Abstract
Purpose
This preliminary study on lingual–alveolar contact pressures (LACP) in people with amyotrophic lateral sclerosis (ALS) had several aims: (a) to evaluate whether the protocol induced fatigue, (b) to compare LACP during speech (LACP-Sp) and during maximum isometric pressing (LACP-Max) in people with ALS (PALS) versus healthy controls, (c) to compare the percentage of LACP-Max utilized during speech (%Max) for PALS versus controls, and (d) to evaluate relationships between LACP-Sp and LACP-Max with word intelligibility.
Method
Thirteen PALS and 12 healthy volunteers produced /t, d, s, z, l, n/ sounds while LACP-Sp was recorded. LACP-Max was obtained before and after the speech protocol. Word intelligibility was obtained from auditory–perceptual judgments.
Results
LACP-Max values measured before and after completion of the speech protocol did not differ. LACP-Sp and LACP-Max were statistically lower in the ALS bulbar group compared with controls and PALS with only spinal symptoms. There was no statistical difference between groups for %Max. LACP-Sp and LACP-Max were correlated with word intelligibility.
Conclusions
It was feasible to obtain LACP-Sp measures without inducing fatigue. Reductions in LACP-Sp and LACP-Max for bulbar speakers might reflect tongue weakness. Although confirmation of results is needed, the data indicate that individuals with high word intelligibility maintained LACP-Sp at or above 2 kPa and LACP-Max at or above 50 kPa.
The extent to which tongue strength is an important factor in the speech changes that occur from amyotrophic lateral sclerosis (ALS) is not well understood or agreed upon. A principal limiting factor in furthering the understanding of the relation between tongue strength and articulation in people with neurodegenerative disease has been reliance on nonspeech assessments of strength rather than on a metric of strength during speech itself. The main focus of the current study is to report preliminary data on lingual–alveolar contact pressure during speech (LACP-Sp) in ALS participants using measures obtained for six alveolar consonants.
Because ALS can include both upper motor neuron (UMN) and lower motor neuron (LMN) neurodegeneration, clinical features in the limbs as well as the head and neck can vary markedly. In terms of speech, people with ALS (PALS) are often described as having a mixed flaccid-spastic dysarthria (Darley, Aronson, & Brown, 1969). However, the type of dysarthria is expected to vary among PALS depending on where motor neuron damage is greatest. If the disease is mainly affecting UMNs, then spastic dysarthria symptoms are expected to predominate, whereas flaccid symptoms are expected when LMNs are disproportionately involved (Duffy, 2005). Muscle weakness can be present with UMN and with LMN involvement. Regardless of the type of dysarthria, identifiable changes to speech are expected in over 90% of PALS as the disease progresses (Chen & Garrett, 2005). Speech intelligibility is reduced, and 80% or more of PALS may ultimately need an alternative communication method to function in daily activities (Beukelman, Fager, & Nordness, 2011).
Using either pressure or force as an index of strength, several studies have documented reductions in isometric tongue strength in individuals with ALS (DePaul & Brooks, 1993; Dworkin, Aronson, & Mulder, 1980; Easterling, Antinoja, Cashin, & Barkhaus, 2013; Solomon, Clark, Makashay, & Newman, 2008). The reduction in isometric tongue strength may not be limited to those with bulbar symptoms, as evidenced in the study by Easterling et al. (2013). Their results indicated reductions in isometric pressure for participants with spinal and bulbar symptoms (but see Langmore and Lehman [1994], who found differences only for those with bulbar symptoms).
The literature is generally consistent, indicating that tongue strength during isometric pressing is reduced in PALS, particularly for those with bulbar symptoms. However, there is insufficient evidence in the current literature to support the conclusion that tongue weakness is a direct or principal cause of the articulatory degradation that is often present in PALS. Logic dictates that some minimally necessary strength is needed to move the mass of the tongue within the oral cavity in a timely manner to reach intended locations or to achieve a given shape to produce the intended speech sound. The specific value for the minimally necessary strength has not yet been determined, but ultimately, a paretic tongue would be incapable of producing useable speech. A point of contention, however, is whether the articulator weakness that is happening at something less than catastrophic levels is a cause of the degradation in phoneme production.
Skepticism about tongue strength as a meaningful parameter in articulatory changes in PALS arises from several sources. First, there is a reliance on measuring tongue strength in nonspeech oromotor movements. It is well established that nonspeech tasks often do not reflect the movements of the lips and tongue for speech production (Bunton, 2008; Forrest & Iuzzini, 2008; R. D. Kent, 2004) and that neural controls between speech and nonspeech movements differ (Memarian et al., 2012; Salmelin & Sams, 2002). Weismer (2006) eloquently presented the case against use of nonspeech tasks when trying to understand speech motor control, with a portion of the case notably focused on the concept of task specificity when selecting the behavior to be studied. That is, if the intention is to learn about and understand speech, experimental procedures should utilize speech stimuli.
A second source of skepticism is that the limited data regarding the relationship between articulator strength in isometric (i.e., nonspeech) tasks and speech measures such as intelligibility in PALS are not encouraging. DePaul and Brooks (1993) and Langmore and Lehman (1994) have reported that the association between nonspeech strength measures and speech measures such as intelligibility in PALS is weak, with stronger associations between articulator movement speed and speech measures. Green and colleagues have summarized that articulator speed of movement does decrease in PALS, and this reduction may be a sensitive predictor of the motor changes that occur (Green et al., 2013; Kuruvilla, Green, Yunusova, & Hanford, 2012; Mefferd, Green, & Pattee, 2012; Yunusova et al., 2010).
A third reason for skepticism is that speech production is a low-force task utilizing a small proportion of the physiological strength range. Early speculation was that less than 20% of a speaker’s maximum capabilities were needed for speech production (Müller, Milenkovic, & MacLeod, 1985), and more recent studies suggest that speech occurs even lower in the physiological range (< 10%) for healthy adults (Searl, 2007; Searl, Evitts, & Davis, 2007). This allows for the possibility that maximum isometric strength could be decreased substantially while still allowing sufficient force or contact pressure generation for speech production. Several studies have indicated that PALS can produce useable, intelligible speech even though the neurodegeneration from the disease has already begun or continues to progress. For example, a decline in speaking rate often precedes a noticeable drop in speech intelligibility (Ball, Willis, Beukelman, & Pattee, 2001), with the rate reduction attributable to an increase in vowel durations and pause time (Green, Beukelman, & Ball, 2004; Tjaden & Turner, 2000; Yorkston, Strand, Miller, Hillel, & Smith, 1993).
Overall, compelling evidence that tongue strength reduction is a contributing cause of the articulatory deficits in PALS is absent. Despite that, strength continues to receive attention as a potential therapeutic target in ALS, but mostly with a focus on the limbs (Bello-Haas et al., 2007; Drory, Goltsman, Goldman Reznik, Mosek, & Korczyn, 2001); see Plowman (2015) for a review relevant to speech. One limiting factor in supporting or refuting articulator strength as a meaningful parameter in the production of speech in PALS is that essentially no information is available about the strength utilized during speech production itself. The ability to index strength during speech has been difficult because available transducers placed in the mouth have been large enough to disturb speech. We have demonstrated use of a miniature transducer to measure articulatory contact pressures in adults without neurological disease (Searl, 2003; Searl et al., 2007; Searl & Evitts, 2013) and in adults after total laryngectomy (Searl, 2007). The arrangement allows for relatively quick adaptation to a thin pseudopalate and limited disturbance to the speech as detected acoustically or by auditory–perceptual judgement (Searl, 2003; Searl, Evitts, & Davis, 2006). Task specificity as presented by Weismer (2006) was a guiding principle for completing the current study, wherein we felt it was possible to gain information on articulatory activity during speech rather than relying on isometric pressing tasks.
Prior to investment of significant resources into a large-scale study, we attempted to address a few modest aims to either encourage or discourage more intensive study of tongue strength during speech in PALS. Aim 1 was to determine whether PALS demonstrate lingual fatigue from participating in the speech task that we devised. We were unsure how long it might take the patients to complete the task or if it would induce tongue fatigue. Aim 2 was to compare LACP-Sp between PALS and healthy volunteers. The hypothesis was that LACP-Sp would be lower for PALS. This was a speculative hypothesis. Others have reported reductions in size and speed of articulator movements in patients with various neurological diseases, including ALS (Hirose, Kiritani, & Sawashima, 1982; R. D. Kent, Netsell, & Bauer, 1975; Kuruvilla et al., 2012; Mefferd et al., 2012; Yunusova, Weismer, Westbury, & Lindstrom, 2008; Yunusova et al., 2010). We reasoned that reductions in speed and amplitude of articulatory movement could lessen the impact pressures between tongue and palate. We chose to study consonants, as opposed to vowels, because many consonants involve contact between tongue and palate, and some magnitude of contact pressure is presumably required for air pressure buildup and/or release or to hold a site of constriction for frication noise to be generated. In addition, disruptions to phonetic features point to disruptions in consonant production in ALS. For example, R. D. Kent et al. (1990) and J. F. Kent et al. (1992) reported several alterations to phoneme production in men and women with ALS. Although not all of the phonetic alterations were directly relatable to tongue–palate contact, one of the more common disturbances involved changes in stop versus fricative distinction, implying the possibility of tongue function change during the consonant. Weismer, Mulligan, and DePaul (1986) (as cited in J. F. Kent et al., 1992) also noted that spirantization of stops has been detected in PALS, which might be consistent with altered tongue–palate contact. Last, a body of work involving acoustic analysis of speech in PALS, particularly one that focused on formant transition duration and slope, is indicative of articulatory impairments of phoneme production within a syllable unit, with a common finding being a shallower F2 slope consistent with a decreased rate of articulator movement (J. F. Kent et al., 1992; Weismer, Jeng, Laures, Kent, & Kent, 2001; Weismer, Martin, Kent, & Kent, 1992; Yunusova et al., 2012).
Aim 3 was to compare the maximum isometric pressure during LACP (LACP-Max) between PALS and healthy volunteers. This aim was included primarily as a means of linking our results to the small but extant literature on tongue strength in ALS that has used isometric tasks. On the basis of that literature, we expected PALS to have lower LACP-Max. Aim 4 was to evaluate differences in the percentage of the maximum physiological range utilized during speech (%Max) for PALS versus healthy volunteers. As with Aim 2, we considered this a speculative aim, but one that may be of interest in future studies focused on issues such as sense of effort during speaking. The hypothesis was that %Max would be higher for the PALS with bulbar symptoms compared with the controls and the PALS with spinal-only symptoms. That is, we expected those with bulbar symptoms to be working higher within their physiologic range (i.e., disproportionate decrease in LACP-Max compared with LACP-Sp). The final aim, Aim 5, was to determine if there is a relationship between the contact pressures (LACP-Sp and LACP-Max) and word intelligibility. The hypothesis was that both contact pressure measures would be significantly correlated to intelligibility. This supposition was based principally on the idea that tongue contact pressures may be related to overall bulbar disease severity, and overall disease severity, in turn, is likely related to intelligibility. Whereas a future goal is to more directly address the role of tongue strength as a causal factor for articulatory changes in PALS, this initial study was designed to first determine whether the measure was obtainable from PALS, compare speech contact pressures to healthy controls, and to assess whether the measure had any relevance to more global aspects of ALS speech such as intelligibility.
Method
Participants
Two groups participated. The first were PALS (n = 13) who had a firm clinical diagnosis of ALS from the treating neurologist on the ALS team at the authors’ institution. The diagnosis for the team is based on the El Escorial criteria (Brooks, 1994) and includes patient history, physical and neurological exam, and electrophysiological testing as well as additional tests as deemed necessary by the neurologist. Inclusion criteria were as follows: (a) ≥40 years of age, (b) functional hearing for conversation in a quiet room (self-reported), and (c) use of verbal communication for at least 25% of communication needs (self-reported). Individuals were excluded if they were dependent on augmentative or alternative communication devices for more than 75% of their communication (self-assessed), were ventilator dependent, had a positive history of stroke, head injury, or other neurological conditions besides ALS, or had undergone surgeries to the head and neck that might negatively affect speech. Demographics and other information about this group are in Table 1. Information about their speech is given in Table 2. The determination of clinical bulbar symptoms was derived from the clinic notes by the treating neurologist and speech-language pathologist (SLP) on the ALS team. The ALS participants were classified into two groups: (a) those with only spinal symptoms noted clinically (ALS-S), and (b) those with bulbar symptoms ± spinal symptoms (ALS-B). This categorization was based on the clinical notes of the SLP on the ALS team. Two measures were used to describe the overall severity of the speech deficit: a clinical rating of dysarthria severity using the scale from Yorkston et al. (1993), and speaking rate (words per minute). The clinical rating of dysarthria severity was logged in the patient’s clinical record by the SLP on the ALS team. ALS Functional Rating Scale scores were not available from the clinical notes for this group of participants. For speaking rate, sentences that were recorded for LACP-Sp measures were displayed in PRAAT software (Boersma & Weenink, 2011), and the sentence duration was measured. The mean words per minute were calculated for each participant using all recorded samples. Speaking rate and dysarthria severity data are given in Table 2, along with additional descriptors of speech extracted from the ALS team report from the SLP. This team assessment happened within 2 weeks of data collection. The type of dysarthria present also was taken from this team report.
Table 1.
Demographics and medical information for the participants with amyotrophic lateral sclerosis (ALS). Severity of bulbar symptoms was from the clinical notes of the speech-language pathologist.
| Participant | Age (years) | Sex | Months since ALS Diagnosis | Clinical bulbar symptoms? | Comorbidities | Taking Riluzole? | PEG? | FVC% |
|---|---|---|---|---|---|---|---|---|
| B1 | 65 | M | 46 | Dysarthria (mild–moderate) | Hypertension, hypercholesterolemia | N | Y | 58 |
| Dysphagia (mild–moderate) | ||||||||
| B2 | 64 | F | 25 | Dysarthria (mild) | Fibromyalgia | N | N | 74 |
| Dysphagia (mild) | ||||||||
| B3 | 71 | M | 32 | Dysarthria (mild–moderate) | Ventricular tachycardia, hypothyroidism | N | N | 66 |
| Dysphagia (mild) | ||||||||
| B4 | 71 | F | 44 | Dysarthria (minimal) | Hypothyroidism | Y | Y | 30 |
| Dysphagia (severe) | ||||||||
| B5 | 77 | F | 7 | Dysarthria (moderate) | Asthma, hypercholesterolemia | N | Y | 46 |
| Dysphagia (severe) | ||||||||
| B6 | 68 | M | 56 | Dysarthria (mild–moderate) | — | N | Y | 43 |
| Dysphagia (mild–moderate) | ||||||||
| B7 | 44 | F | 80 | Dysarthria (moderate–severe) | — | Y | N | 47 |
| Dysphagia (minimal) | ||||||||
| B8 | 57 | M | 18 | Dysarthria (mild–moderate) | Hypertension | N | N | 63 |
| Dysphagia (minimal) | ||||||||
| S1 | 64 | M | 13 | — | — | N | N | 75 |
| S2 | 46 | M | 18 | — | — | N | N | 104 |
| S3 | 71 | M | 33 | — | Hypertension, restless leg syndrome, asthma, diabetes, hypercholesterolemia | N | N | 61 |
| S4 | 57 | F | 13 | — | Asthma, hypercholesterolemia, hypothyroidism | N | N | 73 |
| S5 | 58 | M | 12 | — | Hypercholesterolemia, Hypertension | Y | N | 97 |
| M | 63 | 8 M | 31 months | 8 with bulbar symptoms | ||||
| SD | 9 | 5 F | 48 months | 5 without bulbar symptoms |
Note. PEG = percutaneous endoscopic gastrostomy, FVC% = forced vital capacity as percentage of predicted value, B = ALS subject with bulbar symptoms, S = ALS subject with spinal symptoms.
Table 2.
Measures and descriptions of speech for each participant.
| Participant | Speaking rate (wpm) | ALS severity scale–speech a | Clinical speech characteristics b | Dysarthria type c | Word intelligibility (%) |
|---|---|---|---|---|---|
| C1 | 161 | — | — | — | 97.7 |
| C2 | 155 | — | — | — | 98.8 |
| C3 | 198 | — | — | — | 98.2 |
| C4 | 180 | — | — | — | 97.1 |
| C5 | 174 | — | — | — | 98.8 |
| C6 | 179 | — | — | — | 99.4 |
| C7 | 224 | — | — | — | 99.4 |
| C8 | 172 | — | — | — | 98.2 |
| C9 | 159 | — | — | — | 98.8 |
| C10 | 214 | — | — | — | 98.8 |
| C11 | 215 | — | — | — | 97.7 |
| C12 | 177 | — | — | — | 99.4 |
| B1 | 99 | 6 | Reduced loudness, imprecise articulation, strained-breathy voice, tongue atrophy and fasciculation, mild hypernasality, reduced speaking rate | Mixed (flaccid >spastic) | 71.9 |
| B2 | 136 | 8 | Reduced loudness, imprecise articulation, strained voice, tongue fasciculation | Mixed (spastic > flaccid) | 83.0 |
| B3 | 106 | 6 | Imprecise articulation, breathy voice, tongue atrophy, and fasciculation | Flaccid | 69.6 |
| B4 | 151 | 8 | Imprecise articulation, mono-loudness | Spastic | 85.4 |
| B5 | 96 | 6 | Hoarse voice, reduced loudness, slow rate, mild hypernasality, imprecise articulation, tongue atrophy, and fasciculation | Mixed (flaccid > spastic) | 77.2 |
| B6 | 71 | 5 | Breathy voice, imprecise articulation, decreased loudness, slow rate | Mixed (spastic = flaccid) | 64.9 |
| B7 | 62 | 4 | Imprecise articulation, slow rate, moderate hypernasality | Flaccid | 48.5 |
| B8 | 121 | 7 | Imprecise articulation, slow rate, strained voice, hypernasality, tongue atrophy, and fasciculation | Mixed (spastic > flaccid) | 81.3 |
| S1 | 155 | 10 | No speech changes noted; sensation of phlegm in throat | — | 95.3 |
| S2 | 139 | 10 | No speech or swallow changes noted | — | 93.0 |
| S3 | 160 | 10 | No speech or swallow changes noted | — | 92.1 |
| S4 | 132 | 10 | No speech or swallow changes noted | — | 94.7 |
| S5 | 131 | 10 | No speech or swallow changes noted | — | 91.8 |
Note. wpm = words per minute, ALS = amyotrophic lateral sclerosis, C = control, B = bulbar, S = spinal.
Yorkston, Strand, Miller, Hillel, & Smith, 1993 (10-point scale with 10 = normal and 1 = nonvocal).
Extracted from the participant’s clinical record.
When mixed dysarthria was noted, the predominant type was indicated, or the types were marked as equal.
A group of healthy adults that closely matched the age and gender distribution of the ALS group comprised the control group. Demographics for the control group are given in Table 3. Informed written consent was obtained from all participants, and the study was approved by the Human Subjects Research Committee at the authors’ institution.
Table 3.
Demographics for the healthy volunteers (controls).
| Participant | Age (years) | Sex | Comorbidities |
|---|---|---|---|
| 1 | 61 | F | — |
| 2 | 66 | M | Shingles |
| Diabetes | |||
| 3 | 56 | F | Hypercholesterolemia |
| 4 | 56 | M | — |
| 5 | 47 | F | Migraines |
| 6 | 78 | F | Hypercholesterolemia |
| Diabetes | |||
| 7 | 73 | F | Hypercholesterolemia |
| 8 | 68 | M | Depression |
| Restless leg syndrome (RSL) | |||
| 9 | 66 | M | Hypercholesterolemia |
| Hypertension | |||
| Benign prostatic hyperplasia | |||
| 10 | 72 | M | Hypertension |
| Atrial fibrillation | |||
| Coronary artery disease | |||
| 11 | 73 | M | Diabetes |
| Hypercholesterolemia | |||
| 12 | 77 | F | Hypothyroidism |
| Hypertension | |||
| M | 66 | 6 M | |
| SD | 10 | 6 F |
Instrumentation for Obtaining LACP
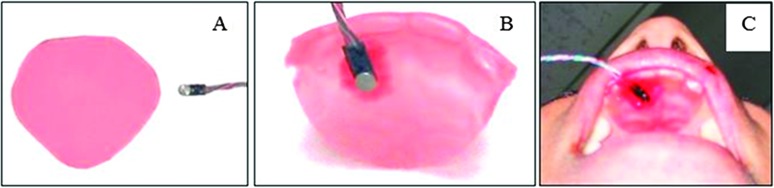
LACP-Sp and LACP-Max were measured by placing a miniature pressure transducer (Entran EPI-BO, Measurement Specialties, Toulouse, France) inside the mouth on a thin wax mold of the four upper incisors and the hard palate immediately posterior to these teeth. The transducer had a 2-mm-diameter sensing surface with a housing unit that was 2 mm × 6 mm × 1 mm with four wire leads (Figure 1). Response characteristics have been described previously (Searl, 2003). The wax appliance was made from base-plate wax sheets used in dentistry to line dentures. The material was first cut into a curvilinear triangle with enough width to span from upper lateral incisor to upper lateral incisor. The wax was held over a hot air stream from a heat gun to soften. The softened wax was then molded to cover the front four teeth and conform to the hard palate by pressing with the fingers and tongue. The result was a palatal mold extending 15–18 mm from where the central incisors emerge from the alveolar ridge and spanning from the right to the left lateral incisors (Figure 1). The transducer was attached to the palatal mold with a thin film of red dental wax. The position of the transducer was 4 mm posterior to the central incisor and 4 mm to the right of midline (adjusted as need to accommodate rugae in the region). In this manner, the middle and posterior hard palate were not covered, but a firm placement of the transducer was achieved. The transducer was calibrated prior to each data collection session following standard procedures by applying a known pressure so that voltage outputs could be converted to absolute pressures in kilopascals (kPa). The unilateral transducer position allowed for detection of tongue–palate pressure for all six experimental phonemes, including /s/ and /z/, where midline contact is not anticipated.
Figure 1.
Transducer and palatal mold used to obtain the articulatory contact pressure measures. (A) Base plate wax cut into curvilinear triangle before custom molding. (B) Transducer attached to palatal mold with red dental wax. (C) Palatal–transducer arrangement in the oral cavity.
Output from the transducer was amplified and routed to one channel of a digital recording system (PowerLab 8/35, ADInstruments, Colorado Springs, CO), where it was low-pass filtered at 50 Hz, displayed, and archived for later analysis. A second channel of the recording system was used to record an audio signal from a headset microphone (AKG C410) positioned 6 cm from the corner of the mouth at a 45° azimuth.
Stimuli for LACP-Sp
The six lingual–alveolar consonants in spoken English were targeted for study: /t, d, s, z, l, n/. Each consonant was placed in the initial position of a real word produced in a carrier phrase, “a [target word] is ____” (Table 4). The final word in each carrier phrase varied in order to produce a linguistically meaningful sentence, but the syllables immediately preceding and following the target word were held constant. Each sentence was produced five times in a fully randomized sequence. The phoneme set consisted of sounds for which alveolar ridge contact was anticipated. The set included stops (± voice), fricatives (± voice), a nasal, and a glide. The transducer placement off midline was intentional to capture tongue-to-palate contact for this set of sounds, including /s/ and /z/, for which midline contact was not expected.
Table 4.
Stimulus list with the consonant of interest underlined in each phrase.
| Stimulus list |
|---|
| “a tug is down” |
| “a sock is down” |
| “a lock is down” |
| “a dock is tall” |
| “a zig is down” |
| “a knock is loud” |
Procedures for Obtaining LACP-Sp
Data were gathered in one visit lasting approximately 60 min. The ALS participants were encouraged to select a time of day when they typically felt most rested and alert. After obtaining informed consent, the custom wax mold was constructed, and the transducer was attached. The instrumentation was left in the mouth for several minutes to allow acclimation. During this time, the participant was encouraged to create oral suction in the mouth and press on the wax with the tongue and lips to help it closely conform to the palate and teeth. The fit was visually inspected by research personnel to avoid situations of poor fit or slippage off the teeth.
The participant was seated in front of a computer screen on which instructions and stimuli were presented using E-Prime software. Three tongue press tasks were completed (described in the section below), followed by the speech stimuli presented in random order, and finally three more tongue presses. Additional tasks involving the lips and bilabial sound production were also part of the protocol, but those data are not analyzed here. The pace of stimulus presentation was under the control of research personnel. In general, a 3-s interval between each sentence was targeted, but this was at times extended to accommodate saliva swallows, spontaneous comments from the subject, and so forth. Research personnel monitored all tasks and productions, asking for repetitions if there were errors due to misreading. Participants were faced away from the data collection monitor. The average time to complete this sequence was 12 min for the PALS and 7 min for the controls.
Procedure for LACP-Max
Participants completed three LACP-Max trials before completing the speech stimuli and three after completing the speech stimuli. They were instructed to push as forcefully as they could with their tongue up against the anterior palate and to hold that maximal press until instructed to stop. Research personnel timed the press task and signaled the participant to stop after 5 s. Others have utilized a shorter duration for isometric tongue press tasks (C. L. Lazarus et al., 2000; Stierwalt & Youmans, 2007). Our initial testing with a few PALS indicated that some participants demonstrated a pressure curve similar to the anticipated curve, namely, a rapid pressure rise and then a gradual decline; however, some PALS did not reach their maximum contact pressure until 3 or more seconds into the pressing task. Because the intention was to obtain the maximum isometric pressure, we opted for the longer press time in order to capture that maximum value for those who built pressure in a time frame extending beyond 2–3 s. Verbal encouragement to “press hard” was offered repeatedly during the trials. Participants were not allowed to view the transducer output during the task. A 30-s rest interval was enforced between trials. The reason for obtaining three trials before and three after the speech stimuli was to help gauge the extent to which the person may have become fatigued from the start of the speech task to the end. A bite block was not utilized for this task. Results from Solomon and Munson (2004) indicated that there was no difference in maximum isometric tongue elevation between a no-bite-block and a 2-mm-bite-block condition; as bite-block size increased to 5 mm, 10 mm, and 15 mm, maximum pressure decreased. They recommended that, when measuring maximum isometric pressure of tongue elevation, a bite block should not be used, or if one is used, it should be of limited height.
Word Intelligibility Assessment
Speech intelligibility was assessed using the Word Intelligibility Test (Yorkston, Beukelman, & Hakel, 1996). The software for this test was used to generate a unique “word in phrase” list that was printed out for each participant. The carrier phrase for each word was, “Say ____ again.” The signal from the headset microphone was routed to a digital audio recorder to record the productions for later playback and judgment by listeners.
Word intelligibility was determined through an auditory–perceptual listening experiment. The listeners were three female graduate students between the ages of 22 and 25 years. They were not involved in any other aspect of data collection or measurement and had only minimal, incidental contact with individuals with dysarthria. The digital audio recordings of the word intelligibility for each participant (ALS and controls) were presented in random order to each listener. Prior to presentation, the intensity of the audio signal was normalized using PRAAT software in order to limit audibility as a factor in the intelligibility assessment. A listener was seated in a sound booth and listened to each carrier phrase played through a speaker with the volume adjusted to a listener-selected comfort level. The phrase structure of the participant recordings (“Say ____ again.”) was shown to them on paper. It was explained that they should listen for the target word and orthographically write down what they heard. A listener was allowed to replay a sentence as needed, although they were instructed to try to make an initial judgment about the word on the first playing as often as possible. There were 57 sentences per subject (n = 25) for a total of 1,425 judgments. A subset of the stimuli in the generated word lists consisted of duplicates for assessing reliability (approximately 5% of the stimulus set or 71 productions). Each participant’s set of sentences was judged independently by each listener. The average percent words intelligible was calculated for a participant by averaging the three listener’s scores.
Measurements
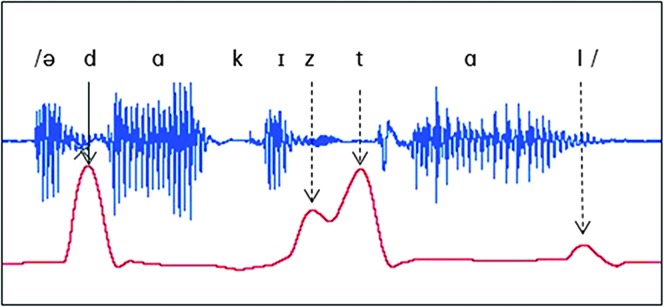
The primary measure was the peak contact pressure during the target consonant (LACP-Sp). This was measured using the peak analysis module in LabChart software (v7, ADInstruments, Colorado Springs, CO). The audio channel recording was synched with the pressure tracing (Figure 2), and research personnel viewed and played the synched recording in LabChart to help identify the target phoneme location. The pressure pulse was bracketed, the peak analysis routine was engaged, and the value was recorded in kilopascals to the nearest hundredth. Because the baseline of the transducer can drift slightly over time, a local baseline immediately preceding each sentence was obtained by taking the average pressure (kPa) for a 0.5-s block that preceded the start of the phrase by 0.5 s (and which was confirmed by research personnel to be free from any talking or swallowing). The difference between the local baseline and the peak pressure on the consonant was calculated and recorded as the LACP-Sp for that production. For each participant, a mean LACP-Sp for each consonant was computed by averaging the peak pressures across that person’s five trials of the consonant.
Figure 2.
Articulatory contact pressure tracing (bottom red) and the acoustic waveform of the stimulus, “a dock is tall,” with the peak pressure of interest marked with a solid arrow (dashed arrows show pressure pulses for other alveolar consonants not targeted for measurement).
LACP-Max was measured for each participant’s six pressure tracings on the maximum isometric pressing task. The peak analysis subroutine was used to obtain this value, which was then subtracted from the local baseline (as above, mean pressure for 0.5-s segment preceding the pressure pulse of interest). The maximum value of the six trials was recorded as the participant’s LACP-Max. In addition, the average LACP-Max from the three trials before the speech task and the average for the three trials after the speech task were computed for later comparison to help gauge whether fatigue may have occurred as a function of completing the speech task. Computation of %Max was calculated per subject and phoneme as follows: (mean LACP-Sp for a given consonant/LACP-Max) × 100.
Analysis
Descriptive statistics (means and standard deviations) were calculated per phoneme for LACP-Sp, %Max, and LACP-Max. A paired t-test was used to compare the average LACP-Max before the speech stimuli recording to the average after the speech protocol in order to assess the possibility that the speech protocol was fatiguing for the participant. Kruskal–Wallis nonparametric procedures were used to determine whether there were group differences in LACP-Sp among the non-ALS, ALS-B, and ALS-S groups. Separate Kruskal–Wallis statistics were calculated for each of the six phonemes, both for the LACP-Sp and for the %Max measure. A Kruskal–Wallis statistic was also computed for LACP-Max. When the Kruskal–Wallis test was statistically significant, post hoc testing was completed for paired comparisons using the Mann–Whitney U statistic. In addition, effect sizes for all paired comparisons were calculated using Cohen’s d. An alpha of .05 was considered to be statistically significant. A correction to the alpha level was not applied given the preliminary nature of the study. The rationale for not adjusting the alpha level was that the statistical test result along with the effect size estimate should allow identification of potential differences worthy of more in-depth investigation. The third aim required calculation of Spearman’s rank order correlation coefficients to assess relationships between LACP-Sp or LACP-Max and word intelligibility. Correlations were computed for the six phonemes individually and as a grand mean across phonemes. Scatterplots with Loess fit lines are also presented for these data.
Intraclass correlation coefficients (ICC) were utilized to assess intra- and intermeasurement reliability. For intrameasurement reliability of peak pressures, 20% of each participant’s productions were remeasured by the laboratory personnel who did the original measures. A 2-week interval between obtaining the original and the repeated measurements was enforced. The ICC evaluating absolute differences from Time 1 to Time 2 was .990, with a 95% confidence interval of .988–.992. The mean difference between the first and second measurement was 0.11 kPa. A second laboratory assistant also measured 20% of each participant’s sentences to assess intermeasurement reliability. The ICC was .980, with a 95% confidence interval from .974 to .985. The mean difference between laboratory personnel was 0.18 kPa. Both intrameasurement reliability and intermeasurement reliability were judged to be high on the basis of these outcomes.
Results
Assessment of Fatigue
To address the possibility that completion of the study tasks induced fatigue in the tongue for the ALS participants, analysis was completed to compare the average LACP-Max from the three isometric trials obtained before reading the speech stimuli to the average of the three trials completed at the end of the speech protocol. Means and standard deviations per group are given in Table 5. A paired t-test assessing the ALS participants had a t value of 1.252, which was not statistically significant (p = .237). Likewise, there was no statistical difference for the controls (t = .416, p = .685).
Table 5.
Maximum isometric lingual–alveolar contact pressure (LACP-Max; SD in parentheses) before and after the speech protocol.
| Recording | Control | ALS-Bulbar | ALS-Spinal |
|---|---|---|---|
| Before speech protocol | 67.94 (14.77) | 32.39 (21.11) | 64.80 (11.43) |
| After speech protocol | 66.24 (14.67) | 35.68 (16.12) | 67.57 (16.47) |
Note. ALS = amyotrophic lateral sclerosis.
LACP-Sp Results
Group means, standard deviations, and statistical results comparing LACP-Sp across groups are listed in Table 6. Kruskal–Wallis results comparing across control, ALS-B, and ALS-S groups were statistically significant for the phonemes /t/, /d/, /s/, and /z/, but not /l/ or /n/. Post hoc comparisons for each of the phonemes (also in Table 6) revealed that the ALS-B group had statistically significantly lower LACP-Sp than the control group for /t, d, s, z/. The control group values were 59% greater for /t, d/ and 72%–76% greater for /z, s/ than the pressure recorded from the ALS-B group. The ALS-S group and the control group did not differ for any phoneme. The ALS-B group had significantly lower LACP-Sp than the ALS-S group for /t, d, z/ but not /s/. The ALS-S group values for /t, d, z/ were 71%–82% larger than those from the ALS-B group.
Table 6.
Mean (SD) lingual–alveolar contact pressure during speech (LACP-Sp) by group and phoneme, and M (SD) maximum isometric LACP (LACP-Max) by group, with statistical results including omnibus testing (Kruskal–Wallis), post hoc comparisons (Mann–Whitney U), and effect size (Cohen’s d).
| Task | Control | ALS-Bulbar | ALS-Spinal | Kruskal–Wallis results |
α for Mann–Whitney U post-hoc comparisons [Cohen’s d] |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | α | Control vs. bulbar | Control vs. spinal | Bulbar vs. spinal | |||||
| /t/ | M | 3.61 | 1.48 | 5.65 | 12.01 | 2 | .002 | .007 | .082 | .003 |
| (SD) | (1.85) | (1.25) | (2.01) | [1.2] | [−1.1] | [2.1] | ||||
| /d/ | M | 3.60 | 1.46 | 4.99 | 10.66 | 2 | .005 | .004 | .328 | .006 |
| (SD) | (2.44) | (1.09) | (1.88) | [0.9] | [−0.6] | [1.9] | ||||
| /s/ | M | 3.21 | 0.91 | 2.61 | 11.41 | 2 | .003 | >.001 | .377 | .171 |
| (SD) | (1.49) | (0.63) | (2.89) | [1.5] | [0.4] | [0.6] | ||||
| /z/ | M | 2.78 | 0.67 | 3.64 | 14.37 | 2 | .001 | >.001 | .721 | .003 |
| (SD) | (1.47) | (0.29) | (3.26) | [1.4] | [−0.6] | [0.9] | ||||
| /l/ | M | 2.02 | 1.48 | 3.25 | 1.96 | 2 | .375 | .596 | .377 | .202 |
| (SD) | (1.67) | (0.97) | (2.39) | [0.3] | [−0.7] | [0.7] | ||||
| /n/ | M | 3.31 | 1.79 | 3.85 | 4.26 | 2 | .119 | .082 | .506 | .127 |
| (SD) | (2.25) | (1.81) | (1.64) | [0.7] | [−0.2] | [1.3] | ||||
| LACP-Max | M (SD) | 67.94 (14.77) | 35.68 (16.12) | 67.57 (16.47) | 11.07 | 2 | .004 | .001 [2.2] | .879 [0.0] | .019 [1.9] |
Note. Bold denotes those statistical results that were statistically significant. ALS = amyotrophic lateral sclerosis.
LACP-Max Results
LACP-Max did differ across groups as indicated by a statistically significant Kruskal–Wallis χ2 value of 11.06 with a p = .004 (see Table 6). Mann–Whitney post hoc comparisons between groups revealed that LACP-Max for the control group was significantly higher than that for the ALS-B group (67.94 kPa vs. 35.68 kPa). The ALS-B and ALS-S groups also differed (35.68 kPa vs. 67.57 kPa). The ALS-S and control groups did not differ in LACP-Max.
LACP-Sp as a Percentage of LACP-Max (%Max)
The %Max for each phoneme is reported in Table 7. None of the Kruskal–Wallis χ2 values was statistically significant. The %Max means were all less than 10% of the LACP-Max, ranging from 2% to 8%.
Table 7.
Mean (SD) percentage of maximum isometric lingual–alveolar contact pressure (LACP-Max) utilized during speech (%Max), with statistical results including omnibus testing (Kruskal–Wallis) and effect size (Cohen’s d). No post hoc comparisons were completed because all omnibus tests were nonsignificant.
| Phoneme | Control | Bulbar | Spinal | Kruskal–Wallis results |
Effect size estimate (Cohen’s d) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | α | Control vs. bulbar | Control vs. spinal | Bulbar vs. spinal | |||||
| /t/ | M | 5.99 | 5.35 | 8.38 | 5.274 | 2 | .072 | −0.2 | 1.0 | 1.3 |
| (SD) | (4.45) | (3.02) | (2.41) | |||||||
| /d/ | M | 5.71 | 5.96 | 7.33 | 2.981 | 2 | .225 | 0.1 | 0.7 | 0.6 |
| (SD) | (5.52) | (3.14) | (2.33) | |||||||
| /s/ | M | 5.07 | 4.90 | 4.00 | 1.549 | 2 | .461 | −0.1 | −0.3 | −0.2 |
| (SD) | (3.47) | (2.85) | (4.26) | |||||||
| /z/ | M | 4.14 | 2.32 | 5.64 | 5.674 | 2 | .059 | −2.0 | 0.3 | 0.7 |
| (SD) | (2.30) | (0.90) | (4.89) | |||||||
| /l/ | M | 3.73 | 5.77 | 5.03 | 1.868 | 2 | .393 | 0.6 | 0.3 | −0.2 |
| (SD) | (4.00) | (3.59) | (3.75) | |||||||
| /n/ | M | 5.35 | 5.45 | 5.71 | .871 | 2 | .647 | 0.0 | 0.2 | 0.1 |
| (SD) | (4.91) | (2.87) | (2.28) | |||||||
Correlations Among LACP-Sp, LACP-Max, and Word Intelligibility
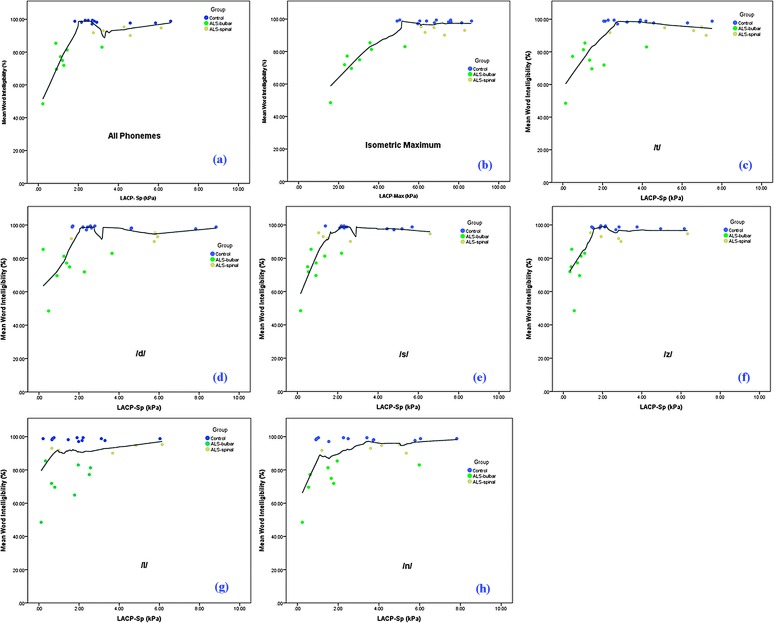
Word intelligibility percentages are listed in Table 2. Scatterplots of LACP-Sp and LACP-Max relative to percent word intelligibility are shown in Figure 3. The Spearman’s correlation values and associated probabilities are in Table 8. Two sets of correlation values are reported: one that includes all three participant groups, and another calculated just with the ALS participants. The second set of correlations was included because the word intelligibility scores for the control group, as expected, were at or near the ceiling (i.e., 100%). Excluding the healthy volunteers for the second set allowed better assessment of the relationship between LACP-Sp and LACP-Max among those participants who had not reached the intelligibility ceiling.
Figure 3.
Scatterplots of lingual–alveolar contact pressure during speech (LACP-Sp) and maximum isometric lingual–alveolar contact pressure (LACP-Max) with word intelligibility. Separate plots are provided for each phoneme, as well as all phonemes combined. Participant group is indicated by marker color (black = healthy volunteer group, green = amyotrophic lateral sclerosis (ALS) bulbar group, tan = ALS spinal group). Solid lines = Loess fit line. Plots of word intelligibility to specific pressure were as follows: (a) LACP-Sp for all phonemes combined, (b) LACP-Max, (c) phoneme /t/, (d) phoneme /d/, (e) phoneme /s/, (f) phoneme /z/, (g) phoneme /l/, (h) phoneme /n/.
Table 8.
Spearman’s rank order correlation coefficients, R 2, and associated probabilities for lingual–alveolar contact pressure during speech (LACP-Sp; phonemes combined and individually) and maximum isometric LACP (LACP-Max) as related to word intelligibility.
| All three groups considered |
ALS groups only |
|||||
|---|---|---|---|---|---|---|
| Spearman’s rho | R 2 | p | Spearman’s rho | R 2 | p | |
| All phonemes combined | .417 | .174 | .038 | .797 | .635 | .001 |
| /t/ | .416 | .173 | .038 | .753 | .567 | .003 |
| /d/ | .429 | .184 | .032 | .703 | .494 | .007 |
| /s/ | .603 | .364 | .001 | .720 | .518 | .006 |
| /z/ | .576 | .332 | .003 | .775 | .601 | .002 |
| /l/ | .156 | .024 | .456 | .538 | .289 | .058 |
| /n/ | .320 | .102 | .119 | .659 | .434 | .014 |
| LACP-Max | .623 | .388 | .001 | .852 | .726 | <.001 |
Note. ALS = amyotrophic lateral sclerosis.
Considering all three groups and all six phonemes combined, LACP-Sp was significantly positively correlated to single-word speech intelligibility (r = .417, p = .038). For individual phonemes, significant correlations were found for /t/, /d/, /s/, and /z/. Variance in word intelligibility explained by LACP-Sp ranged from 17% to 18% for the stop consonants and 33% to 36% for the sibilants. In addition, there was a significant positive correlation between LACP-Max and single-word intelligibility (r = .623, p = .001), with 39% of the variance in word intelligibility accounted for by LACP-Max.
When just the ALS participants were included, all of the correlation values increased. Using the mean pressure for all six phonemes combined, there was a statistically significant correlation to word intelligibility of r = .797 and an R 2 of approximately 63%. The correlations for the individual phoneme LACP-Sp values ranged from a low of .538 on /l/ to a high of .775 on /z/. LACP-Max was strongly correlated to word intelligibility when just analyzing the ALS participants, with r = .852 (R 2 = 72.6%).
The scatterplots provide additional insight into these relationships. The plots for /l/ and /n/ reveal a large range of LACP-Sp values with notable overlap across groups despite apparent differences in word intelligibility. For the remaining phonemes, when LACP-Sp dropped to a value between 1.5 and2.0 kPa, there was a corresponding drop in the word intelligibility scores. Furthermore, this pressure range appears to provide relatively clear distinction between the ALS-B group and the other two groups. Participant ALS-B2 was somewhat of an outlier in these plots, with LACP-Sp values that fell clearly within the range of the controls and ALS-S participants. B2 also had a high word intelligibility score, relatively speaking, within the ALS-B group, as well as the second highest speaking rate in the ALS-B group. Participant ALS-B2 had the highest LACP-Max in the ALS-B group at 53 kPa. The scatterplot for LACP-Max and word intelligibility indicated a distinction between the ALS-B group and the other two groups at approximately 50 kPa (with participant ALS-B2 being the exception).
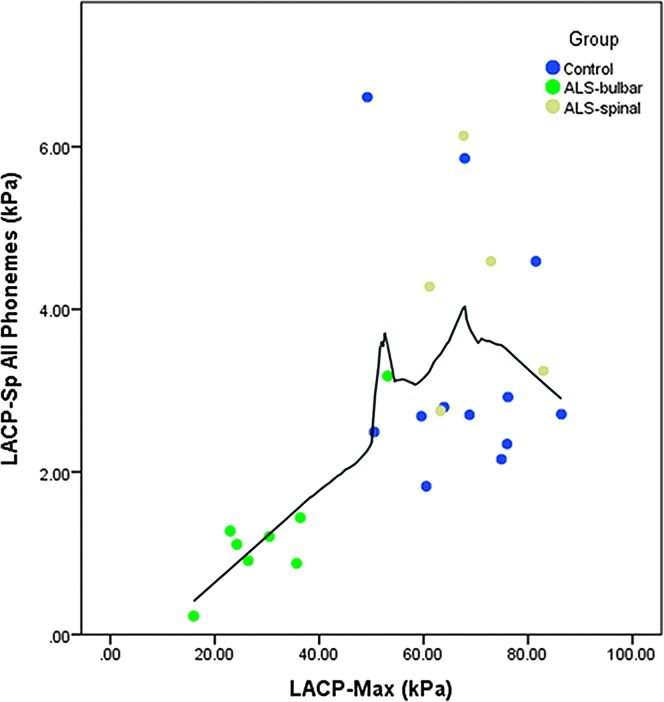
Informal inspection of LACP-Sp and LACP-Max values for the ALS-B group suggested a linear relationship between the two pressure measures. This prompted calculation of an additional Spearman’s rank order correlation that was not originally planned. The correlation value for LACP-Sp to LACP-Max with all three groups included was .612 (p = .001). When only the ALS participants were considered, the correlation rose to .846 (p < .001). The scatterplot in Figure 4 shows a rather striking, linear relationship between LACP-Sp and LACP-Max when LACP-Max is less than approximately 50 kPa. When LACP-Max is greater than 50 kPa, the relationship appears to weaken or become nonexistent. A factor of note is that the ALS-B participants, with the exception of B2, comprised the group of data points below 40 kPa in Figure 4.
Figure 4.
Scatterplot of lingual–alveolar contact pressure during speech (LACP-Sp) with maximum isometric lingual–alveolar contact pressure (LACP-Max). Solid line = Loess fit line.
Discussion
This prospective, cohort comparison study of individuals with and without ALS provides the first preliminary data set detailing alterations in LACP generation during speech production for PALS. Discussions regarding each of the specific aims and hypotheses tested are delineated below. Overall, the primary findings were that (a) the speaking task did not appear to induce tongue weakness as measured by LACP-Max at the end of the recording, (b) LACP-Sp was significantly smaller for the ALS-B but not the ALS-S participants compared with healthy volunteers, (c) LACP-Max also was significantly smaller for the ALS-B but not the ALS-S group compared with healthy volunteers, (d) %Max did not differ between the ALS and the control groups; and (e) LACP-Sp and LACP-Max were correlated with word intelligibility.
Fatigue for the Speaking Task
The average LACP-Max just before starting the speaking task did not differ from the average LACP-Max obtained just after completing the protocol. Although this represents a rather simple assessment of the possible effect of the task on tongue strength, the finding provides some assurance that the participants did not become fatigued from completing the task. More in-depth and differently designed studies are needed to directly explore lingual fatigue in PALS and the effect on speech production.
LACP-Sp Implications
The mean LACP-Sp generated by the healthy participants in this study ranged from 2.02 kPa to 3.61 kPa, which are consistent with pressures previously reported for adults without speech deficits (Brown, McGlone, & Proffit, 1973; McGlone, Proffit, & Christiansen, 1967; Searl, 2003; Searl et al., 2007; Searl & Evitts, 2013). Overall, the literature indicates that phoneme contact pressures between the anterior tongue and palate range from about 2 to 7 kPa, with highest values on stops and lowest values on fricatives, nasals, and glides.
We hypothesized that the ALS-B speakers would have lower LACP-Sp compared with the other two groups. Our hypothesis was only partially supported because differences occurred for /t, d, s, z/ when comparing ALS-B and control participants, but no difference was found for /l/ and /n/. The group differences (ALS-B vs. control) for the stop and fricative phonemes were not only statistically significant, but they also had large effect sizes (Cohen’s d > 0.9 for these paired comparisons). A notable feature is that the effect size for the group comparison for /n/ was also fairly large (d = 0.7), even though the statistical difference did not emerge when comparing ALS-B and control participants. Overall, a conservative conclusion from these data is that the obstruent phonemes differ between ALS-B and control participants. It may be the case that weakness of the tongue is, in fact, evident during the production of these phonemes, which require relatively greater constriction of the vocal tract. Although there are some exceptions, the preliminary data suggested that a minimum of 2 kPa for LACP-Sp is produced by healthy controls and ALS-S speakers, but values below 2 kPa are common for ALS-B speakers when producing /t, d, s, z/. For the other phonemes (nasal and liquid), LACP-Sp may simply have less relevance. When looking only at the healthy volunteers, it is apparent that LACP-Sp for /l/ and /n/ ranged widely from nearly 0 kPa to 8 kPa, with many having values below 2 kPa. This suggests that there is not a specific minimum pressure value to be expected even among healthy adults.
The results comparing the ALS-B and ALS-S groups essentially parallel those of the ALS-B versus control group. The stop and fricative phonemes had lower contact pressures in the ALS-B group that were statistically significant and had large effect sizes. The exception was /s/, which did not differ significantly but had a moderate effect size. The phonemes /l/ and /n/ did not differ, although effect sizes were moderate to large. Additional research to confirm the outcomes is needed, but these preliminary results again suggest that a 2 kPa level of LACP-Sp could differentiate those with and those without intelligibility changes. This is most evident in the scatterplots for /z/ and /t/. It will be of interest to determine whether such measures could serve as a surrogate measure for bulbar ALS involvement, perhaps allowing for early identification of bulbar symptoms and/or as a sensitive measure of tracking change in bulbar symptoms over time, disease progression, or intervention approach. Perhaps LACP-Sp on obstruents could be used in a manner similar to that already proposed for speaking rate for predicting an impending drop in intelligibility (Ball et al., 2001) or kinematic measures to track ALS disease progression (Yunusova et al., 2010). Tracking of LACP-Sp within a subject over time will be necessary to confirm whether the measure can serve in this capacity.
The ALS-S participants did not differ significantly from the control participants on any phoneme comparison, but the group means, direction of differences, and effect size estimates require comment. For five of six phonemes, the ALS-S group had a mean contact pressure that was greater than the control group mean. Of these five phonemes (/t, d, z, l, n/), the Cohen’s d values ranged from 0.6 to 1.1, indicating moderate to large effect sizes. Given the unequal group sizes, rather large standard deviations in the ALS-S data, and the fairly small number of ALS-S participants in total, reduced statistical power may explain the lack of a statistically significant difference in the Kruskal–Wallis analysis. Prudence dictates caution in interpreting the results, although an elevation in the group means in the ALS-S group argues for additional study. It may be that these PALS are engaging in some form of compensation during speech production to retain speech that is understandable, even if other aspects of their functioning that might affect speech are declining. The kinematic data from Yunusova et al. (2010) identified a transient increase in jaw speed that occurred just prior to a drop in speed for two of their three participants. They suggested that ALS speakers might engage in a compensatory jaw speed adjustment if tongue function were declining. If such compensation occurs in the jaw movement, one potential outcome might be an increase in the impact force such as we measured as LACP-Sp in our ALS-S speakers who still had preserved intelligibility.
LACP-Max Implications
Several studies have reported LACP-Max for adults without speech disorders, and the group averages range from approximately 55 kPa to 70 kPa, depending on the study in question (Clark, O’Brien, Calleja, & Corrie, 2009; C. Lazarus, Logemann, Huang, & Rademaker, 2003; Solomon, 2004; Stierwalt & Youmans, 2007). The healthy volunteers and the ALS-S participants in the current study had comparable maximum values that fell within this expected range. The fact that the ALS-S group mean (and standard deviation) was nearly identical to the control group suggested that the spinal group was not demonstrating a reduction in tongue strength during this task, which is consistent with the clinical impressions from the treating neurologist and SLP that indicated the absence of bulbar symptoms. Easterling et al. (2013), however, reported lower mean maximum pressing values for 14 participants with spinal symptoms. This may represent a sampling issue (small in both studies with large standard deviations) or possibly differences in instrumentation and instructions. Easterling et al. utilized the Iowa Oral Performance Instrument (IOPI Medical, LLC, Redmond, WA), a 10-s rest interval between trials (vs. 30 s in the current study), and it was not clear if verbal encouragement was used during pressing as it was in our study.
The group mean value for LACP-Max for the ALS-B participants was approximately 45% lower than that for the control and ALS-S groups. This result is consistents with findings of force reduction in other studies of PALS that used an isometric pressing task such as Langmore and Lehman (1994) and DePaul and Brooks (1993). In addition, Easterling et al. (2013) found a statistically significant difference in maximum pressing ability of the tongue between bulbar and spinal ALS participants when tracked at three points in time spaced approximately three months apart. However, the group mean value for the ALS-B participants in their study was notably lower than the mean for our ALS-B participants (22 kPa at time one in Easterling et al. vs. 35 kPa for our ALS-B participants). Again, this might be reflective of sampling differences (large standard deviations with small N) or instrumentation differences. Overall, these findings confirmed that at least the ALS-B participants had weaker tongues in a maximum isometric pressing task. Inspection of the scatterplot of LACP-Max in Figure 4 reveals that the group of bulbar speakers in the current study had maximum pressures below 40 kPa (with one exception), whereas all of the ALS-S and control participants were above 40 kPa. We are tracking the ALS-S participants over time in an attempt to determine whether their LACP-Max drops and indications of bulbar disease arise.
LACP-Sp as a Percentage of LACP-Max (%Max)
The expectation was that individuals with ALS showing bulbar symptoms would have an increase in %Max compared with the healthy participants. This was not the case, as evidenced by the lack of statistical difference in %Max among any of the three groups. Overall, this suggests a proportional reduction in LACP-Sp and LACP-Max for the ALS-B participants. Although speculative, it is intriguing to consider the possibility that the speech system, even in the presence of ALS disease, may have an effort (internal sense) or output (pressure, force, etc.) goal that remains somewhat constant and is based not on an absolute value but rather one that is proportional to an individual’s physiologic range. Speech is a low-force task, and work by Solomon and Robin (2005) has indicated that the perception of effort during tongue movements (nonspeech in this case) relative to pressure generation was more sensitive at the extremes of the effort range. That is, the tongue may be more sensitive to effort differences at the low (and high) end of the tongue pressure physiologic range, allowing for a %Max target to be reliably attained. Of course, it is also possible that LACP-Sp is not a motor goal of the system but rather just the outcome of other movement parameters such as speed and displacement of the tongue. Simultaneous measurement of the kinematics and the contact pressures would be helpful, but this is not yet feasible as far as the authors are aware.
Relationships Among LACP-Sp, LACP-Max, and Word Intelligibility
LACP-Sp and LACP-Max were significantly and positively correlated with single-word intelligibility, supporting our original hypothesis. When only the ALS participants were considered in the correlation analysis, LACP-Sp (all phonemes combined) accounted for approximately 64% of the variance in word intelligibility scores. What is not known from these results is whether the LACP-Sp reduction in ALS-B speakers was a direct cause for the word intelligibility decline. With the feasibility of obtaining LACP-Sp in PALS now known, attention can be directed toward this issue. It is possible that LACP-Sp is merely correlated to intelligibility because the measure is associated with overall ALS bulbar disease severity, with the latter being the underlying cause of the speech intelligibility decline. Moving forward, it will be important to obtain auditory perceptual judgment of the specific experimental phonemes from which LACP-Sp is obtained (rather than an intelligibility measure involving a wide range of phonemes and constructions), control or account for ALS disease severity, and track changes in LACP-Sp and phonetic accuracy over time within participants. At the moment, we are only able to conclude that there is a correlation between LACP-Sp and word intelligibility, but it is possible that this relationship is really driven by a third variable such as overall disease severity.
We anticipated that LACP-Sp would be more strongly correlated than LACP-Max to word intelligibility, but this was not the case. In the correlational analysis, LACP-Max accounted for about 9% more of the variance in word intelligibility than did LACP-Sp. One might expect that a measure taken from speech production would be more directly relevant to a measure of speech intelligibility. However, it is unlikely that a single measure taken during speech will explain a significant amount of the variation in a measure of speech intelligibility because of the many ways by which intelligibility can be degraded (or maintained). A multisignal assessment of the speech production process, as advocated for ALS patients by Green et al. (2013), will almost assuredly provide a more robust explanation for variations in any number of clinical measures that might be of interest, such as intelligibility. In addition, the LACP-Sp measures were taken from six phonemes in a single context (initiating a consonant–vowel–consonant and preceded by a central vowel). The speech intelligibility measure utilized here was based on words that included many more consonants and vowels in varied arrangement in words. In some ways, it is perhaps surprising that the strength of the LACP-Sp relationship to word intelligibility was as strong as it was. As argued above, LACP-Max may simply be a reflection of the overall bulbar disease. As a post hoc assessment, we did compute Spearman’s rank order correlations between ALS speech severity ratings from the clinical documentation (Table 2) and both LACP-Sp and LACP-Max. The correlations were strong at .823 for LACP-Sp and .922 for LACP-Max. Langmore and Lehman (1994) also reported that maximum force generation in 10 ALS participants was significantly correlated to severity of dysarthria. DePaul and Brooks (1993) reported that isometric tongue force measures were significantly related to scores of speech severity in 10 men with ALS but were not significantly correlated with speech intelligibility. Until these relationships among LACP-Sp, LACP-Max, disease severity, and intelligibility at the phoneme level can be disentangled, we can only conclude that the measured pressures do correlate with word intelligibility.
Although individual phonetic contrasts and acoustic analysis were not included in the present study, prior data have identified phoneme production issues in PALS that are clearly related to tongue function. J. F. Kent et al. (1992) identified a set of phonetic contrast errors that occurred more frequently than others in women with ALS: stop versus nasal consonant, alveolar consonant versus palatal consonant, and stop versus affricate production, among others. The interpretation offered to explain some of these contrast errors was altered lingual function for fricatives and altered manner of production for lingual consonants. Measures of F2 slope between the vowel and adjacent consonants suggested not only slower rate of speaking (longer duration of the slope) but also a shallower slope consistent with slowed articulatory movement. Although not identical, similar phonetic errors were reported for men with ALS (R. D. Kent et al., 1990). The question remains whether slower movements and less displacement of the tongue during phoneme production in PALS (Weismer et al., 1986; Yunusova et al., 2010, 2012) cause LACP-Sp to be reduced in those with bulbar symptoms or whether reduced lingual strength causes the alterations in lingual speed and displacement. Although it may not be possible to obtain the kinematic measures simultaneous with LACP-Sp, it should be possible to take both sets of measures within a speaker very close in time and, with enough trials, to begin understanding the relationship in more detail.
The scatterplots of LACP-Sp and LACP-Max relative to word intelligibility offer perhaps the most useful information from this data set. The LACP-Sp plots are suggestive of a critical minimum LACP-Sp for stops and fricatives (about 1.5 kPa) occurring in speakers with high word intelligibility scores. When LACP-Sp dropped below approximately 1.5 kPa, word intelligibility declined. Tracking LACP-Sp and intelligibility within a speaker over time should allow for more refined understanding of this relationship and how it changes over time. If this relationship can be confirmed, and a critical LACP-Sp range can be identified for maintaining high intelligibility, the measure might have some value in clinical situations to predict impending decline in speech that requires a shift in clinical care. LACP-Max might also have such a use after further confirmation with a larger subject pool. The data here suggest that individuals with LACP-Max values that are greater than 50 kPa generally have high word intelligibility, and those with values below that threshold show a decline in intelligibility.
Conclusions and Limitations
Overall, it is feasible to obtain LACP-Sp measures from PALS, and the pressure measures in those with bulbar symptoms differ from patients without bulbar symptoms and healthy controls. An ultimate goal is to determine whether tongue strength reduction has a causal relationship to impairment at the level of phoneme production. These preliminary data and the established feasibility of obtaining the strength index measure during speech production offer a degree of encouragement to pursue this line of investigation.
Although a long-range goal is to utilize speech tasks rather than isometric tasks to understand the role of tongue strength during speech production in PALS, the study design here was principally to establish that the measures could be obtained and to describe how the speech values compare to healthy volunteers. This design limitation was intentional at this early stage, but it does hinder drawing conclusions regarding this question: Does strength matter for articulation production in PALS? The correlational results that are offered at a minimum suggest that strength (LACP-Sp and LACP-Max) is related to word intelligibility. Future work will need to focus on LACP-Sp relative to auditory–perceptual judgments and/or acoustic analysis of the target phonemes from which LACP-Sp is measured. In addition, serial recordings of LACP-Sp and the corresponding judgment of phonetic accuracy within speakers will be important, with careful attention devoted to also accounting for overall ALS disease severity. Manipulation of LACP-Sp in PALS while also tracking alterations to phonetic integrity (perceptual and/or acoustic) could also be investigated.
The small sample size requires caution in generalizing the results to the ALS population at large. Another limitation of the study was use of a single pressure transducer site. ALS differentially alters various regions of the tongue, as documented by measures of muscle fiber degeneration (DePaul, Waclawik, Abbs, & Brooks, 1998), as well as muscle atrophy and fibrosis, with greater alterations in the anterior compared with the posterior region of the tongue (Cha & Patten, 1989). Movement-coupling of tongue regions during speech in PALS is reported to be more restricted in the middle-posterior tongue than in the anterior tongue (Kuruvilla et al., 2012). The magnitude of LACP-Sp might also vary as a function of the tongue–palate contact measurement location.
If the intention is to obtain a metric of speech intelligibility that is reflective of a person’s functional communication, the choice of single word intelligibility as opposed to sentence-level intelligibility may be debated. However, word intelligibility and sentence intelligibility in dysarthric speakers have been reported to be highly correlated (Yorkston & Beukelman, 1978). In addition, acoustic measures of F2 slope and vowel space, which reasonably relate to articulatory movement of the tongue, are correlated to not just single word intelligibility but also scaled sentence intelligibility in people with dysarthria resulting from ALS and Parkinson’s disease (Weismer et al., 2001). Word intelligibility might be justified on the basis of such studies, but a clearer choice with perhaps stronger ecological validity would be sentence-level intelligibility.
Acknowledgment
This work was supported by a Clinical and Translational Science Award grant from National Center for Advancing Translational Science (NCATS) awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research UL1TR000001 (formerly UL1RR033179). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or NCATS.
Funding Statement
This work was supported by a Clinical and Translational Science Award grant from National Center for Advancing Translational Science (NCATS) awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research UL1TR000001 (formerly UL1RR033179).
References
- Ball L. J., Willis A., Beukelman D. R., & Pattee G. L. (2001). A protocol for identification of early bulbar signs in amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 191(1–2), 43–53. https://doi.org/10.1016/S0022-510X(01)00623-2 [DOI] [PubMed] [Google Scholar]
- Bello-Haas V. D., Florence J. M., Kloos A. D., Scheirbecker J., Lopate G., Hayes S. M., … Mitsumoto H. (2007). A randomized controlled trial of resistance exercise in individuals with ALS. Neurology, 68(23), 2003–2007. https://doi.org/10.1212/01.wnl.0000264418.92308.a4 [DOI] [PubMed] [Google Scholar]
- Beukelman D., Fager S., & Nordness A. (2011). Communication support for people with ALS. Neurology Research International, 2011, 714693. https://doi.org/10.1155/2011/714693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P., & Weenink D. (2011). PRAAT: Doing phonetics by computer (Version 5.3) [Computer program]. Retrieved on August 15, 2011, from http://www.praat.org/
- Brooks B. R. (1994). El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. Journal of the Neurological Sciences, 124(Suppl), 96–107. [DOI] [PubMed] [Google Scholar]
- Brown W. S. Jr., McGlone R. E., & Proffit W. R. (1973). Relationship of lingual and intraoral air pressures during syllable production. Journal of Speech and Hearing Research, 16, 141–151. https://doi.org/10.1044/jshr.1601.141 [DOI] [PubMed] [Google Scholar]
- Bunton K. (2008). Speech versus nonspeech: Different tasks, different neural organization. Seminars in Speech and Language, 29(4), 267–275. https://doi.org/10.1055/s-0028-1103390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha C. H., & Patten B. M. (1989). Amyotrophic lateral sclerosis: Abnormalities of the tongue on magnetic resonance imaging. Annals of Neurology, 25(5), 468–472. https://doi.org/10.1002/ana.410250508 [DOI] [PubMed] [Google Scholar]
- Chen A., & Garrett C. G. (2005). Otolaryngologic presentations of amyotrophic lateral sclerosis. Otolaryngology and Head and Neck Surgery, 132(3), 500–504. https://doi.org/10.1016/j.otohns.2004.09.092 [DOI] [PubMed] [Google Scholar]
- Clark H. M., O’Brien K., Calleja A., & Corrie S. N. (2009). Effects of directional exercise on lingual strength. Journal of Speech, Language, and Hearing Research, 52, 1034–1047. https://doi.org/10.1044/1092-4388(2009/08-0062) [DOI] [PubMed] [Google Scholar]
- Darley F., Aronson A., & Brown J. (1969). Motor speech disorders. Philadelphia, PA: W. B. Saunders Company. [Google Scholar]
- DePaul R., & Brooks B. R. (1993). Multiple orofacial indices in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 36, 1158–1167. https://doi.org/10.1044/jshr.3606.1158 [DOI] [PubMed] [Google Scholar]
- DePaul R., Waclawik A. J., Abbs J. H., & Brooks B. R. (1998). Histopathological characteristics in lingual muscle tissue in ALS: Perspectives on the natural history of the disease. In Cannito M. P., Yorkston K. M., & Beukelman D. R. (Eds.), Neuromotor speech disorders: Nature, assessment, and management (pp. 69–84). Baltimore, MD: Brookes. [Google Scholar]
- Drory V. E., Goltsman E., Goldman Reznik J., Mosek A., & Korczyn A. D. (2001). The value of muscle exercise in patients with amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 191(1–2), 133–137. https://doi.org/10.1016/s0022-510x(01)00610-4 [DOI] [PubMed] [Google Scholar]
- Duffy J. (2005). Motor speech disorders: Substrates, differential diagnosis, and management (2nd ed.). St. Louis, MO: Mosby. [Google Scholar]
- Dworkin J. P., Aronson A. E., & Mulder D. W. (1980). Tongue force in normals and in dysarthric patients with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 23, 828–837. https://doi.org/10.1044/jshr.2304.828 [DOI] [PubMed] [Google Scholar]
- Easterling C., Antinoja J., Cashin S., & Barkhaus P. E. (2013). Changes in tongue pressure, pulmonary function, and salivary flow in patients with amyotrophic lateral sclerosis. Dysphagia, 28(2), 217–225. https://doi.org/10.1007/s00455-012-9436-7 [DOI] [PubMed] [Google Scholar]
- Forrest K., & Iuzzini J. (2008). A comparison of oral motor and production training for children with speech sound disorders. Seminars in Speech and Language, 29(4), 304–311. https://doi.org/10.1055/s-0028-1103394 [DOI] [PubMed] [Google Scholar]
- Green J. R., Beukelman D. R., & Ball L. J. (2004). Algorithmic estimation of pauses in extended speech samples of dysarthric and typical speech. Journal of Medical Speech-Language Pathology, 12(4), 149–154. [PMC free article] [PubMed] [Google Scholar]
- Green J. R., Yunusova Y., Kuruvilla M. S., Wang J., Pattee G. L., Synhorst L., … Berry J. D. (2013). Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotrophic Lateral Sclerosis Frontotemporal Degeneration, 14(7–8), 494–500. https://doi.org/10.3109/21678421.2013.817585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose H., Kiritani S., & Sawashima M. (1982). Velocity of articulatory movements in normal and dysarthric subjects. Folia Phoniatrica, 34(4), 210–215. [DOI] [PubMed] [Google Scholar]
- Kent J. F., Kent R. D., Rosenbek J. C., Weismer G., Martin R., Sufit R., & Brooks B. R. (1992). Quantitative description of the dysarthria in women with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 35, 723–733. https://doi.org/10.1044/jshr.3504.723 [DOI] [PubMed] [Google Scholar]
- Kent R. D. (2004). The uniqueness of speech among motor systems. Clinical Linguistics & Phonetics, 18(6–8), 495–505. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15573486 [DOI] [PubMed] [Google Scholar]
- Kent R. D., Kent J. F., Weismer G., Sufit R. L., Rosenbek J. C., Martin R. E., & Brooks B. R. (1990). Impairment of speech intelligibility in men with amyotrophic lateral sclerosis. Journal of Speech and Hearing Disorders, 55, 721–728. https://doi.org/10.1044/jshd.5504.721 [DOI] [PubMed] [Google Scholar]
- Kent R. D., Netsell R., & Bauer L. L. (1975). Cineradiographic assessment of articulatory mobility in the dysarthrias. Journal of Speech and Hearing Disorders, 40, 467–480. https://doi.org/10.1044/jshd.4004.467 [DOI] [PubMed] [Google Scholar]
- Kuruvilla M. S., Green J. R., Yunusova Y., & Hanford K. (2012). Spatiotemporal coupling of the tongue in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 55, 1897–1909. https://doi.org/10.1044/1092-4388(2012/11-0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmore S. E., & Lehman M. E. (1994). Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 37, 28–37. https://doi.org/10.1044/jshr.3701.28 [DOI] [PubMed] [Google Scholar]
- Lazarus C., Logemann J. A., Huang C. F., & Rademaker A. W. (2003). Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatrica et Logopedica, 55(4), 199–205. https://doi.org/71019 [DOI] [PubMed] [Google Scholar]
- Lazarus C. L., Logemann J. A., Pauloski B. R., Rademaker A. W., Larson C. R., Mittal B. B., & Pierce M. (2000). Swallowing and tongue function following treatment for oral and oropharyngeal cancer. Journal of Speech, Language, and Hearing Research, 43, 1011–1023. https://doi.org/10.1044/jslhr.4304.1011 [DOI] [PubMed] [Google Scholar]
- McGlone R. E., Proffit W. R., & Christiansen R. L. (1967). Lingual pressures associated with alveolar consonants. Journal of Speech and Hearing Research, 10(3), 606–615. https://doi.org/10.1044/jshr.1003.606 [DOI] [PubMed] [Google Scholar]
- Mefferd A. S., Green J. R., & Pattee G. (2012). A novel fixed-target task to determine articulatory speed constraints in persons with amyotrophic lateral sclerosis. Journal of Communication Disorders, 45(1), 35–45. https://doi.org/10.1016/j.jcomdis.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarian N., Ferrari P., Macdonald M. J., Cheyne D., De Nil L. F., & Pang E. W. (2012). Cortical activity during speech and non-speech oromotor tasks: A magnetoencephalography (MEG) study. Neuroscience Letters, 527(1), 34–39. https://doi.org/10.1016/j.neulet.2012.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E. M., Milenkovic P. H., & MacLeod G. E. (1985). Perioral tissue mechanics during speech production. In DeLisi C. & Eisenfeld J. (Eds.), Proceedings of the Second IMACS International Symposium on Biomedical Systems Modeling (pp. 363–371). Amsterdam, the Netherlands: International Association for Mathematics and Computers in Simulation. [Google Scholar]
- Plowman E. K. (2015). Is there a role for exercise in the management of bulbar dysfunction in amyotrophic lateral sclerosis? Journal of Speech, Language, and Hearing Research, 58, 1151–1166. https://doi.org/10.1044/2015_JSLHR-S-14-0270 [DOI] [PubMed] [Google Scholar]
- Salmelin R., & Sams M. (2002). Motor cortex involvement during verbal versus non-verbal lip and tongue movements. Human Brain Mapping, 16(2), 81–91. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11954058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searl J. (2003). Comparison of transducers and intraoral placement options for measuring lingua–palatal contact pressure during speech. Journal of Speech, Language, and Hearing Research, 46, 1444–1456. https://doi.org/10.1044/1092-4388(2003/112) [DOI] [PubMed] [Google Scholar]
- Searl J. (2007). Bilabial contact pressure and oral air pressure during tracheoesophageal speech. Annals of Otology, Rhinology and Laryngology, 116(4), 304–311. [DOI] [PubMed] [Google Scholar]
- Searl J., & Evitts P. (2013). Tongue-palate contact pressure, oral air pressure, and acoustics of clear speech. Journal of Speech, Language, and Hearing Research, 56, 826–839. https://doi.org/10.1044/1092-4388(2012/11-0337) [DOI] [PubMed] [Google Scholar]
- Searl J., Evitts P., & Davis W. J. (2006). Perceptual and acoustic evidence of speaker adaptation to a thin pseudopalate. Logopedica Phoniatrica Vocology, 31(3), 107–116. https://doi.org/10.1080/14015430500390961 [DOI] [PubMed] [Google Scholar]
- Searl J., Evitts P., & Davis W. J. (2007). Articulatory contact pressure between the tongue and palate during normal speech production. Journal of Medical Speech-Language Pathology, 15(3), 279–292. [Google Scholar]
- Solomon N. P. (2004). Assessment of tongue weakness and fatigue. International Journal of Orofacial Myology, 30, 8–19. [PMC free article] [PubMed] [Google Scholar]
- Solomon N. P., Clark H. M., Makashay M. J., & Newman L. A. (2008). Assessment of orofacial strength in patients with dysarthria. Journal of Medical Speech-Language Pathology, 16(4), 251–258. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25264422 [PMC free article] [PubMed] [Google Scholar]
- Solomon N. P., & Munson B. (2004). The effect of jaw position on measures of tongue strength and endurance. Journal of Speech, Language, and Hearing Research, 47, 584–594. https://doi.org/10.1044/1092-4388(2004/045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon N. P., & Robin D. A. (2005). Perceptions of effort during handgrip and tongue elevation in Parkinson’s disease. Parkinsonism & Related Disorders, 11(6), 353–361. https://doi.org/10.1016/j.parkreldis.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierwalt J. A., & Youmans S. R. (2007). Tongue measures in individuals with normal and impaired swallowing. American Journal of Speech-Language Pathology, 16, 148–156. https://doi.org/10.1044/1058-0360(2007/019) [DOI] [PubMed] [Google Scholar]
- Tjaden K., & Turner G. (2000). Segmental timing in amyotrophic lateral sclerosis. Journal of Speech, Language, & Hearing Research, 43(3), 683–696. https://doi.org/10.1044/jslhr.4303.683 [DOI] [PubMed] [Google Scholar]
- Weismer G. (2006). Philosophy of research in motor speech disorders. Clinical Linguistics & Phonetics, 20(5), 315–349. https://doi.org/10.1080/02699200400024806 [DOI] [PubMed] [Google Scholar]
- Weismer G., Jeng J. Y., Laures J. S., Kent R. D., & Kent J. F. (2001). Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopedica, 53(1), 1–18. https://doi.org/52649 [DOI] [PubMed] [Google Scholar]
- Weismer G., Martin R., Kent R. D., & Kent J. F. (1992). Formant trajectory characteristics of males with amyotrophic lateral sclerosis. The Journal of the Acoustical Society of America, 91(2), 1085–1098. [DOI] [PubMed] [Google Scholar]
- Weismer G., Mulligan M., & DePaul R. (1986). Selected acoustic characteristics of the dysarthria associated with ALS in young adults. Paper presented at the Clinical Dysarthria Conference, Tucson, AZ. [Google Scholar]
- Yorkston K., & Beukelman D. R. (1978). A comparison of techniques for measuring intelligibility of dysarthric speech. Journal of Communication Disorders, 11(6), 499–512. [DOI] [PubMed] [Google Scholar]
- Yorkston K., Beukelman D., & Hakel M. (1996). Speech Intelligibility Test. Lincoln, NE: Institute for Rehabilitation Science and Engineering at Madonna Rehabilitation Hospital, Communication Disorders Software. [Google Scholar]
- Yorkston K., Strand E., Miller R., Hillel A., & Smith K. (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology, 1(1), 35–46. [Google Scholar]
- Yunusova Y., Green J. R., Greenwood L., Wang J., Pattee G. L., & Zinman L. (2012). Tongue movements and their acoustic consequences in amyotrophic lateral sclerosis. Folia Phoniatrica et Logopedica, 64(2), 94–102. https://doi.org/10.1159/000336890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y., Green J. R., Lindstrom M. J., Ball L. J., Pattee G. L., & Zinman L. (2010). Kinematics of disease progression in bulbar ALS. Journal of Communication Disorders, 43(1), 6–20. https://doi.org/10.1016/j.jcomdis.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y., Weismer G., Westbury J. R., & Lindstrom M. J. (2008). Articulatory movements during vowels in speakers with dysarthria and healthy controls. Journal of Speech, Language, and Hearing Research, 51, 596–611. https://doi.org/10.1044/1092-4388(2008/043) [DOI] [PubMed] [Google Scholar]