Abstract
Background
We aimed to identify the prevalence of cardiovascular risk factors, and investigate preventive cardiovascular medication use and achievement of targets as per Dutch cardiovascular risk management guidelines among human immunodeficiency virus (HIV)-positive and HIV-negative individuals.
Design
The design was a cross-sectional analysis within an ongoing cohort study.
Methods
Data on medication use and cardiovascular disease prevalence were available for 528 HIV-positive and 521 HIV-negative participants. We identified cardiovascular risk factors and applied cardiovascular risk management guidelines, mainly focusing on individuals eligible for (a) primary prevention because of high a priori cardiovascular risk, or for (b) secondary prevention.
Results
One hundred and three (20%) HIV-positive and 77 (15%) HIV-negative participants were classified as having high cardiovascular risk; 53 (10%) HIV-positive and 27 (5%) HIV-negative participants were eligible for secondary prevention. Of HIV-positive individuals 57% at high cardiovascular risk and 42% of HIV-positive individuals eligible for secondary prevention had systolic blood pressures above guideline-recommended thresholds. Cholesterol levels were above guideline-recommended thresholds in 81% of HIV-positive individuals at high cardiovascular risk and 57% of HIV-positive individuals eligible for secondary prevention. No statistically significant differences were observed between HIV-positive and HIV-negative participants regarding achievement of targets, except for glycaemic control (glycated haemoglobin ≤ 53 mmol/mol) among individuals using diabetes medication (90% vs 50%, p = 0.017) and antiplatelet/anticoagulant use for secondary prevention (85% vs 63%, p = 0.045), which were both superior among HIV-positive participants.
Conclusions
Cardiovascular risk management is suboptimal in both HIV-positive and HIV-negative individuals and should be improved.
Keywords: Human immunodeficiency virus, cardiovascular disease, prevention, hypertension, dyslipidaemia
Introduction
Cardiovascular disease (CVD) is highly prevalent in human immunodeficiency virus (HIV)-positive populations.1,2 Primary and secondary CVD prevention reduce the burden of CVD.3 Optimal cardiovascular risk management is essential, especially in populations with an increased cardiovascular risk such as the HIV-positive population. In The Netherlands, the Dutch Cardiovascular Risk Management (CVRM) guidelines are used to identify patients at high cardiovascular risk and to provide guidance to physicians on how best to reduce this risk.4,5 These Dutch guidelines are quite comparable to other European CVRM guidelines.6
Several studies have reported suboptimal CVD prevention in the general population,5,7,8 as well as in HIV-positive populations.9–13 To our knowledge, none of the studies in HIV-positive populations provided an overview of all facets of cardiovascular risk management in a contemporary and largely virally suppressed HIV-positive population and highly comparable HIV-negative controls.
The aims of this study were (a) to identify the prevalence of cardiovascular risk factors, and (b) to investigate the use of preventive cardiovascular medication and achievement of targets as per Dutch CVRM guidelines among HIV-positive and HIV-negative individuals.
Methods
Study participants and design
The AGEhIV Cohort Study is an ongoing prospective comparative cohort study (see details of the AGEhIV Cohort Study Group listed in the Supplementary Material, Appendix). Between 2010–2012, 598 HIV-positive individuals were recruited from the HIV outpatient clinic of the Academic Medical Center, Amsterdam, The Netherlands. Five hundred and fifty HIV-negative controls were recruited from the Amsterdam Cohort Studies on HIV/AIDS and among individuals attending the sexual health clinic at the Amsterdam Public Health Service with the aim of being highly comparable to the HIV-positive group regarding demographic and behavioural characteristics. All participants were age ≥45 years and had laboratory-confirmed presence or absence of HIV-1 infection. Participants with available data on CVD prevalence and medication use were included in this analysis. The study was approved by the local ethics review board (ClinicalTrials.gov identifier NCT01466582) and all participants provided written informed consent.
Study protocol
The study protocol has been described in detail previously.2 Participants attend biennial study visits, in which they undergo a standardised screening for age-related comorbidities, organ dysfunction and risk factors.
Brachial blood pressure was measured three times while seated after a five-minute rest using an automated device (Omron 705-IT). Blood samples were collected to measure lipid levels, glycated haemoglobin (HbA1c), glucose, plasma creatinine, CD4 T-cell count, and HIV-1 RNA levels. Participants completed a standardised questionnaire regarding demographic characteristics, medication use, medical history, family history, smoking status, alcohol/recreational drug use and physical activity. Self-reported CVD diagnoses were validated using hospital records for HIV-positive participants, and general practitioners’ records for controls.2
Definitions
Systolic blood pressure (SBP) and diastolic blood pressure were calculated using the mean of the second and third blood pressure measurement. We used SBP targets as per Dutch CVRM guidelines (see Supplementary Material, Table).4
Diabetes mellitus (DM) was considered present if HbA1c was ≥ 48 mmol/mol, and/or blood glucose was elevated (non-fasting ≥ 11.1 mmol/l, fasting ≥ 7.0 mmol/l), and/or if using diabetes medication.14 Glycaemic control was defined as HbA1c ≤ 53 mmol/mol, while on diabetes medication.15
We defined CVD as a validated diagnosis of angina pectoris, myocardial infarction, ischaemic cerebrovascular disease, or peripheral arterial disease. Rheumatic disease was considered present in participants with a validated diagnosis of rheumatic disease but only in those using antirheumatic agents.
The World Health Organization definition for obesity was used (body mass index (BMI) ≥ 30 kg/m2). We classified individuals as physically inactive when they did not meet the Dutch healthy physical activity guidelines (moderate physical activity ≥5 days/week for ≥30 min, and/or heavy physical activity ≥3 days/week for ≥20 min).16 Heavy alcohol use was defined as alcohol intake ≥5 units/day for males or ≥3 units/day for females.
The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation.
Cardiovascular risk management guidelines (Supplementary Material, Table)
Cardiovascular risk management was evaluated as per Dutch CVRM guidelines.4
Primary prevention
In individuals without prior CVD, a 10-year risk of cardiovascular mortality and morbidity was estimated based on age, sex, smoking, presence of DM and/or rheumatic disease, SBP, and total cholesterol/high-density lipoprotein cholesterol ratio (TC/HDL-ratio) using the Systemic Coronary Risk Evaluation (SCORE) risk equation adjusted for national data.17 Age-specific conversion factors for the Dutch population were used to translate the cardiovascular mortality risk to a cardiovascular mortality and morbidity risk (i.e. 35-–45 years: 5, 45–65 years: 4, and ≥65 years: 3). The estimated 10-year risk is expressed as a percentage, which is subsequently categorised as low (<10%), moderate (10–20%), or high (≥20%). Recommendations for preventive treatment depend on the estimated cardiovascular risk and the presence of additional risk factors.
Secondary prevention
Antiplatelet medication is recommended in all individuals with prior CVD; recommendations regarding prescription of other preventive cardiovascular medication depend on the type of event.
All individuals on preventive cardiovascular medication
Blood pressure control was defined as SBP ≤140 mm Hg, and cholesterol control as low-density lipoprotein cholesterol (LDL-c)≤2.5 mmol/l among individuals using antihypertensive or lipid-lowering medication, respectively (regardless of CVD status or risk).
Statistical analysis
Stata software (version 12.1; StataCorp, College Station, Texas, USA) was used for statistical analyses. A cross-sectional analysis was performed in participants with complete data regarding CVD status and co-medication use. Missing data of other cardiovascular risk factors were handled by multiple imputation. Group comparisons were performed using Fisher’s exact, Wilcoxon rank sum or nonparametric trend tests, as appropriate. Since treatment recommendations for individuals at high 10-year cardiovascular risk and moderate 10-year cardiovascular risk plus additional cardiovascular risk factors are identical, we combined these individuals into a single group to determine the prevalence of cardiovascular risk factors, preventive medication use and achievement of targets. The current analysis aimed to provide a complete overview of cardiovascular risk management, and therefore did not focus on any one particular primary endpoint.
As a sensitivity analysis we conducted a complete case analysis excluding participants with missing data regarding cardiovascular risk factors.
Results
Cohort characteristics (Table 1)
Table 1.
Baseline characteristics of human immunodeficiency virus (HIV)-positive and HIV-negative participants.
| HIV-positives (n = 528) | HIV-negatives (n = 521) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 53 (48–60) | 52 (48–58) | 0.17a |
| Male gender | 468 (89%) | 444 (85%) | 0.12b |
| African descent | 64 (12%) | 30 (6%) | <0.001b |
| Men who have sex with men | 394 (75%) | 364 (70%) | 0.10b |
| Educational level | <0.001c | ||
| No or primary education only | 34 (7%) | 9 (2%) | |
| Secondary or vocational education | 268 (51%) | 221 (43%) | |
| Higher or academic education | 220 (42%) | 289 (56%) | |
| Cardiovascular disease | |||
| Prevalence of cardiovascular disease | 53 (10%) | 27 (5%) | 0.003b |
| Use of antihypertensive medicationd | 121 (23%) | 72 (14%) | <0.001b |
| Use of lipid lowering medicatione | 79 (15%) | 42 (8%) | <0.001b |
| Use of diabetes medicationf | 20 (4%) | 14 (3%) | 0.38b |
| HIV specific characteristics | |||
| Known duration of HIV-infection (years) | 12 (6–17) | ||
| Using cART at enrolment | 501 (95%) | ||
| Using protease inhibitor at enrolmentg | 213 (40%) | ||
| Using abacavir at enrolmentg | 69 (14%) | ||
| HIV-1 viral load <40 copies/ml among cART-treated individuals within 4 months before or at enrolment | 483 (97%) | ||
| Prior diagnosis of AIDS | 163 (31%) | ||
| Nadir CD4 cell count (cells/mm3) | 180 (80–260) | ||
| Current CD4 cell count (cells/mm3) | 568 (434–745) | ||
AIDS: acquired immune deficiency syndrome; cART: combination antiretroviral therapy.
Data are presented as median (interquartile range) or number (%). Type of test used: aWilcoxon rank sum test, bFisher’s exact test, cNon-parametric trend test.
Antihypertensive medication included diuretics, beta-blockers, calcium antagonists, angiotensin-converting-enzyme inhibitors and angiotensin receptor blockers.
Lipid-lowering medication included statins, fibrates and other lipid-lowering medication.
Diabetes medication included both oral diabetes medication and insulin.
Current use of protease inhibitors and abacavir was only described among HIV-positive individuals using cART at enrolment.
Data on prior CVD and medication use were available for 528 of 598 (88%) HIV-positive participants and 521 of 550 (95%) controls. Excluded participants were more often women (23% vs 13%), and more often of African descent (29% vs 9%). Excluded HIV-positive participants had a lower median nadir CD4 (135 vs 180 cells/mm3).
Participants included in the current analysis had a median age of 53 years, 87% were male, of whom 83% were men who have sex with men. HIV-positive individuals were more often of African descent than controls (12% vs 6%, p < 0.001), and their educational level was lower (42% of HIV-positive vs 56% of HIV-negative individuals had completed higher or academic education, p < 0.001). The prevalence of CVD was higher among HIV-positive individuals (10% vs 5%, p = 0.003).
The median known duration of HIV-infection among HIV-positive participants was 12 years. Ninety-five percent were on combination antiretroviral therapy (cART) at enrolment, of whom 97% had HIV-RNA below 40 copies/ml within four months before or at enrolment. Median nadir CD4 was 180 cells/mm3 and median current CD4 was 568 cells/mm3.
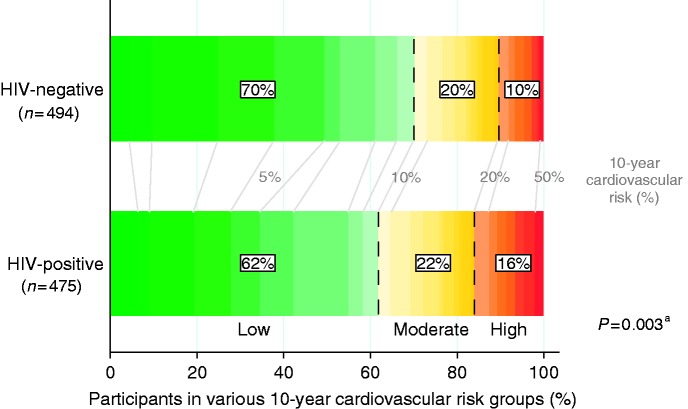
Predicted 10-year cardiovascular risk among participants without prior CVD (Figure 1)
Figure 1.
Predicted 10-year cardiovascular risk among human immunodeficiency virus (HIV)-positive and HIV-negative participants without prior cardiovascular disease (CVD). The figure shows the proportion of participants in low (<10%, green), moderate (10–20%, yellow), and high (≥20%, orange/red) predicted 10-year cardiovascular risk groups as calculated using the Systemic Coronary Risk Evaluation (SCORE) risk equation adjusted for national data. The percentages between the bars (coloured grey) indicate the absolute 10-year cardiovascular risk.
aNon-parametric trend test.
Four hundred and seventy-five of 528 (90%) HIV-positive participants and 494 of 521 (95%) controls had not experienced prior CVD. Of these, 294 (62%) HIV-positive individuals and 347 (70%) controls had a low 10-year cardiovascular risk, and 105 (22%) HIV-positive participants and 96 (20%) controls had a moderate 10-year cardiovascular risk, of whom 27 HIV-positive individuals and 26 controls had additional cardiovascular risk factors. A high 10-year cardiovascular risk was more common among HIV-positive individuals than among controls: 76 (16%) vs 51 (10%), p = 0.003.
Prevalence of cardiovascular risk factors (Table 2)
Table 2.
Prevalence of cardiovascular risk factors among human immunodeficiency virus-positive (HIV+) and HIV-negative (HIV–) participants.
| All |
Primary prevention, stratified by cardiovascular risk group |
Secondary prevention overall |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lowa |
Moderateb |
Highc |
|||||||||||||
| HIV+ (n = 528) | HIV– (n = 521) | p | HIV+ (n = 294) | HIV– (n = 347) | p | HIV+ (n = 78) | HIV– (n = 70) | p | HIV+ (n = 103) | HIV– (n = 77) | p | HIV+ (n = 53) | HIV– (n = 27) | p | |
| SBP above thresholdd | 84 (16%) | 61 (12%) | 0.05 | 3 (1%) | 0 (0%) | 0.10 | 0 (0%) | 1 (1%) | 0.47 | 59 (57%) | 46 (60%) | 0.76 | 22 (42%) | 14 (52%) | 0.48 |
| Distribution SBP (mm Hg) among those above thresholdd | 156 (145–170) | 153 (146–167) | 0.39 | − | − | − | − | − | − | 156 (147–169) | 150 (145–164) | 0.16 | 154 (145–164) | 160 (151–169) | 0.30 |
| Lipid levels above thresholde | 118 (22%) | 81 (16%) | 0.006 | 1 (0.3%) | 0 (0%) | 0.46 | 4 (5%) | 0 (0%) | 0.12 | 83 (81%) | 62 (81%) | 1.00 | 30 (57%) | 19 (70%) | 0.33 |
| Distribution LDL-c (mmol/l) among those above thresholdd | 3.6 (3.0– 4.0) | 3.7 (3.2–4.3) | 0.13 | − | − | − | − | − | − | 3.6 (3.0– 4.1) | 3.8 (3.2–4.3) | 0.11 | 3.6 (3.1–4.0) | 3.4 (3.2–4.2) | 0.86 |
| Diabetes | 31 (6%) | 20 (4%) | 0.15 | 1 (0.3%) | 0 (0%) | 0.46 | 1 (1%) | 1 (1%) | 1.00 | 21 (20%) | 14 (18%) | 0.85 | 8 (15%) | 5 (19%) | 0.75 |
| Current smoker | 169 (32%) | 129 (25%) | 0.009 | 79 (27%) | 70 (20%) | 0.05 | 27 (35%) | 22 (31%) | 0.73 | 45 (44%) | 28 (36%) | 0.36 | 18 (34%) | 9 (33%) | 1.00 |
| Obesity (BMI ≥ 30 kg/m2) | 41 (8%) | 51 (10%) | 0.28 | 16 (5%) | 19 (5%) | 1.00 | 2 (3%) | 2 (3%) | 1.00 | 18 (17%) | 19 (25%) | 0.27 | 5 (9%) | 11 (41%) | 0.002 |
| Physical inactivityf | 294 (56%) | 245 (47%) | 0.005 | 151 (51%) | 154 (44%) | 0.08 | 49 (63%) | 26 (37%) | 0.003 | 58 (56%) | 43 (56%) | 1.00 | 36 (68%) | 22 (81%) | 0.29 |
| Heavy alcohol useg | 26 (5%) | 37 (7%) | 0.15 | 14 (5%) | 22 (6%) | 0.49 | 3 (4%) | 4 (6%) | 0.71 | 7 (7%) | 8 (10%) | 0.42 | 2(4%) | 3(11%) | 0.33 |
BMI: body mass index; LDL-c: low-density lipoprotein cholesterol; SBP: systolic blood pressure; TC/HDL-ratio: total cholesterol to high-density lipoprotein cholesterol ratio.
Data are presented as number (%) or median (interquartile range). Fisher’s exact test was used to test for group differences.
Low cardiovascular risk is defined as a predicted 10-year cardiovascular risk <10%.
Moderate cardiovascular risk is defined as a predicted 10-year cardiovascular risk 10–20% without additional cardiovascular risk factors.
High cardiovascular risk is defined as a 10-year cardiovascular risk ≥20% or 10-year cardiovascular risk 10–20% with additional cardiovascular risk factors.
Blood pressure is above the threshold when (a) SBP > 180 mm Hg in participants with primary prevention low 10-year cardiovascular risk or moderate 10-year cardiovascular risk participants with no additional risk factors; or (b) SBP > 160 mm Hg in participants aged ≥80 years with primary prevention high 10-year cardiovascular risk; or (c) SBP > 140 mm Hg in participants aged <80 years with primary prevention high 10-year cardiovascular risk, or those eligible for secondary prevention;
Plasma lipid levels are above the threshold when (a) TC/HDL-ratio >8 (regardless of cardiovascular risk), or (b) LDL-cholesterol >2.5 mmol/l in participants with primary prevention high 10-year cardiovascular risk, or eligible for secondary prevention;
Physical inactivity was defined as not meeting the Dutch healthy physical activity guidelines (i.e. ‘Combinorm’: performing moderate physical activity ≥5 days per week for ≥30 min, and/or heavy physical activity ≥3 days per week for ≥20 min);
Heavy alcohol use was defined as alcohol intake ≥5 units/day for males, or ≥3 units/day for females.
Physical inactivity, smoking, plasma lipid levels and/or SBP above the guideline-recommended thresholds were the most frequently observed cardiovascular risk factors in both groups, with a higher prevalence among HIV-positive individuals.
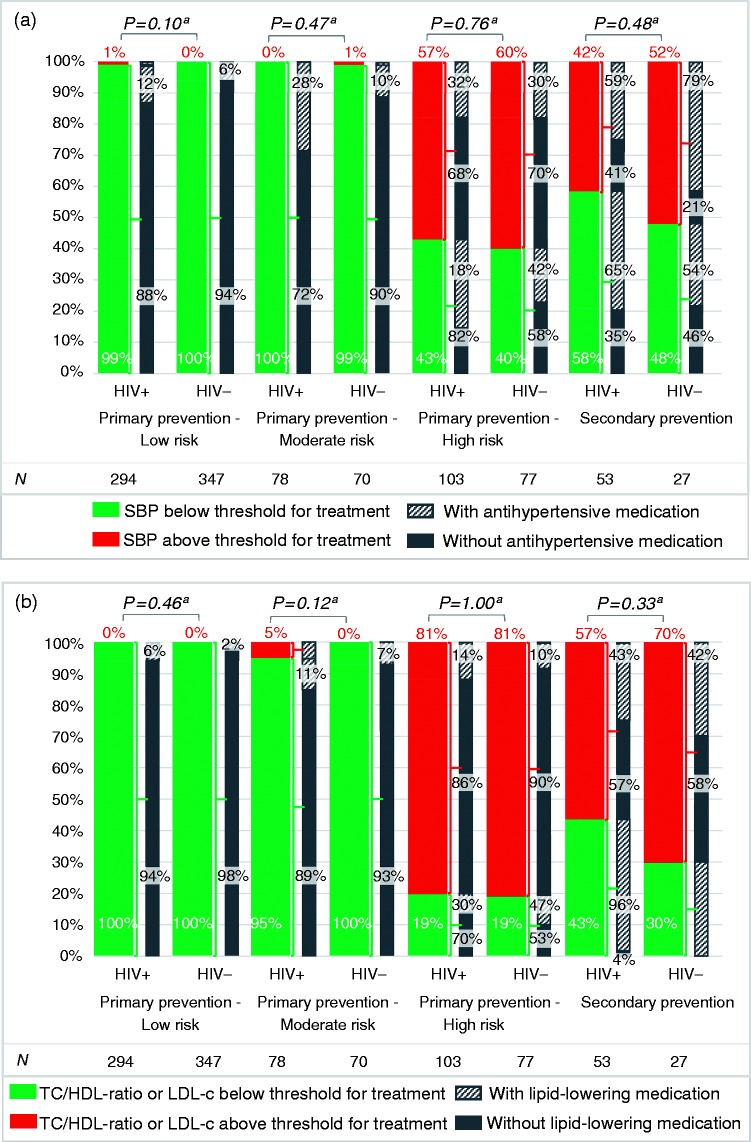
Hypertension: achievement of targets (Figure 2(a))
Figure 2.
Proportions of human immunodeficiency virus (HIV)-positive (HIV+) and HIV-negative (HIV–) individuals (a) below/above the recommended systolic blood pressure (SBP) and (b) cholesterol thresholds, and proportions using antihypertensive and lipid-lowering medication; (a) illustrates the proportion of individuals below (green) or above (red) the recommended SBP threshold for treatmentb among HIV-positive and HIV-negative participants, with the bars in grey indicating the proportion of individuals who do (upward diagonal lines) or do not (solid fill) use antihypertensive medication, stratified by cardiovascular risk group; (b) shows the proportion of individuals below (green) or above (red) the recommended cholesterol thresholds for treatmentc among HIV-positive and HIV-negative participants, with the bars in grey demonstrating the proportion of individuals who do (upward diagonal lines) or do Figure 2. Continued not (solid fill) use lipid-lowering medication, stratified by cardiovascular risk group. The numbers below the graph show the number of participants within each group. Data are presented as percentages. High risk: 10-year cardiovascular risk≥20% or 10-year cardiovascular risk 10–20% with additional cardiovascular risk factors; low risk: 10-year cardiovascular risk<10%; moderate risk: 10-year cardiovascular risk 10–20% without additional cardiovascular risk factors. LDL-c: low-density lipoprotein cholesterol; TC/HDL-ratio, total cholesterol/ high-density lipoprotein cholesterol ratio.
aFisher’s exact test; group comparison of HIV-positive versus HIV-negative individuals below or above the recommended threshold for treatment in each risk stratum.
bSBP thresholds differ between and within the different subgroups: (a) SBP > 180 mm Hg in participants with primary prevention low 10-year cardiovascular risk or moderate 10-year cardiovascular risk participants with no additional risk factors; or (b) SBP > 160 mm Hg in participants aged ≥80 years with primary prevention high 10-year cardiovascular risk; or (c) SBP > 140 mm Hg in participants aged <80 years with primary prevention high 10-year cardiovascular risk, or those eligible for secondary prevention;
cPlasma lipid levels are above the threshold when (a) TC/HDL-ratio>8 (regardless of cardiovascular risk), or (b) LDL-cholesterol>2.5 mmol/l in participants with primary prevention high 10-year cardiovascular risk, or eligible for secondary prevention.
Primary prevention
The majority of participants with a low/moderate cardiovascular risk had a SBP below the threshold for treatment. Of the participants at high cardiovascular risk 59 of 103 (57%) HIV-positive individuals and 46 of 77 (60%) controls had an indication for (intensification of) antihypertensive treatment (p = 0.76), of whom 40 (68%) and 32 (70%), respectively were not on antihypertensive medication.
Secondary prevention
Twenty-two of 53 (42%) HIV-positive individuals and 14 of 27 (52%) controls eligible for secondary prevention had an indication for (intensification of) antihypertensive treatment (p = 0.48), of whom nine (41%) and three (21%), respectively did not use antihypertensive medication. Beta-blockers were not used in 20 of 35 (57%) HIV-positive individuals and nine of 18 (50%) controls with prior coronary heart disease (p = 0.77).
All individuals on antihypertensive medication
Among all individuals using antihypertensive medication, regardless of CVD status or risk, blood pressure control was achieved in 61 of 121 (50%) HIV-positive individuals and 34 of 72 (47%) controls (p = 0.77).
Dyslipidaemia: achievement of targets (Figure 2(b))
Primary prevention
The majority of participants with a low/moderate cardiovascular risk had a TC/HDL-ratio below the threshold for treatment. Of the participants at high cardiovascular risk 83 of 103 (81%) HIV-positive individuals and 62 of 77 (81%) controls had an indication for (intensification of) lipid-lowering therapy (p = 1.00), of whom 71 (86%) and 56 (90%), respectively were not using lipid-lowering medication.
Secondary prevention
Thirty of 53 (57%) HIV-positive individuals and 19 of 27 (70%) controls eligible for secondary prevention had an indication for (intensification of) lipid-lowering therapy (p = 0.33), of whom 17 (57%) and 11 (58%), respectively, did not use lipid-lowering medication. Lipid-lowering medication was not used in 10 of 35 (29%) HIV-positive individuals and five of 18 (28%) controls with prior coronary heart disease (p = 1.00).
All individuals on lipid-lowering medication
Among all individuals using lipid-lowering medication, regardless of CVD status or risk, cholesterol control was achieved in 36 of 79 (46%) HIV-positive individuals and 23 of 42 (55%) controls (p = 0.35).
Glycaemic control
Primary prevention
Twenty-three of 475 (5%) HIV-positive individuals and 15 of 494 (3%) controls without a history of CVD had diabetes (p = 0.19), of whom 10 (43%) and 5 (33%), respectively did not use diabetes medication.
Secondary prevention
Diabetes was present in eight of 53 (15%) HIV-positive individuals, and five of 27 (19%) controls eligible for secondary prevention (p = 0.75), of whom one (13%) and one (20%), respectively did not use diabetes medication.
All individuals on diabetes medication
Among all participants using diabetes medication, regardless of CVD status or risk, glycaemic control was achieved in 18 of 20 (90%) HIV-positive individuals and seven of 14 (50%) controls (p = 0.017).
Use of antiplatelet/anticoagulant medication (secondary prevention)
Forty-five of 53 (85%) HIV-positive individuals and 17 of 27 (63%) controls in need of secondary prevention used antiplatelet/anticoagulant medication (p = 0.045).
Sensitivity analysis
A complete case analysis revealed results similar to those reported above (data not shown).
Discussion
Management of modifiable cardiovascular risk factors was suboptimal in both HIV-positive and HIV-negative individuals; this is surprising given the recent emphasis in the literature on CVD in HIV-positive individuals.1,2
Physical inactivity, smoking, dyslipidaemia and hypertension were the most prevalent cardiovascular risk factors. High prevalences of these risk factors have been described previously in HIV-positive populations.1,11–13,18–20 In our study, these risk factors were more frequently observed among HIV-positive participants, which also translated into higher predicted cardiovascular risk.
The main objective of our study was to investigate the use of preventive cardiovascular medication and achievement of targets as per Dutch CVRM guidelines in HIV-positive and HIV-negative individuals. A substantial proportion of those at high cardiovascular risk or with prior CVD had an indication for (intensification of) cardiovascular treatment. Suboptimal cardiovascular risk management has been previously reported both in the general and HIV-positive population.5,7–13,18,19,21–26 These studies in HIV-positive populations had smaller proportions of individuals with suppressed viraemia on cART and often focused on one aspect of cardiovascular risk management, whereas our study assessed management more comprehensively. Although over 90% of HIV-positive individuals in our study population had suppressed viraemia – and were thus adherent to cART – we found it striking that (effective) use of cardiovascular medication was poor. Compared to controls, HIV-positive individuals had an equally suboptimal achievement of cholesterol and blood pressure targets, despite biennial monitoring at the HIV outpatient clinic. Healthcare consumption is unknown among controls, some of whom might not be in care nor aware of their cardiovascular risk. This could potentially have resulted in lower levels of achievement of targets.
No data were available concerning patient or care-provider barriers to effective cardiovascular risk management. Contra-indications or adverse events might be a reason to quit preventive medication, in particular statins. Statins are generally well tolerated, but it remains uncertain whether their safety and efficacy is similar in HIV-positive individuals.27,28 Inadequate cardiovascular risk assessment by physicians, uncertainty about applying guidelines to specific populations, lack of time, polypharmacy and patient-related barriers such as side-effects, reduced motivation when perceived cardiovascular risk is lower than estimated risk, and pill burden may also contribute.29–31
Our study has a number of limitations, including that part of the collected data (i.e. cardiovascular medication use and various cardiovascular risk factors) was self-reported. Though we validated all self-reported diagnoses of CVD,2 medication use was not validated systematically. Among HIV-positive individuals with an indication for cardiovascular medication and no self-reported medication use, we validated medication prescription using hospital records, and found under-reporting in 3% of participants. Medical charts were not available for cardiovascular medication review in HIV-negative controls.
Blood pressure, glucose and lipid levels might be overestimated due to the cross-sectional design and use of non-fasting blood samples. Also, the cross-sectional design of the analysis may not account for small changes in Dutch CVRM guidelines (updated in 2011) or initial steps of cardiovascular risk management. In addition, no data were collected regarding smoking cessation attempts. However, we performed an additional analysis using two-year follow-up data (data not shown), showing no major changes. Furthermore, the SCORE risk equation was developed in the general population and does not account for HIV-serostatus. Since CVD prevalence is higher among HIV-positive compared to HIV-negative individuals after adjustment for traditional risk factors,1,2 SCORE might have underestimated cardiovascular risk in HIV-positive individuals. Lastly, the analysis did not have a single primary endpoint but aimed to provide an overview of different aspects of cardiovascular risk management. Values of p should therefore be interpreted with caution.
Our study also has a number of strengths. First of all, data collection was executed in a systematic and detailed manner. Thereby, our study provides a complete overview of cardiovascular risk management in a contemporary, largely virologically suppressed HIV-positive population. Furthermore, enrolment of HIV-negative controls with similar demographic and behavioural characteristics is a major strength, which allows us to better study any HIV-specific effects over and above those exerted by demographic and lifestyle factors. Of note, due to the specific procedure for the selection of the HIV-negative control group these results are not likely to be representative of the general population in The Netherlands.
Although women and people from African descent were underrepresented in this study, we expect the reported results to be generalisable to other high-income settings with unrestricted access to cART. Although one should realise that national guidelines were applied in this analysis, the Dutch guidelines are rather comparable to other European guidelines.6,32 The American College of Cardiology (ACC)/American Heart Association (AHA) guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults is different in several aspects, for example, it has abandoned dose titration to achieve specific LDL-c or non- high-density lipoprotein cholesterol (HDL-c) targets based on the absolute risk, and uses a distinct risk equation.33 The results regarding cholesterol control might therefore not be similarly relevant in settings where the ACC/AHA guideline is used, but undertreatment of dyslipidaemia remains an important observation even in those settings.
Conclusion
In summary, we conclude that cardiovascular risk, management of blood pressure and cholesterol, and use of cardiovascular preventive medication can be much improved in both HIV-positive and HIV-negative individuals. Since HIV-positive individuals have a higher prevalence and risk of developing CVD, suboptimal cardiovascular risk management is especially worrisome in these individuals, and requires the attention of HIV physicians and general practitioners, as well as HIV-positive individuals themselves.
Supplementary Material
Acknowledgements
The authors wish to thank Judith Schouten, Eveline Verheij, Sebastiaan Verboeket, Barbara Elsenga, Aafien Henderiks, Maartje Dijkstra, Jane Berkel, Sandra Moll, Marjolein Martens, Maja Totté, Laura del Grande and Tessa Kruijer for running the AGEhIV study programme and capturing their data with such care and passion. They thank Aafien Henderiks and Hans-Erik Nobel for their advice on logistics and organisation at the Academic Medical Center. They thank Yolanda Ruijs-Tiggelman, Lia Veenenberg-Benschop, Sima Zaheri, Mariska Hillebregt and Ahmed el Berkaoui at the HIV Monitoring Foundation for their contributions to data management.They also thank all HIV-physicians and HIV-nurses at the Academic Medical Center, and the Public Health Service Amsterdam personnel for their efforts to include the HIV-positive and HIV-negative participants into the AGEhIV Cohort Study.In addition, the authors thank all study participants without whom this research would not be possible.
This study was presented in part at the 20th International Workshop on HIV and Hepatitis Observational Databases, Budapest, Hungary, April 2016, and the International Workshop on Co-morbidities and Adverse Drug Reactions in HIV, New York City, USA, September 2016.
Author contribution
MV, MP and PR contributed to the conception and design of the study. RZ, FW, KK and MP contributed to data acquisition. RZ, FW and KK contributed to data analysis. RZ, FW, IV, KK, JH, MP and PR contributed to data interpretation. RZ drafted the manuscript. MV, FW, IV, KK, JH, MP and PR critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RZ has received travel grants from Bristol-Myers Squibb and Gilead Sciences, and was a speaker at an event sponsored by Gilead Sciences for which her institution received remuneration. MV through his institution has received independent scientific grant support from Janssen and MSD. He has served on scientific advisory boards for Abbvie, Bristol-Myers Squibb, Gilead, and Johnson and Johnson. He serves on the data safety monitoring committee for ViiV Healthcare. He has received travel grants from Bristol-Myers Squibb, Pfizer, Gilead Sciences, and Johnson and Johnson. FW has received travel grants from Gilead Sciences, ViiV Healthcare, Boehringer Ingelheim, Abbvie, and Bristol-Myers Squibb. KK has received travel grants from Gilead Sciences and ViiV Healthcare, and was a speaker at an event sponsored by Gilead Sciences and served on a scientific advisory board for Gilead Sciences for which her institution received remuneration. PR through his institution has received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals Inc., Merck & Co, Bristol-Myers Squibb and ViiV Healthcare; he has served on scientific advisory board for Gilead Sciences; he serves on data safety monitoring committee for Janssen Pharmaceuticals Inc.; chaired a scientific symposium by ViiV Healthcare, for which his institution has received remuneration. For the remaining authors no conflicting interests were declared.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by The Netherlands Organization for Health Research and Development (ZonMW) together with AIDS Fonds (grant numbers 300020007 and 2009063, respectively). Additional unrestricted scientific grants were received from Gilead Sciences; ViiV Healthcare; Janssen Pharmaceutica N.V.; Bristol-Myers Squibb and Merck & Co.None of these funding bodies had a role in the design or conduct of the study, the analysis and interpretation of the results, the writing of the report, or the decision to publish.Ilonca Vaartjes was supported by a grant from the Dutch Heart Foundation (grant DHF project ‘Facts and Figures’).
References
- 1.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173: 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV cohort study. Clin Infect Dis 2014; 59: 1787–1797. [DOI] [PubMed] [Google Scholar]
- 3.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1: CD004816–CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutch College of General Practitioners. M84: NHG-Standaard Cardiovasculair Risicomanagement, https://www.nhg.org/standaarden/volledig/cardiovasculair-risicomanagement (2011, accessed 14 December2016).
- 5.Balder JW, Scholtens S, de Vries JK, et al. Adherence to guidelines to prevent cardiovascular diseases: The LifeLines cohort study. Neth J Med 2015; 73: 316–323. [PubMed] [Google Scholar]
- 6.Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011; 217: 3–46. [DOI] [PubMed] [Google Scholar]
- 7.Kotseva K, De Bacquer D, De Backer G, et al. Lifestyle and risk factor management in people at high risk of cardiovascular disease. A report from the European Society of Cardiology European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE) IV cross-sectional survey in 14 European regions. Eur J Prev Cardiol 2016; 23: 2007–2018. [DOI] [PubMed] [Google Scholar]
- 8.Achelrod D, Gray A, Preiss D, et al. Cholesterol- and blood-pressure-lowering drug use for secondary cardiovascular prevention in 2004–2013 Europe. Eur J Prev Cardiol 2017; 24: 426–436. [DOI] [PubMed] [Google Scholar]
- 9.Clement ME, Park LP, Navar AM, et al. Statin utilization and recommendations among HIV- and HCV-infected veterans: A cohort study. Clin Infect Dis 2016; 63: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freiberg MS, Leaf DA, Goulet JL, et al. The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid lowering medication. J Gen Intern Med 2009; 24: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinsch N, Neuhaus K, Esser S, et al. Are HIV patients undertreated? Cardiovascular risk factors in HIV: Results of the HIV-HEART study. Eur J Prev Cardiol 2012; 19: 267–274. [DOI] [PubMed] [Google Scholar]
- 12.Myerson M, Poltavskiy E, Armstrong EJ, et al. Prevalence, treatment, and control of dyslipidemia and hypertension in 4278 HIV outpatients. J Acquir Immune Defic Syndr 2014; 66: 370–377. [DOI] [PubMed] [Google Scholar]
- 13.Shahmanesh M, Schultze A, Burns F, et al. The cardiovascular risk management for people living with HIV in Europe: How well are we doing? AIDS 2016; 30: 2505–2518. [DOI] [PubMed] [Google Scholar]
- 14. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35: S64–S71. [DOI] [PMC free article] [PubMed]
- 15.Dutch College of General Practitioners. M01: NHG-Standaard Diabetes mellitus type 2, https://www.nhg.org/standaarden/samenvatting/diabetes-mellitus-type-2 (2013, accessed 14 December 2016).
- 16.Combinorm (Dutch healthy physical activity guideline), https://www.volksgezondheidenzorg.info/sport/kernindicatoren/beweeg-en-zitgedrag#definitie–node-normen-en-adviezen-voor-sport-en-bewegen/ (accessed 14 December 2016).
- 17.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur Heart J 2003; 24: 987–1003. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein KA, Armon C, Buchacz K, et al. Provider compliance with guidelines for management of cardiovascular risk in HIV-infected patients. Prev Chronic Dis 2013; 10: E10–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suchindran S, Regan S, Meigs JB, et al. Aspirin use for primary and secondary prevention in human immunodeficiency virus (HIV)-infected and HIV-uninfected patients. Open Forum Infect Dis 2014; 1: ofu076–ofu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schäfer J, Young J, Calmy A, et al. High prevalence of physical inactivity among patients from the Swiss HIV Cohort Study. AIDS Care 2017, pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 21.De Socio GV, Ricci E, Parruti G, et al. Statins and aspirin use in HIV-infected people: Gap between European AIDS Clinical Society guidelines and clinical practice: The results from HIV-HY study. Infection 2016; 44: 589–597. [DOI] [PubMed] [Google Scholar]
- 22.Manner IW, Baekken M, Oektedalen O, et al. Hypertension and antihypertensive treatment in HIV-infected individuals. A longitudinal cohort study. Blood Press 2012; 21: 311–319. [DOI] [PubMed] [Google Scholar]
- 23.Monroe AK, Fu W, Zikusoka MN, et al. Low-density lipoprotein cholesterol levels and statin treatment by HIV status among multicenter AIDS cohort study men. AIDS Res Hum Retroviruses 2015; 31: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nüesch R, Wang Q, Elzi L, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV-infected patients: Swiss HIV Cohort Study (SHCS). J Acquir Immune Defic Syndr 2013; 62: 396–404. [DOI] [PubMed] [Google Scholar]
- 25.Schulte-Hermann K, Schalk H, Haider B, et al. Impaired lipid profile and insulin resistance in a cohort of Austrian HIV patients. J Infect Chemother 2016; 22: 248–253. [DOI] [PubMed] [Google Scholar]
- 26.Hanna DB, Jung M, Xue X, et al. Trends in nonlipid cardiovascular disease risk factor management in the Women’s Interagency HIV Study and association with adherence to antiretroviral therapy. AIDS Patient Care STDs 2016; 30: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med 2009; 150: 301–313. [DOI] [PubMed] [Google Scholar]
- 28.Boccara F, Miantezila Basilua J, Mary-Krause M, et al. Statin therapy and low-density lipoprotein cholesterol reduction in HIV-infected individuals after acute coronary syndrome: Results from the PACS-HIV lipids substudy. Am Heart J 2017; 183: 91–101. [DOI] [PubMed] [Google Scholar]
- 29.Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation 2005; 111: 499–510. [DOI] [PubMed] [Google Scholar]
- 30.Van Peet PG, Drewes YM, Gussekloo J, et al. GPs’ perspectives on secondary cardiovascular prevention in older age: A focus group study in the Netherlands. Br J Gen Pract 2015; 65: e739–e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz M, Laurinavicius AG, Franco FGM, et al. Calculated and perceived cardiovascular risk in asymptomatic subjects submitted to a routine medical evaluation: The perception gap. Eur J Prev Cardiol 2015; 22: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 32.European AIDS Clinical Society (EACS) treatment guidelines, version 8.1, http://www.eacsociety.org/files/guidelines_8.1-english.pdf (October 2016, accessed 14 December 2016).
- 33.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S1–S45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.