Abstract
Purpose
The purpose of this study was to describe the auditory phenotype of a large cohort with Smith–Magenis syndrome (SMS), a rare disorder including physical anomalies, cognitive deficits, sleep disturbances, and a distinct behavioral phenotype.
Method
Hearing-related data were collected for 133 individuals with SMS aged 1–49 years. Audiogram data (97 participants) were used for cross-sectional and longitudinal analyses. Caregivers completed a sound sensitivity survey for 98 individuals with SMS and a control group of 24 unaffected siblings.
Results
Nearly 80% of participants with interpretable audiograms (n = 76) had hearing loss, which was typically slight to mild in degree. When hearing loss type could be determined (40 participants), sensorineural hearing loss (48.1%) occurred most often in participants aged 11–49 years. Conductive hearing loss (35.2%) was typically observed in children aged 1–10 years. A pattern of fluctuating and progressive hearing decline was documented. Hyperacusis was reported in 73.5% of participants with SMS compared with 12.5% of unaffected siblings.
Conclusions
This study offers the most comprehensive characterization of the auditory phenotype of SMS to date. The auditory profile in SMS is multifaceted and can include a previously unreported manifestation of hyperacusis. Routine audiologic surveillance is recommended as part of standard clinical care.
Smith–Magenis syndrome (SMS) is a rare congenital disorder commonly caused by a microdeletion of chromosome 17p11.2 that contains the retinoic acid induced 1 gene (RAI1) or, less often, by a mutation in RAI1 (Gropman, Elsea, Duncan, & Smith, 2007). Since its initial description in 1982, SMS has been characterized by physical anomalies, cognitive deficits, sleep disturbances, and a distinct behavioral phenotype (Smith, Dykens, & Greenberg, 1998a; Smith et al., 1986). The estimated incidence of SMS is 1:15,000–25,000 births worldwide, affecting boys and girls from various racial and ethnic groups (Elsea & Girirajan, 2008; Greenberg et al., 1991; Smith et al., 1998a). SMS is thought to be underdiagnosed, despite a distinct phenotype, in part because diagnostic signs in early childhood are subtle and may be overlooked (Greenberg et al., 1991; Gropman, Duncan, & Smith, 2006; Smith et al., 1998a). Clinical recognition typically leads to diagnosis of SMS, but molecular cytogenetic testing or fluorescence in situ hybridization is required to confirm the microdeletion or gene sequencing to identify a mutation in RAI1 (Smith et al., 2012).
Individuals with SMS often exhibit mild cranial facial anomalies, including midface hypoplasia, brachycephaly, relative prognathism and dental anomalies, synophrys (confluent eyebrow), frontal bossing, and low or posteriorly rotated ears (Greenberg et al., 1991; Greenberg et al., 1996; Smith et al., 1986; Tomona, Smith, Guadagnini, & Hart, 2006). Although those affected by SMS have varying degrees of intellectual disability, most exhibit mild or moderate cognitive dysfunction (Greenberg et al., 1991; Martin, Wolters, & Smith, 2006). Speech–language skills are impaired in children with SMS, and expressive language lags behind receptive language (Elsea & Girirajan, 2008). Vocal polyps and nodules often occur (Greenberg et al., 1996) with up to 82% exhibiting a hoarse, deep voice in later years (Greenberg et al., 1991). Swallowing and feeding difficulties and delayed gross and fine motor skills occur frequently (Gropman et al., 2006).
More than 90% of patients with SMS exhibit self-injurious behaviors, such as head banging, biting, hitting, picking of nails (onychotillomania), and inserting foreign objects into body orifices (polyembolokoilamania; Dykens & Smith, 1998; Martin et al., 2006). Maladaptive behaviors, such as attention seeking, impulsiveness, hyperactivity, and aggression, are common. Distinct stereotypic behaviors include self-hugging or hand licking and hand flipping in states of joy or excitement. Temper tantrums and attention deficits correlate with the syndrome's known sleep disturbances (Dykens & Smith, 1998), which the vast majority of those with SMS manifest (Boudreau et al., 2009; Greenberg et al., 1991, 1996; Gropman, Duncan, & Smith, 2005; Gropman et al., 2006).
The clinical presentation of SMS is distinct but not dissimilar from other syndromes involving facial dysmorphisms and behavioral manifestations. Because of the phenotypic overlap with Down, Angelman, and Prader–Willi syndromes, SMS may go unrecognized in infancy (Gropman et al., 2006). During childhood, delayed speech and language and stereotypic behaviors may lead to the concomitant diagnosis of autism spectrum disorder (Gropman et al., 2006; Laje et al., 2010).
In the original delineation of SMS, both conductive and sensorineural hearing losses were noted in six of nine cases although audiometric data were not included (Smith et al., 1986). Subsequent clinical series (Girirajan et al., 2006; Greenberg et al., 1991; Potocki, Shaw, Stankiewicz, & Lupski, 2003) and meta-analyses (Edelman et al., 2007; Gamba et al., 2011) describing the overall clinical phenotype of persons with SMS list hearing loss as a frequent manifestation, occurring in approximately two thirds of reported cases. The loss has been historically considered mild in most cases (Greenberg et al., 1996) and attributed to a high prevalence of chronic and recurrent otitis media associated with SMS (Elsea & Girirajan, 2008; Smith, Dykens, & Greenberg, 1998b). Frequent otitis media may be related to Eustachian tube dysfunction and craniofacial anomalies, such as a shallow nasopharynx, observed in persons with SMS (Di Cicco et al., 2001).
Although there is sufficient evidence that hearing loss is part of the SMS phenotype, there has been a lack of detail regarding audiometric findings, and operational definitions of degree and type of hearing loss have not been specified. This prevents a clear understanding of the hearing loss associated with SMS and obstructs attempts to replicate methodology and findings across studies. In clinical and educational settings, it is useful to have an understanding of the syndrome's typical audiometric characteristics, especially in the context of patients who have many comorbid conditions and who may be difficult to test. Furthermore, we know very little about the progression or resolution of hearing loss over time in persons with SMS, which has important implications for surveillance and intervention. We hypothesize that hearing will change over time and, in particular, that there may be fluctuations in hearing as bouts of otitis media wax and wane.
Hyperacusis, an inappropriate or exaggerated intolerance to sound that is not typically uncomfortable or threatening (Klein, Armstrong, Greer, & Brown, 1990), was identified as a potential problem for individuals with SMS in the early stages of our work (Smith et al., 2007). Autism spectrum disorder, which can co-occur with SMS (Laje et al., 2010), has a known association with sound sensitivity (Rosenhall, Nordin, Sandstrom, Ahlsen, & Gillberg, 1999), and persons with SMS may be similarly affected.
The purpose of the current study was to expand and characterize comprehensively the auditory phenotype of SMS in a large and diverse cohort and, for the first time, report on longitudinal outcomes for hearing in a subset of patients. A secondary goal was to explore and further characterize the manifestation of hyperacusis in individuals with SMS.
Method
Participants
Retrospective and prospective audiological data were collected as part of a multidisciplinary natural history study from the National Human Genome Research Institute at the National Institutes of Health (NIH) in Bethesda, Maryland. The Institutional Review Board at the National Human Genome Research Institute approved this study, and informed consent was obtained from participants' guardians. One hundred twenty-seven participants were enrolled in protocol NCT00013559, 01-HG-0109 at the NIH Clinical Center or the off-site SMS research clinic at Camp Breakaway, San Remo, New South Wales, Australia, between 2001 and 2014. Audiograms from six additional adult participants with SMS seen at the care facility Elwyn (Pennsylvania) were provided for inclusion in this study by the fourth author (BMF) with appropriate guardian consent.
Audiometric Assessments
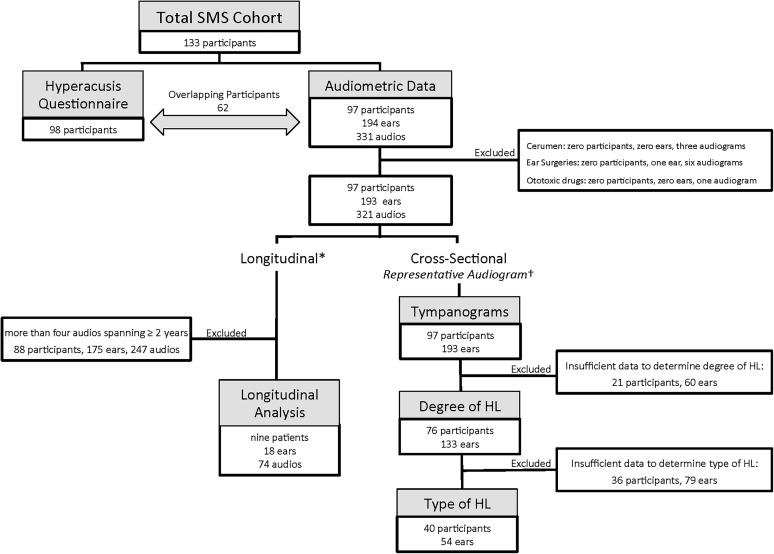
The audiometric portion of our study included 97 participants (56 female and 41 male participants) with confirmed SMS (17p11.2 microdeletion: n = 92, RAI1 mutation: n = 5) ranging in age from 3 months to 49 years (M = 10.5 years; Mdn = 8 years). A total of 331 audiograms from 194 ears were analyzed (see Figure 1).
Figure 1.
Flowchart detailing the data exclusion process for individuals with Smith–Magenis syndrome (SMS) and final sample sizes used in various analyses. HL = hearing loss. *Required at least four audiograms over, minimally, a 2-year period. †On the basis of the most recent and most complete audiogram.
Audiometric evaluations at the NIH were conducted using age- and ability-appropriate methods and, when possible, consisted of air conduction (250–8000 Hz) and bone conduction (250–4000 Hz) pure-tone thresholds and tympanometry. Previous and subsequent audiometric assessments conducted at outside facilities also were included. In order to assess the auditory phenotype directly attributable to SMS, audiometric data from ears with cerumen impaction, prior otologic surgeries other than myringotomies with pressure-equalizing tubes, and exposure to potentially ototoxic drugs were excluded from all analyses (see Figure 1, upper right box). Analyses of degree and type of hearing loss did not include data that were considered unreliable by the examining audiologist or data that were incomplete. Our initial acquisition of audiometric data for 97 participants, 194 ears, was reduced as outlined in Figure 1. Audiometric threshold data from participants who met inclusionary criteria ranged from a single test (n = 32) to multiple tests spanning 2 to 28 years (n = 44).
Classifications and criteria for degree and type of hearing loss were adapted using a framework from Mazzoli et al. (2003) and are presented in Table 1. Degree of hearing loss was calculated for each ear using a four-frequency (500, 1000, 2000, and 4000 Hz) pure-tone average (4F-PTA) by air conduction. When thresholds were obtained in the sound field, results were assumed to represent sensitivity in only the better-hearing ear. Because our cohort was predominantly pediatric, 15 dB HL was selected as the upper limit of normal hearing acuity (Clark, 1981).
Table 1.
Classifications and criteria for degree and type of hearing loss, tympanometry and hearing symmetry, and longitudinal hearing change.
| Classification | Criteria | |
|---|---|---|
| Degree of HL a | ||
| None | ≤ 15 dB HL | |
| Slight | > 15 and ≤ 25 dB HL | |
| Mild | > 25 and ≤ 40 dB HL | |
| Moderate | > 40 and ≤ 70 dB HL | |
| Severe | > 70 and ≤ 95 dB HL | |
| Profound | > 95 dB HL | |
| Type of HL b | ||
| None | AC ≤ 15 dB HL | |
| Conductive | AC >15 dB HL; BC ≤ 15 dB HL; ABG > 10 dB | |
| Mixed | BC > 15 dB HL; ABG > 10 dB | |
| Sensorineural | AC > 15 dB HL; ABG ≤ 10 dB | |
| Unknown | AC > 15 dB HL; unknown BC | |
| Tympanometry | Adult | Child (3–5 years) |
| Normal c | 0.3–1.4 cc; > −100 daPa | 0.2–0.9 cc; > −100 daPa |
| Immobile (flat) | No mobility, no peak | No mobility, no peak |
| Eustachian tube Dysfunction | < −100 daPa | < −100 daPa |
| Hypermobility | > 1.4 cc, > −100 daPa | > 0.9 cc, > −100 daPa |
| Hypomobility | < 0.3 cc, > −100 daPa | < 0.2 cc, > −100 daPa |
| HL asymmetry | > 10-dB difference in at least two consecutive frequencies (of 0.25, 0.5, 1, 2, 4, and 8 kHz) with better-hearing ear > 15 dB 4F-PTA (AC). | |
| Longitudinal change d | ||
| Stable | No significant fluctuation, improvement, or progression | |
| Improvement | 10-dB improvement at any three frequencies, 15-dB improvement at any two frequencies, or ≥ 20-dB improvement at one frequency | |
| Progressive | 10-dB decline at any three frequencies, 15-dB decline at any two frequencies, or ≥ 20-dB decline at any one frequency | |
| Fluctuating | Interim audiogram shows 10-dB change at any three frequencies, 15-dB change at any two frequencies, or ≥ 20-dB change at any one frequency, and final audiogram does not show significant improvement or progression from baseline | |
| Fluctuating/improvement | Interim audiogram shows 10-dB change at any three frequencies, 15-dB change at any two frequencies, or ≥ 20-dB change at any one frequency, and final audiogram shows significant improvement from baseline | |
| Fluctuating/progressive | Interim audiogram shows 10-dB change at any three frequencies, 15-dB change at any two frequencies, or ≥ 20-dB change at any one frequency, and final audiogram shows significant progression from baseline | |
Note. Three frequencies (0.5, 1, and 2 kHz). Four frequencies (0.5, 1, 2, and 4 kHz). HL = hearing loss; AC = air conduction; BC = bone conduction; ABG = air–bone gap; PTA = pure-tone average. 4F-PTA = four-frequency pure-tone average.
On the basis of 4F-PTA by AC.
On the basis of three-frequency pure-tone average for AC and BC or, less commonly, by speech threshold in ears with some degree of HL by 4F-PTA, ABG.
Required a minimum of four audiograms spanning at least 2 years.
If a participant had hearing loss by 4F-PTA, type of hearing loss was determined by calculating the difference between the three-frequency (500, 1000, and 2000 Hz) pure-tone averages for air and bone conduction (i.e., air–bone gap). When bone conduction thresholds were not obtained, type of hearing loss was categorized as unknown. In cases with air–bone gaps and non–ear-specific unmasked bone conduction thresholds, hearing loss type was assigned to the better-hearing ear. This conservative approach allowed identification of seven ears with evidence of conductive hearing loss that otherwise would have been labeled unknown. In cases with missing or incomplete pure-tone data, air–bone gaps for speech thresholds were used as a proxy to determine type of hearing loss (n = 8 ears).
A representative audiogram, defined as the most recent and most complete audiometric evaluation, was designated for each participant and used for all cross-sectional analyses. The same audiogram was used to determine hearing symmetry, defined in Table 1. Longitudinal hearing sensitivity in the worse-hearing ear, on the basis of the 4F-PTA by air conduction, was evaluated for participants with four or more ear-specific audiograms spanning at least 2 years. Longitudinal changes in hearing, including progressions, fluctuations, improvements, and stability, were categorized using definitions shown in Table 1 (Madden, Halsted, Benton, Greinwald, & Choo, 2003). Tympanometry was classified using peak admittance and middle ear pressure according to age-appropriate reference ranges (Margolis & Heller, 1987, see Table 1).
Hyperacusis Questionnaire
Parents or guardians completed a two-page loudness sensitivity questionnaire, adapted from a survey developed to assess sound sensitivity in persons with Williams syndrome (Cohen, Marriage, & Rosen, 2006), for 98 participants with SMS (58 female and 40 male participants) aged 8 months to 49 years (M = 10.75 years, Mdn = 9.5 years). Questionnaires also were completed for 24 unaffected siblings (9 female and 15 male siblings) aged 3 to 21 years (M = 9.83 years, Mdn = 8.5 years). To reduce the possibility of parental bias, questionnaires were completed first for children with SMS and then distributed at a later time for the unaffected siblings. Questionnaires were initially distributed to the guardians of all previously enrolled participants via the postal service in 2006 and subsequently completed via an online portal or during the NIH visit. Although the survey referred to loudness sensitivity, we use sound sensitivity, noise intolerance, and hyperacusis interchangeably.
Current or past sound sensitivity was determined using a yes/no format with a multiple choice follow-up question addressing if the intolerance to sound had digressed, progressed, or remained unchanged over time. Caretakers also were asked about known contextual or emotional triggers and possible palliative strategies. Guardians were instructed to “check all that apply” to a list of 15 reactions their child(ren) may exhibit following a distressing sound(s). A Likert scale, with 0 representing no problem and 10 representing major problem, was used to rate the person's overall tolerance of sound as well as his or her current and past tolerance of 25 common environmental sounds.
Data Analyses
Data were analyzed using Microsoft Excel (version 14.5.1) and GraphPad Prism 6.0b (La Jolla, CA). Descriptive statistics were computed for hearing loss type and degree, tympanometry, and patterns of hearing loss progression. Simple linear regressions were applied to the cross-sectional reference audiogram data to approximate hearing loss progression by both air and bone conduction as a function of age. Outliers for age were determined using Grubbs' test, and coefficients of determination were calculated with and without identified outliers. Associations in the prevalence of hyperacusis for persons with SMS and typically developing siblings were investigated using a Fisher's exact test. Differences in severity of sound sensitivity between groups were explored using a Mann–Whitney U test. Statistical significance was set at p ≤ .05.
Results
Cross-Sectional Analysis of Auditory Function
Degree and type of hearing loss for individuals with sufficient audiological data are presented in Tables 2 and 3. Due to young age, cognitive impairments, and behavioral problems, sufficient pure-tone data to determine degree and type of hearing loss were not obtained on all participants, ears, or visits (see Figure 1).
Table 2.
Degree of hearing loss for 133 ears, 76 participants.
| Classification of HL degree | Total group (76 participants, 133 ears) | Age groups |
|||
|---|---|---|---|---|---|
| 1–5 years (20 participants, 26 ears) | 6–10 years (24 participants, 44 ears) | 11–20 years (24 participants, 47 ears) | 21–49 years (8 participants, 16 ears) | ||
| None | 37 (27.8) | 9 (34.6) | 14 (31.8) | 10 (21.3) | 4 (25) |
| Slight | 34 (25.6) | 5 (19.2) | 14 (31.8) | 11 (23.4) | 4 (25) |
| Mild | 42 (31.6) | 11 (42.3) | 8 (18.2) | 19 (40.4) | 4 (25) |
| Moderate | 18 (13.5) | 1 (3.8) | 8 (18.2) | 5 (10.6) | 4 (25) |
| Severe | 2 (1.5) | 0 | 0 | 2 (4.3) | 0 |
| Profound | 0 | 0 | 0 | 0 | 0 |
Note. Data are presented as n = ears (%). HL = hearing loss.
Table 3.
Type of hearing loss for 54 ears, 40 participants.
| Classification of HL type | Total (60 participants, 96 ears) | Age groups |
|||
|---|---|---|---|---|---|
| 1–5 years (15 participants, 17 ears) | 6–10 years (18 participants, 30 ears) | 11–20 years (20 participants, 37 ears) | 21–49 years (7 participants, 12 ears) | ||
| Unknown | 42 a (43.4) | 14 (82.4) | 14 (46.7) | 10 (27) | 4 (33.3) |
| Known | 54 b (55.7) | n = 3 | n = 16 | n = 27 | n = 8 |
| Conductive | 19 (35.2) | 3 (100) | 11 (68.8) | 4 (14.8) | 1 (12.5) |
| Mixed | 9 (16.7) | 0 | 2 (12.5) | 6 (22.2) | 1 (12.5) |
| Sensorineural | 26 (48.1) | 0 | 3 (18.8) | 17 (63) | 6 (75) |
Note. Data are presented as n = ears (%). HL = hearing loss.
42 ears, 38 participants.
54 ears, 40 participants.
Normal hearing sensitivity occurred in 27.8% of all ears (n = 133) and was present more often in the 1- to 5-year (34.6%) and 6- to 10-year (31.8%) age groups compared with older groups (see Table 2). Hearing loss occurred in 72.2% of ears across all age groups, ranging from slight to severe in degree and affecting 78.9% (n = 60) of individuals in at least one ear. Slight or mild hearing loss was observed most often (57.2%) and was consistently documented across all age groups. Moderate hearing loss was seen in only 13.5% of ears. Severe hearing loss was rare and was observed in one ear of two 19-year-old participants.
Sensorineural was the most common type of hearing loss and was documented in 48.1% of the 54 ears (40 participants) for which type could be determined (see Table 3). Although sensorineural hearing loss was not observed in the youngest age group and was rarely seen in those aged 6–10 years, it increased in frequency in those aged 11–20 years (63%) and 21–49 years (75%). Conductive hearing loss affected 35.2% of ears. It was more prevalent in those aged 1–5 and 6–10 years compared with those in the older age groups. Mixed hearing loss affected 16.7% of all ears with a known type of hearing loss and was not observed in the 1- to 5-year age group. Although type of hearing loss was unknown most often for the 1- to 5-year age group (82.4%), the unclassified type of hearing loss occurred in all age groups.
Symmetric hearing was observed in 26 of 57 participants (45.6%) who provided audiological data for both ears. Asymmetric hearing loss was observed in 10 participants (17.5%), and unilateral hearing loss was documented in another set of 10 participants (17.5%), with 11 participants (19.4%) having bilateral normal hearing thresholds (data not shown).
Tympanograms were obtained for 193 ears of 97 participants (see Table 4). Flat tympanograms were the most frequent finding (43.7%) and were associated with presumed middle ear effusion (24.4%), patent pressure-equalizing tubes (15.2%), and tympanic membrane perforation (4.1%). In the 1- to 5- and 6- to 10-year age groups, flat tympanograms occurred in 62% and 53.6% of ears, respectively. In both of these younger groups, flat tympanograms were most often caused by presumed middle ear effusion. In the older age groups, 11–20 and 21–49 years, normal tympanometry was more common, occurring in 60.8% and 50% of ears, respectively.
Table 4.
Tympanometry for 193 ears, 97 participants.
| Tympanogram classification | All ages (97 participants, 193 ears) | Age groups |
|||
|---|---|---|---|---|---|
| 1–5 years (33 participants, 66 ears) | 6–10 years (28 participants, 56 ears) | 11–20 years (26 participants, 51 ears) | 21–49 years (10 participants, 20 ears) | ||
| Normal | 74 (38.3) | 16 (24.2) | 17 (30.4) | 31 (60.8) | 10 (50) |
| Immobile (flat) | |||||
| Tubes | 29 (15.2) | 16 (24.2) | 12 (21.4) | 0 | 1 (5) |
| Perforation | 8 (4.1) | 3 (4.5) | 2 (3.6) | 3 (5.9) | 0 |
| Effusion | 47 (24.4) | 22 (33.3) | 16 (28.6) | 6 (11.8) | 3 (15) |
| ETD | 24 (12.4) | 7 (10.6) | 7 (12.5) | 7 (13.7) | 3 (15) |
| Hypermobile | 3 (1.6) | 0 | 2 (3.6) | 1 (1.9) | 0 |
| Hypomobile | 8 (4.1) | 2 (3) | 0 | 3 (5.9) | 3 (15) |
Note. ETD = Eustachian tube dysfunction.
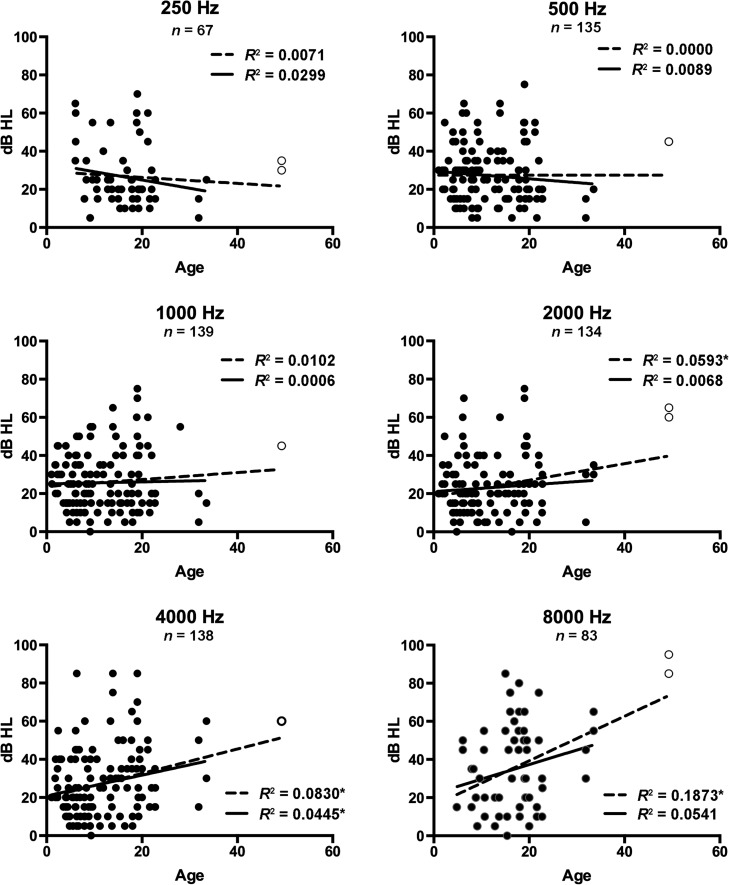
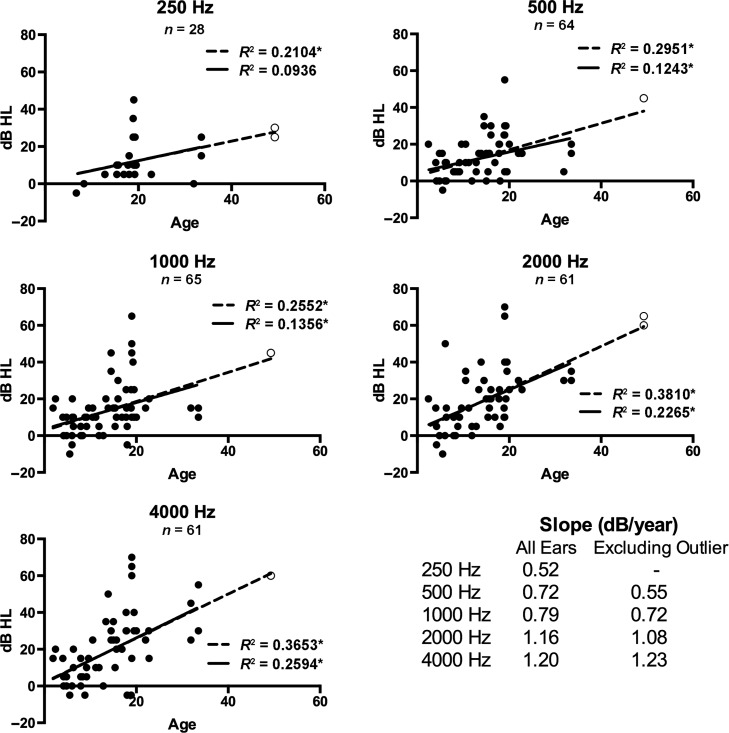
Simple linear regressions approximating change in air conduction pure-tone thresholds by age at individual test frequencies from all representative audiograms are presented in Figure 2. When all participants with SMS were included, significant hearing loss progression with age was observed at 2000, 4000, and 8000 Hz (p ≤ .05). Reanalysis of the data following removal of an age-based outlier (49 years) continued to reveal a significant coefficient of determination for 4000 Hz (p ≤ .05) with a slope of 0.54 dB per year. Significant progression of bone conduction thresholds with age was observed at 500, 1000, 2000, and 4000 Hz (p ≤ .05), independent of whether the age-based outlier was included or removed (see Figure 3). Threshold progression by bone conduction occurred at a rate of 0.55, 0.72, 1.08, and 1.23 dB per year at 500, 1000, 2000, and 4000 Hz, respectively (outlier removed).
Figure 2.
Bivariate scatterplots and linear regression lines for air conduction pure-tone thresholds for individual ears against age. Linear regressions for all ears are represented by the dotted line, and the subset excluding an outlier by age (open circle) is represented by the solid line. The coefficient of determination (R 2) is shown for each frequency. *Significant at p ≤ .05.
Figure 3.
Bivariate scatterplots and linear regression lines for bone conduction pure-tone thresholds for individual ears against age. Linear regressions for all ears are represented by the dotted line, and the subset excluding an outlier by age (open circle) is represented by the solid line. The coefficient of determination (R 2) is shown for each frequency. *Significant at p ≤ .05. Slopes for the significant linear regressions in dB per year are shown as an inset in the lower right corner.
Longitudinal Analysis of Hearing Sensitivity
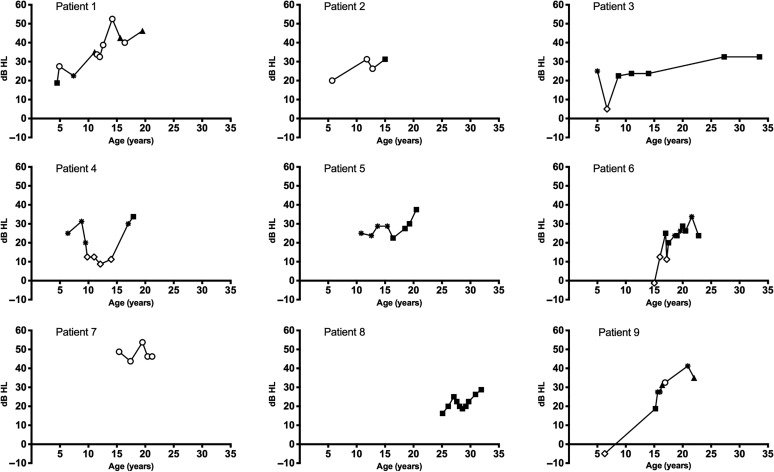
Longitudinal analyses of hearing were based on audiometric data from nine participants/18 ears (four female and five male participants) with four or more audiograms spanning 2 or more years. The 4F-PTA by air conduction and type of hearing loss for the worse-hearing ear over time are presented in Figure 4, and longitudinal categorizations of air conduction threshold changes on the basis of the full audiogram over time are presented in Table 5. Fluctuating, progressive hearing loss occurred most often, affecting eight individuals (88.9%) and 11 ears (61.1%). For seven of the participants, hearing fluctuation was associated with waxing and waning in the size of air–bone gaps. Progression of hearing loss was related to a decline in sensory hearing (bone conduction) thresholds (data not shown) with a sensorineural or mixed hearing loss at the last audiogram in eight of the nine participants with longitudinal data (see Figure 4).
Figure 4.
Four-frequency pure-tone averages by air conduction plotted longitudinally for the worse-hearing ear and type of hearing loss for the nine individuals with Smith–Magenis syndrome who had at least four threshold audiograms over, minimally, a 2-year period. Hearing loss type is indicated by symbols as follows: normal hearing = open diamond; conductive = open circle; mixed = filled triangle; sensorineural = filled square; unknown type = asterisk.
Table 5.
Categorical longitudinal change in pure-tone thresholds by air conduction.
| Patient | Duration of observation (years) | Age at first and last audiogram | Right ear | Left ear |
|---|---|---|---|---|
| 7 | 5.8 | 15 years, 5 months to 21 years, 2 months | Improving | Fluctuating improving |
| 8 | 6.8 | 25 years, 1 month to 31 years, 11 months | Stable | Fluctuating progressive |
| 6 | 7.8 | 15 years, 0 months to 22 years, 10 months | Fluctuating progressive | Fluctuating progressive |
| 2 | 9.3 | 5 years, 8 months to 15 years, 0 months | Fluctuating progressive | Progressive |
| 5 | 9.7 | 10 years, 10 months to 20 years, 6 months | Fluctuating progressive | Progressive |
| 4 | 11.5 | 6 years, 5 months to 17 years, 11 months | Fluctuating progressive | Fluctuating progressive |
| 1 | 15.0 | 4 years, 6 months to 19 years, 6 months | Fluctuating progressive | Fluctuating progressive |
| 9 | 15.7 | 6 years, 4 months to 22 years, 0 months | Fluctuating progressive | Progressive |
| 3 | 28.5 | 5 years, 0 months to 33 years, 6 months | Fluctuating | Fluctuating progressive |
Hyperacusis Questionnaire
Results of the loudness sensitivity questionnaire for participants with SMS are summarized in Table 6. Sound sensitivity was reported significantly more often in those with SMS (73.5%) as compared with their typically developing siblings (12.5%; Fisher's exact, p = .0001). Of the 26 participants with SMS who did not have oversensitivity or distress to loud sounds at the time of the questionnaire, five had a past history of hyperacusis (19.2%). Although typically developing siblings had a lower prevalence of hyperacusis, parents of the siblings reported one third (n = 7) had previously exhibited sound sensitivity. Of the 10 siblings with some degree of current or past hyperacusis, this resolved or improved for 70%, became worse for 10%, and remained unchanged for 20%. In contrast, participants with SMS rarely had resolution of the hyperacusis (1.4%); more often, sound sensitivity remained unchanged (58.3%).
Table 6.
Results of sound sensitivity questionnaire.
| Question | SMS | Siblings |
|---|---|---|
| Shows current oversensitivity or distress to sound a* | 72/98 (73.5) | 3/24 (12.5) |
| If not current, ever shown oversensitivity or distress | 5/26 (19.2) | 7/21 (33.3) |
| The problem has | ||
| Cleared up | 1/72 (1.4) | 4/10 (40) |
| Improved | 26/72 (36.1) | 3/10 (30) |
| Become worse | 3/72 (4.1) | 1/10 (10) |
| Remained unchanged | 42/72 (58.3) | 2/10 (20) |
| Triggers intolerance to sound (top 2) | ||
| Mood | 14/21 (66.7) | — |
| Tiredness | 10/21 (47.6) | — |
| Responses to distressing sounds (top 5) | ||
| Covers ears with hands | 62/75 (82.7) | — |
| Gets upset (but may not cry) | 45/75 (60) | — |
| Gets anxious or tense | 42/75 (56) | — |
| Cries | 32/75 (42.7) | — |
| Says something like “I don't like” | 34 /75 (45.3) | — |
| Awaken from sleep | 30/73 (41.1) | — |
| Anything make child's reaction worse (top 2) | 46/73 (63) | — |
| Mood | 23/46 (50) | — |
| Tiredness | 33/46 (71.7) | — |
| If warned, does child cope better | 45/75 (60) | — |
| If familiar, does child show less distress | 38/72 (52.8) | — |
| Anything help (top 3) | 46/72 (63.9) | — |
| Warning/explanation | 21/46 (45.7) | — |
| Avoidance/headphones | 22/46 (47.8) | — |
| Familiarity | 5/46 (10.9) | — |
| Severity of problem, median, range b * | 4, 0–10 | 0, 0–5 |
| Rating of distressing sounds, median, range | ||
| Fireworks | 6, 0–10 | 0, 0–3 |
| Balloon burst | 4, 0–10 | 0, 0–1 |
| Sudden shout | 3, 0–10 | 0, 0–4 |
| Jackhammer | 3, 0–10 | 0, 0–3 |
| Loud music | 2, 0–10 | 0, 0–5 |
| Thunder | 2, 0–10 | 0, 0–5 |
| Median number of distressing sounds (range) out of 24 | 10 (0–24) | 0.5 (0–5) |
Note. SMS = Smith–Magenis syndrome.
Data presented as number of “yes” responses/total number responding to question (% “yes”).
Data presented as median, range of Likert scores. — indicates data not reported.
p ≤ .05.
Hyperacusis in participants with SMS was usually triggered or made worse by mood or tiredness. Frequent reactions within the SMS cohort were “covers ears with hands” (82.7%), “gets upset (but may not cry)” (60%), and “gets anxious or tense” (56%). Parents of children with SMS also reported 41% would awaken from sleep due to sudden loud noises.
Both familiar and novel sounds were similarly distressing for individuals with SMS. Guardians reported that palliative strategies helped the child to prepare and cope; examples shown in Table 6 include explanations and warnings before exposure, dampening of sound through use of headphones or earplugs, and complete avoidance of the upsetting sounds.
The median severity of hyperacusis in the SMS group was 4 (on the basis of a Likert scale of 0–10 on which 0 = no problem and 10 = very major problem), which was significantly higher compared with that of the typically developing siblings who had a median score of 0 (Mann–Whitney U, p = .0001). Of the 24 sounds listed, participants with SMS reacted negatively to a median of 10 sounds versus siblings who were distressed by a median of 0.5 sounds.
Discussion
We have provided a detailed description of the prevalence of hearing loss and audiometric characteristics observed in persons with SMS in a large cohort using clearly defined criteria for degree and type of hearing loss. Our data show that hearing loss is a frequent manifestation of the SMS phenotype. On the basis of the representative audiogram, hearing loss affects up to 78.9% of individuals in at least one ear and occurs at a slightly higher rate than the previously reported prevalence of 62%–68% (Edelman et al., 2007; Gamba et al., 2011; Girirajan et al., 2006; Greenberg et al., 1991, 1996; Potocki et al., 2003). In addition, when we reviewed all audiograms of our 76 participants, 90.7% had hearing loss on at least one assessment. It is possible that our cutoff of 15 dB HL for normal hearing is more stringent than that used in other studies, accounting for the higher prevalence of hearing loss in our cohort. Similar to previous observations, hearing loss of a mild or lesser degree occurred most often; however, thorough comparison is not possible because degree of hearing loss is not operationally defined or adequately addressed in previous reports of hearing status in persons with SMS (Edelman et al., 2007; Gamba et al., 2011; Girirajan et al., 2006; Greenberg et al., 1991, 1996; Potocki et al., 2003).
Categorical hearing sensitivity on the basis of 4F-PTA criteria (see Table 1) could not be calculated for 21 persons in our original cohort of 97 individuals. Of these, 13 participants provided three-frequency pure-tone average (three persons, four ears) or speech thresholds (10 persons, 17 ears) that indicated the presence of hearing loss in 66.7% (14 ears), ranging from slight to moderate. Although these findings were determined using less than a 4F-PTA, results were comparable to those with sufficient data to determine hearing loss degree.
We observed a higher prevalence of sensorineural hearing loss than conductive hearing loss in our cohort, in contrast to prior reports (Greenberg et al., 1991; Potocki et al., 2003). The age of individual participants who received an audiometric evaluation in prior studies was not fully disclosed, nor were criteria for hearing loss type defined (Edelman et al., 2007; Gamba et al., 2011; Greenberg et al., 1991, 1996); therefore, a thorough comparison to address these discrepancies is not currently possible. We acknowledge that many of our participants, particularly the younger ones, did not have sufficient pure-tone data to identify hearing loss type but had tympanometric evidence of middle ear effusion and that we may have underestimated the prevalence of conductive hearing loss.
Middle ear dysfunction was frequent in our cohort of persons with SMS. This observation is consistent with earlier reports (Di Cicco et al., 2001; Edelman et al., 2007; Greenberg et al., 1996). More than half of the ears tested in the current study exhibited abnormal middle ear function, most frequently characterized by flat tympanograms (43.7%). Within our smaller group of individuals with known degree and type of hearing loss, however, the prevalence of flat tympanograms dropped to 27.5%. In comparison, flat tympanograms were observed in approximately 50% of ears for those with an unknown type of hearing loss. On the basis of this finding, we anticipate that a majority of participants with an unknown type of hearing loss may have conductive hearing loss and/or middle ear disease. Routine and careful assessment of middle ear function is important and, for most persons with SMS, routine surveillance by an otologist will be necessary.
Although cross-sectional analysis of hearing sensitivity by frequency revealed significant progression with age at only 4000 Hz by air conduction, sensory hearing (i.e., bone conduction) progressively declined with age at the majority of test frequencies (500, 1000, 2000, and 4000 Hz). These findings are corroborated by the longitudinal data (see Figure 4, Table 5), which most commonly showed a pattern of fluctuation and progression over time with a tendency for closure of air–bone gaps and progression of the sensory hearing loss component. To our knowledge, this is the first longitudinal analysis of hearing in persons with SMS, and future prospective studies including more adult participants over the age of 30 years are needed to refine and expand our understanding of long-term hearing outcomes in this population. Conservative management should consider that the risk for hearing loss in those with SMS is high and that the hearing may fluctuate and decline over time.
Fluctuating and minimal hearing loss can have a deleterious impact on language skills, auditory processing, academic performance, and social–emotional development (Tharpe & Bess, 1999; Yoshinaga-Itano, Johnson, Carpenter, & Stredler-Brown, 2008), all of which are affected by SMS itself. Although it is not possible to determine the exact interaction between the comorbid conditions characteristic of SMS, it is important that hearing status be established and taken into consideration in the development of management strategies and individualized educational programs. In particular, attention should be directed toward enhancing the auditory signal to ensure reception of speech. This could prove to be challenging in persons with SMS who may not tolerate physical devices, such as hearing aids; in these cases, systems such as sound-field amplification for the whole classroom may provide a solution.
Hyperacusis is an emotional response to distressing sounds and is not a pathology of the auditory pathway, specifically. Individuals with SMS who are prone to behavioral problems, emotional disturbances, and a range of sensory processing difficulties, including distractibility and difficulty functioning in the presence of noise (Hildenbrand & Smith, 2012), may be more inclined to react disproportionately to sound. Moreover, the sleep disturbance observed in SMS may potentiate any underlying propensity for a disproportionate emotional response to noise (Job, 1996). To our knowledge, this is the first article to specifically address hyperacusis in persons with SMS. Sound sensitivity in the general population ranges from 9% to 22% (Andersson, Lindvall, Hursti, & Carlbring, 2002; Rubenstein, Ahlqwist, & Bengtsson, 1996), which is comparable to the prevalence (12.5%) of hyperacusis reported in the typically developing siblings in this study. In comparison, hyperacusis was reported in 73.5% of individuals with SMS. There is no standard assessment tool for identifying hyperacusis, and there is a potential bias in our data if guardians of individuals with SMS perceived a suggestion that their child might react differently to sound than a typically developing sibling. Nonetheless, we propose that individuals with SMS are at risk for hyperacusis and that parents and families should be counseled regarding this topic. Identification of hyperacusis as a problem should result in and guide efforts to develop strategies to modify the environment and mitigate negative reactions to sounds.
Because we had such a small number of participants with RAI1 mutations and measured degree of hearing loss (four persons), a statistical comparison of hearing status to those with a chromosomal microdeletion was not conducted. In our cohort, hearing loss was less common among those with RAI1 mutations (37.5% of ears) as compared with those with 17p11.2 microdeletions (74.4% of ears, data not shown). Although these findings are similar to those observed in eight persons with RAI1 mutations by Edelman et al. (2007), a larger cohort of individuals is needed to better understand the role of RAI1 in the auditory system. Examining individual differences and overlap in the proteins encoded in the 17p11.2 region could lead to a better understanding of the effects of haploinsufficiency of MYO15A, an autosomal recessive nonsyndromic deafness gene (DFNB3) located in the 17p11.2 region (Liburd et al., 2001) and other genes that might influence auditory function.
Caveats to this study include an ascertainment bias toward young patients recruited in the initial years of the natural history study as well as families recruited at Camp Breakaway in Australia. Data from most of our older patients came from a single facility (Elwyn) with onsite access to audiology services. Longitudinal data may be inherently biased toward those with identified hearing loss seeking repeat hearing tests.
The American Speech-Language-Hearing Association criterion of 15 dB HL used as the upper limit of normal hearing (Clark, 1981) is conservative. Because even minimal hearing loss can affect classroom performance, communication abilities, and social skills (Bess, Dodd-Murphy, & Parker, 1998; Yoshinaga-Itano et al., 2008), we felt this was most appropriate for our largely pediatric cohort to ensure attention to this aspect of the phenotype.
SMS is a rare syndrome with a complex phenotype that includes a principal and unique behavioral component. As a consequence, large cohort studies are difficult, and persons with SMS can be challenging to test, often rendering data sets incomplete. Our analysis of hearing within a large cohort of persons with SMS expands current understanding of the auditory phenotype. In addition, understanding why hearing loss develops in persons with SMS and in individuals with a 17p11.2 microdeletion or, more generally, a contiguous gene deletion syndrome should contribute to understanding genotypic factors in the auditory pathway. It is important that parents or guardians, clinicians, and educators become aware of the possibilities and characteristics of hearing loss associated with SMS. Audiologic surveillance should be a standard part of clinical care for individuals with SMS throughout their life to facilitate early identification and intervention when necessary.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Department of Health and Human Services. A 2001 National Institutes of Health Clinical Center Bench-to-Bedside grant awarded to principal investigator/coauthor, A. C. M. Smith established the SMS multidisciplinary research team that laid the foundation for this research. The first author, M. A. Brendal, was supported by the National Institutes of Health Academy Enrichment Program within the Office of Intramural Training & Education. The authors would like to thank the individuals who took part in the study, their families, and their clinicians for dedicating their time. The authors also extend gratitude to Jennifer Bentley and Kethia Harding for their contributions to earlier phases of this study. We appreciate the careful review of the manuscript by Tracy Fitzgerald and Talah Wafa.
Funding Statement
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Department of Health and Human Services. A 2001 National Institutes of Health Clinical Center Bench-to-Bedside grant awarded to principal investigator/coauthor, A. C. M. Smith established the SMS multidisciplinary research team that laid the foundation for this research. The first author, M. A. Brendal, was supported by the National Institutes of Health Academy Enrichment Program within the Office of Intramural Training & Education.
References
- Andersson G., Lindvall N., Hursti T., & Carlbring P. (2002). Hypersensitivity to sound (hyperacusis): A prevalence study conducted via the Internet and post. International Journal of Audiology, 41, 545–554. [DOI] [PubMed] [Google Scholar]
- Bess F. H., Dodd-Murphy J., & Parker R. A. (1998). Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear and Hearing, 19, 339–354. [DOI] [PubMed] [Google Scholar]
- Boudreau E. A., Johnson K. P., Jackman A. R., Blancato J., Huizing M., Bendavid C., … Magenis R. E. (2009). Review of disrupted sleep patterns in Smith–Magenis syndrome and normal melatonin secretion in a patient with an atypical interstitial 17p11.2 deletion. American Journal of Medical Genetics Part A, 149A, 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. G. (1981). Uses and abuses of hearing loss classification. ASHA: A Journal of the American Speech-Language-Hearing Association, 23, 493–500. [PubMed] [Google Scholar]
- Cohen M., Marriage J., & Rosen S. (2006). Hearing impairment and sensitivity to sound in Williams syndrome. Paper presented at the Association for Research in Otolaryngology, Baltimore, MD. [Google Scholar]
- Di Cicco M., Padoan R., Felisati G., Dilani D., Moretti E., Guerneri S., & Selicorni A. (2001). Otorhinolaringologic manifestation of Smith–Magenis syndrome. International Journal of Pediatric Otorhinolaryngology, 59, 147–150. [DOI] [PubMed] [Google Scholar]
- Dykens E. M., & Smith A. C. M. (1998). Distinctiveness and correlates of maladaptive behaviour in children and adolescents with Smith–Magenis syndrome. Journal of Intellectual Disability Research, 42, 481–489. [DOI] [PubMed] [Google Scholar]
- Edelman E. A., Girirajan S., Finucane B., Patel P. I., Lupski J. R., Smith A. C. M., & Elsea S. H. (2007). Gender, genotype, and phenotype differences in Smith–Magenis syndrome: A meta-analysis of 105 cases. Clinical Genetics, 71, 540–550. [DOI] [PubMed] [Google Scholar]
- Elsea S. H., & Girirajan S. (2008). Smith–Magenis syndrome. European Journal of Human Genetics, 16, 412–421. [DOI] [PubMed] [Google Scholar]
- Gamba B. F., Vieira G. H., Souza D. H., Monteiro F. F., Lorenzini J. J., Carvalho D. R., & Morreti-Ferreira D. (2011). Smith–Magenis syndrome: Clinical evaluation in seven Brazilian patients. Genetics and Molecular Research, 10, 2664–2670. [DOI] [PubMed] [Google Scholar]
- Girirajan S., Vlangos C. N., Szomju B. B., Edelman E., Trevors C. D., Dupuis L., … Elsea S. H. (2006). Genotype-phenotype correlation in Smith–Magenis syndrome: Evidence that multiple genes in 17p11.2 contribute to the clinical spectrum. Genetics in Medicine, 8, 417–427. [DOI] [PubMed] [Google Scholar]
- Greenberg F., Guzzetta V., Montes de Oca-Luna R., Magenis R. E., Smith A. C., Richter S. F., … Lupski J. R. (1991). Molecular analysis of the Smith–Magenis syndrome: A possible contiguous-gene syndrome associated with del(17)(p11.2). American Journal of Human Genetics, 49, 1207–1218. [PMC free article] [PubMed] [Google Scholar]
- Greenberg F., Lewis R., Potocki L., Glaze D., Parke J., Killian J., … Lupski J. R. (1996). Multi-disciplinary clinical study of Smith–Magenis syndrome (deletion 17p11.2). American Journal of Medical Genetics, 62, 247–254. [DOI] [PubMed] [Google Scholar]
- Gropman A. L., Duncan W., & Smith A. C. M. (2005). Sleep disturbance in Smith–Magenis syndrome. Annals of Neurology, 58, Suppl 9, S128. [Google Scholar]
- Gropman A. L., Duncan W. C., & Smith A. C. M. (2006). Neurologic and developmental features of the Smith–Magenis syndrome (del 17p11.2). Pediatric Neurology, 34, 337–350. [DOI] [PubMed] [Google Scholar]
- Gropman A. L., Elsea S., Duncan W. C., & Smith A. C. M. (2007). New developments in Smith–Magenis syndrome (del 17p11.2). Current Opinion in Neurology, 20, 125–134. [DOI] [PubMed] [Google Scholar]
- Hildenbrand H. L., & Smith A. C. M. (2012). Analysis of the sensory profile in children with Smith–Magenis syndrome. Physical & Occupational Therapy in Pediatrics, 32, 48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job R. F. S. (1996). The influence of subjective reactions to noise on health effects of the noise. Environment International, 22, 93–104. [Google Scholar]
- Klein A. J., Armstrong B. L., Greer M. K., & Brown F. R. III (1990). Hyperacusis and otitis media in individuals with Williams syndrome. Journal of Speech and Hearing Disorders, 55, 339–344. [DOI] [PubMed] [Google Scholar]
- Laje G., Morse R., Richter W., Ball J., Pao M., & Smith A. C. M. (2010). Autism spectrum features in Smith–Magenis syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C, 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liburd N., Ghosh M., Riazuddin S., Naz S., Khan S., Ahmed Z., … Friedman T. B. (2001). Novel mutations of MYO15A associated with profound deafness in consanguineous families and moderately severe hearing loss in a patient with Smith–Magenis syndrome. Human Genetics, 109, 535–541. [DOI] [PubMed] [Google Scholar]
- Madden C., Halsted M., Benton C., Greinwald J., & Choo D. (2003). Enlarged vestibular aqueduct syndrome in the pediatric population. Otology & Neurotology, 24, 625–632. [DOI] [PubMed] [Google Scholar]
- Margolis R. H., & Heller J. W. (1987). Screening tympanometry: Criteria for medical referral. Audiology, 26, 197–208. [DOI] [PubMed] [Google Scholar]
- Martin S. C., Wolters P. L., & Smith A. C. M. (2006). Adaptive and maladaptive behavior in children with Smith–Magenis syndrome. Journal of Autism and Developmental Disorders, 36, 541–552. [DOI] [PubMed] [Google Scholar]
- Mazzoli M., Van Camp G., Newton V., Giarbini N., Declau F., & Parving A. (2003). Recommendations for the description of genetic and audiological data for families with nonsyndromic hereditary hearing impairment. Audiological Medicine, 1, 148–150. [Google Scholar]
- Potocki L., Shaw C. J., Stankiewicz P., & Lupski J. R. (2003). Variability in clinical phenotype despite common chromosomal deletion in Smith–Magenis syndrome [del(17)(p11.2p11.2)]. Genetics in Medicine, 5, 430–434. [DOI] [PubMed] [Google Scholar]
- Rosenhall U., Nordin V., Sandstrom M., Ahlsen G., & Gillberg C. (1999). Autism and hearing loss. Journal of Autism and Developmental Disorders, 29, 349–357. [DOI] [PubMed] [Google Scholar]
- Rubenstein B., Ahlqwist M., & Bengtsson C. (1996). Hyperacusis, headache, temporomandibular disorders and amalgam fillings: An epidemiological study. In Feich G. E. & Vernon J. A. (Eds.), Proceedings of the Fifth International Tinnitus Seminar (pp. 657–658), Portland, OR: American Tinnitus Association. [Google Scholar]
- Smith A. C. M., Bentley J., Zalewski C., Morse R., Introne W. J., & Brewer C. C. (2007, October). Hyperacusis in persons with Smith Magenis syndrome: Expanding the SMS phenotype. Paper presented at the American Society of Human Genetics, San Diego, CA. [Google Scholar]
- Smith A. C. M., Boyd K. E., Elsea S. H., Finucane B. M., Haas-Givler B., Gropman A., … Potocki L. (2012). Smith–Magenis Syndrome. In Pagon R. A., Adam M. P., Ardinger H. H., Bird T. D., Dolan C. R., Fong C. T., Smith R. J. H., & Stephens K. (Eds.), GeneReviews. Seattle, WA: University of Washington; Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK1310/ [Google Scholar]
- Smith A. C. M., Dykens E., & Greenberg F. (1998a). Behavioral phenotype of Smith–Magenis syndrome (del 17p11.2). American Journal of Medical Genetics, 81, 179–185. [DOI] [PubMed] [Google Scholar]
- Smith A. C. M., Dykens E., & Greenberg F. (1998b). Sleep disturbance in Smith–Magenis syndrome (del 17 p11.2). American Journal of Medical Genetics, 81, 186–191. [PubMed] [Google Scholar]
- Smith A. C. M., McGavran L., Robinson J., Waldstein G., Macfarlane J., Zonona J., … Magenis E. (1986). Interstitial deletion of (17)(p11.2p11.2) in nine patients. American Journal of Medical Genetics, 24, 393–414. [DOI] [PubMed] [Google Scholar]
- Tharpe A. M., & Bess F. H. (1999). Minimal, progressive, and fluctuating hearing losses in children. Characteristics, identification, and management. Pediatric Clinics of North America, 46, 65–78. [DOI] [PubMed] [Google Scholar]
- Tomona N., Smith A. C. M., Guadagnini J. P., & Hart T. C. (2006). Craniofacial and dental phenotype of Smith–Magenis syndrome. American Journal of Medical Genetics Part A, 140A, 2556–2561. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C., Johnson C., Carpenter K., & Stredler-Brown A. (2008). Outcomes of children with mild bilateral hearing loss and unilateral hearing loss. Seminars in Hearing, 29, 196–211. [Google Scholar]