Abstract
In primates, during times of need, calling for help is a universal experience. Calling for help recruits social support and promotes survival. However, calling for help also can attract predators, and it is adaptive to inhibit calls for help when a potential threat is perceived. Based on this, we hypothesized that individual differences in calling for help would be related to the activity of brain systems that mediate goal-directed behavior and the detection of threat. By using high-resolution positron emission tomography in rhesus monkeys undergoing social separation, we demonstrate that increased [18F]-fluoro-2-deoxy-d-glucose uptake in the right dorsolateral prefrontal cortex and decreased uptake in the amygdala independently predict individual differences in calling for help. When taken together, these two regions account for 76% of the variance in calling for help. This result suggests that the drive for affiliation and the perception of threat determine the intensity of an individual's behavior during separation. These findings in monkeys are relevant to humans and provide a conceptual neural framework to understand individual differences in how primates behave when in need of social support.
Keywords: anxiety, monkey, positron emission tomography
Stable attachment bonds are fundamentally important to the success of social primates. Moreover, when attachment bonds are disrupted, separated individuals are more vulnerable to threatening environmental stressors, including predation. During separation, individuals experience varying levels of distress that motivate them to engage in behaviors that increase their likelihood of reunion. Vocalizations expressed during separation are vital because they signal the need for social support and help other group members locate the individual in need.
Extensive studies in rhesus monkeys have characterized the parameters of separation-induced vocalizations, or coo calls, as well as the neurochemical mechanisms that modulate their expression (1). Furthermore, it has been suggested that these high-pitched vocalizations are analogous to human cries (2). Rhesus monkeys are particularly well suited to study mechanisms underlying individual differences in human responses to separation because of similarities between the two species in social behavior, the expression of psychopathology, and in brain systems, such as the prefrontal cortex, that are involved in the regulation of emotion (3).
In any given situation, the degree to which an individual calls for help is related to numerous factors. These factors include not only the amount of distress that an individual experiences and the importance of social support at that time but also the risks related to calling for help. Research from our laboratory demonstrates that when separated monkeys are exposed to a potential threat, they decrease their calling for help (1). In the process of assessing the extent to which potential predatorial threat is real, the adaptive response is to engage in behaviors that reduce the likelihood of detection. Calling for help at this time would reveal the separated individual's location to not only conspecifics but also to potential predators. Current environmental factors, along with individual sensitivity to loss and threat, are prominent factors in determining individual differences in separation responses (1).
Despite the importance of social attachments and the universal experience of calling for help during attachment bond disruption, little is known about the neural circuitry that underlies calling for help. In this study, we quantified the frequency of coo calls emitted by rhesus monkeys undergoing social separation and correlated individual differences in cooing with regional brain activity determined by [18F]-fluoro-2-deoxy-d-glucose (FDG) small-animal high-resolution positron emission tomography (PET). This technology allows for an unprecedented spatial resolution of 8 mm3 in functional imaging studies of primates. Studies in human infants and children demonstrate that increased calling for help or crying is associated with increased right frontal brain electrical activity, compared with left frontal brain electrical activity (4, 5). Therefore, we predicted that monkeys that emitted the most coo calls would be those with the highest levels of activity in the right prefrontal cortex. Furthermore, findings from recent lesion studies in rhesus monkeys led us to hypothesize that individual differences in amygdala activity would be negatively correlated with cooing (6–8).
Methods
Subjects. Twenty-five male rhesus monkeys (Macaca mulatta) ranging in age from 2.2 to 4.6 years (mean age, 3.1 years) and weighing between 3.2 and 7.4 kg (mean weight, 5.0 kg) were the subjects. The monkeys were pair-housed and maintained on a 12-h light/dark cycle at the Wisconsin National Primate Research Center and Harlow Primate Laboratory (both of the University of Wisconsin). Animals were given water ad libitum and fed monkey chow every morning. Animal housing and experimental procedures were in accordance with institutional guidelines.
PET Scan Protocol. Monkeys received an FDG injection immediately before 30 min of separation from their cage mates. Injections were administered through a 19-gauge i.v. catheter placed in the saphenous vein and always consisted of <10 mCi (1 Ci = 37 GBq) of FDG. To minimize nonspecific effects, animals were adapted to handling and injection procedures for 5 days before the study. FDG was used in comparison with other shorter-lived tracers because FDG uptake reflects metabolic activity and most of the FDG uptake occurs within 30 min of injection (9, 10). Furthermore, the procedure associated with the assessment of FDG uptake allows for the assessment of brain activity in relation to behaviors that occur in unrestrained, freely behaving primates. Importantly, the time course of FDG uptake coincides with that of the separation response. The behaviors occurring during social separation were videotaped and assessed by trained raters (1). After the 30-min separation period, the animals were anesthetized with ketamine (15 mg/kg) delivered intramuscularly, placed on isofluorothane gas anesthesia (1–2%), and scanned in the microPET P4 scanner (Concorde Microsystems, Knoxville, TN) (11–15).
PET Processing. To facilitate intersubject comparisons, each PET scan was smoothed, transformed into a standard space (16), and globally scaled to match a mean value, by using a standard voxel-wise analysis methodology (17). Smoothing was performed by using a 4-mm full-width/half-maximum Gaussian filter to account for small variations in gyral anatomy and to enforce a normal distribution (18). The transformation into a standard space involved a coregistration of each PET scan to a lab-specific PET template. The PET-scan-to-PET-template coregistrations were performed by using a 12-parameter affine transformation followed by a 60-parameter nonlinear transformation (19, 20). Each final image was carefully inspected, and identifiable landmarks were found to overlap across subjects within ≈1.5 mm. Because acquiring blood samples would have interfered with naturalistic behaviors occurring during FDG uptake, it was necessary to globally normalize the intensity of each PET scan. By using accepted methods (21), images were globally scaled based on a partial brain region of interest that excluded regions not collected for all subjects.
Data Analysis. Four monkeys were scanned that did not have their prefrontal areas within the PET scanner field of view. Because prefrontal areas were of particular interest, analyses were performed excluding these subjects, leaving 21 subjects. Additionally, in some animals, we were unable to acquire posterior brain areas. Therefore, for all subjects, data from areas >20 mm posterior to the center of the anterior commissure were excluded from analyses. The excluded regions included portions of visual and parietal cortices.
A brain-wide voxel-wise search, controlling for age, was performed to identify brain regions that were significantly correlated with individual differences in the frequency of cooing (thresholded at P < 0.005, two-tailed, uncorrected). Because the frequency of cooing was not normally distributed, cooing data were square-root-transformed to normalize the distribution. Average values across contiguous suprathreshold voxels were extracted for each subject. All further analyses used these average cluster values and accounted for age. Multiple regression analyses performed between significant clusters and individual differences in cooing behavior to examine the data for possible outliers and to determine R2 values. Brain regions were defined by using the Paxinos et al. atlas (16), based on the Walker classification system (22).
Hierarchical multiple linear regression analyses were used to investigate how brain activity from different brain regions combined to predict individual differences in cooing behavior (as suggested by ref. 23). The relative contribution of significant clusters was determined by computing the change in the R2 statistic after first entering age and other brain regions into a linear regression model (24). The significance of this change was calculated by using the change in the F statistic of the regression model (24).
Results
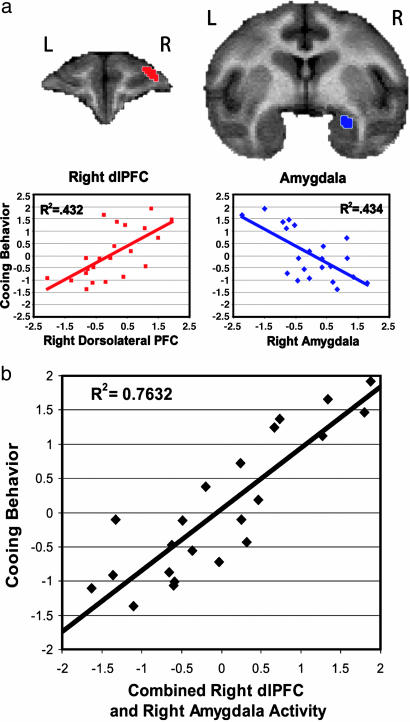
A brain-wide search revealed a positive correlation between the frequency of cooing and activity in the right dorsolateral prefrontal cortex (dlPFC) (area 9/46; R2 = 0.431, P < 0.001) (Fig. 1a) and a negative correlation between cooing and brain activity in a cluster that included dorsal right amygdala and the temporal pole. Because a local maximum existed within the amygdala, and because of concerns that activity in the temporal pole can be influenced by activity in nonbrain metabolic tissues, a specific amygdala cluster was defined by selecting significant voxels within an independently drawn region of interest around the amygdala (defined by N.H.K. and S.E.S.). This region significantly correlated with the frequency of cooing (R2 = 0.433, P < 0.001) (Fig. 1a) and was used for further amygdala analyses. No significant correlations were found between individual differences in right dlPFC and dorsal right amygdala activity (P < 0.83). The relation between dlPFC activity and cooing was lateralized as the correlation between cooing, and dlPFC activity in the right hemisphere was significantly greater than that for the left hemisphere (P < 0.05, one-tailed). In contrast, the correlation between cooing and amygdala activity did not significantly differ between hemispheres (P > 0.08, one-tailed).
Fig. 1.
Right dlPFC and right amygdala predict 76% of cooing behavior. (a) As demonstrated in the coronal sections above, right dlPFC (Left) (area 9/46; defined in ref. 16) and right amygdala (Right) regions are correlated with individual differences in cooing frequency (square-root transformation of coos per min, controlling for age). Regions were extracted by using a whole-brain voxel-wise search (thresholded at P < 0.005, two-tailed, uncorrected) and overlaid on a rhesus monkey MRI template. The scatter plots demonstrate the relationship between cooing frequency (standardized residual variance of the square-root transformation of coos per min accounting for age) and brain activity (standardized residual FDG concentration, accounting for age) in the right dlPFC and right amygdala. (b) Scatter plot representing the significant relationship between cooing (standardized residual variance of the square-root transformation of coos per min, accounting for age) and an individual subject's activity in the right dlPFC and right amygdala (standardized combined residuals of FDG concentration, accounting for age).
To examine whether other behaviors correlated with activity in these brain regions, we examined whether threat-related freezing behavior and locomotion were correlated with right amygdala and right dlPFC activity. Freezing duration was positively correlated with right amygdala activity (r2 = 0.38, P < 0.05, one-tailed), whereas freezing duration and right dlPFC activity were not significantly correlated (P < 0.67). Locomotion was not significantly correlated with either the right dlPFC (P < 0.99) or the right amygdala (P < 0.37), demonstrating the specificity of the brain–behavior correlations.
A hierarchical regression was performed to understand the unique and overlapping contributions of the right dlPFC and right amygdala to individual differences in cooing. Results demonstrated that significant contributions from the right dlPFC [F-change (1,19) = 26.44, P < 0.001] and right amygdala [F-change (1,19) = 26.50, P < 0.001] explained 76.3% of the variance, with each area uniquely explaining ≈33% of the variance [F-change (2,19) = 30.623, P < 0.001] (Fig. 1b). This result was confirmed by using maximum voxel values of the right amygdala and right dlPFC clusters [right dlPFC, F-change (1,19) = 27.51, P < 0.001; right amygdala: F-change (1,19) = 27.00, P < 0.001; explaining 76.2% of the variance in cooing behavior: F-change (2,19) = 30.483, P < 0.001]. Table 1 displays the other brain regions that were significantly correlated with the frequency of cooing. None of these regions, or their maximum voxels, significantly accounted for additional variance beyond that contributed by the right dlPFC and right amygdala.
Table 1. Significant clusters correlated with cooing.
| Distance from anterior commissure
|
|||||||
|---|---|---|---|---|---|---|---|
| Correlation | Region | Hemisphere | Volume, mm3 | Maximum T value | x | y | z |
| Positive | Parietal areas PE and PEa (MIP) | Left | 113 | 5.42 | -11.875 | -20 | 20 |
| Parietal area PGM | Left | 20 | 3.58 | -1.25 | -21.875 | 13.125 | |
| dlPFC | Right | 16 | 3.75 | 11.25 | 20.625 | 11.875 | |
| Negative | Amygdala and temporal pole | Right | 67 | 4.55 | 11.875 | 5 | -6.875 |
| Lateral geniculate | Right | 48 | 4.48 | 14.375 | -11.25 | -3.125 | |
Although not hypothesized, the relation between cooing and activity in the parietal region PE/PEa (middle intraparietal area) is of interest. Because the PE/PEa region has been linked to the dlPFC in visual–attentional mechanisms (for a review, see ref. 25), we examined the relation between activity in the dlPFC and the PE/PEa regions and found that they were significantly correlated (r2 = 0.62, P < 0.005).
Discussion
Separation induces a state of loss and distress that motivates the production of calls for help. The ultimate goal of calling for help is to be reunited with a supportive conspecific. Although the dlPFC has traditionally been associated with working memory (26), more recently, the dlPFC has been proposed to act as an interface among emotion, cognition, and attention, and in maintaining emotional states that function to facilitate goal-directed behavior (27–30). The current findings, demonstrating a positive correlation between individual differences in cooing with dlPFC activity, are consistent with the functions of dlPFC. Moreover, the correlation between dlPFC and parietal areas PE/PEa (middle intraparietal area) may represent the circuitry underlying the recruitment of attentional processes important in maintaining the mental representations associated with attachment (25). Other evidence from humans and monkeys demonstrates right asymmetric PFC activation in association with negative emotional states (4, 5, 31). In addition, an FDG study in six young rhesus monkeys demonstrated activation of the right dlPFC during maternal separation (10). Because we found that activity in the right, and not the left, dlPFC was correlated with cooing, it is plausible that hemispheric specialization exists in relation to maintaining a distressed emotional state and/or a mental representation that facilitates separation-induced goal-directed responses.
At any given moment during separation, an individual's level of distress, need for reattachment, and perceived degree of environmental threat are primary determinants of how much it calls for help. The levels of distress and associated need for reattachment increases coo calls, whereas these levels are decreased by the perception of possible predatorial threat (1). Amygdala activity is associated with threat detection (32), and under some circumstances, individual differences in amygdala activity reflect an individual's degree of perceived threat. The findings from this study of a negative relation between amygdala activity and cooing and a positive relation between amygdala activity and freezing provide the neural basis for the observation that threat is an important factor modulating calls for help. Furthermore, recent studies in rhesus monkeys demonstrate that selective amygdala lesions decrease fearfulness and at the same time increase cooing (6–8).
It will be important to understand the neurochemical systems in the dlPFC and amygdala that mediate the influences of attachment (i.e., opiate, oxytocin, vasopressin, and dopamine systems) and threat-detection (i.e., GABA and corticotropin-releasing factor) on calling for help. Anatomical studies in monkeys and humans demonstrate the presence of these systems in the amygdala and/or prefrontal cortex (33–36). Furthermore, numerous rodent studies demonstrate involvement of the opiate, vasopressin, and oxytocin systems in behaviors associated with attachment, and alterations in these systems affect separation-induced vocalizations (37–40). Studies in nonhuman primates provide similar evidence for the opiate system (1). Both the opiate and dopamine systems are involved in attention, reward prediction, and reinforcement processes that are likely important in determining individual differences in the strength of social bonds (41, 42). Neurotransmitters that modulate the intensity of a perceived threat include GABA and corticotropin-releasing factor. Within the amygdala, an increase in GABA activity dampens (43), whereas an increase in corticotropin-releasing factor activity heightens the response to a threat (44).
To summarize, we found that 76.3% of the variance in calling for help was accounted for by individual differences in increased activity in right dlPFC and decreased activity in the amygdala. Furthermore, amygdala and dlPFC activity were not significantly correlated, and these regions independently contributed to individual differences in cooing. These results suggest that at least two separate neural networks, one reflecting distress driven goal directed behavior and the other reflecting threat detection, are involved in modulating calls for help. In primates, calling for help during periods of separation or loss is a universal experience, because maintaining social bonds is critical for survival. Some individuals are more reactive to separation, whereas others are less affected. These findings provide insights into the neural circuitry that mediates these individual differences and offer an excellent model to pursue research on the functional neurochemical underpinnings of these individual differences.
Acknowledgments
We thank H. Van Valkenberg, T. Johnson, J. King, and the staff at the Harlow Center for Biological Psychology and the National Primate Research Center at the University of Wisconsin for their technical support. This work was supported by National Institute of Mental Health Grants MH46729 (to N.H.K.), MH52354 and MH69315 (both to R.J.D.); the HealthEmotions Research Institute; and Meriter Hospital (Madison, WI).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: dlPFC, dorsolateral prefrontal cortex; PET, positron emission tomography; FDG, [18F]-fluoro-2-deoxy-d-glucose.
References
- 1.Kalin, N. H. & Shelton, S. E. (1989) Science 243, 1718–1721. [DOI] [PubMed] [Google Scholar]
- 2.Newman, J. D. (1985) in Infant Crying, eds. Lester, B. M. & Boukydis, C. F. Z. (Plenum, Plainview, NY), pp. 307–325.
- 3.Kalin, N. H. & Shelton, S. E. (2003) Ann. N.Y. Acad. Sci. 1008, 189–200. [DOI] [PubMed] [Google Scholar]
- 4.Buss, K. A., Malmstadt, J. R., Dolski, I., Kalin, N. H., Goldsmith, H. H. & Davidson, R. J. (2003) Behav. Neurosci. 117, 11–20. [DOI] [PubMed] [Google Scholar]
- 5.Davidson, R. J. & Fox, N. A. (1989) J. Abnorm. Psychol. 98, 127–131. [DOI] [PubMed] [Google Scholar]
- 6.Kalin, N. H., Shelton, S. E. & Davidson, R. J. (2004) J. Neurosci. 24, 5506–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery, N. J., Capitanio, J. P., Mason, W. A., Machado, C. J., Mendoza, S. P. & Amaral, D. G. (2001) Behav. Neurosci. 115, 515–544. [PubMed] [Google Scholar]
- 8.Bauman, M. D., Lavenex, P., Mason, W. A., Capitanio, J. P. & Amaral, D. G. (2004) J. Cognit. Neurosci. 16, 1388–1411. [DOI] [PubMed] [Google Scholar]
- 9.Phelps, M. E., Huang, S. C., Hoffman, E. J., Selin, C., Sokoloff, L. & Kuhl, D. E. (1979) Ann. Neurol. 6, 371–388. [DOI] [PubMed] [Google Scholar]
- 10.Rilling, J. K., Winslow, J. T., O`Brien, D., Gutman, D. A., Hoffman, J. M. & Kilts, C. D. (2001) Biol. Psychiatry 49, 146–157. [DOI] [PubMed] [Google Scholar]
- 11.Chatziioannou, A. F., Silverman, R. W., Meadors, K., Farquhar, T. H. & Cherry, S. R. (2000) IEEE Trans. Nucl. Sci. 47, 422–427 [Google Scholar]
- 12.Chatziioannou, A. F., Cherry, S. R., Shao, Y., Silverman, R. W., Meadors, K., Farquhar, T. H., Pedarsani, M. & Phelps, M. E. (1999) J. Nucl. Med. 40, 1164–1175. [PMC free article] [PubMed] [Google Scholar]
- 13.Cherry, S. R., Shao, Y., Silverman, R. W., Meadors, K., Siegel, S., Chatziioannou, A., Young, J. W., Jones, W., Moyers, J. C., Newport, D., et al. (1997) IEEE Trans. Nucl. Sci. 44, 1167–1171. [Google Scholar]
- 14.Farquhar, T. H., Chatziioannou, A. F. & Cherry, S. R. (1998) IEEE Trans. Med. Imaging 17, 1073–1080. [DOI] [PubMed] [Google Scholar]
- 15.Knoess, C., Siegel, S., Smith, A., Newport, D., Richerzhagen, N., Winkeler, A., Jacobs, A., Goble, R. N., Graf, R., Wienhard, K. & Heiss, W. D. (2003) Eur. J. Nucl. Med. Mol. Imaging 30, 737–747. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos, G., Huang, X.-F. & Toga, A. W. (2000) The Rhesus Monkey Brain in Stereotaxic Coordinates (Academic, San Diego).
- 17.Friston, K. J., Holmes, A. P., Worsley, K. J., Poline, J. P., Frith, C. D. & Frackowiak, R. S. J. (1995) Hum. Brain Mapp. 2, 189–210. [DOI] [PubMed] [Google Scholar]
- 18.Worsley, K. J., Marret, S., Neelin, P. & Evans, A. C. (1996) Hum. Brain Mapp. 4, 74–90. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson, M., Bannister, P., Brady, J. & Smith, S. (2002) NeuroImage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- 20.Woods, R. P., Grafton, S. T., Holmes, C. J., Cherry, S. R. & Mazziotta, J. C. (1998) J. Comput. Assist. Tomogr. 22, 139–152. [DOI] [PubMed] [Google Scholar]
- 21.Camargo, E. E., Szabo, Z., Links, J. M., Sostre, S., Dannals, R. F. & Wagner, H. N., Jr. (1992) J. Cereb. Blood Flow. Metab. 12, 281–290. [DOI] [PubMed] [Google Scholar]
- 22.Walker, A. E. (1940) J. Comp. Neurol. 73, 59–86. [Google Scholar]
- 23.Kosslyn, S. M., Cacioppo, J. T., Davidson, R. J., Hugdahl, K., Lovallo, W. R., Spiegel, D. & Rose, R. (2002) Am. Psychol. 57, 341–351. [PubMed] [Google Scholar]
- 24.Tabachnick, B. G. & Fidell, L. S. (2001) Using Multivariate Statistics (Allyn & Bacon, Needham Heights, MA), 4th Ed.
- 25.Corbetta, M. & Shulman, G. L. (2002) Nat. Rev. Neurosci. 3, 201–215. [DOI] [PubMed] [Google Scholar]
- 26.Smith, E. E. & Jonidies, J. (1999) Science 283, 1657–1661. [DOI] [PubMed] [Google Scholar]
- 27.Davidson, R. J. & Irwin, W. (1999) Trends Cognit. Sci. 3, 11–21. [DOI] [PubMed] [Google Scholar]
- 28.Miller, E. K. & Cohen, J. D. (2001) Annu. Rev. Neurosci. 24, 167–202. [DOI] [PubMed] [Google Scholar]
- 29.Gray, J. R., Braver, T. S. & Raichle, M. E. (2002) Proc. Natl. Acad. Sci. USA 99, 4115–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallis, J. D. & Miller, E. K. (2003) Eur. J. Neurosci. 18, 2069–2081. [DOI] [PubMed] [Google Scholar]
- 31.Davidson, R. J., Kalin, N. H. & Shelton, S. E. (1992) Biol. Psychiatry 32, 438–451. [DOI] [PubMed] [Google Scholar]
- 32.Davis, M. & Whalen, P. J. (2001) Mol. Psychiatry 6, 13–34. [DOI] [PubMed] [Google Scholar]
- 33.Amaral, D. G., Price, J. L., Pitkanen, A. & Carmichael, S. T. (1992) in The Amygdala, ed. Aggleton, J. (Wiley–Liss, New York), pp. 1–67.
- 34.Duanis, J. B., Lechworth, S. R., Simm-Selley, L. J., Smith, H. R., Childer, S. R. & Porrino, L. J. (2001) J. Comp. Neurol. 433, 471–485. [DOI] [PubMed] [Google Scholar]
- 35.Loup, F., Tribollet, E., Dubois-Dauphin, M. & Dreifuss, J. J. (1991) Brain Res. 555, 220–232. [DOI] [PubMed] [Google Scholar]
- 36.Young, L. J., Tolockzo, D. & Insel, T. R. (1999) J. Neuroendocrinol. 11, 291–297. [DOI] [PubMed] [Google Scholar]
- 37.Insel, T. R. (1997) Am. J. Psychiatry 154, 726–735. [DOI] [PubMed] [Google Scholar]
- 38.Winslow, J. T. & Insel, T. R. (1993) Eur. J. Pharmacol. 233, 101–107. [DOI] [PubMed] [Google Scholar]
- 39.Winslow, J. T., Hearn, E. F., Ferguson, J., Young, L. J., Matzuk, M. M. & Insel, T. R. (2000) Horm. Behav. 37, 145–155. [DOI] [PubMed] [Google Scholar]
- 40.Herman, B. H. & Panksepp, J. (1978) Pharmacol. Biochem. Behav. 9, 213–220. [DOI] [PubMed] [Google Scholar]
- 41.Young, L. J. & Wang, Z. (2004) Nat. Neurosci. 7, 1048–1054. [DOI] [PubMed] [Google Scholar]
- 42.Sawaguchi, T. & Goldman-Rakic, P. S. (1994) J. Neurophysiol. 71, 515–528. [DOI] [PubMed] [Google Scholar]
- 43.Davis, M., Rainnie, D. & Cassell, M. (1994) Trends Neurosci. 17, 208–214. [DOI] [PubMed] [Google Scholar]
- 44.Swiergel, A. H., Takahashi, L. K. & Kalin, N. H. (1993) Brain Res. 623, 229–234. [DOI] [PubMed] [Google Scholar]