Abstract
Circular RNAs (circRNAs) represent a newly identified class of non-coding RNA molecules, which interfere with gene transcription by adsorbing microRNAs (miRNAs). CircRNAs serve important roles in disease development and have the potential to serve as a novel class of biomarkers for clinical diagnosis. However, the role of circRNAs in the occurrence and development of gastric cancer (GC) remains unclear. In the present study, the expression profiles of circRNAs were compared between GC and adjacent normal tissues using a circRNA microarray, following which quantitative polymerase chain reaction (qPCR) was used to confirm the results of the circRNA microarray. Compared with the adjacent, normal mucosal tissues, 16 circRNAs were upregulated and 84 circRNAs were downregulated in GC. A total of 10 circRNAs were selected for validation in three pairs of GC and adjacent noncancerous tissues. The qPCR results were consistent with the findings of the microarray-based expression analysis. Of the circRNAs studied, only circRNA-0026 (hsa_circ_0000026) exhibited significantly different expression in GC (2.8-fold, P=0.001). Furthermore, online Database for Annotation, Visualization and Integrated Discovery annotation was used to predict circRNA-targeted miRNA-gene interactions. The analysis revealed that circRNA-0026 may regulate RNA transcription, RNA metabolism, gene expression, gene silencing and other biological functions in GC. In conclusion, differential expression of circRNAs may be associated with GC tumorigenesis, and circRNA-0026 is a promising biomarker for GC diagnosis and targeted therapy.
Keywords: gastric cancer, circular RNA, microarray, biomathematics, differential expression
Introduction
Gastric cancer (GC) is one of the most common malignant tumors worldwide and is globally the second leading cause of cancer-associated mortality, being particularly prevalent in China (1–3). Despite a steadily declining incidence of GC, it was still estimated that ~498,000 Chinese individuals would succumb to GC in 2015 (3). A detailed molecular understanding of GC pathogenesis is required, in order to improve the prognosis of patients with this complex disease (4). In addition, an improved understanding of GC pathogenesis would be advantageous for translating molecular findings into clinical use, helping to identify novel biomarkers and treatment targets, and developing personalized therapies for individual patients with GC in the future (4).
Noncoding RNAs (ncRNAs) are defined as RNAs that do not encode proteins, these include ribosomal RNAs, transfer RNAs and small nuclear RNAs, as well as the more recently discovered microRNAs (miRNAs), long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs), which have critical regulatory roles in cancer biology (5–7). CircRNAs represent a specific type of ncRNA that is present in the cytoplasm of eukaryotic cells and is predominantly produced by pre-mRNAs through variable shearing processes (8–11).
CircRNAs are a novel type of RNA that, unlike linear RNA, forms a covalently closed continuous loop, and in some cases is highly represented in the eukaryotic transcriptome (8–11). The majority of circRNAs are composed of exonic sequences, which are conserved in various species, and often exhibit tissue/developmental-stage-specific expression (8–11). Since circRNAs are not sensitive to digestion by RNases, they are more conserved and stable than linear RNA (10,11), which highlights clear advantages in using circRNAs as novel diagnostic markers (10,11). In addition, recent studies have indicated that circRNAs may act as competitive endogenous RNAs (ceRNAs) to sequester miRNAs of a particular family, thereby serving as competitive inhibitors that suppress the ability of a miRNA to bind its mRNA targets (12,13). Therefore, it has been hypothesized that circRNAs potentially regulate disease progression by sequestering a miRNA associated with a particular disease (12,13).
Previous studies have demonstrated that circRNAs serve an important role in the regulation of various cancer pathways (8–11). Ghosal et al (14) performed a Gene Ontology (GO) enrichment analysis on a set of protein-coding genes in the miRNA-circRNA interactome for individual diseases, in order to study the enrichment of genes associated with particular biological processes. The results demonstrated that 194 and 68 genes involved in various biological processes were associated with cervical cancer and GC, respectively. Li et al (15) discovered that hsa_circ_002059 was significantly downregulated in GC and was potentially involved in GC development. However, no direct biological evidence has indicated that circRNAs are associated with GC.
To explore the underlying molecular regulation of circRNAs in GC, the present study examined circRNA expression using a microarray analysis, in order to acquire circRNA profiles in GC and adjacent normal tissues. Subsequently, a quantitative polymerase chain reaction (qPCR) analysis was conducted to confirm the results. In addition, a bioinformatics analysis was performed to predict the biological functions of the differentially expressed circRNAs in GC.
Materials and methods
Patient specimens
Three sets of primary GC tissue samples (the GC group) and paired, adjacent noncancerous tissues (the control group), which were ≥5 cm away from cancerous tissue, were obtained from patients who had undergone curative surgical resection at the Affiliated Hospital of Hainan Medical University (Haikou, China) from June 2014 to July 2014. Histological diagnoses were made from formalin-fixed, paraffin-embedded tissues by two pathologists. The clinicopathological characteristics were obtained from medical records, including gender, age, tumor size, tumor location, histological type, Lauren classification, differentiation grade and surgical record. None of the patients received neoadjuvant therapy. Written informed consent was obtained from the patients for the use of their samples for research, and the research protocols were approved by the Ethics Committee of the Affiliated Hospital of Hainan Medical University.
RNA isolation
A total of 100 mg tissue was taken from each GC and paired normal mucosal tissues, and were homogenized using a TissueLyser II BioRobot Universal system (Qiagen GmbH, Hilden, Germany). Total RNA was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA was purified using the RNeasy Mini kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Subsequently, RNA quality and quantity were measured using a NanoDrop spectrophotometer (ND-1000; NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA), and RNA integrity was determined by gel electrophoresis.
CircRNA microarray
The Arraystar Human Circular RNA Microarray V2.0 (Arraystar, Inc., Rockville, MD, USA) is designed for global profiling of human circRNAs. This microarray is comprised of ~13,617 circRNAs with stringent experimental support, which was carefully and comprehensively collected from circRNA studies and landmark publications (10,11). Each circRNA is represented by a circular splice junction probe, which can reliably and accurately detect the circRNA, even in the presence of its linear counterparts. A random primer-based labeling system is coupled with RNase R sample pretreatment to ensure specific and efficient labeling of circRNAs. RNA spike-in controls were included to monitor labeling and hybridization efficiencies. Microarray hybridization and bioinformatic analysis were performed by KangChen Bio-tech, Inc. (Shanghai, China).
Labeling and hybridization
RNA from three pairs of selected samples was subjected to microarray analysis according to the manufacturers protocol (Arraystar, Inc.). Briefly, total RNA was digested with RNase R (Epicentre; Illumina, Inc., Madison, WI, USA) to remove linear RNAs and enrich for circRNAs. Subsequently, the enriched circRNAs were amplified and transcribed into fluorescent cRNA using a random priming method (Arraystar Super RNA Labeling kit; Arraystar, Inc.). The Cy3-labeled cRNAs were purified using the RNeasy Mini kit (Qiagen GmbH). Each labeled cRNA (1 µg) was fragmented by adding 5 µl 10X blocking agent and 1 µl 25X fragmentation buffer, after which the mixture was incubated at 60°C for 30 min and was then diluted in 25 µl 2X hybridization buffer. A total of 50 µl hybridization solution was dispensed into the gasket slide, which was assembled onto the circRNA expression microarray slide. The slides were incubated for 17 h at 65°C in a hybridization oven (Agilent Technologies, Inc., Santa Clara, CA, USA). The hybridized arrays were washed, fixed and scanned using an Agilent Microarray Scanner system (catalog no. G2505C; Agilent Technologies, Inc.).
Data analysis
Scanned images were imported into Agilent Feature Extraction software version 11.0.1.1 (Agilent Technologies, Inc.) for raw data extraction. Quantile normalization of raw data and subsequent data processing were performed using the R software package (www.arraystar.com/arraystar-human-circular-rna-microarray). Following quantile normalization of the raw data, low-intensity filtering was performed and circRNAs having the ‘P’ or ‘M’ flags (‘All Targets Value’) in ≥3 out of 6 samples were retained for further analyses. Subsequently, samples were clustered hierarchically with Cluster software version 2.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm) to evaluate the robustness of the formed clusters, using the correlation-centered metric and average linkage-clustering algorithm. When comparing profile differences between the GC and control groups, the fold-change between the groups for each circRNA was computed. The statistical significance of each difference was estimated by Student's t-test. CircRNAs exhibiting a ≥2-fold change in expression (P<0.05) were considered to be significantly differentially expressed.
Biomathematical analyses
The present study initially identified circRNAs that exhibited a >2-fold change in expression by microarray analysis. Subsequently, circRNA/miRNA interactions were predicted using Arraystar in-house generated miRNA target-prediction software (Arraystar, Inc.), based on TargetScan (16) and miRanda (17). Differentially expressed circRNAs were used as seeds to enrich for a circRNA-miRNA-gene network, based on a cutoff value determined using miRNA support-vector regression (mirSVR), as described by Wang et al (18). The predicted gene functions in the networks were annotated using GO (http://www.geneontology.org/) and the Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/).
Validation by reverse transcription (RT)-qPCR
Total RNA from three GC specimens and matched, adjacent normal tissues were reverse transcribed to cDNA using the FastQuant RT kit with gDNase (Tiangen Biotech Co., Ltd., Beijing, China) in 20-µl reactions. Triplicate qPCR assays were performed in 20-µl reactions using the FastFire qPCR PreMix (SYBR-Green) kit (Tiangen Biotech Co., Ltd.) according to the manufacturer's protocol. The thermal cycling conditions were as follows: Initial denaturation at 95°C for 60 sec, 40 cycles of amplification at 95°C for 20 sec, annealing and extension at 60°C for 30 sec. GAPDH was used as an internal control for PCR amplification. The sequences of circRNAs were obtained from the circBase database (http://circbase.org/), and PCR primers were designed in divergent orientation, in order to be capable of amplifying the reverse splice site of the circRNA, using Primer Premier software version 6.0 (Premier Biosoft International, Palo Alto, CA, USA) and were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) (Table I). The data were analyzed using the 2−ΔΔCq method (19). Validation was performed by determining the ratio of differential cellular expression/differential microarray expression, and amplification products were analyzed by 1.5% agarose gel electrophoresis and stained with 0.1% GeneGreen Nucleic Acid gel stain (Tiangen Biotech Co., Ltd.) for band size consistency. The qPCR data were analyzed by Student's t-test using SPSS software version 18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Table I.
Quantitative polymerase chain reaction primers and product sizes.
| Amplicon | Primers | Accession no. | Genomic location | Gene symbol | Tm (°C) | Length (bp) |
|---|---|---|---|---|---|---|
| GAPDH(human) | F: 5′GGGAAACTGTGGCGTGAT3′ | NM_001289746 | Chr 12 | GAPDH | 60 | 299 |
| R: 5′GAGTGGGTGTCGCTGTTGA3′ | ||||||
| hsa_circ_0000144 | F: 5′GAAGGTAGGACAAATGGAAGTT3′ | NM_052931 | Chr 1 | SLAMF6 | 60 | 136 |
| R: 5′GAATCTGCTTAGTTCTACCTCTC3′ | ||||||
| hsa_circ_0023642 | F: 5′ATGACAAACTGACGGAAAAGGAG3′ | NM_003369 | Chr 11 | UVRAG | 60 | 64 |
| R: 5′AACCAAGGGCAACAGCAATG3′ | ||||||
| hsa_circ_0032821 | F: 5′AGATAGAAAGGCAGGAGCAG3′ | NM_152446 | Chr 14 | CEP128 | 60 | 146 |
| R: 5′TGTTCAGTCTCCAAGCAAAG3′ | ||||||
| hsa_circ_0005529 | F: 5′AGTCCCTGCGCCTCATCTTG3′ | NM_018668 | Chr 15 | VPS33B | 60 | 101 |
| R: 5′CGCCGCTCTAGCACCTTTCT3′ | ||||||
| hsa_circ_0061274 | F: 5′CAGCCTTCTCAATTTTCTTTC3′ | NM_003489 | Chr 21 | NRIP1 | 60 | 96 |
| R: 5′AGTCTTCAGATTCCCTGTCCT3′ | ||||||
| hsa_circ_0000026 | F: 5′CCATCCCCTTATTCAGCACAT3′ | NM_001198803 | Chr 1 | EIF4G3 | 60 | 132 |
| R: 5′TCCAAACTTCAGTTTCCTCATCA3′ | ||||||
| hsa_circ_0040039 | F: 5′CAGGATACTTGTTCAGGGTTGC3′ | NM_006750 | Chr 16 | SNTB2 | 60 | 201 |
| R: 5′TTGGTGCTGTTCTGGTGTTTT3′ | ||||||
| hsa_circ_0041732 | F: 5′GCTCACATGCCCACCCATTA3′ | NM_019013 | Chr 17 | FAM64A | 60 | 127 |
| R: 5′CAGCCACTTGGTGCCACTTT3′ | ||||||
| hsa_circ_0068610 | F: 5′GACAATGCTGCTTTCCCTTTC3′ | NM_003234 | Chr 3 | TFRC | 60 | 154 |
| R: 5′CCAGTAACCGGATGCTTCACA3′ | ||||||
| hsa_circ_0005927 | F: 5′TGAATTTGGAGGTTCTATCTACCAG3′ | NM_001135694 | Chr 8 | VDAC3 | 60 | 162 |
| R: 5′CCTTCAATTTCCCACTCTTCTTT3′ |
circ, circular RNA; F, forward; R, reverse; Chr, chromosome; Tm, melting temperature; bp, base pairs.
Results
circRNA microarray analysis
The present study investigated the alterations in circRNA expression profiles between the GC group and the control group. To obtain consistent biological information, paired samples from 3 patients with similar clinical data were selected for microarray analysis. All GC cases were of the diffuse type (Lauren classification), male, stage IIIA (TNM staging system), and aged 55–58 (average age, 56.7 years). Total RNA was subsequently extracted, and circRNA expression in the groups was analyzed using an Arraystar microarray.
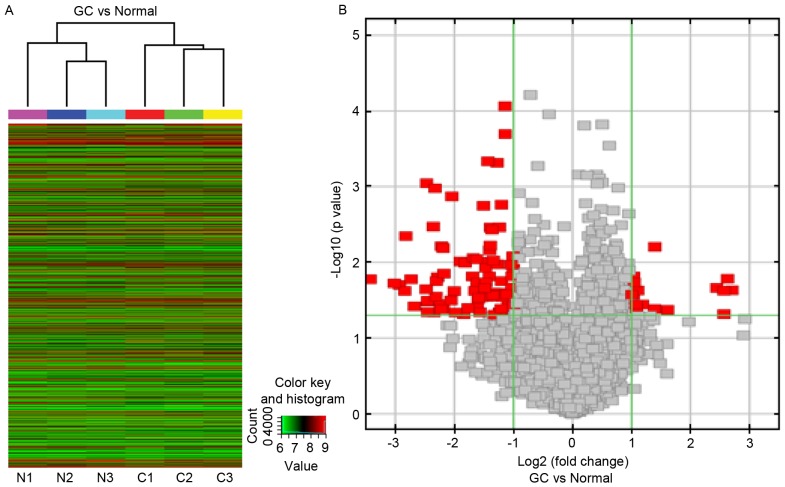
To determine whether circRNA profiles were informative with regards to tissue type, an unsupervised, hierarchical cluster analysis was conducted based on the circRNA expression levels in 3 GC specimens and adjacent normal tissues. The results of hierarchical clustering showed distinguishable circRNA expression profiling among 6 samples. Two main clusters were formed, the GC group cluster and the control group cluster (Fig. 1A). These data indicated that circRNAs have a different expression pattern in GC compared with in normal gastric mucosa.
Figure 1.
Expression profile of circRNAs in GC tissue, as determined by microarray analysis. (A) Unsupervised hierarchical cluster analysis, based on the expression levels of circRNAs. CircRNA microarray expression profiles from three sets of matched GC tissue samples (C1-C3) and adjacent normal tissues samples (N1-N3). CircRNAs in red indicate overexpression; those in green indicate reduced expression. (B) Volcano plot analysis based on circRNA expression levels. The vertical green lines correspond to 2.0-fold increased or decreased expression, and the horizontal green line represents P<0.05. Therefore, the red points in the plot represent differentially expressed circRNAs with statistical significance. circRNA, circulating RNA; GC, gastric cancer.
The circRNA chip detected >2,000 circRNAs expressed in GC tissues and adjacent normal tissues, and 100 circRNAs (86 exonic circRNAs, 12 intronic circRNAs and 2 antisense circRNAs) that were ≥2-fold differentially expressed between the GC and the matching, adjacent normal tissues (Fig. 1B), which were distributed across all 22 autosomes. A total of 16 circRNAs were significantly upregulated and 84 were significantly downregulated in the GC group, compared with the control group. The circRNAs were ranked according to fold-changes in expression between the groups. The top 5 upregulated and downregulated circRNAs are presented in Table II.
Table II.
Biological information regarding the top 5 upregulated and downregulated circRNAs.
| Aliasa | Fold change | P-value | circRNA type | Chr | Best transcript | Gene symbol |
|---|---|---|---|---|---|---|
| Upregulated | ||||||
| hsa_circ_0023642 | 6.450 | 0.023 | Exonic | chr11 | uc009yuh.1 | UVRAG |
| hsa_circ_0000144 | 6.16 | 0.016 | Antisense | chr1 | NM_001184714 | SLAMF6 |
| hsa_circ_0061274 | 5.92 | 0.023 | Exonic | chr21 | uc002yjx.2 | NRIP1 |
| hsa_circ_0032821 | 5.91 | 0.048 | Exonic | chr14 | uc001xux.2 | CEP128 |
| hsa_circ_0005529 | 5.43 | 0.022 | Exonic | chr15 | uc002bqp.1 | VPS33B |
| Downregulated | ||||||
| hsa_circ_0040039 | 10.78 | 0.017 | Exonic | chr16 | uc002ewu.3 | SNTB2 |
| hsa_circ_0000026 | 7.62 | 0.020 | Exonic | chr1 | uc001bec.3 | EIF4G3 |
| hsa_circ_0041732 | 7.16 | 0.024 | Exonic | chr17 | uc002gcu.2 | FAM64A |
| hsa_circ_0005927 | 7.08 | 0.004 | Exonic | chr8 | uc022aul.1 | VDAC3 |
| hsa_circ_0092341 | 6.66 | 0.017 | Intronic | chr6 | NM_001164446 | C6orf132 |
Alias refers to the circRNA ID in circBase. circRNA, circular RNA; Chr, chromosome; Best transcript, is transcribed from the same gene position with circular RNA, the sequence information is most similar to circular RNA.
Microarray validation by RT-qPCR
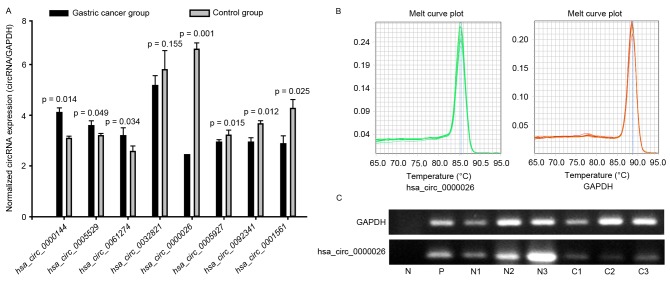
To validate the expression profiles of circRNAs in three matched pairs of GC and normal specimens, the top 5 down- and upregulated circRNAs were further studied by qPCR (Fig. 2). The primers were specifically capable of amplifying the back splice sites of each circRNA, and the PCR results were validated by gel electrophoresis and melt-curve analysis (Fig. 2B and C); melting curves for qPCR assays of circRANs showed a single peak indicated the specificity of the PCR results, and the sizes of the PCR products were in accordance with the anticipated sizes, based on the primer designs. The expression levels of 7 selected circRNAs were consistent with those measured by microarray analysis; however, 2 circRNAs were not detected, perhaps due to the extremely low expression levels in the 6 samples, and differential expression was not detected with 1 circRNA (P>0.05; Fig. 2A). Of these, only circRNA-0026 expression was significantly different between the GC and control samples (2.8-fold, P=0.001), and all other differences were less than 1.5 fold.
Figure 2.
RT-qPCR was used to detect the expression in matched GC tissues and adjacent normal tissues. circRNA expression was validated by performing RT-qPCR (in triplicate) with three pairs of GC samples. (A) RNA expression levels were normalized to expression of the reference gene GAPDH, which did not show differential expression between the sample groups. A total of 3 upregulated circRNAs and 5 downregulated circRNAs were validated. Only circRNA_000026 was significantly differentially expressed between the 2 groups (2.8-fold, P=0.001). All other differences were <1.5-fold. (B) Melt curve plot of GAPDH and hsa_circ_000026. (C) qPCR products of hsa_circ_000026 were subjected to gel electrophoresis. N, negative control; P, positive control; C1-C3, GC tissue; N1-N3, normal tissue adjacent to GC tissue; GC, gastric cancer; circRNA, circular RNA; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Biomathematical analyses
It has previously been indicated that circRNAs regulate gene expression by targeting miRNAs and blocking their biological functions (7,10). In the present study, miRNA and circRNA sequences were aligned, and 5 miRNAs with the highest mirSVR were identified for each differentially expressed circRNA using miRNA target-prediction software, based on TargetScan and miRanda. All differentially expressed circRNAs were annotated in detail, with the circRNA/miRNA interaction information. As a result, 327 miRNAs (including 221 miRNA families) were identified and ranked according to their predicted number of interactions with different circRNAs, as described by Wang et al (18); the top 10 miRNA families consisted of the miR-29, miR-30, miR-15, miR-146, miR-16, miR-181, miR-135, miR-23, miR-19 and miR-let-7 families. Bioinformatics analysis revealed that these miRNAs were abnormally expressed in various malignant tumors, including GC (20), colon cancer (21), breast cancer (22), lung cancer (23) and others (24–26), thus suggesting their involvement in the occurrence and development of malignant tumors. Subsequently, candidate target genes were identified based on miRNA/mRNA sequence pairing (mRNA-dependent cutoff value, −0.50) using TargetScan online tools, and a circRNA-0026-targeted circRNA-miRNA-mRNA/gene network was constructed. It was demonstrated that 13 miRNAs and 578 genes were targeted in the network (miRNA-dependent cutoff value, −0.14; mRNA-dependent cutoff value, −0.50). To investigate the functions of the predicted network genes, GO analysis was performed using the DAVID tool. Genes in the top 10 annotated clusters were involved in regulating transcription, including RNA metabolic processes, gene expression, gene silencing and other biological functions (Table III).
Table III.
Gene Ontology term enrichment in the circular RNA0026-microRNA-mRNA/gene network.
| Term | Gene count | P-value | Fold enrichment |
|---|---|---|---|
| Regulation of RNA metabolic process | 67 | 8.0×10−8 | 1.9 |
| Regulation of transcription, DNA-dependent | 65 | 1.90×10−7 | 1.9 |
| Regulation of transcription | 84 | 3.4×10−7 | 1.7 |
| Transcription | 69 | 3.9×10−6 | 1.7 |
| Negative regulation of gene expression | 21 | 1.5×10−3 | 2.2 |
| Negative regulation of transcription | 17 | 1.5×10−2 | 1.9 |
| Negative regulation of macromolecule biosynthetic process | 19 | 1.7×10−2 | 1.8 |
| Negative regulation of cellular biosynthetic process | 19 | 2.1×10−2 | 1.8 |
| Negative regulation of biosynthetic process | 19 | 2.5×10−2 | 1.7 |
| Negative regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolic process | 17 | 3.5×10−2 | 1.7 |
| Negative regulation of nitrogen compound metabolic process | 17 | 3.9×10−2 | 1.7 |
| Regulation of protein kinase cascade | 10 | 4.9×10−2 | 2.1 |
| Negative regulation of transcription from RNA | 10 | 6.8×10−2 | 2 |
| Polymerase II promoter | |||
| Negative regulation of transcription, DNA-dependent | 12 | 7.8×10−2 | 1.8 |
| Negative regulation of RNA metabolic process | 12 | 8.4×10−2 | 1.7 |
| Cell activation | 10 | 9.7×10−2 | 1.8 |
| Anti-apoptosis | 8 | 9.8×10−2 | 2 |
Discussion
Despite a steadily declining incidence, GC remains the second leading cause of cancer-associated mortality in China due to its highly malignant nature (1,4). Over the last decade, the understanding of the molecular pathogenesis of GC has advanced considerably; however, much remains unclear (1,4). In recent years, mounting evidence has suggested that ncRNAs serve important roles in cellular metabolism and in regulatory processes, including development, proliferation, differentiation and apoptosis (18,25). Aberrantly expressed ncRNAs, such as lncRNA, miRNAs and circRNAs, serve key roles in tumor pathogenesis by regulating numerous tumor signaling pathways, including epidermal growth factor receptor (EGFR) (26,27), Notch (28), mammalian target of rapamycin (mTOR) (29), nuclear factor (NF)-κB (30) and Wnt (31).
CircRNAs represent a class of widespread ncRNAs that are considered ceRNAs, since they are capable of regulating each other by competing for binding to shared miRNAs (32,33). Previous studies have demonstrated that circRNAs serve crucial roles in fine-tuning miRNA-mediated regulation of gene expression by sequestering miRNAs (6,33), and their aberrant expression may be associated with human diseases (6,12–15,32). For example, the circRNA ciRS-7 contains >60 miR-7-binding sites, thereby acting as an endogenous miRNA “sponge” to adsorb and thereby quench normal miR-7 functions (33). Considering the widespread involvement of miR-7 as a key regulator of various cancer pathways, including EGFR (34–36), Raf1 (36), NF-κB (37), mTOR (38,39), AKT (34,39) among others (6,40), ciRS-7 may serve as a crucial factor in tumor development (6,39). Increasing evidence has indicated that the aberrant expression of circRNAs may promote cancer pathogenesis by adsorbing cancer-associated miRNAs (6,40). Some synthetic circRNAs have exhibited marked anticancer effects (41,42), indicating that circRNAs have diagnostic and therapeutic potential (6,40–42).
Recent findings have reported that circRNAs are potentially involved in GC (14–15). Ghosal et al (14) performed a GO enrichment analysis on the protein-coding genes in the miRNA-circRNA interactome of individual diseases, in order to study the enrichment of genes associated with particular biological processes; a total of 68 genes involved in various biological processes were revealed to be associated with GC. Li et al (15) demonstrated that hsa_circ_002059 was significantly downregulated in GC, suggesting its potential as a novel and stable biomarker for GC diagnosis.
The present study identified 100 circRNAs with ≥2-fold differential expression between matched GC and normal tissues, as determined by circRNA chip analysis. The qPCR results confirmed the abnormal expression of these circRNAs in GC. To further analyze the role of these circRNAs in GC pathogenesis, bioinformatics analysis was performed to identify the miRNAs with the highest mirSVRs for each differentially expressed circRNA, based on predicted base pairing between circRNAs and miRNAs. A total of 327 candidate miRNAs (from 221 miRNA families) were identified, and the top 10 miRNA families in terms of the number of potential circRNA interactions were miR-29, miR-30, miR-15, miR-146, miR-16, miR-181, miR-135, miR-23, miR-19 and miR-let-7. Previous studies have provided evidence that these miRNAs are abnormally expressed in various types of cancer, including GC (20), colorectal cancer (21), breast cancer (22), lung cancer (23) and others (24–26), and are involved in tumor cell proliferation and drug resistance, as well as the occurrence, development, invasion and metastasis of tumors (20–26). In agreement, the results of the present study demonstrated that these differentially expressed circRNAs may be involved in the pathogenesis of GC.
Although >7,000 circRNAs have been identified in human tissues (43), and sponge-like activity is the main function of some circRNAs (33), to date only ciRS-7 (6) and circRNA transcripts from the sex determining region Y gene (33,44) have been reported to function as molecular sponges against their target miRNAs. In the present study, circ_000026 was significantly downregulated in GC tissues compared with paired, adjacent noncancerous tissues (P=0.001), as determined using a circRNA chip and qPCR analysis. To further understand the biological function of circ_000026, the top 5 miRNAs with the highest mirSVRs were identified (including miR-23a, miR-23b, miR-581, miR-146a and miR-450a), and the circ_000026-miRNA-gene network was predicted using TargetScan and miRanda. The DAVID tool was used to enrich for GO terms associated with circRNA-producing genes. The analysis revealed that the circ_000026-targeted miRNA-mRNA network may regulate transcription, RNA metabolism, gene expression and gene silencing, among other functions. These results suggested that circRNAs not only act as miRNA sponges, but also may potentially regulate RNA and protein production, which is in agreement with the findings from previous studies (45,46).
In conclusion, the present study demonstrated that circRNAs are aberrantly expressed in GC. Significant downregulation of circ_000026 expression in GC tissues was confirmed, suggesting its potential involvement in GC tumorigenesis and its potential use as a novel biomarker for GC diagnosis and targeted therapy. In the future, a longitudinal study is required to investigate the potential of circRNAs as biomarkers for GC diagnosis and targeted therapy.
Acknowledgements
The present study was supported by the National Natural Science Foundation (grant no. 81260321). circRNA microarray results were deposited in the National Center for Biotechnology Information Gene Expression Omnibus database under accession number GSE78092.
Glossary
Abbreviations
- ceRNA
competitive endogenous RNA
- circRNA
circular RNA
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- GC
gastric cancer
- GO
Gene Ontology
- lncRNA
long noncoding RNA
- miRNA
microRNA
- mirSVR
miRNA support vector regression
- ncRNA
non-coding RNA
- qPCR
quantitative polymerase chain reaction
References
- 1.Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149:1153–1162.e3. doi: 10.1053/j.gastro.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 5.Wilusz JE. Long noncoding RNAs: Re-writing dogmas of RNA processing and stability. Biochim Biophys Acta. 2016;1859:128–138. doi: 10.1016/j.bbagrm.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 7.Salzman J. Circular RNA expression: Its potential regulation and function. Trends Genet. 2016;32:309–316. doi: 10.1016/j.tig.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett SP, Wang PL, Salzman J. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife. 2015;4:e07540. doi: 10.7554/eLife.07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 11.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/annotation/f782282b-eefa-4c8d-985c-b1484e845855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Li C, Tan C, Liu X. Circular RNAs: A new frontier in the study of human diseases. J Med Genet. 2016;53:359–365. doi: 10.1136/jmedgenet-2016-103758. [DOI] [PubMed] [Google Scholar]
- 13.Zhao ZJ, Shen J. Circular RNA participates in the carcinogenesis and the malignant behavior of cancer. RNA Biol. 2017;14:514–521. doi: 10.1080/15476286.2015.1122162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosal S, Das S, Sen R, Basak P, Chakrabarti J. Circ2Traits: A comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:283. doi: 10.3389/fgene.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquinelli AE. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 18.Wang YH, Yu XH, Luo SS, Han H. Comprehensive circular RNA profiling reveals that circular RNA100783 is involved in chronic CD28-associated CD8(+)T cell ageing. Immun Ageing. 2015;12:17. doi: 10.1186/s12979-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Tan X, Fu SW. May Circulating microRNAs be gastric cancer diagnostic biomarkers. J Cancer. 2015;6:1206–1213. doi: 10.7150/jca.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas J, Ohtsuka M, Pichler M, Ling H. MicroRNAs: clinical relevance in colorectal cancer. Int J Mol Sci. 2015;16:28063–28076. doi: 10.3390/ijms161226080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertoli G, Cava C, Castiglioni I. MicroRNAs: New biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122–1143. doi: 10.7150/thno.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inamura K, Ishikawa Y. MicroRNA in lung cancer: Novel biomarkers and potential tools for treatment. J Clin Med. 2016;5 doi: 10.3390/jcm5030036. pii: E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heneghan HM, Miller N, Kerin MJ. MiRNAs as biomarkers and therapeutic targets in cancer. Curr Opin Pharmacol. 2010;10:543–550. doi: 10.1016/j.coph.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Song YX, Wang ZN. Non-coding RNAs in gastric cancer. Gene. 2015;560:1–8. doi: 10.1016/j.gene.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Deng G, Sui G. Noncoding RNA in oncogenesis: A new era of identifying key players. Int J Mol Sci. 2013;14:18319–18349. doi: 10.3390/ijms140918319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pak MG, Lee CH, Lee WJ, Shin DH, Roh MS. Unique microRNAs in lung adenocarcinoma groups according to major TKI sensitive EGFR mutation status. Diagn Pathol. 2015;10:99. doi: 10.1186/s13000-015-0339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng N, Cai W, Ren S, Li X, Wang Q, Pan H, Zhao M, Li J, Zhang Y, Zhao C, et al. Long non-coding RNA UCA1 induces non-T790M acquired resistance to EGFR-TKIs by activating the AKT/mTOR pathway in EGFR-mutant non-small cell lung cancer. Oncotarget. 2015;6:23582–23593. doi: 10.18632/oncotarget.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji J, Tang J, Deng L, Xie Y, Jiang R, Li G, Sun B. LINC00152 promotes proliferation in hepatocellular carcinoma by targeting EpCAM via the mTOR signaling pathway. Oncotarget. 2015;6:42813–42824. doi: 10.18632/oncotarget.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajbhandari R, McFarland BC, Patel A, Gerigk M, Gray GK, Fehling SC, Bredel M, Berbari NF, Kim H, Marks MP, et al. Loss of tumor suppressive microRNA-31 enhances TRADD/NF-κB signaling in glioblastoma. Oncotarget. 2015;6:17805–17816. doi: 10.18632/oncotarget.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Zhang L, Li W, Deng J, Zheng J, An M, Lu J, Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 34.Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, Purow B. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 35.Suto T, Yokobori T, Yajima R, Morita H, Fujii T, Yamaguchi S, Altan B, Tsutsumi S, Asao T, Kuwano H. MicroRNA-7 expression in colorectal cancer is associated with poor prognosis and regulates cetuximab sensitivity via EGFR regulation. Carcinogenesis. 2015;36:338–345. doi: 10.1093/carcin/bgu242. [DOI] [PubMed] [Google Scholar]
- 36.Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284:5731–5741. doi: 10.1074/jbc.M804280200. [DOI] [PubMed] [Google Scholar]
- 37.Zhao XD, Lu YY, Guo H, Xie HH, He LJ, Shen GF, Zhou JF, Li T, Hu SJ, Zhou L, et al. MicroRNA-7/NF-κB signaling regulatory feedback circuit regulates gastric carcinogenesis. J Cell Biol. 2015;210:613–627. doi: 10.1083/jcb.201501073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Liu J, Liu C, Naji A, Stoffers DA. MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic β-cells. Diabetes. 2013;62:887–895. doi: 10.2337/db12-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55:1852–1562. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Yang J, Zhou P, Le Y, Zhou C, Wang S, Xu D, Lin HK, Gong Z. Circular RNAs in cancer: Novel insights into origins, properties, functions and implications. Am J Cancer Res. 2015;5:472–480. [PMC free article] [PubMed] [Google Scholar]
- 41.Bak RO, Hollensen AK, Mikkelsen JG. Managing microRNAs with vector-encoded decoy-type inhibitors. Mol Ther. 2013;21:1478–1485. doi: 10.1038/mt.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haraguchi T, Ozaki Y, Iba H. Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res. 2009;37:e43. doi: 10.1093/nar/gkp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-Y. [DOI] [PubMed] [Google Scholar]
- 45.Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martineau Y, Derry MC, Wang X, Yanagiya A, Berlanga JJ, Shyu AB, Imataka H, Gehring K, Sonenberg N. Poly(A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Mol Cell Biol. 2008;28:6658–6667. doi: 10.1128/MCB.00738-08. [DOI] [PMC free article] [PubMed] [Google Scholar]