Abstract
Sustained phagocytosis requires the continuous replacement of cell-surface membrane from intracellular sources. Depending on the nature of the engulfed particles, a variety of endocytic compartments have been demonstrated to contribute membranes needed for the formation of phagosomes. It has recently been reported that the endoplasmic reticulum (ER) can also fuse with the plasma membrane during phagocytosis [Gagnon, E., Duclos, S., Rondeau, C., Chevet, E., Cameron, P. H., Steele-Mortimer, O., Paiement, J., Bergeron, J. J. & Desjardins, M. (2002) Cell 110, 119–131]. However, there is currently no known mechanistic basis for this fusion process to occur. Here we report that direct ER–plasma membrane fusion during phagocytosis requires the ER resident soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein ERS24/Sec22b and that J774-macrophages react toward the challenge of large (3.0-μm) but not small (0.8-μm) particles by triggering this fusion mechanism, allowing them to access the most abundant endogenous membrane source in the cell, the ER.
Keywords: endoplasmic-reticulum-mediated phagocytosis, SNARE proteins
Macrophages are specialized cells of the immune system that are responsible for ingesting dead cells and eliminating various pathogens. The remarkable fact that these cells are able to phagocytose particles larger than themselves (1) vividly illustrates their reliance on internal membranes for this process (2). The plasma membrane and membranes of endocytic compartments contribute to the forming phagosome (3). Lysosomes are specifically recruited during trypanosome invasion (4), whereas recycling endosomes contribute to Fc-receptor-mediated phagocytosis (5). Still, there are indications that the amount of membrane required to sustain phagocytosis of the largest particles may exceed the capacity of lysosomes or recycling endosomes and result in the disappearance of large portions of these compartments. The vesicle N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) vesicle-associated membrane protein-3 mediates fusion of recycling endosomes with the plasma membrane for phagosome formation, but vesicle-associated membrane protein-3 knockout mice are not impaired in phagocytosis (6), implying that there is an additional source of intracellular membranes for this process. Furthermore, certain engulfed pathogens are known to redirect intracellular trafficking and to wrap themselves in endoplasmic reticulum (ER)-derived membranes (7).
Recently, Gagnon et al. (8) observed by electron microscopy macrophages having ER tubules in close proximity to the bottom of phagocytic cups in the presence of phosphatidylinositol 3-kinase (PI3K) inhibitors and detected ER-resident proteins on isolated phagosomes at different levels of maturation (8, 9). These findings suggest that fusion of ER-derived membranes can occur with the plasma membrane and that PI3Ks are involved in regulating this fusion process. If so, this fusion event and its mechanism would be of considerable interest.
Cellular membrane fusion is mediated by SNAREpins, formed by the cognate partnering of a target-SNARE complex residing in one bilayer and a vesicle-SNARE residing in the other (10–12). Different cognate SNAREpins mediate different fusion events with great specificity (13–16). However, in a rare apparent exception to the SNARE hypothesis, it was observed that the isolated ER-localized SNARE Sec22p (from yeast) could mediate fusion of liposomes by paring with isolated yeast plasma membrane target-SNAREs (Sso1p and Sec9p) (13). An alternative interpretation would be that, rather than being an artifact or an exception to the SNARE hypothesis, this unexpected observation would be the basis for the prediction of a physiological transport pathway from ER to plasma membrane in which Sec22 functions as the vesicle-SNARE.
The present study demonstrates that, in Fc-receptor-mediated phagocytosis, the ER resident SNARE protein ERS24 (the mammalian equivalent to yeast Sec22p) (17), is required early in the phagocytic process. The recruitment of ERS24 to the site of phagocytosis and its function in phagocytosis is selectively triggered by large (3.0-μm) particles. The requirement for ERS24 in phagocytosis is independent of its function in ER–Golgi transport.
Materials and Methods
Cell Culture and Glass-Bead-Loading Assay. J774 cells were obtained from I. Tabas (Columbia University, New York) and were cultured in RPMI medium 1640 supplemented with 10% FCS/100 units/ml penicillin/100 μg/ml streptomycin/10 mM Hepes/1 mM sodium pyruvate/2 mM l-glutamine/4.5 mg/ml glucose. Cells were plated onto 12-mm cover slips for immunofluorescence microscopy or onto 25-mm cover slips for glass bead-loading 48 h before the experiment. For glass-bead-loading, 25-mm cover slips were transferred into live cell imaging chambers, rinsed with PBS, drained, and covered with loading solution [1.25 mg/ml recombinant protein or 1.2 mg/ml Fab fragments with 10 mg/ml rhodamine B isothiocyanate-dextran 10S (Sigma) in 10 mM Hepes/110 mM K-acetate, pH 7.4]. We then added and gently agitated 24 mg of 425- to 600-μm acid-washed glass beads (Sigma). Glass beads were removed by washing with excess PBS, and the cells were incubated for 10 min at 37°C to recover. Latex beads (0.8 μm and 3.0 μm; Sigma) were opsonized with 1 mg/ml technical grade mouse IgGs (Sigma) in PBS for 30 min at room temperature, rinsed with PBS, and diluted in fully supplemented RPMI medium 1640 at a 1:250 ratio for 3.0-μm beads and a 1:1,000 ratio for 0.8-μm beads. Glass-bead-loaded cells were covered with opsonized latex beads, incubated for 10 min at 37°C, rinsed with RPMI medium 1640 to remove excess and unbound latex beads and incubated for 20 min at 37°C. After fixation with 4% paraformaldehyde in PBS, the extracellular beads were stained with anti-mouse antibodies labeled with Alexa Fluor 488 (Molecular Probes). Efficient loading of 20-kDa molecules was tested by loading GST–Alexa Fluor 488 (Fig. 5, which is published as supporting information on the PNAS web site). Confocal pictures (Leica TCS SP confocal microscope; leica confocal 2.5 software; HCX PL APO 63× oil-immersion objective) were taken from loaded and unloaded cells, and the fluorescent intensity of rhodamine B isothiocyanate-dextran in glass-bead-loaded cells was quantified by using National Institutes of Health image software (Fig. 5). In total, >1,000 cells were analyzed for studies with ERS24 cytoplasmic domain (Fig. 1 A, B, and E) and >3,000 cells were analyzed for studies with anti-ERS24 Fab fragments (Fig. 1 C, D, and F). The graphs in Fig. 1 are representatives of at least two independent experiments (error bars indicate standard deviations).
Fig. 1.
ERS24 is required for phagocytosis of large (3.0-μm) particles. J774 cells were glass-bead-loaded with ERS24 cytoplasmic domain, GST, anti-ERS24 Fab fragments, or random Fab fragments and then challenged with mouse IgG-opsonized latex beads. The measured arbitrary fluorescent intensity of rhodaminedextran reflects the extent of loading. The average number of internalized beads per cell was counted. The extent of bead internalization is expressed as a percentage of the value for unloaded, control cells (set at 100%). (A and B) ERS24 cytoplasmic domain decreases the phagocytosis of 3.0-μm beads (A) but not of 0.8-μm beads (B). (C and D) Anti-ERS24 Fab fragments inhibit phagocytosis of 3.0-μm beads (C) but not of 0.8-μm beads (D) in a dose-dependent manner. (E and F) Inhibition of ERS24 function by ERS24 cytoplasmic domain (E) and anti-ERS24 Fab fragments (F) impairs phagocytosis of aggregates of 0.8-μm beads.
Antibodies and Recombinant Proteins. His-6-tagged ERS24 cytoplasmic domain (amino acids 1–192) was cloned into the pET-15b vector (Novagen). The protein was expressed in BL21 bacteria (Novagen) and purified by nickel-agarose affinity columns. GST protein was purchased from Sigma. Rabbit polyclonal anti-ERS24 antibodies were generated against recombinant ERS24 cytoplasmic domain as described in ref. 17. Rabbit IgG was purchased from Sigma. Fab fragments were prepared by using an ImmunoPure Fab kit (Pierce) according to the manufacture's instructions. Recombinant proteins and Fab fragments used for glass-bead-loading assays were first dialyzed against 10 mM Hepes/110 mM K-acetate, pH 7.4, and concentrated by using centricon filters (Amicon/Millipore). Alexa Fluor 488- and Alexa Fluor 594-labeled secondary antibodies were purchased from Molecular Probes.
Cell Treatment with PI3K Inhibitors and Brefeldin A. J774 cells were preincubated for 30 min with 0.1 μM wortmannin (see Fig. 4B), 20 μM LY294002 (see Figs. 3 and 4), or brefeldin A (Sigma) (see Fig. 2) as indicated in the respective figure legends. Opsonized latex beads were then added for 10 min at 37°C, rinsed with RPMI medium 1640 to remove excess and unbound beads, and the cells were further incubated for 20 min at 37°C. After fixation in 4% paraformaldehyde in PBS, cells were permeabilized with 0.1% Triton X-100 in PBS and stained with primary polyclonal anti-ERS24, anti-GOS28, and antirbet1 (18) antibodies, followed by labeling with secondary anti-rabbit antibodies. Confocal sections bearing continuous cytoplasm were obtained.
Fig. 4.
Phagocytosis of clumped 0.8-μm beads but not of individual 0.8-μm beads induces the mobilization of ER-derived membranes to sites of internalization. J774 cells were pretreated with LY29402 (20 μM) for 30 min before phagocytosis was induced by the addition of mouse IgG-opsonized, 0.8-μm latex beads. (A) Fixed cells were permeabilized and stained with antibodies raised against the SNARE proteins rbet1. rbet1 mobilize to sites of internalization induced by phagocytosis of clumped, 0.8-μm particles (Upper) but not individual 0.8-μm beads (Lower). (B) Pretreatment with two different PI3K inhibitors impairs phagocytosis of mouse IgG-opsonized latex beads [3.0-μm(Upper) and 0.8-μm(Lower)]. J774 cells were pretreated with 0.1 μM wortmannin or 20 μM LY294002 for 30 min and then challenged with phagocytosis in the presence of these inhibitors.
Fig. 3.
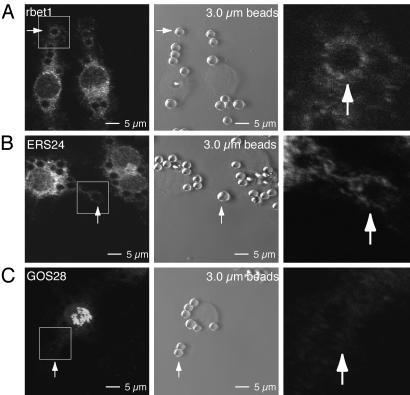
ER-but not Golgi-derived membranes are recruited for phagocytosis of 3.0-μm beads. J774 cells were pretreated with LY294002 (20 μM) for 30 min before phagocytosis was induced by the addition of mouse IgG-opsonized, 3.0-μm latex beads. (Left) Fixed cells were permeabilized and stained with antibodies raised against rbet1 (A), ERS24 (B), and GOS28 (C). A bead at a site of internalization is marked with a white arrow in each image. (Center) Phase-contrast images of immuno stained regions in Left. A bead at a site of internalization is marked with a white arrow in each image. (Right) Magnified images of respective sites of internalization (arrows). (A) The ER-resident SNARE protein rbet1 mobilizes to the sites of internalization induced by phagocytosis of 3.0-μm beads. (B) The SNARE protein ERS24 is recruited to sites of phagocytosis of 3.0-μm beads. (C) The Golgi-resident SNARE protein GOS28 does not mobilize to sites of internalization.
Fig. 2.
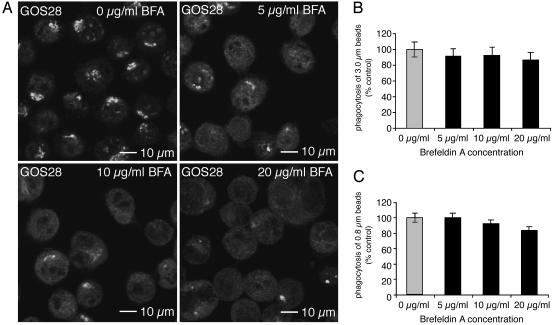
Disruption of the Golgi structure by brefeldin A (BFA) does not affect phagocytosis. J774 cells were treated for 30 min with brefeldin A at the concentrations indicated. (A) The distinct Golgi staining of the SNARE protein GOS28 disappears upon treatment with brefeldin A. (B and C) Pretreatment with brefeldin A does not impair phagocytosis of 3.0-μm(B) and 0.8-μm(C) IgG-opsonized latex beads.
Results and Discussion
The SNARE Protein ERS24 Is Required for Phagocytosis of Large Particles. To test the hypothesis that ERS24 is required for phagocytosis, we antagonized ERS24 function by using a dominant negative cytoplasmic domain of ERS24 (amino acids 1–192) or Fab fragments derived from an affinity-purified polyclonal antibody directed against the cytoplasmic domain of ERS24. These proteins were loaded into the cytoplasm of the macrophage cell line J774 by transient glass-bead rupture (19). Phagocytosis was initiated by challenging J774 cells with large and small IgG-opsonized latex beads (3.0-μm and 0.8-μm diameter, respectively). We opsonized latex beads to obtain efficient phagocytosis of large beads (average, 10 beads per cell). Because small beads tend to clump into aggregates, it was important to dilute the 0.8-μm beads to an extent that minimized the formation of aggregates, which allowed phagocytosis to be meaningfully studied as a function of the particle size of individual beads. To quantify the loading of cells with the cytoplasmic domain of ERS24 or Fab fragments, we added a fluorescent dye to the loading medium and measured the fluorescence intensity of individual cells. Comparison of cells loaded with lower or higher concentration ranges of the ERS24 cytoplasmic domain (low fluorescent intensity, 1–50 arbitrary units; high fluorescent intensity, 100–250 arbitrary units) showed dose-dependent inhibition of phagocytosis of 3.0-μm beads (Fig. 1A). Phagocytosis of the 0.8-μm beads, however, was not affected (Fig. 1B).
Anti-ERS24 Fab fragments have been shown to efficiently block ERS24 function in intra-Golgi transport (18). Introduction of anti-ERS24 Fab fragments into J774 cells also inhibited phagocytosis of large beads in a dose-dependent manner (Fig. 1C) but did not impair uptake of the small beads (Fig. 1D).
To confirm that size is the determinant of the difference in cellular response to the two beads, we took advantage of the tendency of the 0.8-μm beads to form clumps that behave as one large particle. Despite dilution and thorough mixing, a small portion of the 0.8-μm beads are found inside cells in the form of large aggregates (averaging 0.2 clusters per cell), in comparison with ≥10 individual beads per cell in the same cell. Considering the behavior of just these aggregates (≥6 beads per cluster) of small beads separately, it is apparent that aggregates of small beads are inhibited by ERS24 cytoplasmic domain (Fig. 1E) and anti-ERS24 Fab antibody (Fig. 1F). In summary, ERS24 function is required for Fc-receptor-mediated phagocytosis of 3.0-μm beads (and aggregates of 0.8-μm beads) but not individual 0.8-μm beads. Collectively, these results indicate that the requirement for ERS24 function in phagocytosis depends on particle size.
The Requirement of ERS24 in Phagocytosis Is Independent of ERS24 Function in ER–Golgi Transport. ERS24 is required for ER–Golgi transport (17) and intra-Golgi transport (18) where it functions as a subunit of a Golgi-localized target-SNARE (12). Therefore, the observed requirement of ERS24 for phagocytosis might be secondary to the inhibition of the secretory pathway. To address this possibility, we abolished ER–Golgi transport by using brefeldin A, a fungal toxin that causes disassembly of the Golgi apparatus (20, 21). We tested the effect of brefeldin A on phagocytosis in our system because the effective dose varies between cell lines and the phagocytic response can differ depending on the nature of the cell line, the particle tested, and the time frame studied (22–24). We examined the localization of the Golgi-resident SNARE protein GOS28 in the absence and presence of brefeldin A (Fig. 2A). Although 10 μg/ml brefeldin A was sufficient to disrupt the Golgi, pretreatment of J774 cells with even higher concentrations of brefeldin A had no effect on phagocytosis of large (Fig. 2B) or small (Fig. 2C) beads. These results demonstrate that ongoing ER–Golgi and intra-Golgi transport is not required to supply plasma membrane for a burst of phagocytosis. Therefore, the requirement for ERS24 in phagocytosis is independent of its function in the secretory pathway. Based on the results with yeast SNAREs, it is likely that ERS24 could function separately and distinctly as a vesicle-SNARE for fusion with the plasma membrane (13).
Phagocytosis of Large Beads Elicits Recruitment of ERS24 from ER-Derived Membranes to Sites of Internalization. ERS24-mediated ER–plasma membrane fusion in support of phagocytosis would require the recruitment of ERS24-containing ER membranes to sites of internalization. In resting cells, ERS24 is localized in the ER and Golgi (18). To ascertain which ERS24-containing compartment(s) is recruited for phagosome formation, we studied the localization of other SNARE proteins during phagocytosis of 3.0-μm beads. To facilitate the study of nascent phagosomes, we focused on beads localized distal to the cell body because, in the process of phagosomal maturation, the phagosome travels from the rim of a cell toward the perinuclear region (25). As previously described, direct contacts and apparent continuities between the ER and the plasma membrane have been observable only in the presence of PI3K inhibitors (8), presumably because these normally transient intermediates accumulate when these enzymes are inhibited.
A prediction of the proposed recruitment of ER membranes to sites of phagocytosis is that, in the presence of PI3K inhibitors, ER markers should accumulate at those sites. To test this hypothesis, we monitored two ER-localized SNARE proteins (rbet1 and ERS24) (18, 26). rbet1 is detected on ER and intermediate compartment (IC) membranes. ERS24 localizes to ER, intermediate compartment and Golgi membranes. We also monitored GOS28, a Golgi-localized SNARE (27), as a control protein that is not present on ER membranes. During large-particle phagocytosis, in the presence of LY294002 (a PI3K inhibitor), rbet1 (Fig. 3A), and ERS24 (Fig. 3B) showed some accumulation at the sites of internalization. In contrast, GOS28 did not appreciably accumulate (Fig. 3C). We also examined phagocytosis of 0.8-μm beads. Although aggregates of clumped 0.8-μm beads mobilized rbet1-bearing membranes (Fig. 4A) to sites of internalization, individual 0.8-μm beads did not induce mobilization of this SNARE protein. We note that rbet1 and ERS24 accumulation was modest. However, this result is expected, because these proteins are also partially localized to compartments that do not become recruited to sites of phagocytosis. Furthermore, the ER is a large compartment, only a fraction of which is expected to mobilize during phagocytosis. Although a particle size-dependent inhibition of phagocytosis has been observed previously (28), under our experimental conditions, two different PI3K inhibitors, LY294002 (20 μM) and wortmannin (0.1 μM), inhibited phagocytosis of small and large particles to a similar degree (Fig. 4B). Therefore, the differential use of ER membranes in phagocytosis is likely to be independent of PI3K function.
These findings demonstrate that ER-residents ERS24 and rbet1 are recruited during phagocytosis of 3.0-μm particles and suggests that the ER but not the Golgi serves as a selective reserve of membrane to enable the ingestion of large particles.
Conclusion
Our data suggest that J774 macrophages respond to excess demand for membranes in phagocytosis by the recruitment of membranes from the most abundant intracellular source, the ER. These results suggest that membrane fusion occurs between the ER and the plasma membrane. McNew et al. (13) discovered a fusogenic SNARE complex in yeast (sec22p, Sso1p, and Sec9p) that is capable of mediating ER–plasma membrane fusion. In our study, the observed requirement for the mammalian Sec22p homolog ERS24 in phagocytosis confirms the existence of membrane fusion between the ER and the plasma membrane.
Inhibition of ERS24 function by antibodies or excess of ERS24 soluble domain decreases (<30%) but does not abolish phagocytosis. These findings suggest that only when the demand for membrane exceeds the capacity of other sources does the cell revert to the ER. Phagocytosis of large numbers of individual small particles might also require ER membranes, but it is difficult to address this question because of their tendency to clump and their stacked accumulation in the cells (Fig. 6, which is published as supporting information on the PNAS web site). The particle-size-dependent recruitment of ER membranes to sites of phagocytosis is consistent with the requirement for ERS24 in phagocytosis of large (3.0-μm) particles but does not prove ER–plasma membrane fusion. The ER typically harbors more than half of the total cellular membrane and is an obvious candidate in times of large demand (29). However, fusion between the ER and nascent phagosome would inevitably lead to the loss of numerous proteins of the highly specialized ER. Therefore, it is not surprising that only minor portions of ER membranes compared with major portions of endosomal compartments are mobilized for phagocytosis (5). Whether the recruitment of ER-derived membranes in phagocytosis is compensatory or supplementary to the contribution of endocytic compartments in Fc-receptor-mediated phagocytosis remains to be established (5, 8).
How do large particles trigger the mobilization of ER reserves? Given the variety of intracellular membranes potentially available to support phagocytosis, the specific recruitment of particular membranes must be regulated. The repertoire of phagocytic receptors and the resulting complex intracellular signaling allows macrophages to create a tailored response (30). Indeed, the requirement for ER-derived membranes in phagocytosis vary between different systems with respect to cell types and engulfed particles (8, 23). The selection of particular phagocytic receptors as well as the local concentration of activated signaling molecules (determined by the nature of the engulfed particle) could navigate the specific recruitment of internal membranes (31).
Supplementary Material
Acknowledgments
We thank the members of the J.E.R. laboratory, especially Peter Antinozzi, Matthew Beard, Thomas Melia, and Fabienne Paumet, for stimulating discussions and for critically reading the manuscript and William Eng for assistance with antibody purification. T.B. was supported by a postdoctoral fellowship from the Deutsche Forschungs-gemeinschaft.
Abbreviations: SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; ER, endoplasmic reticulum; PI3K, phosphatidylinositol 3-kinase.
References
- 1.Cannon, G. J. & Swanson, J. A. (1992) J. Cell Sci. 101, 907–913. [DOI] [PubMed] [Google Scholar]
- 2.Vicker, M. G. (1977) Exp. Cell Res. 109, 127–138. [DOI] [PubMed] [Google Scholar]
- 3.Heine, J. W. & Schnaitman, C. A. (1971) J. Cell Biol. 48, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tardieux, I., Webster, P., Ravesloot, J., Boron, W., Lunn, J. A., Heuser, J. E. & Andrews, N. W. (1992) Cell 71, 1117–1130. [DOI] [PubMed] [Google Scholar]
- 5.Bajno, L., Peng, X. R., Schreiber, A. D., Moore, H. P., Trimble, W. S. & Grinstein, S. (2000) J. Cell Biol. 149, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen, L. A., Yang, C. & Pessin, J. E. (2002) J. Leukocyte Biol. 72, 217–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Roy, C. R. (2002) Trends Microbiol. 10, 418–424. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon, E., Duclos, S., Rondeau, C., Chevet, E., Cameron, P. H., Steele-Mortimer, O., Paiement, J., Bergeron, J. J. & Desjardins, M. (2002) Cell 110, 119–131. [DOI] [PubMed] [Google Scholar]
- 9.Garin, J., Diez, R., Kieffer, S., Dermine, J. F., Duclos, S., Gagnon, E., Sadoul, R., Rondeau, C. & Desjardins, M. (2001) J. Cell Biol. 152, 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T. H. & Rothman, J. E. (1998) Cell 92, 759–772. [DOI] [PubMed] [Google Scholar]
- 11.Melia, T. J., Weber, T., McNew, J. A., Fisher, L. E., Johnston, R. J., Parlati, F., Mahal, L. K., Sollner, T. H. & Rothman, J. E. (2002) J. Cell Biol. 158, 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parlati, F., McNew, J. A., Fukuda, R., Miller, R., Sollner, T. H. & Rothman, J. E. (2000) Nature 407, 194–198. [DOI] [PubMed] [Google Scholar]
- 13.McNew, J. A., Parlati, F., Fukuda, R., Johnston, R. J., Paz, K., Paumet, F., Sollner, T. H. & Rothman, J. E. (2000) Nature 407, 153–159. [DOI] [PubMed] [Google Scholar]
- 14.Paumet, F., Brugger, B., Parlati, F., McNew, J. A., Sollner, T. H. & Rothman, J. E. (2001) J. Cell Biol. 155, 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parlati, F., Varlamov, O., Paz, K., McNew, J. A., Hurtado, D., Sollner, T. H. & Rothman, J. E. (2002) Proc. Natl. Acad. Sci. USA 99, 5424–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paumet, F., Rahimian, V. & Rothman, J. E. (2004) Proc. Natl. Acad. Sci. USA 101, 3376–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paek, I., Orci, L., Ravazzola, M., Erdjument-Bromage, H., Amherdt, M., Tempst, P., Sollner, T. H. & Rothman, J. E. (1997) J. Cell Biol. 137, 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volchuk, A., Ravazzola, M., Perrelet, A., Eng, W. S., Di Liberto, M., Varlamov, O., Fukasawa, M., Engel, T., Sollner, T. H., Rothman, J. E. & Orci, L. (2004) Mol. Biol. Cell 15, 1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeil, P. L. & Warder, E. (1987) J. Cell Sci. 88, 669–678. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara, T., Oda, K., Yokota, S., Takatsuki, A. & Ikehara, Y. (1988) J. Biol. Chem. 263, 18545–18552. [PubMed] [Google Scholar]
- 21.Mossessova, E., Corpina, R. A. & Goldberg, J. (2003) Mol. Cell 12, 1403–1411. [DOI] [PubMed] [Google Scholar]
- 22.Hackam, D. J., Botelho, R. J., Sjolin, C., Rotstein, O. D., Robinson, J. M., Schreiber, A. D. & Grinstein, S. (2001) J. Biol. Chem. 276, 18200–18208. [DOI] [PubMed] [Google Scholar]
- 23.Henry, R. M., Hoppe, A. D., Joshi, N. & Swanson, J. A. (2004) J. Cell Biol. 164, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, Q., Cox, D., Tseng, C. C., Donaldson, J. G. & Greenberg, S. (1998) J. Biol. Chem. 273, 19977–19981. [DOI] [PubMed] [Google Scholar]
- 25.Toyohara, A. & Inaba, K. (1989) J. Cell Sci. 94, 143–153. [DOI] [PubMed] [Google Scholar]
- 26.Hay, J. C., Hirling, H. & Scheller, R. H. (1996) J. Biol. Chem. 271, 5671–5679. [DOI] [PubMed] [Google Scholar]
- 27.Nagahama, M., Orci, L., Ravazzola, M., Amherdt, M., Lacomis, L., Tempst, P., Rothman, J. E. & Sollner, T. H. (1996) J. Cell Biol. 133, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox, D., Tseng, C. C., Bjekic, G. & Greenberg, S. (1999) J. Biol. Chem. 274, 1240–1247. [DOI] [PubMed] [Google Scholar]
- 29.Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. & Walter, P. (2002) Molecular Biology of the Cell (Garland, New York).
- 30.Aderem, A. & Underhill, D. M. (1999) Annu. Rev. Immunol. 17, 593–623. [DOI] [PubMed] [Google Scholar]
- 31.Niedergang, F. & Chavrier, P. (2004) Curr. Opin. Cell Biol. 16, 422–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.