plates. After a 24-h incubation, the cells were treated with soyasaponin Ag (25–100 µM) and then incubated for 48 h in an atmosphere of 5% CO2 at 37°C. After 48 h, MTT solution (5 mg/ml) was added to each well and the cells were incubated for 4 h at 37°C. The supernatants were removed, and dimethylsulphoxide (DMSO) was added to dissolve the formazan crystals. The absorbance of each well was estimated at 570 nm using an ELISA plate reader (Tecan, Mannedorf, Switzerland). The percentage of living cells was determined relative to the control group.
Melanin content assay
The B16F10 cells (3.5×104) were cultured in 6-well plates at 37°C in an atmosphere of 5% CO2 for 24 h. After 24 h, the cells were treated with α-MSH (20 nM) and medium containing arbutin (Sigma-Aldrich; arbutin was diluted in 0.1 M phosphate buffer and used at 1 and 2 mM concentrations as a positive control) or soyasaponin Ag (25, 50 and 100 µM) for 48 h. After 48 h, the cells were washed with phosphate-buffered saline (PBS), and the cells were detached by incubation with trypsin-EDTA and centrifuged at 3,000 rpm for 5 min. The supernatant was then discarded and the cell pellet was solubilized in 1 N NaOH at 60°C for 2 h. To determine the melanin content, the absorbance was measured at 405 nm using an ELISA plate reader and then normalized to the total protein concentration.
Cellular tyrosinase activity assay
B16F10 cell seeding and treatment methods were the same as those used to measure melanin contents. Briefly, the cells were cultured in 6-well plates, and after 24 h, the cells were treated with α-MSH (20 nM) and soyasaponin Ag-containing medium (25–100 μM) for 48 h. After treatment, the cells were washed twice with cold PBS and lysed with 100 µl of RIPA buffer containing 0.1 mM phenylmethylsulfonyl fluoride (PMSF) for 30 min on ice. The lysates were centrifuged (13,000 rpm for 15 min), 20 µl of the supernatant was mixed with 100 µl of 4% N,N-dimethylformamide, 50 µl of 5 mM L-DOPA and 50 µl of 20.7 mM 3-methyl-2-benzothiazolinone hydrazine (MBTH) in a 96-well plate and then incubated at 37°C for 30 min. To determine the tyrosinase activity, the absorbance was measured at an absorbance wavelength of 505 nm, and then normalized to the total protein concentration.
Western blot analysis
The B16F10 cells were treated with soyasaponin Ag (25–100 µM) for 24 h and then further treated with α-MSH (20 nM) and the soyasaponin sample for a further 24 h. After these treatments, the cells were collected and lysed with RIPA buffer containing 1X solution (50 mM Tris HCl, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS) for 30 min on ice. The cell lysates were centrifuged (10,000 rpm for 10 min), and the protein concentrations were determined using the BCA protein assay. Proteins were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes (GE Healthcare, Buckinghamshire, UK). The membranes were blocked with blocking buffer (Thermo Fisher Scientific, Inc.) and then incubated with the appropriate antibodies. The membranes were washed with wash buffer and incubated with anti-mouse (sc-2371) or anti-rabbit (sc-2379) horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) at 1:1,000 dilution for 1 h. The immunoreactive bands were enhanced using chemiluminescence reagents. The loading control was assessed using an anti-β-actin antibody.
Statistical analysis
All data were obtained in triplicate and are presented as the means ± standard deviation. Values were compared using the Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of soyasaponin Ag on cell viability and melanin synthesis in α-MSH-stimulated B16F10 cells
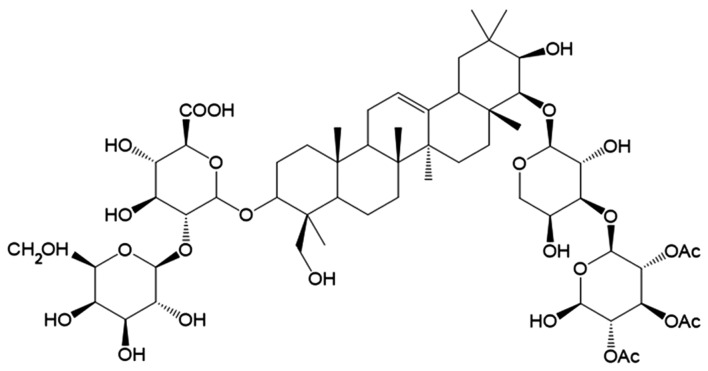
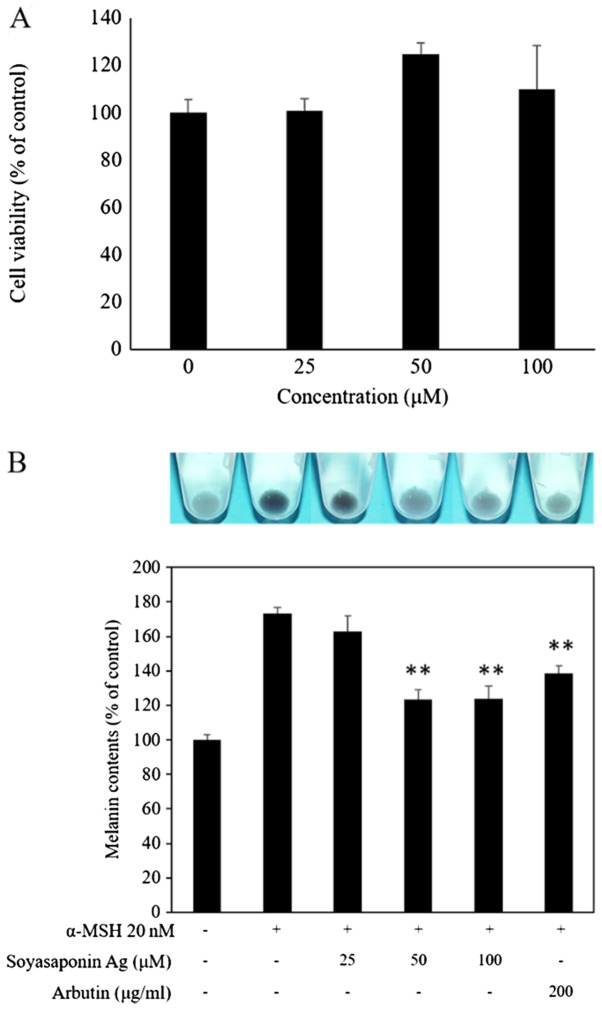
The chemical structure of saponin Ag is shown in Fig. 1. The first step in this study was to determine any potential cytotoxic effects of soyasaponin Ag in view of its potential utilization as a functional food or cosmetic agent. We performed cell viability assays with soyasaponin Ag to determine its in vitro cytotoxicity in murine B16F10 melanoma cells. More specifically, we utilized the MTT colorimetric assay for cell viability. In short, MTT is reduced to purple formazan crystals by the enzymatic activity of the mitochondria. Since for most living cells total mitochondria activity correlates with the number of viable cells, the assay is a simple and reliable method for determining potential cytotoxic effects (21). To determine the effect of soyasaponin Ag on B16F10 cell viability, the cells were treated with 25–100 µM of soyasaponin Ag for 48 h (Fig. 2A). No significant effect on cell viability was detected when using this concentration range of soyasaponin Ag. To determine the effect of soyasaponin Ag (25–100 µM) treatment on α-MSH-induced melanin synthesis, the melanin content of the B16F10 cells was evaluated photometrically. As shown in Fig. 2B, α-MSH markedly upregulated the B16F19 cell melanin content (P<0.05). Of ntoe, treatment with soyasaponin Ag at 50 and 100 µM significantly decreased the cellular melanin content of the α-MSH-stimulated B16F10 cells (P<0.01). In the control experiments, arbutin (positive control), a known melanogenic inhibitor (22) was shown to attenuate the melanin content of stimulated B16F10 cells (Fig. 2B).
Figure 1.
Chemical structure of soyasaponin Ag.
Figure 2.
Effects of soyasaponin Ag on (A) cell viability and (B) melanin content in α-MSH-stimulated B16F10 cells. α-MSH, α-melanocyte-stimulating hormone. **P<0.01.
Effect of soyasaponin Ag on tyrosinase activity in α-MSH-stimulated B16F10 cells
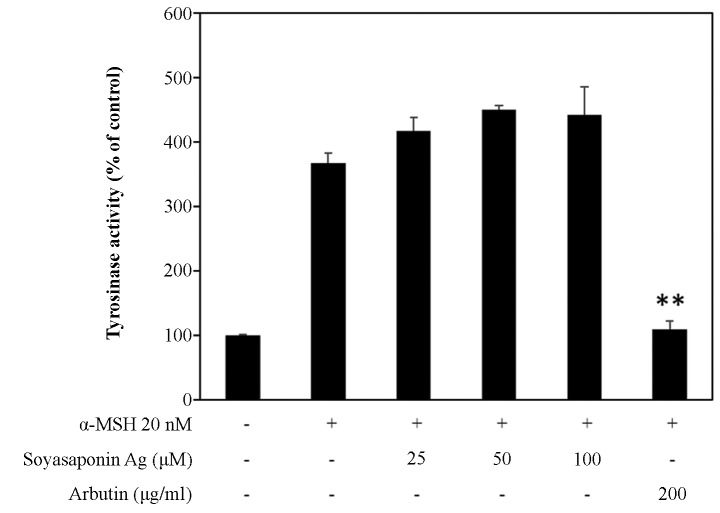
Tyrosinase is known to play a key role in melanin synthesis. Thus, in this study, to investigate the mechanisms underlying the inhibition of melanogenesis, we measured tyrosinase activity in α-MSH-stimulated B16F10 cells (Fig. 3). B16F10 melanoma cells treated with up to 100 μM soyasaponin Ag did not exhibit any inhibition of tyrosinase activity. On the other hand, in the control experiments, arbutin (22), a direct inhibitor of tyrosinase activity, at 200 µg/ml completely inhibited α-MSH stimulated tyrosinase activity to basal levels (Fig. 3).
Figure 3.
Wffects of soyasaponin Ag on the cellular tyrosinase activity of α-MSH-stimulated B16F10 cells. α-MSH, α-melanocyte-stimulating hormone. **P<0.01.
Effects of soyasaponin Ag on the expression of melanin synthesis factors in α-MSH-stimulated B16F10 cells
We then examined the effect of soyasaponin Ag treatment on the the expression of tyrosinase, TRP-1 and TRP-2 in B16F10 cells stimulated with α-MSH. As demonstrated by western blot analysis, soyasaponin did not affect α-MSH-induced tyrosinase and TRP-1 expression (Fig. 4). Of note, treatment of the B16F10 cells with 100 µM of soyasaponin Ag abolished TRP-2 expression (Fig. 4).
Figure 4.
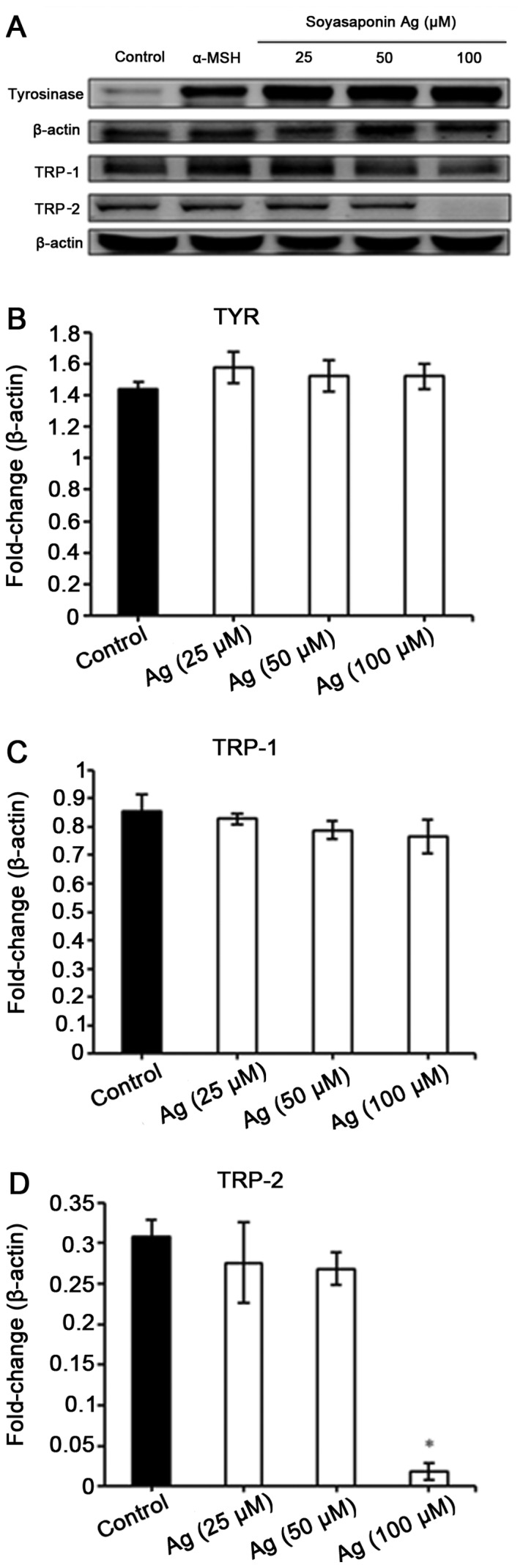
Effects of soyasaponin Ag on the protein levels of melanogenic factors in B16F10 cells. TYR, tyrosinase; TRP, tyrosinase-related protein. *P<0.05.
Discussion
Saponins are beneficial bioactive molecules that are widely distributed in plants and exhibit vast structural and functional diversity; thus, they may be important substances which are worthy of investigation for potential pharmaceutical applications.
The process of melanogenesis is under complex regulatory control by at least 125 genetic loci (23). The enzyme tyrosinase plays a critical role in melanin biosynthesis, and in concert with TRP-1 and TRP-2 is responsible for modifying melanin into different forms (5,24). Thus, the expression level and catalytic activity of these enzymes correlate with melanin production and have been widely investigated with respect to the whitening properties of natural compounds (25). The putative effects of soyasaponin Ag on tyrosinase activity, gene expression, or maturation in melanin biosynthesis during skin pigmentation have not been studied to date, at least to the best of our knowledge. In this study, we demonstrate that soyasaponin Ag inhibits melanogenesis in B16F10 murine melanoma cells via the downregulation of tyrosinase TRP-2 expression. In our initial experiments, we determined that soyasaponin Ag had no cytotoxic effect on B16F10 melanoma cell viability, even at high concentrations. This first indication of the safe use of soyasaponin Agas an anti-melanogenic agent needs however, to be verified in normal melanocytes and in in vivo models.
There are different types of inhibitory mechanisms for melanin synthesis in melanocytes. Indeed, hypopigmenting agents have been classified according to their mechanism of action into three categories: a) regulation of enzyme, which is subdivided into three categories: i) regulation of transcription and maturation of tyrosinase, ii) inhibition of tyrosinase activity, and iii) post-transcriptional control of tyrosinase; b) inhibition of melanosome transfer; and c) additional mechanisms, such as regulation of the melanocyte environment and antioxidant agents (26). Thus, many anti-melanogenic agents, such as kojic acid, hydroquinone and arbutin, are direct inhibitors of tyrosinase enzyme activity (25). Additionally, some compounds exert their anti-melanogenic activities via the suppression of tyrosinase gene expression, although they do not have any direct inhibitory effect on tyrosinase activity in general (27). This type of mechanism was previously identified for acetylsalicylic acid (ASA) in B16 melanoma cells, where ASA exerted a strong dose-dependent inhibitory effect on tyrosinase expression (28). Moreover, ASA did not exert any effect on mushroom tyrosinase activity (28). Other compounds, such as oleoylethanolamide were found to have a more general effect on melanogenesis, inhibiting the expression levels of MITF, TRP-1 and tyrosinase (29). Indeed, MITF plays a crucial role in melanogenesis as the master regulator of tyrosinase, TRP-1 and TRP-2 expression (30). The hydrolyzed ginseng extract, was found to downregulate MITF and TRP-1 expression B16F10 cells, but only indirectly to attenuate cellular tyrosine activity (31). Moreover, the coumarin derivative, osthol, was found to decrease tyrosinase, TRP-1 and TRP-2 expression, but not to modulate tyrosinase activity (32).
The results of the present study indicate that soyasapogenol A treatment decreased the expression of TRP-2 protein and attenuated melanin production. The most likely explanation for these findings is that soyasapogenol A affects MITF activity/expression, which in other studies has been associated with hypopigmentation (33–38). Importantly, a number of studies have reported that not all skin-whitening agents, such as nicotinic acid hydroxamate, glutathione-monoisopropyl ester and plumbagin, simultaneously inhibit TRP-1 and TRP-2 expression (39–41). In addition, the melanin content can be affected by mutation and degradation of the tyrosinase protein (42).
In conclusion, to the best of our knowledge, the present study is the first to demonstrate that soyasapogenol A inhibits melanogenesis by downregulating the protein levels of TRP-1 and TRP-2, resulting in reduced melanin production. Therefore, given the biological properties of soyasapogenol A, we suggest that saponin Ag is a putative skin-whitening agent and may be effective in the treatment of hyperpigmentation disorders, and may also be used in skin-whitening cosmetics. Further investigations are required in order to fully determine the depigmenting mechanisms of action of soyasapogenol A, as well as its anti-melanogenic activity in vivo.
Acknowledgments
This study was carried out with the support of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A09060925).
References
- 1.Videira IF dos S, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regad T. Molecular and cellular pathogenesis of melanoma initiation and progression. Cell Mol Life Sci. 2013;70:4055–4065. doi: 10.1007/s00018-013-1324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine N, Maibach HI. Pigmentation and Pigmentary Disorders A Volume in the Dermatology: Clinical and Basic Science Series. 1st Edition. CRC Press; Boca Raton, FL: 1993. [Google Scholar]
- 5.Costin GE, Hearing VJ. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 6.D'Mello SAN, Finlay GJ, Baguley BC, Askarian-Amiri ME. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millington GWM. Proopiomelanocortin (POMC): The cutaneous roles of its melanocortin products and receptors. Clin Exp Dermatol. 2006;31:407–412. doi: 10.1111/j.1365-2230.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Malek Z, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, Urabe K, Hearing VJ. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA. 1995;92:1789–1793. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prota G. Melanins melanogenesis. Academic Press; San Diego, CA: 1992. Melanin-producing cells; pp. 14–33. [DOI] [Google Scholar]
- 10.Kobayashi T, Imokawa G, Bennett DC, Hearing VJ. Tyrosinase stabilization by Tyrp1 (the brown locus protein) J Biol Chem. 1998;273:31801–31805. doi: 10.1074/jbc.273.48.31801. [DOI] [PubMed] [Google Scholar]
- 11.Choe YS. The effect of basic cosmetic ingredients on the melanogenesis in the B16F10 mouse melanocyte. Konkuk University; Seoul: 2006. [Google Scholar]
- 12.del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165–168. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- 13.Kim SS, Kim MJ, Choi YH, Kim BK, Kim KS, Park KJ, Park SM, Lee NH, Hyun CG. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16 F10 melanoma. Asian Pac J Trop Biomed. 2013;3:617–622. doi: 10.1016/S2221-1691(13)60125-2. discussion 621–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guang C, Chen J, Sang S, Cheng S. Biological functionality of soyasaponins and soyasapogenols. J Agric Food Chem. 2014;62:8247–8255. doi: 10.1021/jf503047a. [DOI] [PubMed] [Google Scholar]
- 15.Shiraiwa M, Kudo S, Shimoyamada M, Harada K, Okubo K. Composition and structure of 'group A saponin' in soybean seed. Agric Biol Chem. 1991;55:315–322. [PubMed] [Google Scholar]
- 16.Shiraiwa M, Harada K, Okubo K. Composition and structure of 'group B saponin' in soybean seed. Agric Biol Chem. 1991;55:911–917. [PubMed] [Google Scholar]
- 17.Zhang W, Popovich DG. Chemical and biological characterization of oleanane triterpenoids from soy. Molecules. 2009;14:2959–2975. doi: 10.3390/molecules14082959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang SH, Ahn EK, Lee JA, Shin TS, Tsukamoto C, Suh JW, Mei I, Chung G. Soyasaponins Aa and Ab exert an anti-obesity effect in 3T3-L1 adipocytes through downregulation of PPARγ. Phytother Res. 2015;29:281–287. doi: 10.1002/ptr.5252. [DOI] [PubMed] [Google Scholar]
- 19.Xiao JX, Huang GQ, Zhang SH. Soyasaponins inhibit the proliferation of Hela cells by inducing apoptosis. Exp Toxicol Pathol. 2007;59:35–42. doi: 10.1016/j.etp.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Park SH, Kim DS, Park SH, Shin JW, Youn SW, Park KC. Inhibitory effect of p-coumaric acid by Rhodiola sachalinensis on melanin synthesis in B16F10 cells. Pharmazie. 2008;63:290–295. [PubMed] [Google Scholar]
- 21.van Meerloo J, Kaspers GJL, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- 22.Maeda K, Fukuda M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J Pharmacol Exp Ther. 1996;276:765–769. [PubMed] [Google Scholar]
- 23.Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- 24.Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991;5:2902–2909. [PubMed] [Google Scholar]
- 25.Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Choi HR, Kim DS, Park KC. Topical hypopigmenting agents for pigmentary disorders and their mechanisms of action. Ann Dermatol. 2012;24:1–6. doi: 10.5021/ad.2012.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung SY, Seo YK, Park JM, Seo MJ, Park JK, Kim JW, Park CS. Fermented rice bran downregulates MITF expression and leads to inhibition of α-MSH-induced melanogenesis in B16F1 melanoma. Biosci Biotechnol Biochem. 2009;73:1704–1710. doi: 10.1271/bbb.80766. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Takahashi H, Iraha R, Toriyama M. Down-regulation of tyrosinase expression by acetylsalicylic acid in murine B16 melanoma. Biol Pharm Bull. 2008;31:33–37. doi: 10.1248/bpb.31.33. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Ren T, Li Y, Cheng A, Xie W, Xu L, Peng L, Lin J, Lian L, Diao Y, et al. Oleoylethanolamide inhibits α-melanocyte stimulating hormone-stimulated melanogenesis via ERK, Akt and CREB signaling pathways in B16 melanoma cells. Oncotarget. 2017 May 23; doi: 10.18632/oncotarget.18097. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasumoto K, Amae S, Udono T, Fuse N, Takeda K, Shibahara S. A big gene linked to small eyes encodes multiple Mitf isoforms: Many promoters make light work. Pigment Cell Res. 1998;11:329–336. doi: 10.1111/j.1600-0749.1998.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 31.Han JS, Sung JH, Lee SK. Antimelanogenesis activity of hydrolyzed ginseng extract (GINST) via inhibition of JNK mitogen-activated protein kinase in B16F10 cells. J Food Sci. 2016;81:H2085–H2092. doi: 10.1111/1750-3841.13380. [DOI] [PubMed] [Google Scholar]
- 32.Beom Kim S, Kim C, Liu Q, Hee Jo Y, Joo Choi H, Hwang BY, Kyum Kim S, Kyeong Lee M. Optimization of extraction conditions for osthol, a melanogenesis inhibitor from Cnidium monnieri fruits. Pharm Biol. 2016;54:1373–1379. doi: 10.3109/13880209.2015.1078382. [DOI] [PubMed] [Google Scholar]
- 33.Kim DS, Hwang ES, Lee JE, Kim SY, Kwon SB, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J Cell Sci. 2003;116:1699–1706. doi: 10.1242/jcs.00366. [DOI] [PubMed] [Google Scholar]
- 34.Koo JH, Rhee KS, Koh HW, Jang HY, Park BH, Park JW. Guggulsterone inhibits melanogenesis in B16 murine melanoma cells by downregulating tyrosinase expression. Int J Mol Med. 2012;30:974–978. doi: 10.3892/ijmm.2012.1057. [DOI] [PubMed] [Google Scholar]
- 35.Kim DS, Kim SY, Chung JH, Kim KH, Eun HC, Park KC. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell Signal. 2002;14:779–785. doi: 10.1016/S0898-6568(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 36.Tachibana M. MITF: A stream flowing for pigment cells. Pigment Cell Res. 2000;13:230–240. doi: 10.1034/j.1600-0749.2000.130404.x. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Guo L, Sun Y, Zhou J, Gu Y, Li Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int J Mol Med. 2010;25:923–927. doi: 10.3892/ijmm_00000423. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Jang JY, Park C, Kim BW, Choi YH, Choi BT. Curcumin suppresses α-melanocyte stimulating hormone-stimulated melanogenesis in B16F10 cells. Int J Mol Med. 2010;26:101–106. [PubMed] [Google Scholar]
- 39.Lin YS, Chuang MT, Chen CH, Chien MY, Hou WC. Nicotinic acid hydroxamate downregulated the melanin synthesis and tyrosinase activity through activating the MEK/ERK and AKT/GSK3β signaling pathways. J Agric Food Chem. 2012;60:4859–4864. doi: 10.1021/jf301109p. [DOI] [PubMed] [Google Scholar]
- 40.Chung BY, Choi SR, Moon IJ, Park CW, Kim YH, Chang SE. The glutathione derivative, GSH monoethyl ester, may effectively whiten skin but GSH does not. Int J Mol Sci. 2016;17:629. doi: 10.3390/ijms17050629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh TI, Yun JM, Park EJ, Kim YS, Lee YM, Lim JH. Plumbagin suppresses α-MSH-induced melanogenesis in B16F10 mouse melanoma cells by inhibiting tyrosinase activity. Int J Mol Sci. 2017;18:320. doi: 10.3390/ijms18020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007;127:751–761. doi: 10.1038/sj.jid.5700683. [DOI] [PubMed] [Google Scholar]