Abstract
Lung cancer is a leading cause of cancer-associated mortality worldwide, including in developing countries such as China. Traditional Chinese medicine may provide a novel insight for the treatment of patients with lung cancer. The present study aimed to uncover the mechanism by which the Chinese herbal medicine, Fuzheng Kang-Ai (FZKA), functions on lung cancer cell metastasis. The results demonstrated that treatment with FZKA markedly inhibited cell viability, migration and invasion of lung cancer cells, as determined by cell viability and Transwell assays. Notably, the activity and expression of matrix metalloproteinase 9 (MMP9) was significantly inhibited by FZKA treatment on lung cancer cells, as determined by an MMP9 activity assay and western blot analysis. Furthermore, FZKA markedly inhibited epithelial-mesenchymal transition (EMT) of lung cancer cells by inhibiting the expression of the mesenchymal markers N-cadherin and vimentin. In addition, activation of the oncoprotein signal transducer and activator of transcription 3 (STAT3) was suppressed following treatment with FZKA. Conversely, overexpression of STAT3 was able to rescue MMP9 activity following FZKA treatment. The present study indicated that FZKA may inhibit lung cancer metastasis via the STAT3/MMP9 pathway and EMT, suggesting that FZKA may serve as a novel promising therapeutic strategy for the treatment of patients with late stage lung cancer.
Keywords: Fuzheng Kang-Ai decoction, lung cancer, STAT3/MMP9 pathway, epithelial-mesenchymal transition
Introduction
Lung cancer is a leading cause of cancer-associated mortality worldwide (1). It is the most common fatal cancer in males and females, and accounts for 29 and 26% of all male and female cancer-associated mortalities worldwide, respectively (2). In China, lung cancer is the most frequently diagnosed cancer in males (22.14%), and is the leading cause of cancer-associated mortality in males (27.21%) and females (21.91%) (3). Despite advances in combination chemotherapy and surgical techniques, the prognosis of non-small cell lung cancer (NSCLC) remains poor; the 5-year survival rate for all stages and subtypes combined remains as low as 11% (4). Metastasis is the predominant cause of mortality in patients with lung cancer; ~90% of patients succumb to metastatic cancer (5). The metastatic process is initiated by dissemination of clonal cells from the primary tumor site, which invade the extracellular matrix (ECM) and surrounding stroma (6).
The matrix metalloproteinase (MMP) family members are involved in degradation of the ECM during normal physiological processes, including embryonic development and tissue remodeling, as well as in disease processes, including tumor metastasis (7). MMP9 is a member of the ECM (8–10). Overexpression of MMP9 has been reported to facilitate metastatic spread of various cancer cells, including lung cancer cells. A recent study demonstrated that MMP9 expression is positively correlated with lung cancer malignancy (11), and suggested that MMP9 is an important factor in the process of lung cancer metastasis. Furthermore, the process of epithelial-mesenchymal transition (EMT) is known to serve an important role in metastasis formation (12). The series of EMT events is predominantly activated in cancer cells acquiring invasive and metastatic properties (13); MMP9 and EMT are critical in the processes associated with cancer metastasis. Signal transducer and activator of transcription 3 (STAT3) is an oncogenic transcription factor known to be involved in cancer cell proliferation and metastasis (14). In numerous types of cancer, STAT3 is constitutively active, leading to continued expression of target genes that promote cell proliferation, survival and invasion. The role of STAT3 in tumorigenesis and cancer cell invasion has been well established in a wide range of human cancers, including lung cancer (15). When activated by upstream signaling pathways, including epidermal growth factor and the interleukin-6/Janus kinase pathway, STAT3 is phosphorylated and then forms homodimers or heterodimers with other members of the STAT family. Subsequently, the activated STAT3 complex translocates into the nucleus to initiate transcription of STAT3 target genes, including MMP9 (16).
Fuzheng Kang-Ai (FZKA) decoction, initially prescribed by Dr. Wanyin Wu (17), has been used to treat patients with NSCLC at the Guangdong Provincial Hospital of Traditional Chinese Medicine (Guangzhou, China) for a decade, and has been shown to exert a positive impact on patient health. Our previous study demonstrated that the efficacy of a combination of gefitinib plus FZKA exhibited better outcome compared with gefitinib alone (17). In addition, FZKA could enhance the disease control rate, and prolong the progression-free survival (PFS) and median survival time (MST) in patients with NSCLC (18,19). Furthermore, our recent study reported that FZKA inhibited lung cancer cell growth through AMP-activated protein kinase α-mediated induction, and an interplay between insulin-like growth factor-binding protein 1 and forkhead box O3a, indicating its therapeutic effect on lung cancer (20). Metastasis is the predominant cause of mortality in patients with lung cancer; however, the mechanism by which FZKA affects lung cancer metastasis remains to be elucidated. The present study identified the inhibitory effects of FZKA on lung cancer cell invasion and migration. In addition, the probable mechanisms by which FZKA inhibited lung cancer cell metastasis were examined, which may provide evidence to support the clinical usage of FZKA decoction to treat patients with NSCLC.
Materials and methods
Cells
Human A549 NSCLC cells were obtained from the Cell Line Bank at the Laboratory Animal Center of Sun Yat-sen University (Guangzhou, China). PC9 and H1650 cells were obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (Shanghai, China). All cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 0.5% penicillin-streptomycin sulfate, and incubated at 37°C with 5% CO2. Cells were counted using a Countstar automated cell counter (Inno-Alliance Biotech, Inc., Wilmington, DE, USA).
Chemicals
Monoclonal antibodies against total STAT3 (cat no. 8232), phosphorylated (p)-STAT3 (cat. no. 4093), vimentin (cat. no. 12826), N-cadherin (cat. no. 13116) and MMP9 (cat. no. 13667) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Lipofectamine 3000 reagent was purchased from Invitrogen (Thermo Fisher Scientific, Inc.). The control (pCMV6-AC) and STAT3 overexpression (pCMV6-AC-STAT3) vectors were obtained from OriGene Technologies, Inc. (Rockville, MD, USA).
FZKA decoction
FZKA decoction is a Chinese herbal medicine that has been used to treat NSCLC at the Guangdong Provincial Hospital of Traditional Chinese Medicine for >10 years. It consists of Taizishen, 30 g; Atractylodes macrocephala Koidz. (Baizhu), 15 g; Astragalus membranaceus (Fisch.) Bge. (Huangqi), 30 g; Oldenlandia diffusa (Willd.) Roxb. (Baihuasheshecao), 30 g; Solanum nigrum L. (Longkui), 30 g; Salvia chinensis Benth (Shi-jianchuan), 30 g; Cremastra appendiculata (D. Don) Makino (Shancigu), 30 g; Coix lachrymal-jobi L. (Yiyiren), 30 g; Akebia quinata (Thunb.) Decne (Bayuezha), 30 g; Rubus parviflolius L. (Shepaole), 30 g; Curcuma kwangsiensis S.G. Lee et C.F. Liang (Ezhu), 15 g; and Glycyrrhiza uralensis Fisch. (Gancao), 10 g (19). All of the components were soaked together for 30 min prior to decoction. The concentrated liquid was finally spray dried into particles by Guangdong One Pharmaceutical Co., Ltd (Guangzhou, Guangdong, China). The FZKA particles were dissolved in RPMI-1640 and filtered using 0.22 µm filters prior to use. The pH value of the cultured cells in media was adjusted to 7.2–7.4 following FZKA addition.
Cell viability assay
Cells were seeded in 6-well plates at a density of 3×105 cells/well. After 24 h of culture, cells were treated with FZKA (0, 1, 2 and 3 mg/ml) and were incubated at 37°C for 24 h; 0 mg/ml FZKA cultured cells were used as the untreated control cells. Subsequently, cells were collected by trypsinization and stained with trypan blue at a concentration of 1:1. The cells were resuspended and were then counted using a Countstar automated cell counter. Cell viability was expressed as a percentage of untreated cells. Data were taken from an average of three independent experiments.
Wound-healing assay
Wound-healing assay was performed to determine the migratory ability of cells. The cells were cultured (4×105) in 6-well plates, and incubated until the cell density reached 90%. Cell monolayers were wounded by scratching with a 200-µl pipette tip, after which the plates were washed twice with PBS to remove detached cells, and were incubated in RPMI-1640 supplemented with 2% FBS containing FZKA (0, 1 and 2 mg/ml). After 12 or 24 h at 37°C, the medium was replaced with PBS and washed twice. The wound gap was observed and images were captured using a fluorescence microscope (Olympus IX71; Olympus Corporation, Tokyo, Japan; magnification, ×40). The distance of the scratch was measured using ImageJ software (version 1.48; National Institutes of Health, Bethesda, MD, USA). The results were obtained from three independent experiments.
Transwell assay
A Transwell plate (Corning Incorporation, Corning, NY, USA; diameter, 10 mm; 8 µm pore polycarbonate membrane) was used to detect the migratory and invasive potential of the cells. In the invasion assay, prior to experimentation, Matrigel (BD Bioscience, San Jose, CA, USA) was diluted 8-fold using PBS and was injected into the upper chamber. In the migration assay, this step was omitted. To the lower chamber, 500 µl cell culture medium supplemented with 30% FBS was added. Subsequently, cells were diluted to 0.5×106/ml, pretreated with FZKA (0, 1 and 2 mg/ml) for 24 h at 37°C, and a 200 µl cell suspension was added into the upper chamber. The Transwell plate was then incubated at 37°C in a 5% CO2 atmosphere for 16 h. Non-migrated cells were removed with a cotton swab, and invaded cells were fixed in 4% paraformaldehyde for 15 min at room temperature prior to staining with crystal violet. Images were captured under ×100 magnification with a fluorescence microscope (Olympus DP72; Olympus Corporation). Subsequently, 200 µl 33% acetic acid was added to the chamber and the eluent was removed into 96-well plates. Absorbance at 570 nm was determined using an ELISA reader (Victor X5; Perkin Elmer, Inc., Waltham, MA, USA). The experiment was repeated at least three times.
MMP9 activity assay
The activity of MMP9 was measured using the SensoLyte® 520 MMP9 assay kit (AnaSpec, Fremont, CA, USA) according to the manufacturer's protocol. The cells were seeded in 6-well plates at a density of 3×105 cells/well and treated with FZKA (0, 1, 2 and 3 mg/ml) for 24 h. Subsequently, the cell culture media supernatant was collected and centrifuged at 1,000 × g for 15 min at 4°C. The MMP containing samples were incubated with APMA (component C) at a final concentration of 1 mM in the assay buffer (Component D) and were incubated for 2 h at 37°C in order to activate pro-MMPs. The working solutions were then prepared by diluting the MMP9 substrate 1:100 in assay buffer. The reagents: 50 µl MMP9 containing sample and 50 µl MMP9 substrate solution, were mixed in a 96-well plate by gentle agitation for 30 sec. The reactions were incubated at 37°C for 1 h and fluorescence intensity was measured at excitation/emission=490/520 nm. The experiment was repeated three times.
Western blot analysis
Cells were seeded in 6-well plates at a density of 3×105 cells/well. Following 24 h of culture, cells were treated with FZKA (0, 1, 1.5 and 2 mg/ml) and were incubated at 37°C for 24 h. Then, the cells were harvested, washed and lysed with 1X radioimmunoprecipitation assay buffer (cat. no. 9806; CST Biological Reagents Company Limited, Shanghai, China). Protein concentration was determined using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Inc.). Equal amounts of protein (40 µg) from cell lysates were solubilized in 5X SDS sample buffer and were separated by 10% SDS-PAGE, prior to being transferred onto polyvinylidene fluoride membranes. Membranes were blocked with 5% non-fat milk in TBS containing 1% Tween-20 and were then incubated with primary antibodies against STAT3 (cat. no. 8232; CST Biological Reagents Company Limited; dilution, 1:1,000), p-STAT3 (cat. no. 4093; CST Biological Reagents Company Limited; dilution, 1:1,000), vimentin (cat. no. 12826; CST Biological Reagents Company Limited; dilution, 1:1,000), N-cadherin (cat. no. 13116; CST Biological Reagents Company Limited; dilution, 1:1,000), MMP9 (cat. no. 13667; CST Biological Reagents Company Limited; dilution, 1:1,000) and GAPDH (cat. no. 5174; CST Biological Reagents Company Limited; dilution, 1:3,000) at 4°C overnight. Subsequently, the membranes were washed and incubated with a secondary antibody against rabbit immunoglobulin G (cat. no. 1706515; Bio-Rad Laboratories, Inc., Hercules, CA, USA; dilution, 1:10,000) for 1 h at room temperature. The membranes were then washed and visualized using enhanced chemiluminescence solution (Merck KGaA, Darmstadt, Germany); the blots were exposed and scanned under the Bio-Rad ChemiDoc XRS+ Chemiluminescence imaging system (Bio-Rad Laboratories, Inc.). The results were analyzed using ImageJ software (version 1.48; National Institutes of Health).
Transient transfection assay
The cells were seeded in 6-well plates (3×105 cells/well) and were allowed to reach 50–60% confluence. The pCMV6-AC and pCMV6-AC-STAT3 vectors were obtained from OriGene Technologies, Inc. In each well, 2 µg pCMV6-AC control or pCMV6-AC-STAT3 constructs were transfected into the cells using Lipofectamine 3000 reagent for 30 h at 37°C, according to the manufacturer's protocol. Subsequently, the cells were treated with 2 mg/ml FZKA for an additional 24 h prior to experimentation.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 statistical software (SPSS, Inc., Chicago, IL, USA). All data are presented as the mean ± standard deviation. Differences between groups were assessed by one-way analysis of variance and a Tukey's post hoc test was used for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Lung cancer cell growth is suppressed by FZKA
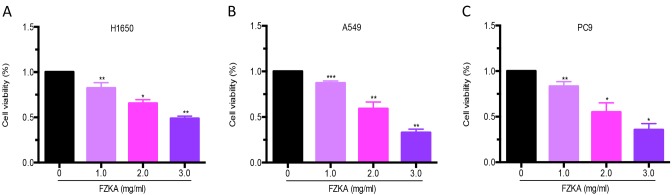
The present study detected the effects of FZKA on lung cancer cell growth. A cell viability assay was performed using trypan blue staining following treatment of lung cancer cells (H1650, A549 and PC9) with various doses of FZKA for 24 h. The results demonstrated that FZKA significantly suppressed the growth of lung cancer cells (>50% following 3 mg/ml FZKA treatment) in a dose-dependent manner (Fig. 1).
Figure 1.
FZKA inhibits lung cancer cell growth. (A) H1650, (B) A549 and (C) PC9 cells were seeded into 6-well plates and treated with FZKA (0, 1, 2 and 3 mg/ml). Cells were stained with trypan blue after 24 h and were counted using an automated cell counter. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control (0 FZKA mg/ml). FZKA, Fuzheng Kang-Ai.
FZKA inhibits migration of lung cancer cells in vitro
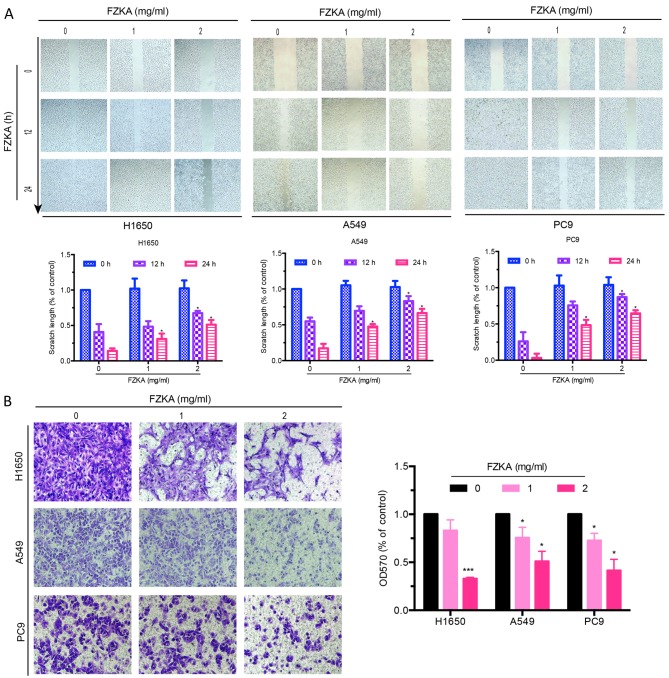
A characteristic of tumor metastasis is the increased migratory ability of tumor cells. The present study conducted a wound-healing assay to determine the effects of FZKA on lung cancer cell migration. As presented in Fig. 2A, the scratch length of all three lung cancer cells was markedly extended by FZKA treatment in a dose-dependent manner, indicating the inhibitory effects of FZKA on lung cancer cell migration. To further verify the inhibitory effects of FZKA on lung cancer cell migration, a Transwell migration assay was used. The results confirmed that FZKA inhibited the migration of lung cancer cells in a dose-dependent manner (Fig. 2B; ~50% decrease in migration following treatment with 2 mg/ml FZKA). These results suggested that FZKA decoction inhibited lung cancer cell migration.
Figure 2.
FZKA inhibits lung cancer cell migration. (A) H1650, A549 and PC9 cells were cultured (4×105) in 6-well plates, and were incubated until cell density reached 90%. Cell monolayers were wounded by scratching with a 200-µl pipette tip and were treated with FZKA (0, 1 and 2 mg/ml). Results are representative of three independent experiments. (B) H1650, A549 and PC9 cells were plated in a Transwell plate. The lower chamber was filled with 500 µl cell culture medium containing 30% fetal bovine serum. Cells were diluted to 0.5×106/ml and were pretreated with FZKA (0, 1 and 2 mg/ml), after which a 200 µl cell suspension was added to the upper chamber and was incubated for 16 h. Magnification, ×40. *P<0.05 and ***P<0.001 vs. control (0 FZKA mg/ml). FZKA, Fuzheng Kang-Ai; OD, optical density.
FZKA inhibits lung cancer cell invasion in vitro
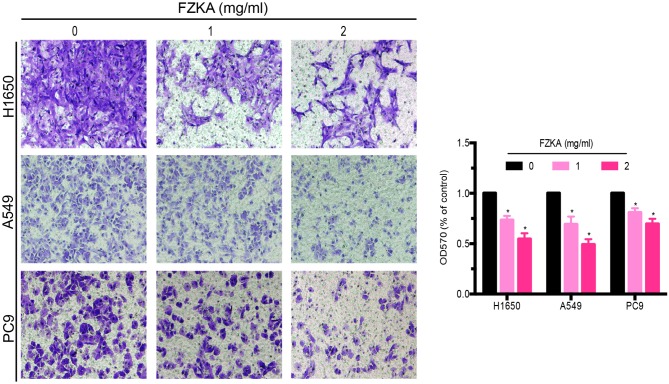
Since treatment with FZKA was able to reduce the migratory capabilities of lung cancer cells, the present study aimed to determine the effects of FZKA on lung cancer cell invasion using a Transwell invasion assay. The results demonstrated that FZKA was also able to inhibit the invasion of the three lung cancer cell lines in a dose-dependent manner (Fig. 3; ~60% decrease in invasion following treatment with 2 mg/ml FZKA). These findings indicated that FZKA may act as a suppressor of lung cancer cell invasion.
Figure 3.
FZKA inhibits lung cancer cell invasion. H1650, A549 and PC9 cells were plated in a Transwell plate. Matrigel was injected into the upper chamber and 500 µl cell culture medium with 30% fetal bovine serum was added to the lower chamber. Cells were diluted to 0.5×106/ml and were pretreated with FZKA (0, 1 and 2 mg/ml), after which a 200 µl cell suspension was added to the upper chamber and was incubated for 16 h. Absorbance was measured at 570 nm using a microplate reader. The experiment was repeated three times. Magnification, ×40. *P<0.05 vs. control (0 FZKA mg/ml). FZKA, Fuzheng Kang-Ai; OD, optical density.
MMP9 activity and expression is downregulated by FZKA
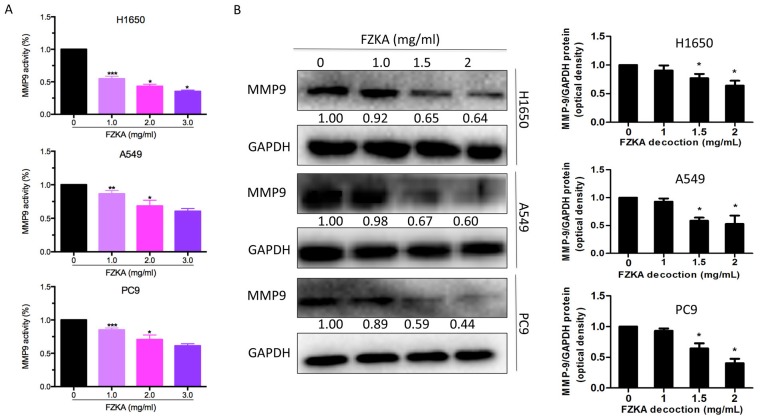
MMP9 is a well-known factor that facilitates cell invasion; therefore, the present study detected MMP9 activity following FZKA treatment using an MMP9 activity assay. The results indicated that MMP9 activity was markedly downregulated by FZKA in a dose-dependent manner in all three lung cancer cell lines (Fig. 4A). Furthermore, the protein expression levels of MMP9 were decreased in the lung cancer cells following FZKA treatment in a dose-dependent manner (Fig. 4B). These data suggested that MMP9 served an important role in FZKA-mediated inhibition of lung cancer cell invasion.
Figure 4.
MMP9 is downregulated by FZKA treatment. (A) H1650, A549 and PC9 cells were seeded into 6-well plates and treated with FZKA (0, 1, 2 and 3 mg/ml) for 24 h. The reactions were measured by fluorescence intensity at excitation/emission=490/520 nm. Data are presented as the mean ± standard deviation of three independent experiments. (B) Protein expression levels of MMP9 were decreased following FZKA treatment (0, 1, 1.5 and 2 mg/ml) for 24 h. Data were measured by ImageJ software. The experiment was repeated three times. *P<0.05, **P<0.01 and ***P<0.001 vs. control (0 FZKA mg/ml). FZKA, Fuzheng Kang-Ai; MMP9, matrix metalloproteinase 9.
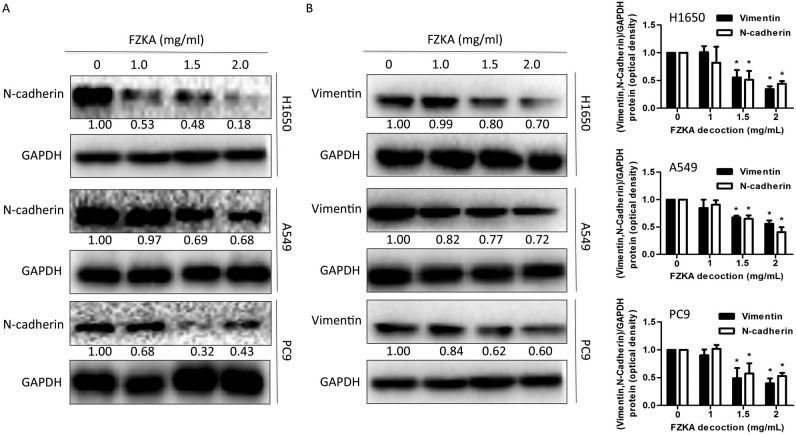
EMT is involved in FZKA-induced inhibition of lung cancer cell metastasis
Previous studies have reported that metastasis and invasion are associated with EMT (21,22). To determine if EMT also mediates the effects of FZKA on lung cancer cells, the present study detected the expression levels of proteins involved in the EMT process following FZKA treatment. The results indicated that the mesenchymal marker N-cadherin and the intermediate filament protein vimentin, which are associated with increased cell motility (23), were downregulated by FZKA treatment in a dose-dependent manner (Fig. 5A and B). These data provided another potential mechanism by which FZKA affected lung cancer metastasis.
Figure 5.
FZKA inhibits epithelial-mesenchymal transition in lung cancer cells. Protein expression levels of (A) N-cadherin and (B) vimentin were detected in lung cancer cells following treatment with FZKA by western blotting. Data were measured by ImageJ software. The experiment was repeated three times. *P<0.05 vs. control (0 FZKA mg/ml). FZKA, Fuzheng Kang-Ai.
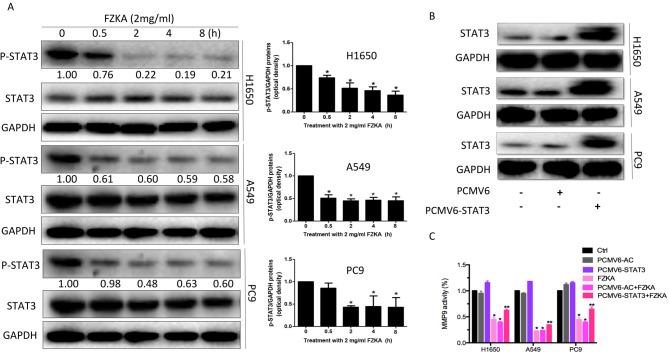
STAT3 may mediate the effects of FZKA on lung cancer cell metastasis
STAT3 is an oncogenic transcription factor, which leads to cell proliferation and invasion. Its activation can induce tumor cell growth, invasion and mesenchymal transition (24). In addition, MMP9 has been reported to be a target of STAT3 (25). In the present study, STAT3 activation was inhibited in a time-dependent manner in lung cancer cells following treatment with FZKA (Fig. 6A). Furthermore, overexpression of STAT3 was able to suppress the FZKA-mediated inhibition of MMP9 activity (Fig. 6B and C), indicating that STAT3 may be an upstream factor of MMP9, which is affected by FZKA treatment in lung cancer cells.
Figure 6.
STAT3 regulates MMP9 activity in lung cancer cells treated with FZKA. (A) Protein expression levels of STAT3 were reduced following treatment with FZKA (2 mg/ml; 0, 0.5, 2, 4 and 8 h). *P<0.05 vs. 0 h. (B) To overexpress STAT3, cells (H1650, A549 and PC9) were seeded into 6-well plates, and transfected with pCMV6-AC (negative control) and pCMV6-AC-STAT3 DNA constructs, prior to treatment with FZKA. STAT3 protein expression was then measured by western blot analysis. (C) MMP9 activity was increased by STAT3 overexpression. Following treatment with FZKA, the FZKA-mediated inhibition of MMP9 activity was significantly suppressed by STAT3 overexpression. *P<0.05 and **P<0.01 vs. control (Ctrl). FZKA, Fuzheng Kang-Ai; MMP9, matrix metalloproteinase 9; STAT5, signal transducer and activator of signaling 3; Ctrl, control.
Discussion
Although advances have been made in the treatment of human cancers, cancer remains a leading cause of human mortality each year. More effective therapies are therefore required for the treatment of patients with cancer. Traditional Chinese medicine popular in Chinese and East Asian societies and serves an active role in modern healthcare systems, including in the treatment of patients with cancer, and therefore may be considered a potential effective strategy for the treatment of human cancers.
The present study was based on our previous clinical and fundamental findings, which indicated that FZKA could sensitize the effects of gefitinib on patients with late stage lung cancer, and prolong the PFS and MST in patients with NSCLC (17–20). In addition, FZKA was reported to inhibit lung cancer cell growth in vivo and in vitro (17–20). The present study aimed to determine the role and mechanisms of FZKA decoction on the process of lung cancer cell metastasis. Initially, the inhibitory role of FZKA in lung cancer cell growth was identified. In addition, the results demonstrated that FZKA significantly inhibited lung cancer cell migration and invasion, as determined by wound-healing and Transwell assays. While the three lung cancer cell lines used in the present study responded to different extents to FZKA decoction, the overall effects of FZKA were consistent in all of the cell lines suggesting that the FZKA decoction had substantial inhibitory effects on human lung cancer cells.
MMP9, which is closely associated with the invasive and metastatic potential of numerous types of solid cancer (26), is critical during the process of FZKA-induced inhibition of lung cancer cell invasion. The present study demonstrated that MMP9 secretion was inhibited by FZKA treatment, as determined using an MMP9 activity assay. MMPs are able to degrade various components of the ECM and basement membrane (27). Once MMP9 is activated, it is able to degrade collagen in the ECM, which increases the metastasis of cancer cells (28). Therefore, the FZKA-induced inhibition of MM9 activity and expression may be considered an important mechanism by which FZKA inhibits lung cancer cell metastasis. Furthermore, STAT3 activation was inhibited by FZKA treatment. Notably, in cells overexpressing STAT3, as mediated by transient transfection, the FZKA-mediated inhibition of MMP9 activity was suppressed to some extent. Aberrant activation of STAT3 contributes to cancer progression in human malignances (29). Therefore, inhibition of STAT3 activation by FZKA may hinder tumor progression. Furthermore, the STAT3/MMP9 pathway has been demonstrated to participate in colon cancer cell invasion (30). The present study obtained similar results suggesting that the STAT3/MMP9 pathway may mediate the inhibitory effects of FZKA decoction on lung cancer cell metastasis.
The present study detected the expression levels of N-cadherin and vimentin, two important proteins involved in the EMT process, both of which were downregulated by FZKA treatment. EMT is characterized by the loss of epithelial characteristics and the acquisition of mesenchymal characteristics. The induction of mesenchymal markers, including N-cadherin and vimentin, are hallmark early- and late-stage events of EMT, respectively (31). The present study demonstrated that FZKA inhibited EMT, as indicated by a decrease in the protein expression levels of N-cadherin and vimentin. EMT is a well-known molecular mechanism associated with cancer metastasis (32). Therefore, FZKA-induced inhibition of EMT in lung cancer cells may be a potential mechanism underlying the effects of FZKA treatment on patients with lung cancer. Since EMT can also be induced by MMPs (33), the mesenchymal markers may be downstream proteins of the STAT3/MMP9 pathway. However, further study is required to verify this.
In conclusion, the present study identified a probable mechanism by which FZKA decoction inhibits lung cancer cell metastasis via the STAT3/MMP9 pathway, thus indicating that FZKA decoction may be considered as a potential effective strategy for the treatment of patients with lung cancer. Briefly, in the present study, FZKA decoction inhibited lung cancer cell migration and invasion in vitro. In addition, the results demonstrated that the STAT3/MMP9 pathway and EMT may mediate the inhibitory effects of FZKA on lung cancer metastasis. These findings provide valid experimental evidence for the clinical usage of FZKA decoction in treating patients with late stage lung cancer.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (grant nos. 81273965 and 81272614), the Canadian Terry Fox Run Foundation for Cancer Research (grant no. YN2014TF01), the Science and Technology Planning Project of Guangdong Province (grant nos. 2016A020226035 and 2014A020221024), and the Administration of Traditional Chinese Medicine of Guangdong Province in China (grant no. 20141104).
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10–21. doi: 10.3978/j.issn.1000-9604.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I, et al. EUROCARE-4 Working Group: Recent cancer survival in Europe: A 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 5.Thomas G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 7.Rhee JS, Coussens LM. RECKing MMP function: Implications for cancer development. Trends Cell Biol. 2002;12:209–211. doi: 10.1016/S0962-8924(02)02280-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Ke ZF, Wang F, Zhang WH, Wang YF, Li SH, Wang LT. GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through upregulating MMP-2 and MMP-9. Cell Physiol Biochem. 2015;35:969–982. doi: 10.1159/000369753. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad R, Shihab PK, Jasem S, Behbehani K. FSL-1 induces MMP-9 production through TLR-2 and NF-κB/AP-1 signaling pathways in monocytic THP-1 cells. Cell Physiol Biochem. 2014;34:929–942. doi: 10.1159/000366310. [DOI] [PubMed] [Google Scholar]
- 10.Yang CQ, Li W, Li SQ, Li J, Li YW, Kong SX, Liu RM, Wang SM, Lv WM. MCP-1 stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell Physiol Biochem. 2014;34:266–276. doi: 10.1159/000362997. [DOI] [PubMed] [Google Scholar]
- 11.Cheng X, Yang Y, Fan Z, Yu L, Bai H, Zhou B, Wu X, Xu H, Fang M, Shen A, et al. MKL1 potentiates lung cancer cell migration and invasion by epigenetically activating MMP9 transcription. Oncogene. 2015;34:5570–5581. doi: 10.1038/onc.2015.14. [DOI] [PubMed] [Google Scholar]
- 12.Sreekumar R, Sayan BS, Mirnezami AH, Sayan AE. MicroRNA control of invasion and metastasis pathways. Front Genet. 2011;2:58. doi: 10.3389/fgene.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia H, Hui KM. MicroRNAs involved in regulating epithelial-mesenchymal transition and cancer stem cells as molecular targets for cancer therapeutics. Cancer Gene Ther. 2012;19:723–730. doi: 10.1038/cgt.2012.58. [DOI] [PubMed] [Google Scholar]
- 14.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 15.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Zhang T, Zou S, Jiang B, Hua D. B7-H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med Rep. 2015;12:5455–5460. doi: 10.3892/mmr.2015.4050. [DOI] [PubMed] [Google Scholar]
- 17.Yang XB, Wu WY, Long SQ, Deng H, Pan ZQ, He WF, Zhou YS, Liao GY, Li QP, Xiao SJ, Cai JZ. Fuzheng Kang'ai decoction combined with gefitinib in advanced non-small cell lung cancer patients with epidermal growth factor receptor mutations: Study protocol for a randomized controlled trial. Trials. 2015;16:146. doi: 10.1186/s13063-015-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu WY, Yang XB, Deng H, Long SQ, Sun LS, He WF, Zhou YS, Liao GY, Chan SM, Shan SP. Treatment of advanced non-small cell lung cancer with extracorporeal high frequency thermotherapy combined with Chinese medicine. Chin J Integr Med. 2010;16:406–410. doi: 10.1007/s11655-010-0535-8. [DOI] [PubMed] [Google Scholar]
- 19.Yang XB, Wu WY, Long SQ, Deng H, Pan ZQ. Effect of gefitinib plus Chinese herbal medicine (CHM) in patients with advanced non-small-cell lung cancer: A retrospective case-control study. Complement Ther Med. 2014;22:1010–1018. doi: 10.1016/j.ctim.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Zheng F, Wu J, Li X, Tang Q, Yang L, Yang X, Wu W, Hann SS. Chinese Herbal Medicine Fuzheng Kang-Ai Decoction inhibited lung cancer cell growth through AMPKα-mediated induction and interplay of IGFBP1 and FOXO3a. Evid Based Complement Alternat Med. 2016;2016:5060757. doi: 10.1155/2016/5060757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan L, Pantel K, Kang Y. Tumor metastasis: Moving new biological insights into the clinic. Nat Med. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: New insights in signaling, development and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lui VW, Wong EY, Ho Y, Hong B, Wong SC, Tao Q, Choi GC, Au TC, Ho K, Yau DM, et al. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int J Cancer. 2009;125:1884–1893. doi: 10.1002/ijc.24567. [DOI] [PubMed] [Google Scholar]
- 25.Guo K, Ma Q, Li J, Wang Z, Shan T, Li W, Xu Q, Xie K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol Cancer Ther. 2013;12:264–273. doi: 10.1158/1535-7163.MCT-12-0809. [DOI] [PubMed] [Google Scholar]
- 26.El-Badrawy MK, Yousef AM, Shaalan D, Elsamanoudy AZ. Matrix metalloproteinase-9 expression in lung cancer patients and its relation to serum mmp-9 activity, pathologic type, and prognosis. J Bronchology Interv Pulmonol. 2014;21:327–334. doi: 10.1097/LBR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 27.Vilen ST, Salo T, Sorsa T, Nyberg P. Fluctuating roles of matrix metalloproteinase-9 in oral squamous cell carcinoma. ScientificWorldJournal. 2013;2013:920595. doi: 10.1155/2013/920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backstrom JR, Tökés ZA. The 84-kDa form of human matrix metalloproteinase-9 degrades substance P and gelatin. J Neurochem. 1995;64:1312–1318. doi: 10.1046/j.1471-4159.1995.64031312.x. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Jove R. The STATs of cancer-new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 30.Ao N, Liu Y, Bian X, Feng H, Liu Y. Ubiquitin-specific peptidase 22 inhibits colon cancer cell invasion by suppressing the signal transducer and activator of transcription 3/matrix metalloproteinase 9 pathway. Mol Med Rep. 2015;12:2107–2113. doi: 10.3892/mmr.2015.3661. [DOI] [PubMed] [Google Scholar]
- 31.Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta. Mol Biol Cell. 2007;18:3533–3544. doi: 10.1091/mbc.E07-03-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]