Abstract
High mobility group box-1 (HMGB-1) has been reported to serve significant roles in various inflammatory diseases. However, the correlation between the circulating level of HMGB-1 and severity of community-acquired pneumonia (CAP) remains unclear. The present study investigated differential alterations in plasma HMGB-1 levels of patients with CAP prior to and following antibiotic treatment, and further analyzed the association between CAP severity and HMGB-1 levels. Furthermore, lipopolysaccharide (LPS)-induced HMGB-1 expression in RAW264.7 macrophages and the relevant signaling pathways were examined. Plasma HMGB-1 levels of 90 patients with CAP and 52 healthy controls were measured using a commercial ELISA. The levels of plasma HMGB-1 were significantly elevated in CAP patients compared with the controls, and antibiotic treatment was effective in reducing HMGB-1 levels. Plasma HMGB-1 correlated with the pneumonia severity index score (r=0.566, P<0.001). Furthermore, LPS-stimulation significantly upregulated HMGB-1 secretion via the c-Jun N-terminal kinase (JNK) signaling pathway in RAW264.7 macrophages, whereas pretreatment with the JNK inhibitor SP600125 markedly downregulated LPS-induced HMGB-1 levels. In conclusion, plasma HMGB-1 levels may serve a role in the diagnosis and clinical assessment of CAP severity. These findings may provide information on novel targets for the treatment of CAP.
Keywords: community-acquired pneumonia, high mobility group box-1, lipopolysaccharide, c-Jun N-terminal signaling pathway
Introduction
High mobility group box-1 (HMGB-1) is a type of nuclear protein named for its high mobility in PAGE, and serves as a classic non-histone protein within nuclei (1). Previous studies largely focused on the intranuclear functions of HMGB-1, including its involvement in nucleosome stability, regulation of gene transcription and reconstruction and restoration of DNA (2–5). In addition, HMGB-1 is highly correlated to formation, invasion and metastasis of cancer cells (6–8).
Previously, HMGB-1 has been classified as a type of cytokine (1,9). Extracellular HMGB-1 induces inflammatory responses and may activate cells of the immune system, including activating the immune response of monocytes (9). Wang et al (10) first revealed that extracellular HMGB-1 serves as a mediator for inflammatory responses, and is a key inflammatory mediator for sepsis. Thereafter, the inflammatory effects of extracellular HMGB-1 attracted considerable interest. Previous studies have demonstrated that extracellular HMGB-1 is a key inflammatory mediator and proinflammatory cytokine present in the pathogenesis of sepsis, arthritis, acute pancreatitis, systemic lupus erythematosus, meningitis, kidney diseases, cardiovascular diseases and other diseases (11–15). In addition, HMGB-1 serves key functions in pulmonary fibrosis and pulmonary inflammation (16–19). Tseng et al (16) revealed that HMGB-1 content in the serum samples of patients in intensive care diagnosed with severe pneumonia serves as a predictor for mortality. Ito et al (17) assessed HMGB-1 content in the serum samples of H1N1 influenza-infected children, and identified a significant increase in HMGB-1 content in the samples of patients diagnosed with severe pneumonia complications. It was hypothesized that HMGB-1 is important in the recovery process from pneumonia. In addition, a previous study has indicated that HMGB-1 content in pneumonia patients who subsequently develop sepsis is significantly increased compared with pneumonia patients without complications (20). However, few studies have examined the correlation and severity of community-acquired pneumonia (CAP) and HMGB-1.
CAP is defined as an acute lung infection occurring in individuals who are not hospitalized (or have been hospitalized for <48 h), and is a common and life-threatening lower respiratory tract infection (21,22). Despite numerous treatment options, the CAP mortality rate remains high. Therefore, identifying biomarkers to assist the early diagnosis of pneumonia is crucial (22). Although previous studies of the authors have aimed to identify the biomarkers of pneumonia (21–24), the correlation of HMGB-1 levels with the severity of pneumonia remains undefined. The present study aimed to examine alterations in HMGB-1 expression and severity of pneumonia in patients prior to and following treatment. Furthermore, the present study examined lipopolysaccharide (LPS)-induced RAW 264.7 macrophages to simulate the pathogenesis of pneumonia and investigate the performance of HMGB-1 and the relevant signaling pathways.
Materials and methods
Subjects
The present study enrolled 144 people (90 CAP patients and 54 healthy controls) from February 2009 to December 2013 from Chung Shan Medical University Hospital (Taichung, Taiwan). Individuals that visited the Department of Family and Community Medicine for a health examination in Chung Shan Medical University Hospital were selected as healthy controls. All CAP patients were empirically treated with antimicrobial agents. All CAP patients were given antibiotics intravenously within the first 48 h. The pretreatment blood samples were obtained prior to patients with CAP receiving treatment protocols, and posttreatment blood samples were obtained within 3 days when the pneumonia had resolved. Blood samples were used to determine white blood cell (WBC) counts, neutrophils, and c-reactive protein (CRP) and plasma concentrations of HMGB-1 prior to and following antibiotic treatment of CAP patients. Blood samples of control subjects were additionally collected and tested. Blood samples were placed in tubes containing EDTA, immediately centrifuged at 2,500 × g, and stored at −80°C. The present study was approved by the Chung Shan Medical University Hospital Institutional Review Board (Taichung, Taiwan) and all participants gave their informed consent. Pneumonia severity was assessed using the Pneumonia Severity Index (PSI) (25).
Measurement of plasma HMGB-1
An ELISA kit (YHB1552Hu; Shanghai YH Biosearch Laboratory, Shanghai, China) was used to measure concentrations of HMGB-1 in blood samples or in the conditional medium of RAW264.7 macrophage. For each plasma sample, 40 µl was directly transferred to microtest strip wells of the ELISA plate combined with a detection antibody and subsequently incubated for 1 h at 37°C. Following 4 washing steps, antibody binding was detected with a horseradish peroxidase (HRP)-conjugated streptavidin secondary antibody for 1 h at 37°C supplied with the kit and developed with a substrate solution. Next, the reaction was stopped, and the optical density was determined at a wavelength of 450 nm using a microplate reader (STNERGY/H4, BioTek Instruments, Inc., Winoosi, VT, USA). Sample results were calculated from a standard curve generated by dilutions of a known amount of recombinant HMGB-1 protein. Each standard or sample was assayed in duplicate.
Cells and cell culture
RAW264.7 macrophages were purchased from the American Type Culture Collection (Manassas, VA, USA) was cultured in Dulbecco's Modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 2 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 400 ng/ml hydrocortisone (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). All cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. For LPS treatment, the conditioned medium was removed from RAW264.7 macrophages and replaced with fresh medium prior to the addition of LPS, following which cells were incubated for the indicated time periods in vitro.
Western blot analysis
Cellular lysates were prepared by suspending cells in a 10 cm dish (density, 2×106 cells) with 200 µl radioimmunoprecipitation assay buffer (Sigma-Aldrich, Merck KGaA) containing protease inhibitors cocktail as described previously (26). The nitrocellulose blot was subsequently incubated with 5% non-fat milk in Tris-buffered saline (20 mM Tris, 137 mM NaCl, pH 7.6) for 1 h to block non-specific binding. Following this, the membrane was incubated with the following rabbit primary antibodies overnight at 4°C: Polyclonal anti-HMGB-1 (6893; 1:500), anti-extracellular signal regulated kinase (ERK) 1/2 (9102; 1:1,000), anti-c-Jun N-terminal kinase (JNK) 1/2 (9258; 1:1,000), p38 (612168; 1:1,000), anti-phosphorylated (p)-ERK (4370; 1:1,000), anti-p-JNK 1/2 (9251; 1:1,000) and anti-p-p38 (4511; 1:1,000), all purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Blots were subsequently incubated with a HRP-conjugated goat anti-rabbit (sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or anti-mouse IgG (sc-2005; Santa Cruz Biotechnology, Inc.) secondary antibody for 1 h at 4°C. β-actin served as an internal control. Proteins were detected using an Enhanced Chemiluminescence commercial kit (RPN 2132; GE Healthcare Life Sciences, Chalfont, UK) and relative optical density was quantified using an AlphaImager® HP system (Alpha Innotech Corporation, San Leandro, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA). All data are expressed as the mean ± standard deviation. N is expressed as percentages for categorical variables. To compare between untreated patients and healthy individuals, the Mann-Whitney U-test and Wilcoxon signed-ranks test were performed for continuous variables. A linear regression analysis was applied for correlations of HMGB-1 with PSI scores of CAP patients. P<0.05 was considered to indicate a statistically significant difference.
Results
Antibiotic treatment reduces CRP levels in CAP patients
Demographic data and clinical characteristics of the participants are presented in Table I. In total, 142 age and gender matched CAP and control participants were included in the analysis. Patients with CAP exhibited significantly increased CRP levels (12.99 vs. 0.47 mg/dl, P<0.001), WBC counts (13,316 vs. 6,301 cells/mm3, P<0.001) and neutrophil counts (10,657 vs. 3,683 cells/mm3, P<0.001) compared with the controls. In these patients, antibiotic treatment significantly reduced median CRP levels (prior to antibiotic treatment, 12.99 mg/dl; following antibiotic treatment, 2.82 mg/dl; P<0.001), WBC counts (prior to antibiotic treatment, 13,316 cells/mm3; following antibiotic treatment, 9,413 cells/mm3; P<0.001), and neutrophil counts (prior to antibiotic treatment, 10,657 cells/mm3; following antibiotic treatment, 6,930 cells/mm3; P<0.001).
Table I.
Laboratory data of controls and patients with CAP prior to and following treatment.
| Clinical variable | C (n=52) | Before antibiotic treatment (n=90) | After antibiotic treatment (n=90) | P UT/Ca | P UT/Tb |
|---|---|---|---|---|---|
| Age | 60.25±10.69c | 65.02±18.10c | =0.085 | ||
| Gender | |||||
| Male | 35 (67.3%) | 65 (72.2%) | =0.536 | ||
| Female | 17 (32.7%) | 25 (27.8%) | |||
| CRP (mg/dl) | 0.47±0.27 | 12.99±6.50 | 2.82±3.16 | <0.001 | <0.001 |
| WBCs (cells/mm3) | 6,301.2±1,827.2 | 13,316.3±4,797.26 | 9,413.7±3,791.4 | <0.001 | <0.001 |
| Neutrophils (cells/mm3) | 3,683.2±1,353.2 | 10,657.3±4,261.7 | 6,930.2±3,718.1 | <0.001 | <0.001 |
| PSI score | |||||
| I | 12 (13.3%) | ||||
| II | 14 (15.5%) | ||||
| III | 24 (26.7%) | ||||
| IV | 33 (36.7%) | ||||
| V | 7 (7.8%) |
CAP, community-acquired pneumonia; CRP, C-reactive protein; WBCs, white blood cells; C, controls; UT, patients with community-acquired pneumonia prior to receiving antibiotic treatment; T, patients with community-acquired pneumonia following antibiotic treatment.
The statistical difference was analyzed by the Mann-Whitney U-test.
The statistical difference was analyzed by the Wilcoxon signed-ranks test.
Data are expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Antibiotic treatment significantly reduces HMGB-1 expression in CAP patients
CAP patients exhibited significantly higher plasma HMGB-1 levels than the controls (11.29±8.44 and 6.00±3.11 ng/ml, respectively, P<0.001). However, antibiotic treatment significantly reduced the expression of HMGB-1 (prior to antibiotic treatment, 11.29±8.44 ng/ml; following treatment, 7.38±5.19 ng/ml; P<0.001; Fig. 1).
Figure 1.
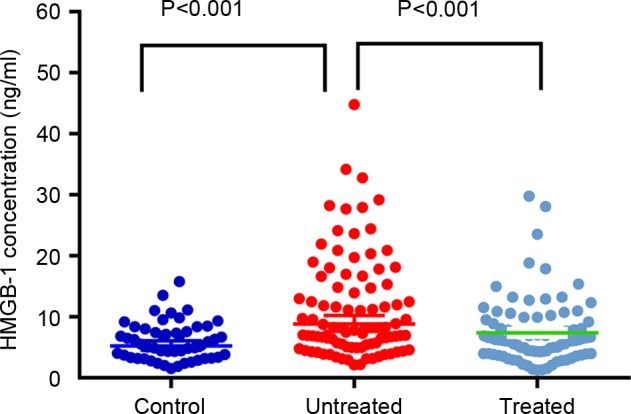
Levels of plasma HMGB-1 in control subjects and patients with CAP prior to and following antibiotic treatment. HMGB-1 levels were significantly elevated in patients with CAP prior to treatment compared with the controls, and significantly decreased in CAP patients following treatment. HMGB-1, high mobility group box-1; CAP, community-acquired pneumonia.
HMGB-1 levels and CAP severity correlations
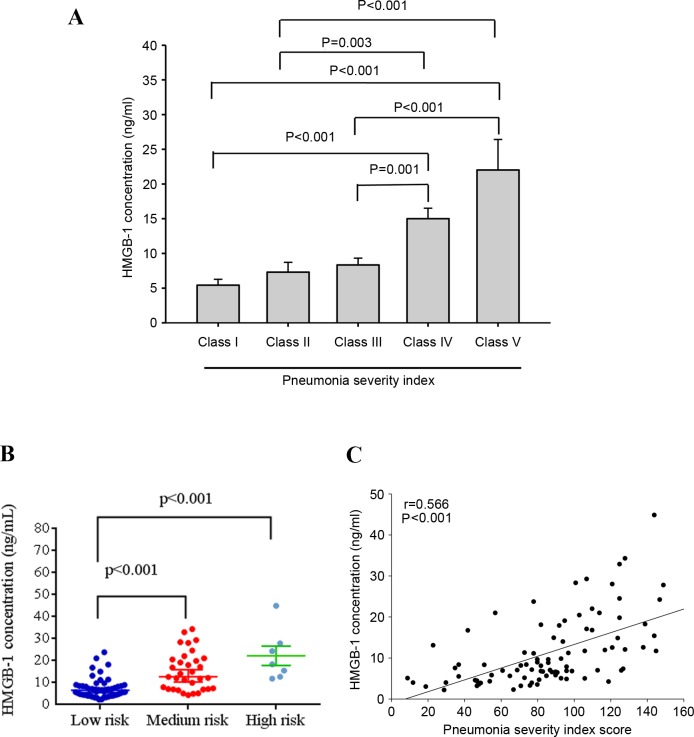
The correlation between HMGB-1 concentration and CAP severity in patients prior to antibiotic treatment was assessed using the PSI scoring system. As presented in Fig. 2A, significant differences in HMGB-1 levels were observed between Class I and Class IV (P<0.001), Class I and V (P<0.001), II and IV (P=0.003), II and V (P<0.002), III and IV (P=0.001), and III and V (P<0.001) patients. Furthermore, significantly different HMGB-1 levels were observed in patients who were classified as low risk compared with those classified as medium risk (P<0.001), and between low risk and high risk patients (P<0.0001) according to PSI scores (Fig. 2B). Furthermore, significant correlations were observed between HMGB-1 levels and PSI scores (Spearman's correlation coefficient: r=0.566; P<0.001; Fig. 2C).
Figure 2.
Levels of HMGB-1 and PSI scores in 90 patients with community acquired pneumonia. (A) Significant differences were observed between Class IV, V, I and II PSI scores. Data are expressed as the mean ± standard deviation. (B) Significant differences were observed between low and medium risk, and low and high risk patients. (C) A significantly positive correlation was observed between plasma HMGB-1 levels and PSI scores. HMGB-1, high mobility group box-1; PSI, pneumonia severity index.
LPS treatment in macrophages
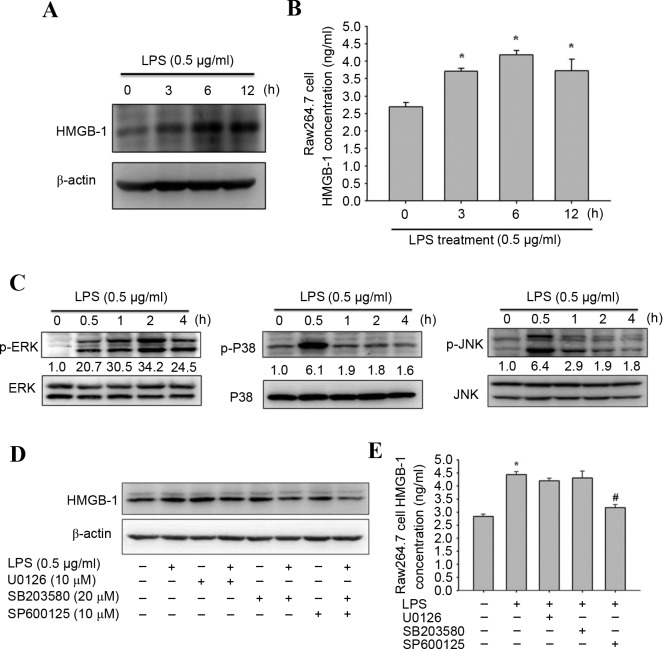
LPS-induced inflammatory responses in RAW264.7 macrophages is a reliable model to study bacterial pneumonia in vitro (27,28). Western blotting and an ELISA assay were used to investigate the expression and secretion of HMGB-1 following LPS treatment. As detected by western blotting, LPS treatment significantly increased HMGB-1 protein expression levels in RAW264.7 macrophages at 3, 6 and 12 h (Fig. 3A). In addition, the ELISA assay revealed that HMGB-1 secretion elevated to 4.18 ng/ml 6 h after LPS induction (Fig. 3B).
Figure 3.
Effects of LPS on HMGB-1 expression and mitogen activated protein kinase signaling pathway proteins. RAW264.7 macrophages were treated with 0.5 µg/ml LPS for 0, 3, 6 or 12 h. (A) Representative western blot images of HMGB-1 protein expression levels. ß-actin served as an internal control. (B) Quantification of ELISA assay to determine HMGB-1 secretion. (C) RAW264.7 macrophages were treated with 0.5 µg/ml LPS for 0, 0.5, 1, 2 or 4 h. Representative western blot images of ERK 1/2, JNK, p38, p-ERK, p-JNK and p-p38 protein expression levels. Total Erk, total p38 and total JNK were the loading controls. (D) Representative western blot images and (E) ELISA assay results of HGMB-1 levels in macrophages following pretreatment with 10 µM U0126, 20 µM SB203580 or 10 µM SP600125 for 30 min, and incubation with 0.5 µg/ml LPS for 6 h. Data are expressed as the mean ± standard deviation (n=3). *P<0.05 vs. untreated group; #P<0.05 vs. LPS-treated group. LPS, lipopolysaccharide; p, phosphorylated; ERK, extracellular signal regulated kinase; JNK, c-Jun N-terminal kinase; HMGB-1, high mobility group box-1.
Mitogen-activated protein kinase (MAPK) serves a crucial role in LPS-induced signal transduction pathways that lead to cytokine synthesis in macrophages (29,30). Thus, the effect of LPS treatment on the expression levels of MAPK signaling pathway proteins were investigated by western blotting to elucidate their underlying mechanisms. LPS treatment significantly increased phosphorylation levels ERK 1/2, p38 and JNK 1/2 in RAW264.7 macrophages (P<0.05; Fig. 3C). To further determine whether LPS-induced HMGB-1 overexpression was caused primarily by the MAPK signaling pathway, its effects on specific inhibitors of ERK 1/2 (U0126), p38 (SB203580) and JNK 1/2 (SP600125) in RAW264.7 macrophages were investigated. As assessed by western blotting, LPS-induced HMGB-1 overexpression was significantly reduced by the JNK 1/2 inhibitor (SP600125; Fig. 3D). In addition, similar results were obtained by ELISA assay (Fig. 3E). Therefore, induction of the JNK 1/2 signaling pathway may induce expression of HMGB-1.
Discussion
In terms of the biological function of HMGB-1, previous studies initially focused on its intranuclear functions (9,31,32). Following identification of the extracellular functions of HMGB-1, a number of studies revealed that HMGB-1 expression levels in plasma may serve as a biomarker for pneumonia and other diseases (13,15,20). Similarly, the present study demonstrated that HMGB-1 is associated with the pathogenesis of CAP. To the best of our knowledge, this is the first report describing the correlation between plasma HMGB-1 expression and CAP severity.
The present study demonstrated a correlation between the severity of CAP and HMGB-1 expression. The PSI and CURB-65 criteria have been used to evaluate hospital length of stay and mortality rates of CAP patients for numerous years (33–35). However, these indicators require large amount of clinical data and the collection of excessive data. Subsequently, numerous traditional and novel biomarkers successfully emerged to serve as a predictor for the severity of CAP, including WBCs, CRP and procalcitonin (23,24). CRP and WBCs are widely employed in clinical tests. However, they are inadequate for evaluating the severity and mortality risks of CAP.
In the past decade, HMGB-1 has been classified as a type of cytokine (1,9). Wang et al (10) demonstrated that, following the peak release of early inflammatory cytokines, macrophages begin to release HMGB-1 (10). Another study has indicated that HMGB-1 is a type of late-phase inflammatory cytokine that has greater clinical significance than early inflammatory cytokines such as tumor necrosis factor (TNF) or interleukin-1 (9). Although the association of TNF with the pathogenesis of CAP has been confirmed, its specificity and correlation with CAP severity are relatively poor (36). Furthermore, anti-HMGB-1 antibody treatment significantly reduces the mortality of LPS-induced mice (37). This indicates that HMGB-1 is a late inflammatory mediator of endotoxin lethality. In addition to clinical specimens, the present study examined LPS-induced macrophages to simulate the pathogenesis of pneumonia. These results revealed significant increases in protein expression levels of HMGB1, which are observed in the JNK 1/2 signaling pathway. RAW264.7 cells are widely used as macrophages for in vitro experiments. Once these cells are stimulated using LPS, they express and release HMGB-1 and numerous other cytokines (38–40). This method was used in the present study to verify the importance of HMGB-1 in pneumonia. In addition to cell models, previous studies have indicated in animal models that processing LPS causes lung damage and increases the concentration of HMGB-1 (41,42), which is consistent with the data from the present study. Thus, in addition to serving as an auxiliary factor for the pneumonia severity, HMGB-1 may become an auxiliary tool for pneumonia treatment in the future. One of the limitations of the present study is the lack of microbial data. Thus, future studies are required to detect the association between different microbial pathogens and HMGB-1.
In conclusion, plasma HMGB-1 levels may be used for predicting CAP severity in Taiwanese populations. Plasma HMGB-1 may additionally be applied to distinguish patients with CAP from healthy participants and evaluate the effects of antibiotic treatment on patients with CAP. These results suggested HMGB-1 as a predictive marker for the clinical diagnosis and severity assessment of CAP.
Acknowledgements
The present study was supported by the Chung Shan Medical University Hospital (grant no. CSH-2015-C-015).
References
- 1.Czura CJ, Wang H, Tracey KJ. Dual roles for HMGB1: DNA binding and cytokine. J Endotoxin Res. 2001;7:315–321. doi: 10.1177/09680519010070041401. [DOI] [PubMed] [Google Scholar]
- 2.Andersson U, Tracey KJ. HMGB1 in sepsis. Scand J Infect Dis. 2003;35:577–584. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 3.Magna M, Pisetsky DS. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med. 2014;20:138–146. doi: 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai J, Wen J, Bauer E, Zhong H, Yuan H, Chen AF. The role HMGB1 in cardiovascular biology: Danger signals. Antioxid Redox Signal. 2015;23:1351–1369. doi: 10.1089/ars.2015.6408. [DOI] [PubMed] [Google Scholar]
- 5.Weber DJ, Allette YM, Wilkes DS, White FA. The HMGB1-RAGE inflammatory pathway: Implications for brain injury-induced pulmonary dysfunction. Antioxid Redox Signal. 2015;23:1316–1328. doi: 10.1089/ars.2015.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang R, Zhang Q, Zeh HJ, III, Lotze MT, Tang D. HMGB1 in cancer: Good, bad, or both? Clin Cancer Res. 2013;19:4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh MJ, Hsieh YH, Lin CW, Chen MK, Yang SF, Chiou HL. Transcriptional regulation of Mcl-1 plays an important role of cellular protective effector of vincristine-triggered autophagy in oral cancer cells. Expert Opin Ther Targets. 2015;19:455–470. doi: 10.1517/14728222.2014.998200. [DOI] [PubMed] [Google Scholar]
- 9.Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002;72:1084–1091. [PubMed] [Google Scholar]
- 10.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 11.Schierbeck H, Pullerits R, Pruunsild C, Fischer M, Holzinger D, Laestadius Å, Sundberg E, Harris HE. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlate with early onset of disease, and are independent of disease duration. J Rheumatol. 2013;40:1604–1613. doi: 10.3899/jrheum.120987. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014;18:257–268. doi: 10.1517/14728222.2014.863876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindström O, Tukiainen E, Kylänpää L, Mentula P, Rouhiainen A, Puolakkainen P, Rauvala H, Repo H. Circulating levels of a soluble form of receptor for advanced glycation end products and high-mobility group box chromosomal protein 1 in patients with acute pancreatitis. Pancreas. 2009;38:e215–e220. doi: 10.1097/MPA.0b013e3181bb59a7. [DOI] [PubMed] [Google Scholar]
- 14.Tang D, Kang R, Cao L, Zhang G, Yu Y, Xiao W, Wang H, Xiao X. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med. 2008;36:291–295. doi: 10.1097/01.CCM.0000295316.86942.CE. [DOI] [PubMed] [Google Scholar]
- 15.Nin JW, Ferreira I, Schalkwijk CG, Jorsal A, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD. Higher plasma high-mobility group box 1 levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12 year follow-up study. Diabetologia. 2012;55:2489–2493. doi: 10.1007/s00125-012-2622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng CC, Fang WF, Leung SY, Chen HC, Chang YC, Wang CC, Chang HC, Lin MC. Impact of serum biomarkers and clinical factors on intensive care unit mortality and 6-month outcome in relatively healthy patients with severe pneumonia and acute respiratory distress syndrome. Dis Markers. 2014;2014:804654. doi: 10.1155/2014/804654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito Y, Torii Y, Ohta R, Imai M, Hara S, Kawano Y, Matsubayashi T, Inui A, Yoshikawa T, Nishimura N, et al. Increased levels of cytokines and high-mobility group box 1 are associated with the development of severe pneumonia, but not acute encephalopathy, in 2009 H1N1 influenza-infected children. Cytokine. 2011;56:180–187. doi: 10.1016/j.cyto.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Higa F, Furugen M, Koide M, Karimata Y, Nabeya D, Iha Y, Kinjo T, Miyagi K, Haranaga S, Hokama A, et al. Clinical evaluation of high mobility group box 1 protein in Legionella pneumophila pneumonia. J Infect Chemother. 2014;20:289–292. doi: 10.1016/j.jiac.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Achouiti A, van der Meer AJ, Florquin S, Yang H, Tracey KJ, van't Veer C, de Vos AF, van der Poll T. High-mobility group box 1 and the receptor for advanced glycation end products contribute to lung injury during Staphylococcus aureus pneumonia. Crit Care. 2013;17:R296. doi: 10.1186/cc13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L, et al. GenIMS Investigators: Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35:1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 21.Chiang TY, Tsao SM, Yeh CB, Yang SF. Matrix metalloproteinases in pneumonia. Clin Chim Acta. 2014;433:272–277. doi: 10.1016/j.cca.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Cheng CW, Chien MH, Su SC, Yang SF. New markers in pneumonia. Clin Chim Acta. 2013;419:19–25. doi: 10.1016/j.cca.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg P, Lindhardt BØ. The role of procalcitonin in adult patients with community-acquired pneumonia-a systematic review. Dan Med J. 2012;59:A4357. [PubMed] [Google Scholar]
- 24.Lippi G, Meschi T, Cervellin G. Inflammatory biomarkers for the diagnosis, monitoring and follow-up of community-acquired pneumonia: Clinical evidence and perspectives. Eur J Intern Med. 2011;22:460–465. doi: 10.1016/j.ejim.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 26.Yang SF, Lee WJ, Tan P, Tang CH, Hsiao M, Hsieh FK, Chien MH. Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget. 2015;6:2736–2753. doi: 10.18632/oncotarget.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, Lakovits K, Mesteri I, Nairz M, Boon L, Spiel A, et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest. 2013;123:3363–3372. doi: 10.1172/JCI67911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Li H, Qiu J, Feng H. Betulin protects mice from bacterial pneumonia and acute lung injury. Microb Pathog. 2014;75:21–28. doi: 10.1016/j.micpath.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Bode JG, Ehlting C, Häussinger D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012;24:1185–1194. doi: 10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Su SC, Hua KF, Lee H, Chao LK, Tan SK, Lee H, Yang SF, Hsu HY. LTA and LPS mediated activation of protein kinases in the regulation of inflammatory cytokines expression in macrophages. Clin Chim Acta. 2006;374:106–115. doi: 10.1016/j.cca.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 31.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, Andersson U, Yang H, Tracey KJ, Andersson J, Palmblad JE. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 33.Abisheganaden J, Ding YY, Chong WF, Heng BH, Lim TK. Predicting mortality among older adults hospitalized for community-acquired pneumonia: An enhanced confusion, urea, respiratory rate and blood pressure score compared with pneumonia severity index. Respirology. 2012;17:969–975. doi: 10.1111/j.1440-1843.2012.02183.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee YT, Chen SC, Shyu LY, Lee MC, Wu TC, Tsao SM, Yang SF. Significant elevation of plasma cathepsin B and cystatin C in patients with community-acquired pneumonia. Clin Chim Acta. 2012;413:630–635. doi: 10.1016/j.cca.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Wang HL, Hsiao PC, Tsai HT, Yeh CB, Yang SF. Usefulness of plasma YKL-40 in management of community-acquired pneumonia severity in patients. Int J Mol Sci. 2013;14:22817–22825. doi: 10.3390/ijms141122817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haugen J, Chandyo RK, Brokstad KA, Mathisen M, Ulak M, Basnet S, Valentiner-Branth P, Strand TA. Cytokine concentrations in plasma from children with severe and non-severe community acquired pneumonia. PLoS One. 2015;10:e0138978. doi: 10.1371/journal.pone.0138978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 38.Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T. Changes in cell culture temperature alter release of inflammatory mediators in murine macrophagic RAW264.7 cells. Inflamm Res. 2007;56:297–303. doi: 10.1007/s00011-007-6161-z. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa A, Iwasaka H, Hagiwara S, Noguchi T. Relationship between HMGB1 and tissue protective effects of HSP72 in a LPS-induced systemic inflammation model. J Surg Res. 2011;169:85–91. doi: 10.1016/j.jss.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol. 2009;76:173–182. doi: 10.1124/mol.109.055137. [DOI] [PubMed] [Google Scholar]
- 41.Ding N, Wang F, Xiao H, Xu L, She S. Mechanical ventilation enhances HMGB1 expression in an LPS-induced lung injury model. PLoS One. 2013;8:e74633. doi: 10.1371/journal.pone.0074633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagiwara S, Iwasaka H, Matsumoto S, Hasegawa A, Yasuda N, Noguchi T. In vivo and in vitro effects of the anticoagulant, thrombomodulin, on the inflammatory response in rodent models. Shock. 2010;33:282–288. doi: 10.1097/SHK.0b013e3181b0ef7b. [DOI] [PubMed] [Google Scholar]