Abstract
The present study aimed to investigate the expression and role of transforming growth factor (TGF) -β-activated protein kinase 1 (TAK1) in human gastric cancer. Immunohistochemistry was performed to investigate the expression of TAK1 in surgical specimens of human gastric cancer tissue and adjacent normal tissue. The association between TAK1 and clinicopathologic factors was analyzed and the association between TAK1 expression and the overall survival rates was evaluated using Kaplan-Meier curves. In addition, the effect of the TAK1 selective inhibitor 5Z-7-oxozeaenol (OZ) on the biological characteristics of MGC803 human gastric cancer cells in vitro were investigated. The role of TAK1 in gastric cancer cell proliferation, apoptosis and invasion were determined by cell proliferation assays, flow cytometry analysis and transwell invasion assays, respectively. The findings of the present study demonstrated that the positive expression rate of TAK1 in gastric cancer and adjacent normal tissues was 70.5 and 25.9%, respectively. Furthermore, TAK1 expression was significantly associated with advanced N stage and pathological stage (P<0.05). Survival analysis of 139 patients with gastric cancer indicated a lower overall survival rate of patients in the TAK1-positive group compared with the TAK1-negative group (P<0.05). In addition, treatment with the TAK1 selective inhibitor OZ reduced the proliferation and invasion abilities of MGC803 cells and significantly reduced the expression levels of phosphorylated-TAK1 (Thr187), nuclear p65, cyclin D1, Bcl-2 apoptosis regulator and matrix metallopeptidase (MMP)9 (P<0.05). OZ treatment significantly increased the expression levels of cytosolic cytochrome c and cleaved caspase 3 and the apoptosis rate in MGC803 cells (P<0.05). In conclusion, these findings suggest that increased TAK1 expression may be involved in the progression of gastric cancer; therefore, TAK1 may be used as a future therapeutic target for gastric cancer treatment.
Keywords: gastric cancer, transforming growth factor-β-activated kinase 1, 5Z-7-oxozeaenol
Introduction
Gastric cancer has one of the highest cancer-associated mortalities worldwide and patients have a particularly high susceptibility to lymph node metastasis (1,2). The incidence of gastric carcinoma has recently increased, which may be due to various environmental and social factors (3), including H. pylori infection, low socioeconomic status and perhaps dietary factors such as low consumption of fruits and vegetables and a high intake of salty and smoked food (4). Although higher overall survival rates for patients with gastric carcinoma are currently observed due to improved early cancer detection and increased use of radical surgery, gastric carcinoma remains the fourth most common cancer and is considered to be the second major cause of cancer-associated deaths globally (5). It is of note that effective methods for early diagnosis, monitoring for metastasis and prognosis are remain to be established for gastric cancer (6). Therefore, the identification of novel therapeutic targets for gastric cancer is required.
Transforming growth factor-β-activated kinase 1 (TAK1) regulates the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, which have important roles in various biological processes, including development, cell survival, immune responses, metabolism and carcinogenesis (7). Previous studies demonstrated that TAK1 functions as a tumor promoter in various tissues, including breast and thyroid cancer (8,9). TAK1 inhibition has also been reported to induce cancer cell death (10,11), indicating that targeting TAK1 may be useful in the development of treatments for gastric cancer. Therefore, in order to develop cancer therapies that target TAK1, it is important to determine the regulation and role of TAK1 in the pathogenesis (12). To the best of our knowledge, the role of TAK1 in gastric cancer has not previously been investigated. 5Z-7-Oxozeaenol, a natural product of fungal origin, was reported to be a TAK1 specific inhibitor (13). Therefore, the present study investigated the expression of TAK1 in gastric cancer and its clinical significance, and further investigated the function of TAK1 in the development and progression of gastric cancer in vitro using 5Z-7-oxozeaenol (OZ), which is a selective TAK1 inhibitor (14).
Materials and methods
Patients
Gastric cancer samples and adjacent normal tissue samples used in the present study were obtained from 139 patients with gastric cancer that underwent resection at The Third Affiliated Hospital, Nanjing University of Traditional Chinese Medicine (Nanjing, China) and Wuxi XiShan People's Hospital (Wuxi, China) between January 2005 and August 2010. Normal gastric mucosa tissue (≤5 cm) adjacent to the tumor was excised and confirmed to be tumor-free following pathological analysis. Every resection specimen was examined by the Department of Pathology, The Third Affiliated Hospital, Nanjing University of Traditional Chinese Medicine (Nanjing, China), to confirm their histology features. The patients consisted of 81 males and 58 females, aged between 30 and 75 years (median, 50 years). The following inclusion criteria were sued for the present study: i) Complete surgical R0 resection of the primary tumor; ii) pathologically confirmed diagnosis of gastric adenocarcinoma; iii) no chemotherapy or radiotherapy administered; and iv) absence of secondary malignancies. All patients provided a signed agreement for participation in the study and the protocol was approved by the Ethics Committees of The Third Affiliated Hospital of Nanjing University of Traditional Chinese Medicine and Wuxi XiShan People's Hospital. Written informed consent was obtained from all patients involved in the present study. The epidemiological, clinical and pathological features of patients included in the present study are summarized in Table I. The clinical outcome of the patients was followed for 1–60 months, from the date of surgery to either the date of mortality or August 30, 2015.
Table I.
Clinicopathological parameters and patients with positive expression of TAK1 in gastric cancer.
| TAK1 expression | ||||
|---|---|---|---|---|
| Clinicopathological parameters | Total | Positive | Negative | P-value (Chi-square test) |
| Gender | 0.96 | |||
| Male | 81 | 57 | 24 | |
| Female | 58 | 41 | 17 | |
| Age, years | 0.41 | |||
| <60 | 62 | 42 | 20 | |
| ≥60 | 77 | 56 | 21 | |
| Tumor size, cm | 0.52 | |||
| <5 | 60 | 44 | 16 | |
| ≥5 | 79 | 54 | 25 | |
| Neural/vascular invasion | 0.51 | |||
| Yes | 42 | 28 | 14 | |
| No | 97 | 70 | 27 | |
| Tumor grade | 0.26 | |||
| I and II | 61 | 40 | 21 | |
| III | 78 | 58 | 20 | |
| T stage | 0.60 | |||
| T1 and T2 | 24 | 18 | 6 | |
| T3 and T4 | 115 | 80 | 35 | |
| N stage | <0.001 | |||
| N0 | 28 | 11 | 17 | |
| N1-N3 | 111 | 87 | 24 | |
| M stage | 0.77 | |||
| M0 | 131 | 92 | 39 | |
| M1 | 8 | 6 | 2 | |
| Pathological stage | <0.001 | |||
| I and II | 47 | 18 | 29 | |
| III and IV | 92 | 80 | 12 | |
TAK1, transforming growth factor-β-activated kinase 1; T, tumor; N, node; M, metastasis.
Immunohistochemistry and scoring
The tissues were fixed for 24 h in 4% paraformaldehyde and embedded in paraffin. The paraffin-embedded tissues were cut into 4 µm sections. Then, tissue sections were deparaffinized in xylene and rehydrated through graded ethanol. Tissues were placed in 0.01 M citrate buffer and incubated at 100°C for 20 min for antigen retrieval. Tissues were blocked with a 3% hydrogen peroxide solution to inhibit endogenous peroxidase activity and washed with PBS, Subsequently, sections were incubated with 5% normal rabbit serum (Abcam, Cambridge, UK) for 30 min at room temperature to block non-specific binding sites. The slides were subsequently incubated with a TAK1 antibody (1:50; catalog no. sc-7162; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight, followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (dilution 1:1,000; catalog no. ab6721; Abcam) for 60 min at room temperature. The sections were then developed in 0.05% diaminobenzidine and counterstained with 0.1% hematoxylin and eosin (H&E) for 5 min at room temperature prior to dehydration and mounting. Evaluation of immunostaining in tumor cells was objectively performed by two pathologists under a light microscope at high magnification (×400). TAK1 staining was determined semi-quantitatively according to the intensity observed (0=no staining; 1=weak staining; 2=moderate staining; and 3=strong staining) and the percentage of positive cells (0, none or <5%; 1, 5–20%; 2, 21–40%; and 3, >40%). Scores of 0–2 were considered to be negative expression and scores of 3–6 were considered to be positive expression. Cells were counted in at least three randomly selected fields (at ×400 magnification) in the tumor areas.
Cell culture
The MGC803 human gastric cancer cell line was purchased from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). MGC803 cells were cultured at 37°C and 5% CO2 and saturation humidity in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), with 10% fetal bovine serum, 100 U/ml penicillin (Thermo Fisher Scientific), and 100 mg/ml streptomycin (Thermo Fisher Scientific, Inc.). The cells adhered to the flask wall and grew into a single-cell monolayer and were passaged every 2–3 days. Cells in the exponential growth phase were harvested for subsequent experiments. There were four experimental groups: Control (without any intervention); vehicle treatment [1% dimethylsulfoxide (DMSO)]; low-dose OZ (3 µM); and high-dose OZ (6 µM). TAK1 kinase inhibitor, OZ was purchased from Tocris Bioscience, Bristol, UK (catalog no. 3604).
MTT assay
Cells were plated in 96-well plates at a density of 5×103/well and treated with 6 µM OZ or vehicle for 24, 48 and 72 h at 37°C. At the aforementioned time points, 20 µl MTT substrate (5 mg/ml) was added to the cells and incubated at 37°C for 4 h. The resulting colored product was made soluble in 200 µl DMSO. Spectrometric absorbance at 490 nm was quantified using a microplate reader. Each cell line was established in quadruplicate wells and repeated three times.
Invasion assay
Cell invasion activity was determined using a BD BioCoat Matrigel Invasion Chamber (8-µm; BD Biosciences, Franklin Lakes, NJ, USA). Briefly, gastric cancer cells were harvested and added to the upper chamber at a cell density of 2×105 cells/ml in RPMI-1640 medium without FBS and treated with 6 µM OZ or DMSO. RPMI-1640 medium with 10% FBS was added to the lower chamber. The chambers were incubated for 48 h at 37°C and 5% CO2. At the end of the incubation period, cells that had invaded through the membrane were subsequently fixed with 4% paraformaldehyde for 15 min and stained with 0.5% crystal violet for 30 min at room temperature. Cells were observed under ×40 magnification with a ZEISS light microscope and counted. Each experiment was performed in triplicate and repeated three times.
Flow cytometry analysis
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was used to analyze the apoptosis rate according to the manufacturer's protocol (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). MGC803 cells were seeded in six-well plates (1×106 cells/well) at 37°C and treated with 6 µM OZ or vehicle for 24, 48 and 72 h. Cells were dissociated using trypsin, then centrifuged at 400 × g for 5 min. Next, cells were washed twice with PBS and centrifuged at 400 × g for 5 min. For apoptosis analysis, the cell pellet was resuspended in 500 µl binding buffer. Then, 5 µl Annexin V-FITC and 5 µl propidiumiodide (PI) was added to the cell suspension, which was gently mixed and incubated at room temperature, and was protected from light, for 15 min. Within 1 h, the cells were analyzed via flow cytometry using a BD FACSCanto II instrument (BD Biosciences, San Jose, CA, USA), and FlowJo software version 9.5.3 (Tree Star, Inc., Ashland, OR, USA).
Western blot analysis
MGC803 cells were treated with 6 µM OZ for 48 h at 37°C. Then, washed twice with PBS and centrifuged at 12,000 × g for 15 min at 4°C. A total cellular protein extraction kit (Beyotime Institute of Biotechnology, Haimen, China) was used to extract the total protein, and the nucleoprotein extraction kit (Beyotime Institute of Biotechnology) was used to extract nucleoprotein, according to the manufacturer's protocol. Isolation of mitochondrial and cytosolic proteins was performed using the Mitochondria/Cytosol Fractionation kit (Bi Yuntian Biological Technology Institution). Protein concentrations were determined using a BCA protein assay (Beyotime Institute of Biotechnology). Cell lysate was boiled for 12 min, and samples (40 µg protein per lane) were separated on 5–20% gradient SDS-PAGE gels. Proteins were subsequently transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA), which were blocked overnight in 5% non-fat milk at 4°C. The membranes were incubated with the following primary antibodies at a dilution of 1:1,000: (p)-TAK1 (Thr187) (catalog no. 4536), pro-caspase 3 (catalog no. 9665), cyt c (catalog no. 11940), cyclin D1 (catalog no. 2978), Bcl-2 apoptosis regulator (Bcl-2; catalog no. 2827), voltage-dependent anion channel (catalog no. 4661), cleaved caspase 3 (catalog no. 9654), matrix metallopeptidase (MMP) 9 (catalog no. 13667), p65 (catalog no. 4764), histone 3 (catalog no. 4499) and β-actin (catalog no. 8457) at 4°C overnight. All primary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). The membranes were then washed with 1x TBS containing 0.1% Tween-20, incubated with anti-rabbit IgG conjugated to HRP (dilution, 1:1,000; Cell Signaling Technology, Inc. catalog no. 7074) for 1 h at room temperature, and washed with 1xTBS containing 0.1% Tween-20 three times for 10 min each. Proteins were visualized using an Enhanced Chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc., catalog no. 32106). The experiments were repeated at least 3 times. Densitometry analysis was performed using ImageJ software version 1.48 (National Institutes of Health).
Statistical analysis
Data are expressed as mean ± standard deviation. SPSS version 19.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Count data were analyzed with a χ2 test. Survival curves of the patients were compared using the Kaplan-Meier method and analyzed by the log-rank test. One-way analysis of variance followed by the Tukey post test was used to analyze differences between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
TAK1 protein expression in gastric cancer tissue and the association with clinical pathology
All gastric cancer tissue specimens and the adjacent normal tissue specimens used in the current study were verified using hematoxylin and eosin staining (Fig. 1). Immunohistochemistry revealed that the cytoplasm of gastric cancer cells appeared yellow or brown in a diffuse pattern, indicating high TAK1 expression; however, TAK1 expression was reduced in the adjacent normal tissues (Fig. 1).
Figure 1.
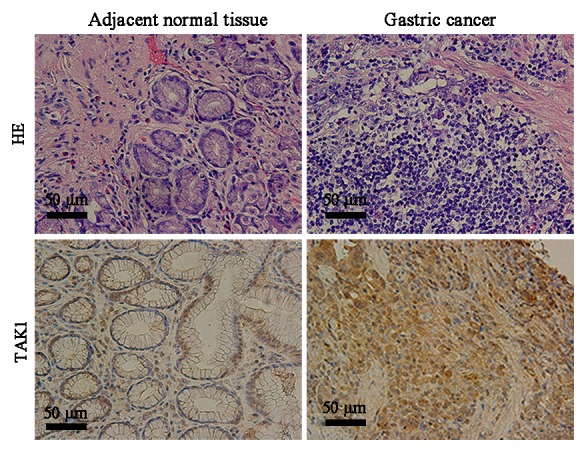
H&E and immunohistochemical staining of gastric cancer tissues and adjacent normal tissues. Upper images present H&E staining results and lower images present results for TAK1 immunohistochemical staining. Scale bar, 50 µM. H&E, hematoxylin and eosin; TAK1, transforming growth factor-β-activated kinase 1.
To investigate the biological significance of TAK1 expression in gastric cancer, the patients were divided into two groups according to TAK1 immunostaining: the TAK1 negative group and the TRAF6 positive group. TAK1 positive expression was quantified as 70.5% in gastric cancer tissue samples and 25.9% in the adjacent normal tissues (P<0.001; Table II). Furthermore, TAK1 expression was positively associated with advanced N stage and pathological stage, indicating that TAK1 protein expression level may be elevated during gastric cancer progression. No significant association was identified between TAK1 protein expression level and gender, age, tumor size, tumor grade, neural or vascular invasion, T stage or M stage (P>0.05; Table I). Kaplan-Meier survival analysis (Fig. 2) demonstrated that the median 5-year survival was 21 months in patients with positive TAK1 expression, which was significantly lower compared with patients with negative TAK1 expression (41 months; P=0.009).
Table II.
Analysis of TAK1 expression level in gastric cancer tissues and adjacent normal tissues.
| TAK1 expression | |||
|---|---|---|---|
| Tissue | Positive (%) | Negative (%) | P-value |
| Gastric cancer | 98 (70.5) | 41 (29.5) | <0.001 |
| Adjacent normal | 36 (25.9) | 103 (74.1) | |
TAK1, transforming growth factor-β-activated kinase 1.
Figure 2.

Overall survival of patients with gastric cancer and TAK1 expression level. Kaplan-Meier curves demonstrated that the 5-year survival rate was significantly higher in patients with negative TAK1 expression compared with patients with positive TAK1 expression. TAK1, transforming growth factor-β-activated kinase 1.
Effect of OZ, the TAK1 inhibitor, on apoptosis
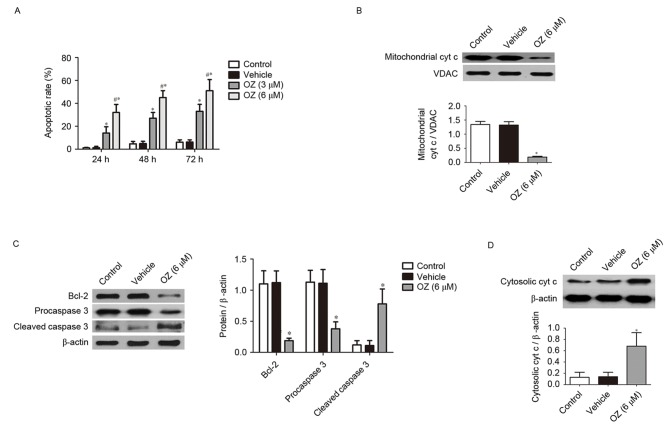
To evaluate the effects of OZ, the TAK1 inhibitor, on MGC803 cells, the present study examined the apoptotic properties of MGC803 cells incubated with OZ. The Annexin V and propidium iodide dual staining revealed that MGC803 cells from the TAK1 inhibitor treatment groups (3 and 6 µM) had a significantly greater percentage of apoptotic cells compared with the vehicle-treated group (P<0.05; Fig. 3A). To further investigate the cellular basis of the apoptotic response observed in the MGC803 cell line, the expression of apoptosis-associated proteins was investigated using western blotting. The aforementioned experiments confirmed that the high dose OZ (6 µM) effectively promoted apoptosis in MGC803 cells, 6 µM OZ was used for the subsequent experiments investigating the apoptotic mechanism. As demonstrated in Fig. 3B-D, OZ treatment significantly reduced the expression of mitochondrial cyt c (P<0.05; Fig. 3B), Bcl-2 (P<0.05; Fig. 3C) and procaspase 3 (P<0.05; Fig. 3C) compared with the vehicle treatment group. Conversely, cleaved caspase 3 (P<0.05; Fig. 3C) and cytosolic cyt c (P<0.05; Fig. 3D) expression levels were significantly greater in the OZ-treated group compared with the vehicle group.
Figure 3.
Effect of OZ on the apoptosis of gastric cancer cells. (A) OZ treatment (3 and 6 µM) significantly increased the apoptosis rate of MGC803 cells compared with the vehicle treatment group. The effect of OZ on the protein levels of (B) mitochondrial cyt c, (C) Bcl-2, procaspase 3 and cleaved caspase 3, and (D) cytosolic cyt c at 48 h after treatment were determined. *P<0.05 vs. vehicle and #P<0.05 vs. 3 µM OZ. OZ, 5Z-7-oxozeaenol; cyt c, cytochrome c; Bcl-2, Bcl-2 apoptosis regulator; VDAC, voltage-dependent anion channel.
Effect of OZ treatment on cell proliferation
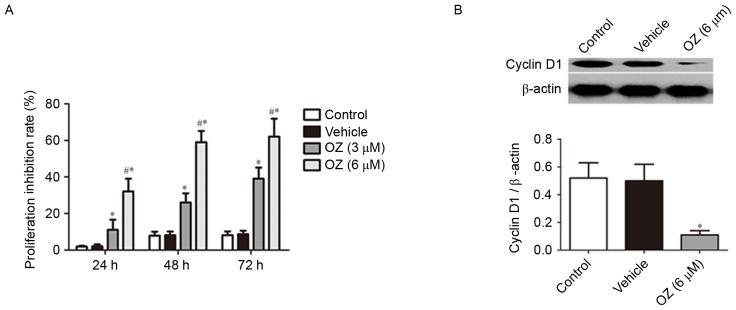
To verify the effect of OZ treatment on tumor growth in MGC803 cells, cell proliferation was examined using an MTT assay. It was revealed that OZ treatment significantly inhibited the growth of MGC803 cells in a time-dependent manner (P<0.05; Fig. 4A) compared with vehicle-treated cells. As cyclin D1 has previously been reported to have an important role in gastric cancer proliferation (15,16), the cyclin D1 protein expression level was examined by western blot analysis. The findings indicated that cyclin D1 expression was significantly downregulated in the OZ treatment group compared with the vehicle-treated group (P<0.05; Fig. 4B).
Figure 4.
Effect of OZ on the proliferation of MGC803 human gastric cancer cells. (A) OZ (3 and 6 µM) significantly inhibited the proliferation of gastric cancer cells compared with the vehicle treatment group. (B) Effect of OZ (6 µM) on the protein expression of cyclin D1 at 48 h post-treatment was determined by western blot analysis. OZ significantly inhibited the protein expression levels of cyclin D1 compared with the vehicle treatment group. *P<0.05 vs. vehicle and #P<0.05 vs. 3 µM OZ. OZ, 5Z-7-oxozeaenol.
Effect of OZ treatment on cell invasion
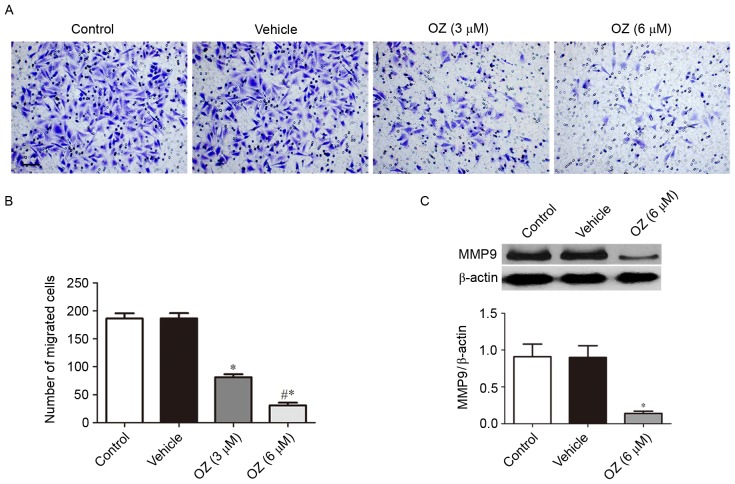
The present study further investigated the effect of OZ treatment on the invasive behavior of MGC803 cells. As presented in Fig. 5A and B, OZ treatment significantly reduced the invasive ability of MGC803 cells compared with the vehicle-treated group (P<0.05). As MMP9 has been previously reported to have an important role in gastric cancer invasion (17), the present study also investigated the expression level of MMP9 protein by western blot analysis and demonstrated that MMP9 expression was significantly downregulated in the OZ treatment group compared with the vehicle treatment group (P<0.05; Fig. 5C).
Figure 5.
Effect of OZ on cell invasion ability of the MGC803 cell line. (A) Representative images and (B) quantification of invasive cells, as determined by the invasion assay. OZ (3 and 6 µM) significantly inhibited gastric cancer cell invasion compared with the vehicle treatment group. (C) Effect of OZ (6 µM) on the protein expression of MMP9 at 48 h post-treatment was determined by western blot analysis. OZ treatment significantly inhibited the protein expression of MMP9 compared with the vehicle treatment group. *P<0.05 vs. vehicle and #P<0.05 vs. 3 µM OZ. OZ, 5Z-7-oxozeaenol; MMP9, matrix metallopeptidase-9.
Effect of OZ treatment on the TAK1/NF-κB signaling pathway
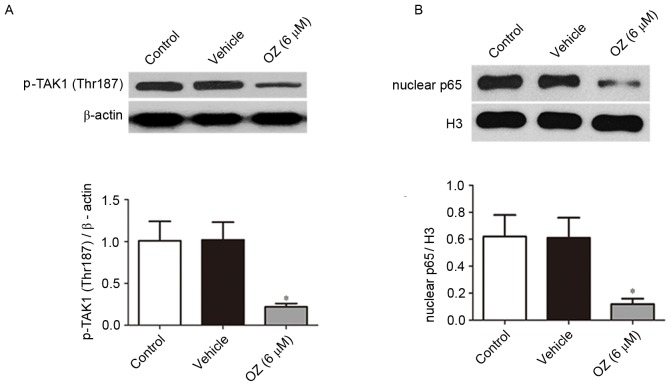
Following treatment with OZ for 48 h, the expression levels of p-TAK1 (Thr187) and nuclear p65 protein were detected by western blot analysis. As presented in Fig. 6, OZ treatment significantly reduced p-TAK1 (Thr187) and nuclear p65 expression levels compared with the vehicle treatment group (both P<0.05).
Figure 6.
Effect of OZ on the protein expression of p-TAK1 (Thr187) and nuclear p65. OZ (6 µM) treatment significantly inhibited the expression of (A) p-TAK1 (Thr187) and (B) nuclear p65 at 48 h post-treatment compared with the vehicle treatment group. *P<0.05 vs. vehicle. OZ, 5Z-7-oxozeaenol; TAK1, transforming growth factor-β-activated kinase 1; p-TAK1, phosphorylated TAK1; Thr, threonine; H3, histone H3.
Discussion
TAK1 is a serine/threonine protein kinase, which belongs to the family of MAPK kinases. TAK1 is a key kinase in the signal pathway of toll-like receptors and the interleukin-1 receptor. Previous reports have demonstrated that the TAK1-mediated signal transduction pathway is a key regulator in signal transduction and the chain reaction of stress responses, inflammation immunity and the occurrence and development of tumors (7,12). In addition, previous studies have demonstrated a high expression of TAK1 in a variety of tumor tissues such as thyroid cancer, non-small cell lung carcinoma and breast cancer, and an association with tumor occurrence, development and invasion (8,9,18). TAK1 regulates the activation of the MAPK and NF-κB signaling pathways (7). Furthermore, abnormal activation of the MAPK signaling pathway is a hallmark of gastric cancer tissues and inhibition of MAPK activity may significantly inhibit the proliferation and invasion of gastric cancer cells, thus promoting apoptosis (19). In addition, the high expression of NF-κB in gastric cancer tissues was significantly associated with poor prognosis of patients with gastric cancer (20,21). Inhibition of the NF-κB signaling pathway may also inhibit the proliferation and invasion of gastric cancer cells, subsequently promoting apoptosis (22). Comprehensive analysis of previous research indicated that TAK1 may also have an important role in the occurrence and development of gastric cancer. The findings of the current study demonstrated that 25.9% of normal (non-neoplastic) gastric mucosae tissue samples exhibited positive TAK1 expression; therefore, it is possible that this regulation occurs at a transcriptional level as TAK1 has a key role in signal transduction in normal tissues (12). TAK1 protein expression was significantly increased in gastric cancer tissues compared with the normal tissues, which was consistent with the findings of a previous study (23). Furthermore, the findings of the present study also demonstrated that TAK1 expression was associated with the advanced N stage and the pathological stage of gastric carcinoma. However, no significant association was identified in terms of gender, age, tumor size, tumor grade, neural or vascular invasion, T and M stage. In addition, the 5-year survival rate of patients with positive TAK1 expression was significantly lower compared with patients with negative TAK1 expression. Therefore, postoperative detection of TAK1 in gastric cancer tumor specimens may be used for a prognosis of the patient.
OZ is a selective inhibitor of TAK1 (13). Several recent studies demonstrated that OZ inhibited the proliferation and invasion of a variety of tumor cells, and promoted apoptosis (8,24–26). Therefore, after confirming high expression of TAK1 in gastric cancer tissues, the present study further investigated the effects of TAK1 on the invasion and apoptosis of gastric cancer cells, and the potential underlying mechanisms using an in vitro culture of MGC803 human gastric carcinoma cells. The present study used a previously reported dose of OZ (8,24,26,27), and the findings indicated that OZ treatment significantly inhibited the invasion and proliferation of gastric cancer cells, whilst promoting apoptosis. A previous report demonstrated that the phosphorylation of threonine 187 at the loci of TAK1 protein kinase was important for the activation of this kinase (28). Previous studies have demonstrated that OZ inhibited the expression of p-TAK1 (Thr187) (29) and significantly reduced the expression of p65 in the nucleus (30,31). Previous studies have demonstrated that cleaved caspase 3 has an important role in the apoptosis of gastric cancer cells. High protein expression level of cleaved caspase 3 significantly promoted the apoptosis of gastric cancer cells, whereas downregulation of Bcl-2 promoted cyt c release and induced the apoptosis of gastric cancer cells (32–35). Importantly, cleaved caspase 3 and Bcl-2 were both regulated by NF-κB signaling pathways (36–38). A previous study also demonstrated that inhibition of TAK1 was associated with the release of cyt c from the mitochondria, which served as an important initial step for apoptosis (39). The findings of the present study indicated that OZ treatment significantly increased cleaved caspase-3 and cytosolic cyt c expression and inhibited the expression of Bcl-2 in gastric cancer cells. These findings may elucidated the underlying mechanism whereby OZ functions as a tumor suppressor by inducing apoptosis. In addition, previous reports indicated that MMP9, which is regulated by NF-κB (40), had an important role in the invasion of gastric cancer cells (17,41). The current study demonstrated that OZ significantly inhibited the expression of MMP9, which may be one of the molecular mechanisms by which OZ inhibited the invasion of gastric cancer cells. NF-κB has also been reported to stimulate the transcription of cyclin D1 (42), which is a key regulator of gastric cancer proliferation (15,16). In the current study, OZ treatment significantly reduced the cyclin D1 expression level. This may be a potential method by which OZ inhibited gastric cancer cell proliferation.
As only one gastric cancer cell line was utilized for mechanistic studies in the current study, the results may be limited and a variety of gastric cancer cell lines are required for future investigation of the relevant mechanisms. Previous studies have revealed that TAK1 may have a biphasic role in tumorigenesis and promote tumor growth during the early development of a tumor and delay metastasis in advanced tumor stages (43,44). Lam et al (43) hypothesized that this discrepancy may be due the influence of other surrounding cell types, such as cancer-associated fibroblasts. Based on these observations, the role of TAK1 in gastric cancer development may require further investigation using in vivo experiments.
In conclusion, the present study demonstrated that TAK1 expression was elevated in gastric carcinoma tissues and was associated with the poor prognosis of patients with gastric cancer. OZ, the specific inhibitor of TAK1, significantly inhibited the proliferation and invasion of gastric cancer cells and promoted cell apoptosis, indicating that TAK1 may be a novel target for the treatment of gastric cancer and that OZ may have the potential to be developed as a novel drug for the treatment of gastric cancer.
Acknowledgements
The present study was supported by the National Natural Science Foundation (grant no. 81470866).
References
- 1.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403–2414. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lordick F, Janjigian YY. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat Rev Clin Oncol. 2016;13:348–360. doi: 10.1038/nrclinonc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomasello G, Ghidini M, Liguigli W, Ratti M, Toppo L, Passalacqua R. Targeted therapies in gastric cancer treatment: Where we are and where we are going. Invest New Drugs. 2016;34:378–393. doi: 10.1007/s10637-016-0330-2. [DOI] [PubMed] [Google Scholar]
- 4.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Meltzer SJ. Gastric cancer in the era of precision medicine. Cell Mol Gastroenterol Hepatol. 2017;3:348–358. doi: 10.1016/j.jcmgh.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SY, Oh SC. Changing strategies for target therapy in gastric cancer. World J Gastroenterol. 2016;22:1179–1189. doi: 10.3748/wjg.v22.i3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Huang HL, Chiang CH, Hung WC, Hou MF. Targeting of TGF-β-activated protein kinase 1 inhibits chemokine (C-C motif) receptor 7 expression, tumor growth and metastasis in breast cancer. Oncotarget. 2015;6:995–1007. doi: 10.18632/oncotarget.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin P, Niu W, Peng C, Zhang Z, Niu J. The role of TAK1 expression in thyroid cancer. Int J Clin Exp Pathol. 2015;8:14449–14456. [PMC free article] [PubMed] [Google Scholar]
- 10.Wu M, Shi L, Cimic A, Romero L, Sui G, Lees CJ, Cline JM, Seals DF, Sirintrapun JS, McCoy TP, et al. Suppression of Tak1 promotes prostate tumorigenesis. Cancer Res. 2012;72:2833–2843. doi: 10.1158/0008-5472.CAN-11-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosman MC, Schepers H, Jaques J, Brouwers-Vos AZ, Quax WJ, Schuringa JJ, Vellenga E. The TAK1-NF-κB axis as therapeutic target for AML. Blood. 2014;124:3130–3140. doi: 10.1182/blood-2014-04-569780. [DOI] [PubMed] [Google Scholar]
- 12.Kilty I, Jones LH. TAK1 selective inhibition: State of the art and future opportunities. Future Med Chem. 2015;7:23–33. doi: 10.4155/fmc.14.138. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Powell F, Larsen NA, Lai Z, Byth KF, Read J, Gu RF, Roth M, Toader D, Saeh JC, Chen H. Mechanism and in vitro pharmacology of TAK1 inhibition by (5Z)-7-Oxozeaenol. ACS Chem Biol. 2013;8:643–650. doi: 10.1021/cb3005897. [DOI] [PubMed] [Google Scholar]
- 14.Fakhouri L, El-Elimat T, Hurst DP, Reggio PH, Pearce CJ, Oberlies NH, Croatt MP. Isolation, semisynthesis, covalent docking and transforming growth factor beta-activated kinase 1 (TAK1)-inhibitory activities of (5Z)-7-oxozeaenol analogues. Bioorg Med Chem. 2015;23:6993–6999. doi: 10.1016/j.bmc.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arici DS, Tuncer E, Ozer H, Simek G, Koyuncu A. Expression of retinoblastoma and cyclin D1 in gastric carcinoma. Neoplasma. 2009;56:63–67. doi: 10.4149/neo_2009_01_63. [DOI] [PubMed] [Google Scholar]
- 16.Seo JH, Jeong ES, Choi YK. Therapeutic effects of lentivirus-mediated shRNA targeting of cyclin D1 in human gastric cancer. BMC Cancer. 2014;14:175. doi: 10.1186/1471-2407-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akter H, Park M, Kwon OS, Song EJ, Park WS, Kang MJ. Activation of matrix metalloproteinase-9 (MMP-9) by neurotensin promotes cell invasion and migration through ERK pathway in gastric cancer. Tumour Biol. 2015;36:6053–6062. doi: 10.1007/s13277-015-3282-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Li Q, He JT, Liu GY. Expression of TAK1/TAB1 expression in non-small cell lung carcinoma and adjacent normal tissues and their clinical significance. Int J Clin Exp Pathol. 2015;8:15801–15807. [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21:11673–11679. doi: 10.3748/wjg.v21.i41.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Tu J, Zhang D, Xu Z, Yang G, Gong L, Yu M. The clinical significance of HER-2 and NF-KB expression in gastric cancer. Hepatogastroenterology. 2013;60:1519–1523. doi: 10.5754/hge13242. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Yu YY, Zhu ZG, Ji YB, Zhang Y, Liu BY, Chen XH, Lin YZ. Effect of NF-kappaB constitutive activation on proliferation and apoptosis of gastric cancer cell lines. Eur Surg Res. 2005;37:105–110. doi: 10.1159/000084541. [DOI] [PubMed] [Google Scholar]
- 22.Uetsuka H, Haisa M, Kimura M, Gunduz M, Kaneda Y, Ohkawa T, Takaoka M, Murata T, Nobuhisa T, Yamatsuji T, et al. Inhibition of inducible NF-kappaB activity reduces chemoresistance to 5-fluorouracil in human stomach cancer cell line. Exp Cell Res. 2003;289:27–35. doi: 10.1016/S0014-4827(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 23.Pak KH, Kim DH, Kim H, Lee DH, Cheong JH. Differences in TGF-b1 signaling and clinicopathologic characteristics of histologic subtypes of gastric cancer. BMC Cancer. 2015;16:60. doi: 10.1186/s12885-016-2091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Li B, Wu H, Ou J, Wei R, Liu J, Cai W, Liu X, Zhao S, Yang J, et al. Synergistic action of 5Z-7-oxozeaenol and bortezomib in inducing apoptosis of Burkitt lymphoma cell line Daudi. Tumour Biol. 2016;37:531–539. doi: 10.1007/s13277-015-3832-1. [DOI] [PubMed] [Google Scholar]
- 25.Hrabe JE, O'Leary BR, Fath MA, Rodman SN, Button AM, Domann FE, Spitz DR, Mezhir JJ. Disruption of thioredoxin metabolism enhances the toxicity of transforming growth factor β-activated kinase 1 (TAK1) inhibition in KRAS-mutated colon cancer cells. Redox Biol. 2015;5:319–327. doi: 10.1016/j.redox.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai PC, Shi L, Liu VW, Tang HW, Liu IJ, Leung TH, Chan KK, Yam JW, Yao KM, Ngan HY, Chan DW. Elevated TAK1 augments tumor growth and metastatic capacities of ovarian cancer cells through activation of NF-κB signaling. Oncotarget. 2014;5:7549–7562. doi: 10.18632/oncotarget.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Cheng J, Vasudevan SA, Patel RH, Liang L, Xu X, Zhao Y, Jia W, Lu F, Zhang H, et al. TAK1 inhibitor 5Z-7-oxozeaenol sensitizes neuroblastoma to chemotherapy. Apoptosis. 2013;18:1224–1234. doi: 10.1007/s10495-013-0864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem. 2005;280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- 29.Choo MK, Kawasaki N, Singhirunnusorn P, Koizumi K, Sato S, Akira S, Saiki I, Sakurai H. Blockade of transforming growth factor-beta-activated kinase 1 activity enhances TRAIL-induced apoptosis through activation of a caspase cascade. Mol Cancer Ther. 2006;5:2970–2976. doi: 10.1158/1535-7163.MCT-06-0379. [DOI] [PubMed] [Google Scholar]
- 30.Cao H, Lu J, Du J, Xia F, Wei S, Liu X, Liu T, Liu Y, Xiang M. TAK1 inhibition prevents the development of autoimmune diabetes in NOD mice. Sci Rep. 2015;5:14593. doi: 10.1038/srep14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Z, Zhu X, Jin R, Wang C, Yan J, Zheng Q, Nanda A, Granger DN, Li G. Roles of the kinase TAK1 in CD40-mediated effects on vascular oxidative stress and neointima formation after vascular injury. PloS One. 2014;9:e101671. doi: 10.1371/journal.pone.0101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo JQ, Li SJ, Guo GX. Long noncoding RNA AFAP1-AS1 promotes cell proliferation and apoptosis of gastric cancer cells via PTEN/p-AKT pathway. Dig Dis Sci. 2017 Apr 27; doi: 10.1007/s10620-017-4584-0. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Tong K, Xin C, Chen W. Isoimperatorin induces apoptosis of the SGC-7901 human gastric cancer cell line via the mitochondria-mediated pathway. Oncol Lett. 2017;13:518–524. doi: 10.3892/ol.2016.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Li Y, Cui P, Zhao Q, Tan BB, Zhang ZD, Liu Y, Jia N. Zerumbone induces gastric cancer cells apoptosis: Involving cyclophilin A. Biomed Pharmacother. 2016;83:740–745. doi: 10.1016/j.biopha.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Shen X, Si Y, Wang Z, Wang J, Guo Y, Zhang X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int J Mol Med. 2016;38:619–626. doi: 10.3892/ijmm.2016.2625. [DOI] [PubMed] [Google Scholar]
- 36.Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol. 2004;10:22–25. doi: 10.3748/wjg.v10.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang MS, Lee HS, Jung EJ, Kim CW, Lee BL, Kim WH. Cell-cycle regulators, bcl-2 and NF-kappaB in Epstein-Barr virus-positive gastric carcinomas. Int J Oncol. 2005;27:1265–1272. [PubMed] [Google Scholar]
- 38.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 39.Buglio D, Palakurthi S, Byth K, Vega F, Toader D, Saeh J, Neelapu SS, Younes A. Essential role of TAK1 in regulating mantle cell lymphoma survival. Blood. 2012;120:347–355. doi: 10.1182/blood-2011-07-369397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park BB, Yoon Js, Kim Es, Choi J, Won Yw, Choi Jh, Lee YY. Inhibitory effects of eupatilin on tumor invasion of human gastric cancer MKN-1 cells. Tumour Biol. 2013;34:875–885. doi: 10.1007/s13277-012-0621-y. [DOI] [PubMed] [Google Scholar]
- 41.Zhang QW, Liu L, Chen R, Wei YQ, Li P, Shi HS, Zhao YW. Matrix metalloproteinase-9 as a prognostic factor in gastric cancer: A meta-analysis. Asian Pac J Cancer Prev. 2012;13:2903–2908. doi: 10.7314/APJCP.2012.13.6.2903. [DOI] [PubMed] [Google Scholar]
- 42.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: Regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/MCB.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam CR, Tan C, Teo Z, Tay CY, Phua T, Wu YL, Cai PQ, Tan LP, Chen X, Zhu P, Tan NS. Loss of TAK1 increases cell traction force in a ROS-dependent manner to drive epithelial-mesenchymal transition of cancer cells. Cell Death Dis. 2013;4:e848. doi: 10.1038/cddis.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omori E, Matsumoto K, Zhu S, Smart RC, Ninomiya-Tsuji J. Ablation of TAK1 upregulates reactive oxygen species and selectively kills tumor cells. Cancer Res. 2010;70:8417–8425. doi: 10.1158/0008-5472.CAN-10-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]