Abstract
Dysregulation of the ubiquitin-proteasome system (UPS) has been implicated in several types of tumorigenesis. Our previous studies have shown the potential role of Cdh1/APC in regulating tumor formation via governing the Skp2-p27-cyclinE/CDK2 axis. In this work, we utilized a xenograft mouse breast cancer model to identify the mechanism by which Cdh1/APC potentially suppresses tumor growth in vivo. Here, we report that depletion of Cdh1 results in a significant enhancement of the breast tumor proliferation, while elevated Cdh1 leads to suppression of breast tumor growth. Analysis of breast tissue arrays has indicated that higher levels of Cdh1 are associated with normal breast epithelial tissues whereas lower Skp2 expression and elevated p27 levels are detected. Conversely, the percentage of breast cancer tissues stained positive for Cdh1 and p27 are significantly lower with higher Skp2 levels. Thus, the E3 ligase, Cdh1/APC, may inhibit breast tumor growth via regulating Skp2-p27 mediated cell cycle progression.
Keywords: Cdh1, APC, Skp2, p27 and breast cancer
Introduction
Abrogated cell cycle regulation often results in tumorigenesis 1. Proper cell cycle progression is ensured by the controlled oscillation of a series of cell cycle machineries. UPS has been tightly linked to the orchestration of several critical cell cycle machineries such as the stage-dependent CDK and their inhibitors 2–6. SCF (Skp1/Cullin/F-box) complex and APC (anaphase-promoting complex) have been implicated as two major ubiquitin protein ligases that govern mitotic progression and transition from G1 to S phase 7–9. Skp2 (an F-box protein) and Cdh1 (a WD-40 family member) function as substrate receptors, which determine substrate specificity as well as control the function for SCF and APC, respectively 7, 8, 10. The major role of SCF-Skp2 is to target p27 for destruction, thus elevating the activity of S-phase driver, cyclinE/CDK2, and promoting G1/S transition 10, 11. The initial function of Cdh1/APC has been addressed in governing cyclin B degradation in late mitosis by stimulating the exit from mitosis and reconstituting the cell for another cycle 12, 13. Recent studies have integrated the Cdh1/APC and SCF-Skp2 in the same G1 regulative pathway as well as in modulating TGF-beta-induced tumor suppression 14–16, where Skp2 is ubiquitylated by Cdh1/APC for proteolysis preventing premature entry of S phase 17, 18. This finding defines a novel function of Cdh1/APC in regulating cell cycle progression from G1 to S phase through regulating the SCF-Skp2-p27 cascade.
Pathological and epigenetic studies have demonstrated that malfunction or mutation in the SCF-Skp2 or Cdh1/APC pathway leads to a variety of cancers 14, 19–23. Skp2 has been suggested to be a biological marker for several types of cancer and can be used to predict the properties for certain types of malignancy 19, 20. Dysfunction of several components in the APC pathway, including APC6, Cdc16, Cdc23, and Cdh1 or Cdc20, is correlated with colon, B-lymphoma, gastric and lung cancer 21–24. The role of Cdh1/APC has also been implicated in mediating TGF-beta-induced tumor suppression 15, 25, 26. Nevertheless, the underlying mechanism by which APC is involved in the aforementioned carcinogenesis remains largely unknown. Exploring whether Cdh1/APC and SCF-Skp2 are important machineries in the same tumorigeneic pathway will be critical in enhancing our understanding of cancer formation.
Our previous work based on cultured tumor cells and tumor immunohistological analysis suggested the role of Cdh1 in regulating Skp2 mediated malignancy 27. To validate the connection between Cdh1 and Skp2 in tumor formation with an in vivo approach we have integrated a xenograft mouse breast cancer model with molecular and tissue array analyses to dissect the role of Cdh1 in tumorigenesis. The results from this work confirmed the function of Cdh1/APC in suppressing tumor expansion via proteolytic regulation of Skp2. These results further suggest that the E3 ligase, Cdh1/APC, may inhibit breast tumor growth through regulating the Skp2-p27 mediated cell cycle progression.
Materials and Methods
Plasmid preparation and construction of small-interfering RNA stable cell lines
pCS2-Myc-Cdh1 and pCS2-HA-Skp2 plasmids were engineered and reported previously 25. Retroviral expression construct pMX-IRES-GFP and construction of Cdh1 using pMX-IRES-GFP were described previously 15. Stable knockdown using small interfering RNA (siRNA) for Cdh1 was reported 26. Construction of Skp2-siRNA stable cell line 1) Skp2 N (amino acid 269–297) and 2) Skp2 C (amino acid 514–532) were engineered using the pSUPER system (Oligoengine, Seattle, WA, USA). Transfection was carried out using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacture’s protocol.
Cell culture
The human breast cancer cell line MCF7, and MDA-MB231 were obtained from the American Type Culture Collection (Manassas, VA, USA) and grown in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% (v/v) fetal bovine serum, penicillin (100 IU/ml; Invitrogen), and streptomycin (100 µg/ml; Invitrogen), following incubation in 5% CO2 at 37°C. MCF10A cells were obtained from the American Type Culture Collection (Manassas) and the cells were maintained routinely in a 1:1 mixture of DMEM/Ham’s F12 medium (Invitrogen) containing 15 mM Hepes (Cambrex Bio Science Walkersville, Inc. Walkersville, MD, USA), 5% horse serum (Invitrogen), 10 µg/ml bovine insulin (Cambrex Bio Science), 20 ng/ml EGF (epidermal growth factor) (Cambrex Bio Science), 100 ng/ml cholera toxin (Cambrex Bio Science), 0.5 µg/ml hydrocortisone (Cambrex Bio Science), 100 IU/ml penicillin (Invitrogen), and 100 µg/ml streptomycin (Invitrogen). Cultures were passaged every 3–4 days by trypsin/EDTA (Invitrogen) detachment.
Antibodies and Reagent
Antibodies used were specific to Cdh1 (Oncogen, CC-43), Skp2 (sc-7164, Santa Cruz, CA, USA), p27 (sc-528, Santa Cruz), tubulin (T-5168, Calbiochem, San Diego, CA, USA) and horseradish peroxidase-conjugated secondary antibody (Promega, Madison, WI, USA). Rat anti-Cdh1 specific antibody was a kind gift from Dr. MW Kirschner (Harvard Medical School). Western blot analysis was performed using an ECL detection kit (Amersham, Buckinghamshire, UK).
Colony formation by soft agar assay
24 hours after transfection, cells were seeded onto soft agar as described previously 28 with slight modification (Dr. Erik Flemington, Department of Pathology, Tulane Cancer Center, USA). Briefly, sterile agarose solution (Sigma) was added to a 6-well plate and made a thin film of agarose on the bottom and side of each well and plate with 2.0×105 cells/well. Colony formation was assessed by microscopic inspection (x 10) and colonies were quantified 7 days after cell seeding.
Bromodeoxyuridine (BrdU) labeling
The proliferative rate of cells grown was measured by assaying 5-bromo-2-deoxyuracil (BrdU) incorporation with a commercially available labeling and detection kit (Rosch Diagnosis, Indianapolis, IN). BrdU-labeled indices were determined by visually scoring nuclei stained with 4',6-diamidino-2-phenylindole in 50–100 cells in independent 10 visual field and thereafter scoring BrdU-positive cells as a percentage of the total cell number 29. Each experiment was repeated at least three times. Values given are the results of mean (+/− S.D) value score.
Immunofluorescence
Immunofluorescence analysis was performed using the following primary antibodies against Cdh1 (rabbit anti-rat) and Skp2 (rabbit anti-mouse). Secondary antibodies were Cy2 (anti-mouse 1:500, Jackson ImmunoResearch, West Grove, PA) and Texas-red (anti-rat 1:100, Jackson ImmunoResearch). Semi-quantification of data was performed using Image-J and Scion Image imaging software (Frederick, MD, USA).
Immunohistochemical staining and clinicopathological analysis
Samples were deparaffinized in xylene and rehydrated in a series of graded alcohols The antigen was retrieved in 0.01 M sodium citrate buffer and sections were then treated with 0.6% hydrogen peroxide 30. Samples were incubated using rat-anti human Cdh1 antibody, rabbit-anti human Skp2 antibody and rabbit-anti human p27 antibody. Sections were thereafter treated with biotinylated mouse-anti rat immunoglobulin (Jackson ImmmunoResearch, West Grove, PA, USA) and donkey-anti rabbit antibody (Vector Laboratories, Burlingame, CA, USA) followed by incubations with avidin-biotin peroxidase complex solution (DAKO Cytomation, Carpinteria, CA, USA) and 3-amino-9-ethylcarbazole solution (DAKO Cytomation). The counterstaining was carried out using Mayer’s hematoxilin (Sigma). Tissue arrays were purchased from US Biomax. (Rockville, MD, USA). For clinicopathological analysis, human breast tissue samples with information of ER, PgR, HER2, axillary lymph nodes status and histological grade were provided from Breast Tissue Bank in the Department of Cancer and Thoracic Surgery, Okayama University, Japan. The specificity and optimal concentration of antibody were verified using the test tissue array slides.
Xenograft experiments in nude mice
We generated breast cancer cell lines (MCF7 and MDA-MB-231) that stably expressed Cdh1 and lacZ (control) with stable knockdown of Cdh1. In an orthotopic model, 1×107 of these cells were separately injected into the mammary fat pads of 8-week old ovariectomized female nude mice (Taconic lab, Hudson, NY, USA). At least 10 mice were tested for each group. Two days before the injection, in case with MCF7 cells, each mouse was supplemented with 17β-estradiol (E2) pellets (0.72 mg/pellet) that were implanted into the left skin on the lateral side of the animal (Innovative Research of America, Sarasota, FL, USA). Tumor growth was monitored when the series of tumor size approached around 500 mm3, then surgically removed. Resected tumors were fixed with paraformaldehyde followed by ethanol. Immunohistochemical evaluation of embedded tissues was carried out after sectioning. Images were captured with SPOT digital cameras (Diagnostic Instrument) equipped on these microscopes.
Scoring of immunohistochemical staining
Staining intensity and subcellular localization were evaluated twice in a blinded manner based on the pre-agreed stain scoring standard from a specialized pathologist (Dr. SY Cheng SY, Department of Pathology, University of Pittsburgh). Staining intensity was scored separately using the following scoring criteria: (a) 0–1, negative or low staining intensity in >50% of tumor cells or moderate to high in <50% of the cells (hereafter referred to as low); and (b) 2–3, moderate to high staining intensity in >50% of tumor cells (hereafter referred to as high) 31.
Statistical analysis
Statistical significance were evaluated with data from at least 3 independent experiments by using a two-sided Student's t-test. Chi-square-test and correlation-test were used for statistical analysis of immunostaining and clinicopathological data. p < 0.05 was considered statistically significant. All data were analyzed with SPSS14.0 (Chicago, IL, USA).
Results
Cdh1/APC pathway is abrogated in cancer cells
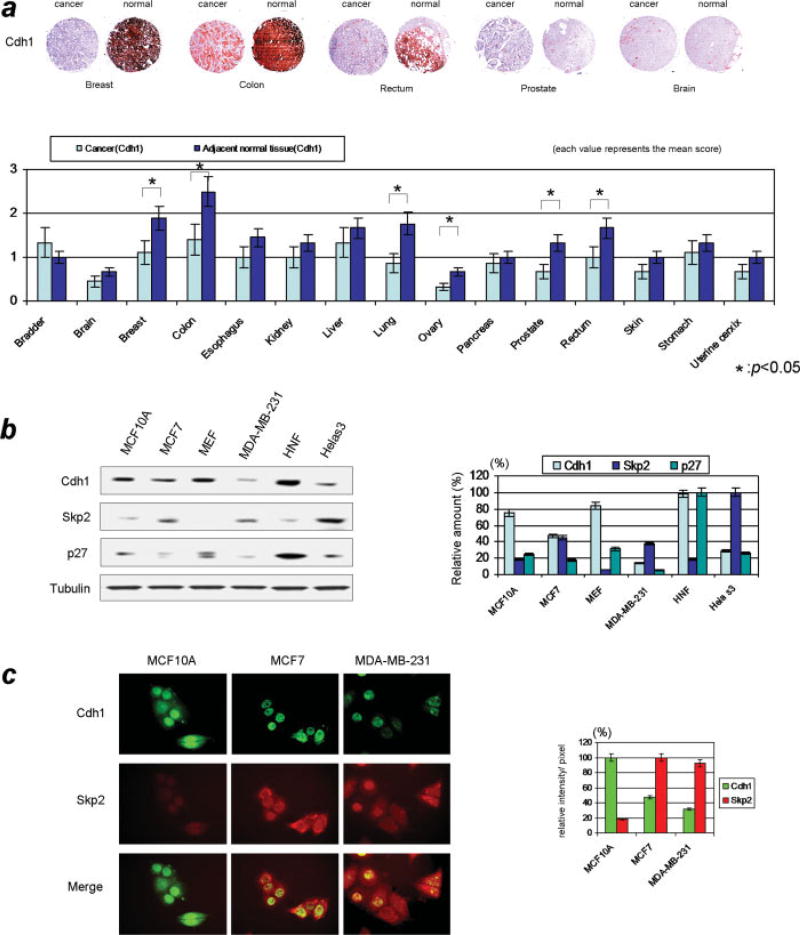
Both Skp2 and p27 are high-turnover proteins governed by the UPS, whose deregulation can result in aberrant protein function and subsequent cancer 19. Previous work have demonstrated that Skp2 is an important molecule that can accelerate the progression to cancer, where Skp2 acts as an oncogenic protein that abrogate the proper G1/S transition via altering p27 19. Current studies have implicated Cdh1/APC in playing a pivotal role in orchestrating the Skp2-p27 axis, thereby preventing premature entry into S phase 17, 18, 25. To address the possible relevance of Cdh1 in tumorigenesis we examined the expression profile of Cdh1 and Skp2 in various human cancer tissues as well as its adjacent normal tissues. As shown in Fig 1a, lower levels of Cdh1 are observed in certain types of malignant tumors, such as breast and colorectal cancer. Significant difference between the IHC score found between cancer and normal adjacent tissues suggest the potential involvement of Cdh1 in cancer. Based on the results of expression profile using various human cancers and in order to correlate the regulatory axis of Cdh1/APC-Skp2-p27 with malignant tumor status, we have compared the protein levels of Cdh1, Skp2 and p27 in breast cancer cells to normal cells. As shown in Fig 1b, protein levels of Cdh1 and p27 were lower in breast tumor cell lines (MCF7, MDA-MB-231) compared with normal breast epithelial cell line (MCF10A) and other non-malignant cell lines; Skp2 protein levels were higher in breast cancer cells but lower in the normal breast cells. No significant difference was observed for APC2 between these cells (Data not shown).
Figure 1. Expression of Cdh1, Skp2, and p27 in breast cancer.
a) Expression of Cdh1 in various human cancer and adjacent normal tissues. Levels of Cdh1 were tested using immunohistochmistry on 15 sets of different human cancers and adjacent normal tissues. Significant differences in the levels of Cdh1 were observed in breast cancer, colon cancer and rectal cancer, where Cdh1 was highly expressed in non-cancer areas while expressed at relatively lower levels in the aforementioned cancer cells.
b) (Right panel) Results from immunoblotting Cdh1 and Skp2/p27 in cultured cells. Levels of Cdh1 and p27 were relatively higher while Skp2 were lower in non-malignant cell lines such as MCF10A human breast epithelial cells, human normal fibroblast cells (HNF) and MEF cells. On the contrary, most of cancer cells (MCF7, MDA-MB-231 and Hela cells) showed lower levels of Cdh1 and p27 and higher levels of Skp2 (Left panel) Quantification of protein expression in these cells. c) Immunocytochemical analysis of Cdh1 and Skp2 in normal breast epithelial and breast cancer cells. Irrespective of malignant and non-malignant cells, Cdh1 was mainly localized in the nucleus. However different levels of expression were observed between cancer and normal cells regarding Cdh1 and Skp2. Lower levels of Cdh1 and higher levels of Skp2 were observed in cancer cells. Results from immunocytochemical analysis were compatible with immunoblotting results.
Focusing on MCF7, MDA-MB-231 and MCF10A cells and wanting to validate the immunoblotting results, we further tested Cdh1 and Skp2 using immunocytochemical analysis. As shown in Fig 1c, Cdh1 was mainly observed in the nucleus of both cancer and normal cells. However, its abundance was distinctly lower in cancer (MCF7 and MDA-MB-231) than in normal breast epithelial cells (MCF10A). Conversely, levels of Skp2 were markedly higher in cancer cells, which is consistent with the results from immunoblotting analysis. Taken together, the results implicate that Cdh1 and Skp2-p27 are involved in breast cancer formation and further suggest that Cdh1 has a potential role as a tumor suppressor regulating the level of Skp2 and subsequently p27.
Cdh1/APC dependent regulation of Skp2-p27 pathway in breast cancer cells
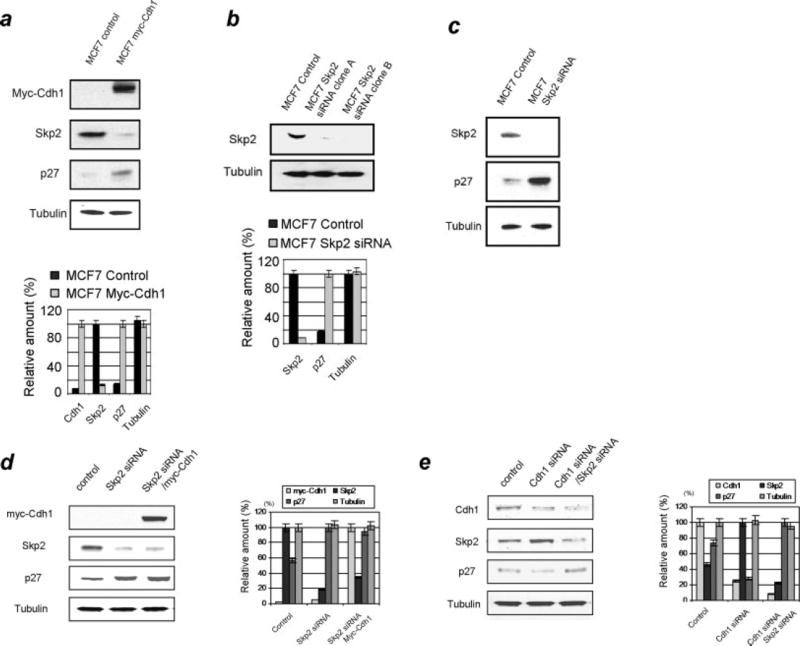
To confirm the role of Skp2 in promoting cellular growth and the potential function of Cdh1 suppressing proliferation 17–19, 32 we examined the effect of Cdh1 overexpression on Skp2 and p27 using immunoblotting analysis of MCF7 cells. As shown in Fig 2a, overexpression of Cdh1 resulted in downregulation of Skp2 and upregulation of p27 suggesting that Cdh1 is an important player in regulating the Skp2-p27 cascade in breast cancer cells. We further examined the correlation of Skp2 and p27 by depleting Skp2 using RNA interference. As shown in Fig 2b, we initially engineered the Skp2 siRNA stable clone based on MCF7 cells and found that p27 levels markedly increased in the absence of Skp2, which suggests that p27 levels were tightly regulated by Skp2 (Fig 2b,c). To assess the correlation between Cdh1 and p27 as mediated through Skp2 we overexpressed Cdh1 in the absence of Skp2 and then measured the alteration of p27. As shown in Fig 2d, no change of p27 was observed when Cdh1 was overexpressed in Skp2 depleted MCF7 cells (Fig 2d), suggesting that Cdh1 regulates the level of p27 through the regulation of Skp2. To further confirm the role of Skp2 in mediating Cdh1 regulated p27 alteration we measured the alteration of Skp2 and p27 in Cdh1 knockdown cells. As shown in Fig 2e, depletion of Cdh1 significantly elevated Skp2 levels and decreased p27 levels, while no significant change in p27 was observed in both Cdh1 and Skp2 knockdown cells. In summary, the above results suggest a Cdh1/APC dependent regulation of Skp2-p27 pathway in breast cancer cells
Figure 2. Overexpression or knockdown of Cdh1 alters the Skp2-p27 cascade in breast cancer cells.
a) (Upper panel) Overexpression of Cdh1 resulted in the downregulation of Skp2 and the upregulation of p27 in breast cancer cells. (Lower panel) Quantification of the expression of Cdh1, Skp2 and p27. b) (Upper panel) Engineering of the Skp2 knockdown clone using shRNA in breast cancer cells. (Lower panel) Quantification of immunoblotting analysis repeated at least three times. c) Depletion of Skp2 resulted in the attenuation of p27 levels. d) Depletion of Skp2 induced upregulation of p27 while additional overexpression of Cdh1 did not alter the levels of p27 in Skp2-knockdown breast cancer cells. e) Depletion of Cdh1 increased the levels of Skp2. However, depletion of both Cdh1 and Skp2 showed no effect on the levels of p27 in MCF7 cells (Representative figures from at least three-independent analysis were shown here.)
Regulation of Skp2-p27 axis by Cdh1/APC results in inhibited cellular proliferation and oncogenic potential in breast cancer cells
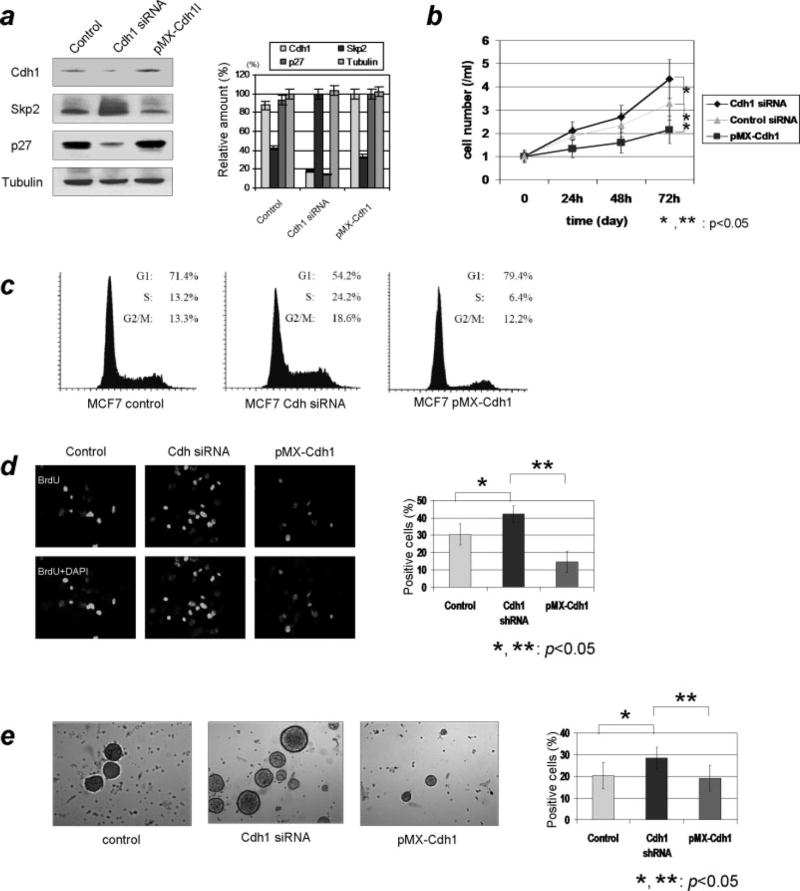
Results based on the above experiments sketch a growth-inhibiting role for Cdh1 through modulating Skp2-p27 axis during the G1/S progression. Given the oncogenic potential of Skp2 and tumor suppressing role of p27, a possible anti-oncogenic role for Cdh1 was expected. To test the potential impact of Cdh1 as a tumor suppressor we examined the effect of Cdh1 depletion on the cell cycle profile and on oncogenic cellular growth. Using MCF7 cells we initially established a stable Cdh1 knockdown as well as a Cdh1 overexpression cell line and then measured Skp2 and p27 levels. As shown in Fig 3a, Skp2 and p27 profiles were consistent with our previous results (Fig 1a and b). Based on the data derived from the above cells we first tested cellular proliferations and found that a significantly higher growth rate was observed in Cdh1 knockdown cells as compared to the control siRNA delivered cells (p<0.05) However, proliferation rate was markedly decreased in Cdh1 overexpressed cells compared with control siRNA transfected cells (p<0.05) (Fig 3b). No significant difference of the cellular proliferation was observed between control siRNA transfected cells and pMX-control transfected cells (data not shown). Moreover, FACS analysis demonstrated that depletion of Cdh1 resulted in a significant increase of the S-phase population compared with the control. On the contrary, the number of S-phase cells was markedly decreased in Cdh1 overexpressed cells (Fig 3c). In addition, as predicted, the results of the BrdU immunofluorescent analysis showed a higher levels of BrdU-positive cells in Cdh1 depleted cells compared with Cdh1 overexpressed cells (Fig 3d). We next analyzed the oncogenic colony formation and the capacity of accelerating cell growth using MCF7 with either overexpressed or depleted Cdh1. As shown in Fig 3e, Cdh1 knockdown cells have an increased capacity for colony formation in soft agar, while both number and size of colony significantly decreased when Cdh1 was overexpressed in MCF7 cells. Taken together, the above results further suggest that regulation of the Skp2-p27 axis by Cdh1/APC results in inhibited cellular proliferation and oncogenic potential in breast cancer cells
Figure 3. Effects of overexpression or knockdown of Cdh1 on cellular proliferation and cell cycle profile in breast cancer cells.
a) Profile of Cdh1 and Skp2-p27 cascade in Cdh1 knockdown cells as well as Cdh1 overexpressed breast cancer cells. Consistent with our previous results knockdown of Cdh1 increased the levels of Skp2 and decrased the expression of p27, whereas overexpression of Cdh1 decreased the levels of Skp2 and increased the expression of p27 in these cells. b) Analysis of cell proliferation for overexpressed and knockdown of Cdh1 status. Knockdown of Cdh1 markedly increased cellular proliferation, while on the other hand overexpression of Cdh1 suppressed cellular growth in MCF7 cells.
c) Impact of cell cycle profile with either knockdown or overexpression of Cdh1. Remarkable elevation of S-phase population was seen in Cdh1-knockdown cells, while overexpression of Cdh1 decreased the population of S-phase cells.
d) Quantification of S-phase population with immunocytochemistry using BrdU. Consistent with the cell cycle profile results, S-phase population increased due to the delivery of Cdh1 siRNA while overexpression of Cdh1 reduced the number of S-phase cells. e) Analysis of oncogenic cellular proliferation using anchorage-independent colony formation assay. The number of colonies was significantly increased by the depletion of Cdh1. However, the number of colonies declined due to the overexpression of Cdh1 in MCF7 breast cancer cells.
The role Cdh1 in suppressing breast tumor growth in mouse xenograft
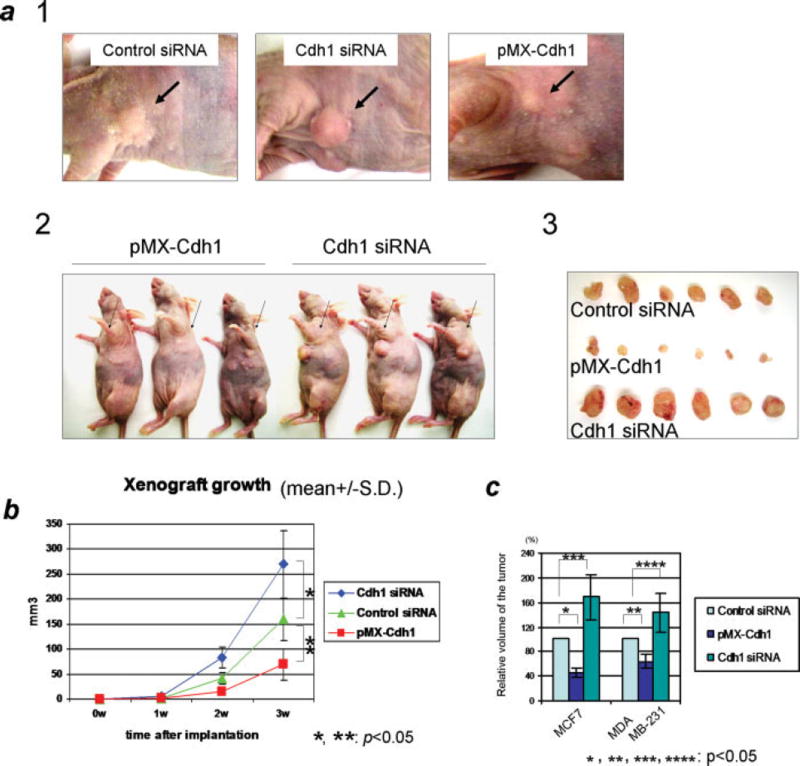
Results from the above experiments revealed that overexpression of Cdh1 suppressed cellular proliferation (Fig 3b) and reduced oncogenic cellular growth in anchorage-independent colony formation assay (Fig 3e). Thus, the hypothesis was that Cdh1 played a role as a suppressor of breast tumorigenesis. However, the potential anti-oncogenic role or significance of Cdh1 had not been confirmed physiologically. Therefore, we decided to characterize the potential tumor suppression function of Cdh1 using a xenograft breast cancer mouse model. Prior to the xenograft experiment using MCF7 cell, we had tested the potential interaction between 17β-estradiol and Cdh1, confirming that 17β-estradiol did not show any impact on the level of Cdh1 (Supplemental Figure S1). As shown in Fig 4a and b, compared with the siRNA scramble control cells, overexpression of Cdh1 markedly reduced the volume of the implanted tumor (p<0.05) while depletion of Cdh1 resulted in significant acceleration of the tumor growth (p<0.05). Similar to MCF7 cells, overexpression of Cdh1 also reduced the tumor growth status in MDA-MB-231 breast cancer cells (Fig 4c). No significant difference of the tumor growth was observed between siRNA scramble control cells and pMX-control cells (data not shown).
Figure 4. Status of Cdh1 affects the tumor growth in xenograft mouse study.
a) 1. Representative picture of the subcutaneous tumors 4 weeks after implantation of breast cancer cells. Remarkable suppression of tumor growth was observed with the implantation of Cdh1-overexpressed cells using the mouse xenograft. 2. Representative picture of the mice 4-weeks after subcutaneously implantated Cdh1-overexpressed and Cdh1-depleted MCF7 cells. 3. Representative picture of surgically removed tumors from nude mice 4-weeks after the implantation of breast cancer cells with overexpression of Cdh1 (middle panel) depletion of Cdh1 (lower panel) compared with control siRNA delivered cells (upper panel). Xenograft tumor growth was markably increased (p<0.05) with the delivery of Cdh1-siRNA, while overexpression of Cdh1 significantly suppressed the tumor growth (p<0.05) in MCF7 breast cancer cells. b) Growth curve of tumors with knockdown or overexpression of Cdh1 as well as control siRNA delivered cells showing that significant differences (p<0.05) of tumor growth were observed between these tumors. c) Summary of the xenograft tumor growth regarding the size of tumors using breast cancer cells. In both MCF7 and MDA-MB-231 breast cancer cells, overexpression of Cdh1 significantly reduced the tumors growth compared with the control siRNA delivered cells (p<0.05), whereas depletion of Cdh1 markedly increased the tumor growth compared with the control (p<0.05).
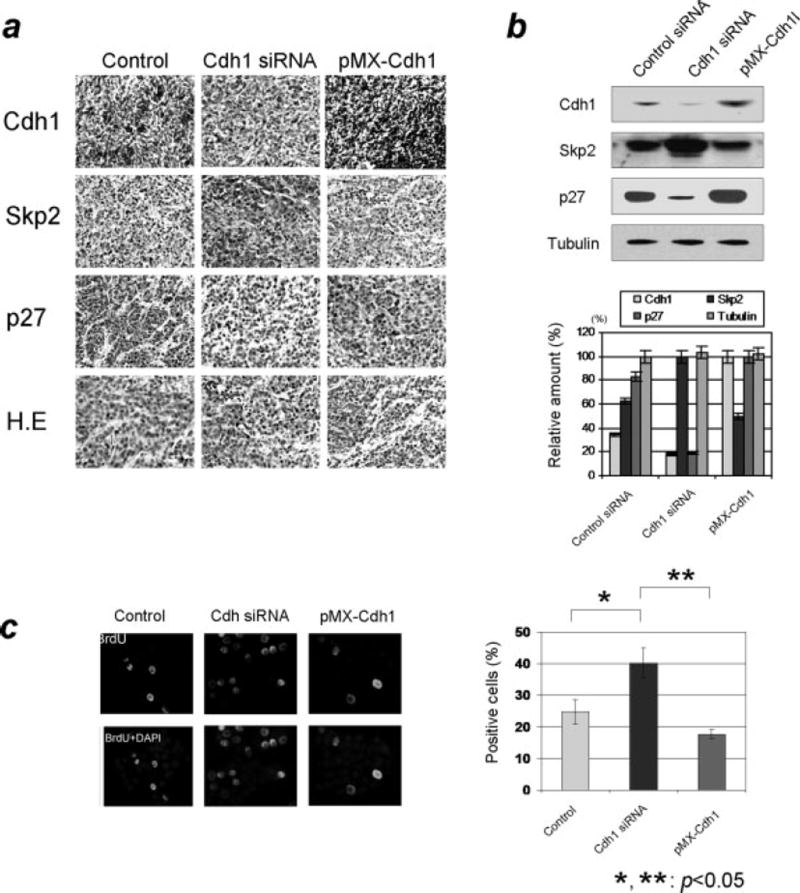
Next, to further validate the status of Skp2-p27 axis in these tumors, we immunohistochemically examined the expression of Skp2 and p27. The results from the immunohistochemical analysis shown in Fig 5a indicate that tumors grown from Cdh1 knockdown cells exhibited higher Skp2 and lower p27 levels, whereas lower levels of Skp2 and higher levels of p27 were detected in tumors with overexpressed Cdh1. Consistent with the immunohistochemical analysis, the results from the immunoblotting assay revealed higher Skp2 and lower p27 levels in Cdh1 knockdown tumors, and lower Skp2 and higher p27 levels in Cdh1 overexpressed tumors (Fig 5b). Moreover, the findings from the BrdU assay on these tumor tissues showed that the population of BrdU positive cells was attenuated in BrdU staining in tumor from Cdh1 overexpressed cells while the number of positive cells increased in tumors from Cdh1 knockdown cells (Fig 5c). In summary, the results from the xenograft experiments strongly suggest an inhibiting role of Cdh1 in breast tumor growth through modulating the Skp2-p27 cascade.
Figure 5. Characterization of the tumors from xenograft mouse study.
a) Immunohistochemical analysis of Cdh1/APC-Skp2-p27 axis in sectioned tumors from the xenograft study. Tumors from Cdh1-overexpressed breast cancer cells were broadly stained positive for Cdh1 and p27 while the cells were mostly negative for Skp2. On the contrary, tumors from Cdh1-knockdown cells appeared to be negative for Cdh1 and p27 while significantly positive for Skp2. b) Evaluation of immunoblotting results using the resected tumors from the xenograft mouse study. Levels of Skp2 markedly increased while levels of Cdh1 and p27 decreased from tumors derived from Cdh1-depleted cells. Levels of Skp2 decreased while levels of p27 increased in tumors from Cdh1-knockdown cells. c) Measurement of S-phase population of the tumor grown in the nude mouse. The population of BrdU positive cells was elevated in the tumors derived from Cdh1-knockdown breast cancer cells.
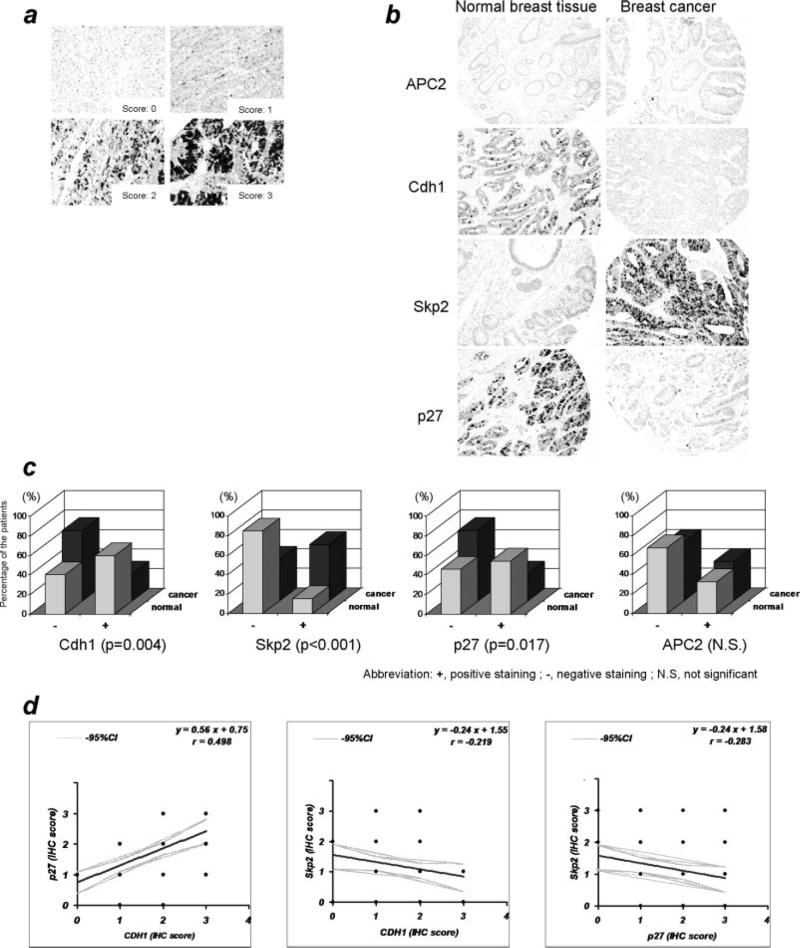
Tissue array analysis of human breast tissue in the Cdh1/APC cascade
Our results regarding the loss of function analyses of Cdh1/APC-Skp2-p27 using RNA interference in combination with the overexpression analyses in breast cancer cells demonstrated that Cdh1 plays a pivotal role in dictating Skp2-p27 function in the cellular proliferation of breast tumor cells. This result suggests that deregulation of Cdh1 could contribute to the aberration of Skp2/p27 seen in breast cancer. To correlate the importance of the molecular pathway of Cdh1/APC-Skp2-p27 in controlling G1/S transition with the pathological relevance in breast cancer, we performed a study using a human tissue array with 325 breast tumors and classified them into a positive or negative group according to an immunohistochemical scoring system (Fig 6a; samples were stained for p27). Representative pictures of immunohistochemical analysis are shown in Fig 6b, exhibiting no significant difference of APC2 expression between normal breast tissue and breast cancer (Fig 6b), yet a significantly higher frequency of positive Cdh1 expression in normal breast tissue with prominent nuclei localization in comparison to breast cancer tissue (Fig 6b). Meanwhile, a higher frequency of positive Skp2 expression was observed in breast cancer tissue, while a lower frequency of p27 expression was measured in the cancer area (Fig 6b). Further, statistical analysis showed no difference in the expression of APC2 between cancer and normal tissue (p=0.483). However, significant differences were examined in Cdh1 (p=0.004), Skp2 (p< 0.001) and p27 (p= 0.017) between breast cancer and normal breast tissue (Fig 6c). The above results show the significant correlation between the expression levels of Cdh1 and p27 (p<0.05) while an inverse correlation was observed between the levels of Cdh1 and Skp2 (p<0.05), where breast cancer cells have a lower expression of Cdh1 and p27 but higher expression of Skp2 (Fig 6d). Overall, results based on the human breast tissue array are consistent with the results from the culture cell studies 17, 18.
Figure 6. Pathological dissection of the Cdh1/APC-Skp2-p27 cascade in breast cancer.
a) Representative picture of immunohistological (IHC) staining scored from human breast tissue b) A significant reduction of Cdh1 protein levels were measured in breast cancer tissue, while abundant Cdh1 protein was detected in normal breast tissue. Expression of APC2, a key subunit of the APC, is at similar levels in normal and breast cancer tissue. Accumulation of Skp2 was observed in breast cancer tissue, while moderate Skp2 protein levels were shown in normal breast tissue. Reduction of p27 protein levels was seen in breast cancer tissue, while abundant p27 protein was visualized in normal breast tissue. c) Summary of normal and breast cancer tissue array showing a statistically significant difference among Cdh1, Skp2 and p27. d) Correlation of the IHC scores among the components of Cdh1/APC-Skp2-p27 cascade from individual patients. Significant correlation (p<0.05) was observed between the scores of Cdh1 and p27, while statistically significant inverse correlation was seen between Cdh1 and Skp2, as well as Skp2 and p27 in human breast cancer tissues.
Pathological relevance of Cdh1 in patients with breast cancer
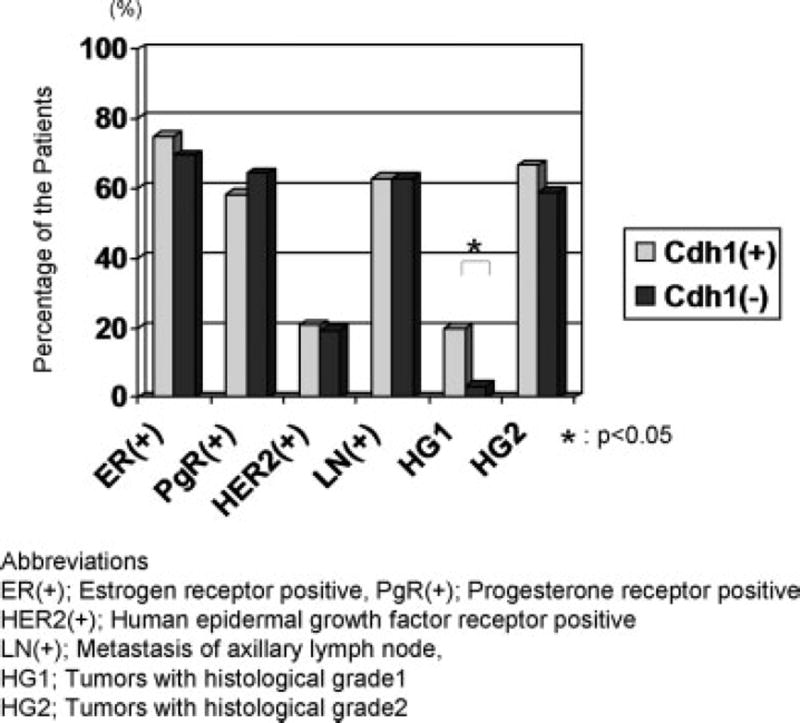
The results from the breast tissue array analysis have demonstrated that Cdh1 regulates the levels of Skp2 and highly suggest that Cdh1 can act as a suppressor of breast cancer. Several biological markers, such as hormone receptor status, have been broadly accepted to predict the patient’s prognosis or to determine the treatment strategy in breast cancer patients. Potential correlations with these existed pathological parameters are often required to evaluate the biological significance of newly identified molecular targets. Therefore, to further validate the clinicopathological significance of Cdh1 in patients with breast cancer we explored the pathological relevance of Cdh1 with other widely-utilized prognostic parameters such as the status of estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor type II receptor (HER2), axillary lymph node status (LN) and histological grade of the tumor (HG). As shown in Fig 7, no remarkable difference was observed in ER, PgR, HER2 and LN status between Cdh1 positive and negative tumors. However, Cdh1 positive tumors had a significant correlation with a higher rate of low histological grade (Grade I) cancer (p<0.05). Given that low histological grade breast cancers are reported to be associated with lower levels of Skp2 and less aggressive biological behavior, the results based on the above study support a tumor suppressive role for Cdh1 in certain aspects of the disease. Consistent with the results of the histological grade of the tumor, significantly better disease-free survival was observed in patient’ with Cdh1-positive breast cancer (Supplemental Figure S4c and d).
Figure 7. Clinical relevance of Cdh1 in patients with breast cancer.
No statistically significant difference was observed in estrogen receptor (ER) status, progesterone receptor (PgR) status, human epidermal growth factor receptor 2 (HER2) status and axillary lymph node (LN), whereas a significant difference in histological grade (HG) was seen between Cdh1 positive and negative breast cancer. Examining the distribution of Cdh1 positive breast cancer in low histological grade (grade 1) tumors has shown a significant correlation between Cdh1 positive breast cancer in low histological grade tumors.
Discussion
Precise and accurate proteolytic regulation of cell cycle machineries by the UPS ensures normal cell cycle progression, while its dysregulation often results in tumorigenesis 2, 13. SCF and APC have been thought to be critical ubiquitin protein ligases that govern mitosis and G1/S progression 13. Dysfunction of the SCF or APC pathway has been implicated in many types of cancer 14, 21–23. Recent studies have demonstrated that an ubiquitylation regulatory cascade exists in the tumorigenesis pathway, where Cdh1/APC regulates SCF-Skp2, modulating the p27-cyclin E/CDK2 axis 17, 18. Our previous work has shown a molecular and pathological connection between the Cdh1/APC regulating SCF-Skp2 and tumorigenesis 27. The present study, using a xenograft mouse model, addresses the mechanism by which Cdh1/APC mediates breast tumorigenesis via modulating the Skp2-p27 pathway. The results from this work confirmed that the tumor suppressing effect of Cdh1/APC is through regulating the Skp2-p27 pathway in breast cancer and thus, advances our understanding of how APC is involved in cancer formation through the cell cycle regulation.
Involvement of Cdh1/APC in breast tumorigenesis through regulating the Skp2-p27 pathway
The protein p27 suppresses aberrant cell cycle progression and is thought to be a tumor suppressor in various types of malignant tumors. Skp2, in association with the SCF complex, catalyzes p27 for degradation. Hence, deregulation of Skp2-p27 often leads to abrogated cell cycle progression, which in turn initiates cancer 17, 18. Results based on cultured cell studies have sketched a framework: Cdh1/APC targets Skp2 for degradation. Given the correlation between aberrant APC subunits and various types of cancer from previous epigenetic research 14, 21–23, this newly demonstrated association of Cdh1/APC and SCF-Skp2 further implicates the potential regulatory role of Cdh1/APC in tumorigenesis. Indeed results of our tissue array analysis show that a marked difference of Cdh1 between normal and cancer were observed in breast, lung and colorectal tissues whereas Skp2 was previously demonstrated as prognostic markers in patients with these malignancies. These observations suggest that Cdh1 play an important role in suppressing the progression of certain type of cancers; Cdh1 does not have a striking impact on cancers in which Skp2 and p27 play a less significant role in disease progression. This hypothesis may be the clue to explain the difference of the expression profile of Cdh1 in various human malignancies. To assess the involvement of Cdh1 in regulating breast tumorigenesis via the Skp2-p27 pathway we conducted analyses of loss of function as well as overexpression of Cdh1 using breast cancer cells to address the biological function of Cdh1 involved in tumorigenesis. Our results suggest that the activity of Cdh1 leads to the downregulation of Skp2 and upregulation of p27. Overexpression or RNA interference of Cdh1 induces significant changes in cellular proliferation, cell cycle profile and the property of oncogenic growth in breast cancer cells, all of which is consistent with the previous notion that Cdh1 regulates Skp2 through the ubiquitin/proteasome system.
Modulating Skp2-p27 is a putative means of APC involved in tumorigenesis
The function of APC has been initially characterized in ensuring separation of duplicated daughter genomes during mitosis with dysfunction of APC often resulting in aneuploidy 33, 34. The results from epigenetic studies have drawn our attention to the correlation of APC with tumorigenesis, where deregulation in components of APC pathway including APC6, Cdc16, Cdc23 and Cdh1 are found in several cancer 21–24. The finding that APC mediates TGF-β signaling targeting SnoN as well as Skp2 has unveiled a potential role for Cdh1/APC involved in tumor inhibition 16, 25. Previous evidence from several lines has established a framework for APC’s involvement in tumor progression. The present results confirm previous results obtained from studies on cultured cells with pathological relevance further suggesting that deregulation of Skp2-p27 may be the mechanism by which APC initiates tumorigenesis. Cdh1 in association with APC could also target additional proteins, other than Skp2, which are potentially involved in tumorigenesis. Among these targets, cyclin B is one of the major Cdh1/APC substrates, which has been implicated in enhancing certain types of malignant tumors. Given that the proteolysis of cyclin B is catalyzed by Cdh1/APC, the potential role of Skp2-p27 axis modulated by the Cdh1/APC in tumorigenesis needs to be addressed. Indeed, cyclin B levels respond to the status of Cdh1 (Supplemental Figure S2a), while cellular proliferation as well as colony formation were more prominent in Cdh1 overexpression cells compared with cyclin B knockdown cells (Supplemental Figure S2b and c). These results suggest that Cdh1/APC could play a tumor suppressing role via several different molecular cascades, supporting previous notions 16. Among these molecular pathways, however, Skp2-p27 axis could be one of the important cascade associated with the tumor progression.
Combination of in vivo dissection and pathological analysis confirms the tumor-inhibiting role of Cdh1/APC
To verify the potential function of Cdh1 in inhibiting tumor cell growth through regulating the Skp2-p27 pathway we performed an in vivo analysis using a xenograft breast cancer mouse model. Overexpression of Cdh1 markedly reduced the size of the implanted tumor, while depletion of Cdh1 resulted in an acceleration of tumor growth. The results based on alteration of Cdh1 using RNA interference or overexpression verified that Cdh1/APC is important in suppressing breast tumor growth. Regulation of Skp2-p27 by Cdh1/APC seems to be significant in controlling tumor expansion in mouse.
The results of our examination of Cdh1, Skp2, p27, and related breast cancer markers such as ER and HER2, on breast tumors using immunohistochemistry provoked the hypothesis that Cdh1 could antagonize tumorigenesis via the downregulation of Skp2, thereby leading to an upregulation of p27 19, 32. Our data, which is composed of the clinicopathological information of over 100 patients, unveil that Cdh1-positive breast cancers are associated with low histological grade tumors. Thus, Cdh1 activity may be linked to the biological characteristic of tumors. Previous demonstrations have revealed that abundant Skp2 levels are often related to higher histological grade tumors resulting in the aggressive behavior of tumors and poor patient prognosis. Therefore our assessment of the clinicopathological relevance of Cdh1 is compatible with prior epigenetic and biological studies suggesting that Cdh1 is a co-activator of the APC complex promoting the proteolysis of Skp2, thereby preventing immature S phase entry and consequently coordinating appropriate cell cycle progression 17, 18. Furthermore, previous reports showing that lower levels of SnoN, an Cdh1/APC substrate in the TGF-β pathway, function as a prognostic marker of breast cancer may support this present finding 31. The current opinion that Cdh1/APC could stabilize p27 through the degradation of Skp2 in response to TGF-β signaling further justifies the prognostic value of Cdh1 protein levels in patients with breast cancer 25.
The in vivo regulatory role of Cdh1/APC in tumorigenesis is consistent with the current paradigm
Dysfunction of the UPS has been strongly linked to carcinogenesis through its disruption of the balance between oncoproteins and tumor suppressor proteins 3–5. Mitotic regulation and G1/S transition are key regulatory sites during the cell cycle. APC and SCF complexes are critical E3 ligases dictating chromatid separation during mitosis and orchestrating cyclinE/CDK2 during G1/S transition. Loss of control of either of these two major ubiquitin-mediated pathways has been correlated to a variety of malignancies 35. Our analyses verified the importance of an ubiquitylation regulatory cascade whose deregulation can result in tumorigenesis; the proper regulation of one E3 ligase (SCF) complex by another E3 ligase (APC) results in controlled G1/S progression. The expression pattern of Cdh1/APC-Skp2-p27 in breast cancer support the notion that this regulatory axis controls cellular proliferation with its abrogation leading to cancinogenesis. The present results from combinatorial studies based on biochemistry, xenograft mouse model system and pathological analysis provide further understanding of Cdh1/APC in breast cancer formation.
Supplementary Material
Our discovery is important in following ways. (1) Using combinatorial approaches, including a xenograft mouse breast caner model, analysis of a human breast cancer tissue and a human patient prognostic analysis, we demonstrate that Cdh1, as a component of ubiquitin protein ligase, could be a prognostic indicator for breast cancer. (2) Given the importance of Cdh1 in the regulation of cell cycle progression and in the modulation of TGF-β-mediated tumor suppression, this work suggests the clinical significance of Cdh1/APC in breast tumorigenesis. It could broadly attract audiences in the field of proteolysis/cell cycle control as well as individuals interested in investigating translational work.
Acknowledgments
We thank Wan laboratory members for critical discussion, and appreciate Shiyuan Cheng, Yong-Tae Kwon and David Roodman laboratories for assisting us with the array analysis and the xenograft experiment. We are grateful to Richard D. Wood for critical discussion of the manuscript. This work is supported by NIH grants CA115943. Y. Wan is a scholar of the American Cancer Society.
References
- 1.Yamasaki L, Pagano M. Cell cycle, proteolysis and cancer. Curr Opin Cell Biol. 2004;16:623–8. doi: 10.1016/j.ceb.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Pagano M. Cell cycle regulation by the ubiquitin pathway. Faseb J. 1997;11:1067–75. doi: 10.1096/fasebj.11.13.9367342. [DOI] [PubMed] [Google Scholar]
- 3.Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–78. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 4.Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer. 2006;6:776–88. doi: 10.1038/nrc1994. [DOI] [PubMed] [Google Scholar]
- 5.Datto M, Wang XF. Ubiquitin-mediated degradation a mechanism for fine-tuning TGF-beta signaling. Cell. 2005;121:2–4. doi: 10.1016/j.cell.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez GJ, Ronai Z. Ubiquitin and SUMO systems in the regulation of mitotic checkpoints. Trends Biochem Sci. 2006;31:324–32. doi: 10.1016/j.tibs.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 8.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 9.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–14. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–25. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 11.Wirbelauer C, Sutterluty H, Blondel M, Gstaiger M, Peter M, Reymond F, Krek W. The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. Embo J. 2000;19:5362–75. doi: 10.1093/emboj/19.20.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson PK. Linking tumor suppression, DNA damage and the anaphase-promoting complex. Trends Cell Biol. 2004;14:331–4. doi: 10.1016/j.tcb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Stewart S, Fang G. Anaphase-promoting complex/cyclosome controls the stability of TPX2 during mitotic exit. Mol Cell Biol. 2005;25:10516–27. doi: 10.1128/MCB.25.23.10516-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Ungermannova D, Jin J, Harper JW, Liu X. Negative regulation of SCFSkp2 ubiquitin ligase by TGF-beta signaling. Oncogene. 2004;23:1064–75. doi: 10.1038/sj.onc.1207204. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Constantinescu SN, Sun Y, Bogan JS, Hirsch D, Weinberg RA, Lodish HF. Generation of mammalian cells stably expressing multiple genes at predetermined levels. Anal Biochem. 2000;280:20–8. doi: 10.1006/abio.2000.4478. [DOI] [PubMed] [Google Scholar]
- 16.Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol Cell. 2001;8:1027–39. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–8. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 18.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–3. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 19.Signoretti S, Di Marcotullio L, Richardson A, Ramaswamy S, Isaac B, Rue M, Monti F, Loda M, Pagano M. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002;110:633–41. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Oliveira AM, Okuno SH, Nascimento AG, Lloyd RV. Skp2 protein expression in soft tissue sarcomas. J Clin Oncol. 2003;21:722–7. doi: 10.1200/JCO.2003.05.112. [DOI] [PubMed] [Google Scholar]
- 21.Wang CX, Fisk BC, Wadehra M, Su H, Braun J. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood. 2000;96:259–63. [PubMed] [Google Scholar]
- 22.Singhal S, Amin KM, Kruklitis R, DeLong P, Friscia ME, Litzky LA, Putt ME, Kaiser LR, Albelda SM. Alterations in cell cycle genes in early stage lung adenocarcinoma identified by expression profiling. Cancer Biol Ther. 2003;2:291–8. doi: 10.4161/cbt.2.3.399. [DOI] [PubMed] [Google Scholar]
- 23.Kim JM, Sohn HY, Yoon SY, Oh JH, Yang JO, Kim JH, Song KS, Rho SM, Yoo HS, Kim YS, Kim JG, Kim NS. Identification of gastric cancer-related genes using a cDNA microarray containing novel expressed sequence tags expressed in gastric cancer cells. Clin Cancer Res. 2005;11:473–82. [PubMed] [Google Scholar]
- 24.Wang Q, Moyret-Lalle C, Couzon F, Surbiguet-Clippe C, Saurin JC, Lorca T, Navarro C, Puisieux A. Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene. 2003;22:1486–90. doi: 10.1038/sj.onc.1206224. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Wu G, Li W, Lobur D, Wan Y. Cdh1-anaphase-promoting complex targets Skp2 for destruction in transforming growth factor beta-induced growth inhibition. Mol Cell Biol. 2007;27:2967–79. doi: 10.1128/MCB.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Glickstein S, Liu W, Fujita T, Li W, Yang Q, Duvoisin R, Wan Y. The anaphase-promoting complex coordinates initiation of lens differentiation. Mol Biol Cell. 2007;18:1018–29. doi: 10.1091/mbc.E06-09-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita T, Liu W, Doihara H, Date H, Wan Y. Dissection of the APCCdh1-Skp2 cascade in breast cancer. Clin Cancer Res. 2008;14:1966–75. doi: 10.1158/1078-0432.CCR-07-1585. [DOI] [PubMed] [Google Scholar]
- 28.Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XJ, Liefer KM, Tsai S, O'Malley BW, Roop DR. Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc Natl Acad Sci U S A. 1999;96:8483–8. doi: 10.1073/pnas.96.15.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo P, Imanishi Y, Cackowski FC, Jarzynka MJ, Tao HQ, Nishikawa R, Hirose T, Hu B, Cheng SY. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166:877–90. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Lundin M, Ristimaki A, Heikkila P, Lundin J, Isola J, Joensuu H, Laiho M. Ski-related novel protein N (SnoN), a negative controller of transforming growth factor-beta signaling, is a prognostic marker in estrogen receptor-positive breast carcinomas. Cancer Res. 2003;63:5005–10. [PubMed] [Google Scholar]
- 32.Masuda TA, Inoue H, Sonoda H, Mine S, Yoshikawa Y, Nakayama K, Nakayama K, Mori M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002;62:3819–25. [PubMed] [Google Scholar]
- 33.Yu H. Regulation of APC-Cdc20 by the spindle checkpoint. Curr Opin Cell Biol. 2002;14:706–14. doi: 10.1016/s0955-0674(02)00382-4. [DOI] [PubMed] [Google Scholar]
- 34.Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 2002;16:2179–206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.