Abstract
Malformations of cortical development are common causes of developmental delay and epilepsy. Some patients have early, severe neurological impairment, but others have epilepsy or unexpected deficits that are detectable only by screening. The rapid evolution of molecular biology, genetics, and imaging has resulted in a substantial increase in knowledge about the development of the cerebral cortex and the number and types of malformations reported. Genetic studies have identified several genes that might disrupt each of the main stages of cell proliferation and specification, neuronal migration, and late cortical organisation. Many of these malformations are caused by de-novo dominant or X-linked mutations occurring in sporadic cases. Genetic testing needs accurate assessment of imaging features, and familial distribution, if any, and can be straightforward in some disorders but requires a complex diagnostic algorithm in others. Because of substantial genotypic and phenotypic heterogeneity for most of these genes, a comprehensive analysis of clinical, imaging, and genetic data is needed to properly define these disorders. Exome sequencing and high-field MRI are rapidly modifying the classification of these disorders.
Introduction
The development of the human cerebral cortex is a complex and tightly organised process. Disruption of any of the overlapping steps that contribute to this process can result in a wide range of developmental disorders. Many of these disorders are recognised as malformations in studies of brain imaging and collectively comprise a class of disorders that we designated as malformations of cortical development (MCD). This term was introduced to include disorders with defective cortical development in which the cortical ribbon itself appears normal (specifically some types of microcephaly, megalencephaly, and heterotopia).1
The classification scheme for MCD is based on the developmental steps at which the process is first disturbed, the underlying genes and biological pathways disrupted, and – when more objective data are not available – imaging features.1–4 This system classifies MCD into three major groups that recapitulate the main developmental steps as malformations of cell proliferation, neuronal migration, or postmigrational cortical organisation and connectivity. The ideal classification should (and eventually will) rely on knowledge of biological pathways, which is not available at present. Indeed, recent advances suggest that boundaries between disorders of neuronal proliferation, migration, or subsequent cortical organisation are fading, as emphasised by the recent identification of a broad range of malformations with mutations in WDR62, DYNC1H1, and TUBG1.5–8 These findings support the notion that MCD-related genes are implicated in many developmental stages that are genetically and functionally interdependent.
In this Review, we address the most difficult issues with classification, review the most common clinical presentations across MCD, provide detailed descriptions of the most common and conceptually important MCD, and discuss the genes associated with these malformations. We address these points from both a general perspective and a more specific phenotype-based approach for the most common of these disorders.
Limits in classification of MCD
Although the classification of MCD has advanced substantially during the past decade, in practice only a few categories are used, including lissencephaly, polymicrogyria, schizencephaly, focal cortical dysplasia (FCD), and periventricular nodular heterotopia. However, emerging evidence suggests that MCD are far more heterogeneous than this classification suggests, especially cases of irregular or pebbled cortical surfaces that are usually classified as polymicrogyria, even when the typical curvilinear microsulci are not seen. One example is so-called bilateral frontoparietal polymicrogyria. Reports both before and after identification of GPR56 as the causal gene of this MCD showed a cobblestone-type cortical malformation associated with defects in the pial basement membrane.9,10 This disorder is now referred to as bilateral frontoparietal cobblestone malformation, but use of the term polymicrogyria persists.
Individuals classified as having polymicrogyria have such diverse clinical courses and outcomes, causes and recurrence risks, associated malformations and syndromes, and imaging and neuropathological abnormalities as to render the term no more specific than that of intellectual disability. Further, the borders between MCD now classified as separate malformations have become blurred, because the new tubulinopathies can present as either lissencephaly-like or a polymicrogyria-like MCD (appendix). Enough data have accumulated to allow reclassification of some polymicrogyria-like CD as malformations due to generalised abnormal transmantle migration, similar to classic lissencephaly.4
MCD would ideally be classified with use of a comprehensive approach that includes data from several different disciplines. However, this system is difficult because specialties such as molecular genetics generate large amounts of new data, whereas other specialties such as neuropathology and developmental neurobiology lag behind, unless surgical removal of affected human tissues is possible or animal models are available.11–14
Brain imaging
Most MCD can be differentiated by moderate-to-high quality MRI scans (figure 1). The key features to look for include distribution and severity of the MCD, the cortical surface and border between white and grey matter (smooth or irregular), cortical thickness, and associated brain malformations. We summarise the key differences in imaging of different MCD in the appendix. Our preliminary experience suggests that ultra-high field 7 Tesla imaging will improve characterisation of localised forms of polymicrogyria and FCD (figure 2), although its usefulness to detect regions of abnormal cortical development after negative standard 3T MRI has not been systematically assessed yet.
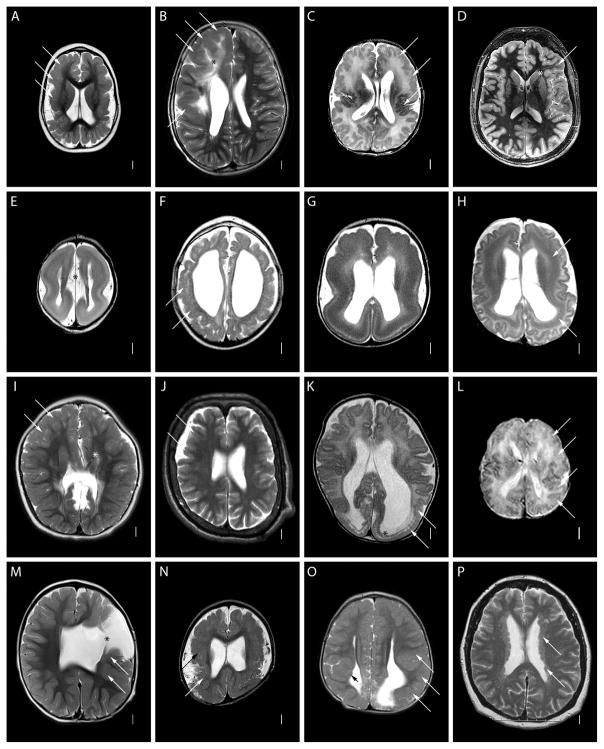
Figure 1.
Axial T2-weighted images at the level of the mid-lateral ventricles showing different malformations of cortical development. Long arrows show representative areas of cortical malformation (A–D, F, I–O), subcortical band heterotopia (H) or periventricular nodular heterotopia (P). The short black arrow shows a small periventricular nodular heterotopia (O). Asterisks denote abnormal white matter (B), focal transmantle dysplasia (D), wide inter hemispheric space due to absent corpus callosum (E), a shunt (I), and an open-lip cleft (M). The malformations of cortical development shown include severe congenital microcephaly with a PMG-like cortical malformation (A), right-sided dysplastic megalencephaly (hemimegalencephaly) (B), megalencephaly and frontal-perisylvian polymicrogyria (C), focal cortical dysplasia type 2b (D), severe lissencephaly with cerebellar hypoplasia and absent corpus callosum (E), PMG-like cortical malformation in a tubulinopathy (F), grade 3 classic lissencephaly (G), diffuse subcortical band heterotopia in a female (H), frontal predominant cobblestone malformation in muscle-eye-brain disease (I), frontal predominant cobblestone malformation in autosomal recessive cutis lax (J), posterior predominant cobblestone mutation in a child with congenital muscular dystrophy (K), peroxisomal cortical malformation in Zellweger syndrome (L), classic schizencephaly with a left frontal open-lip cleft (M), perisylvian polymicrogyria imaged at 7 Tesla (N), posterior periventricular nodular heterotopia with overlying PMG (O), and bilateral diffuse periventricular nodular heterotopia (P).(This is a short form, see Supplementary Text for longer version).
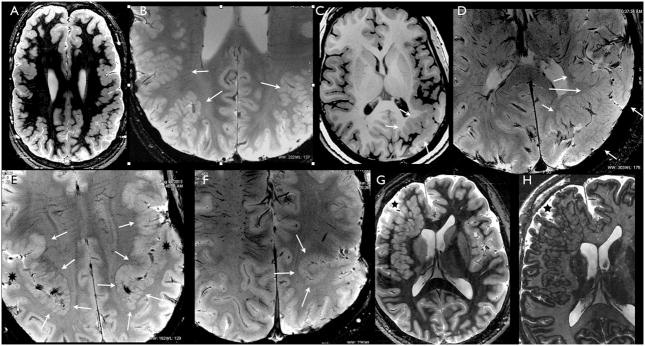
Figure 2.
Axial MRI images at 7T showing different morphological aspects of polymicrogyria in five young adult patients. Images ‘A’ and ‘G’ are inversion recovery (IR) weighed (W), image ‘C’ is T1W, images ‘B’, ‘D–F’ are susceptibility (S) W images; image ‘H’ shows a detail of image ‘G’ and is obtained using the tissue border enhancement by IR acquisition technique.174 ‘A’ shows the pebbled aspects of the grey matter typical of polymicrogyria, with areas of thickening and infoldings, involving the posterior frontal and parietal cortex on both sides, in a young woman. ‘B’, is taken from the same patient as ‘A’, using SW sequences. Detail of the cortex at the parietal lobe level, discloses the underlying structure of the malformed cortex, which is thin and overfolded, with ribbon like aspect. The arrows indicate the areas where these characteristics are more prominent. Note that the cortex has normal thickness and shape in the mesial hemispheric surface. ‘C’ shows gross thickening and abnormal sulcation of the posterior perisylvian and parietal cortex of the left hemisphere in a young adult man. ‘D’, showing a detail of ‘C’ obtained using SW sequences, discloses ribbon like cortical overfolding bordering the abnormal sulci. For comparison, see the normal cortex in the contralateral hemisphere. ‘E’ is obtained using SW in the same patient as in figure 1N. Note the abrupt transition between the abnormally thick and overfolded cortex in the perisylvian borders (arrows) and the normal temporo-parietal cortex (black asterisks). ‘F’ shows abnormal infolding due to abnormal sulcation and cortical thickness in a young man with left unilateral perisylvian polymicrogyria. ‘G’ shows an extensive area of heterotopia involving most of the right frontal lobe in a young man. The macronodular aspect of the heterotopia and the irregular surface of the overlying cortex (black asterisk) make this malformation not easily distinguishable from what is seen, for example, in the left parietal lobe in patients shown in ‘A’ and ‘C’. Tissue border enhancement, used in ‘H’ (showing a detail of ‘G’), helped defining the grey-white matter border and diagnose this malformation as heterotopia even if the overlying cortex contains small gyri and abnormal sulci.
Knowledge of the main morphological features of the normal pattern of cortical development has helped the prenatal diagnosis of some MCD, including regional polymicrogyria at an early sulcation stage with use of either neurosonography15 or fetal MRI.16 Diagnoses can sometimes be made before 24 weeks of gestation, and can help to select the most appropriate genetic testing and counseling. For these reasons, detailed neurosonographic examination or fetal brain MRI is recommended even when minor CNS anomalies, or even non-CNS anomalies, are detected by previous ultrasound.15 However, these imaging techniques also have serious limitations, because of the risk of detection of non-pathological variants, very little association with neuropathological findings, and complexities in provision of appropriate counseling.
Clinical presentation, course, and outcome MCD as a group have highly variable clinical presentations and burden of disability, and can be loosely separated into two large, although overlapping, groups: early diffuse MCD with poor developmental and neurological outcomes, and later-onset MCD with variable outcomes due to patchy brain involvement.
Severe disabilities
Most children with diffuse MCD first come to medical attention because of early feeding problems, seizures, or global developmental delay. Others might be recognised because of abnormally small or large head size, hydrocephalus, or other congenital anomalies. Children with these clinical presentations have severe congenital microcephaly, dysplastic megalencephaly (including hemimegalencephaly), lissencephaly, cobblestone malformation, polymicrogyria-like malformations, or classic polymicrogyria. The most severely disabled children can have severe deficits in language development and social interactions, stereotyped or other involuntary movements, autonomic (especially gastrointestinal) dysregulation, abnormalities in mood, sleep, and attention, and visual and hearing loss. Most have severe neurological disabilities and high risk for a reduced life span.
Less severe disabilities
By contrast, children or adolescents with FCD often have normal neurological skills and are brought to medical attention after the onset of focal epilepsy, which will often be their only problem and the only determinant of their long-term prognosis. Other less severely affected individuals are likely to have mild-to-moderate learning disability, epilepsy of variable severity, and attention deficit. The less severe disabilities are a result of bilateral but patchy malformations such as periventricular nodular or subcortical heterotopia, mild forms of subcortical band heterotopia, or polymicrogyria. The overall burden of neurological disability will depend on these clinical features, which can be combined with any degree of severity. Although few detailed studies comparing the type and distribution of MCD with the outcome have been done,17–25 based on our personal experience of assessing hundreds of children with MCD we have developed general guidelines useful for prediction of the outcomes in children with MCD (table 1).
Table 1.
Presenting signs and predictors for prognosis in MCD
| Neurologic symptoms | Severity | ||
|---|---|---|---|
|
| |||
| Most severe | Intermediate | Least severe | |
| Head size (OFC) | Microcephaly (<−3 SD) | Megalencephaly (>+3) | Normal head size |
| Tone | Spasticity | Hypotonia | Normal tone |
| Seizure onset | Early (0–3 months) | Infancy (3–12 months) | Later (after 1 year) |
| Seizure type | EIEE-ISS-LGS-myoclonic | Nonspecific generalized | Focal, other types |
| MCD distribution | Diffuse | Frontal-perisylvian | Posterior or other |
| MCD symmetry | Bilateral symmetric | Bilateral asymmetric | Unilateral |
Abbreviations: EIEE, early infantile epileptic encephalopathy; ISS, infantile spasms; LGS, Lennox-Gastaut syndrome; MCD, malformations of cortical development; OFC, occipito-frontal circumference; SD, standard deviations.
Genes, biological pathways, and mosaicism
So far, more than 100 genes are reported to be associated with one or more types of MCD. The biological pathways include cell-cycle regulation at many steps (especially mitosis and cell division), apoptosis, cell-fate specification, cytoskeletal structure and function, neuronal migration and basement-membrane function (figures 3, 4), and many inborn errors of metabolism. Metabolic errors include some disorders of mitochondrial and pyruvate metabolism, non-ketotic hyperglycaemia, and protein glycosylation, in addition to many different defects of peroxisomal biogenesis.4
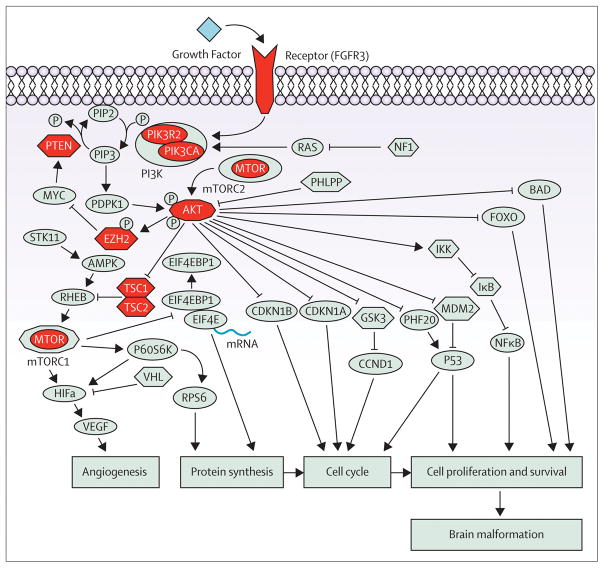
Figure 3.
Schematic representation of the PI3K-AKT-mTOR signaling pathway. Genes encoding proteins with activator effects on the downstream signaling components are represented by ellipsis, genes encoding proteins with inhibitory effect are represented by hexagons and AKT gene family and MTORC1, which may have both activating and repressor effect, are represented by decagons. Genes encoding proteins of the PI3K-AKT-MTOR pathway already implicated in malformations of cortical development in human disease are coloured in red. Protein abbreviations are provided in the appendix.
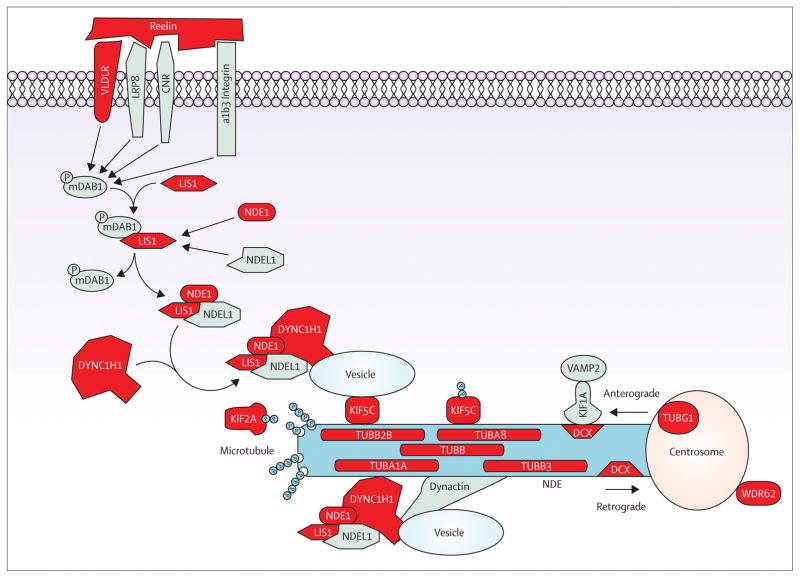
Figure 4.
Reelin-LIS1-tubulins pathway. Schematic representation of the reelin-mediated signaling pathway for neuronal migration. Reelin binding to one of three receptors complexes (CNR, VLDLR/LRP8, or β-integrin) activates mDAB, which mediates the binding of LIS1 with NDE1/NDE1L and DYNC1H1. The interaction of the LIS1-NDE1/NDE1L-DYNC1H1 complex with KIF5C mediates the anterograde transport, whereas its interaction with dynactin mediates the retrograde transport. KIF1A mediates the VAMP2 transport through the interaction with DCX, and KIF2A mediates microtubule depolymerisation. Genes encoding proteins of the reelin-LIS1-tubulins pathway already implicated in malformation of cortical development in human disease are coloured in red. Protein abbreviations are provided in the appendix.
Importantly, a subset of MCD genes – especially those associated with megalencephaly – are associated with postzygotic (ie, mosaic) mutations.12,13,26 Several patients with mosaic mutations of FLNA, LIS1 (also known as PAFAH1B1), or DCX have fairly mild phenotypes.27–31 Also, subcortical nodular heterotopia is frequently unilateral or asymmetrical. We hypothesise that many additional types of megalencephaly, dysplastic megalencephaly (including classic hemimegalencephaly), FCD, lissencephaly, polymicrogyria, and heterotopia will prove to be caused by mosaic mutations.
Megalencephaly, dysplastic megalencephaly, and FCD type 2
Overview
The term megalencephaly refers to an abnormally large brain that exceeds the mean for age and gender by 2 SD (table 2).39 In practice, it is a useful term for syndrome diagnosis for brain sizes more than 3 SD above the mean, because many individuals with so-called benign familial (anatomical) megalencephaly fall just below this range of +3 SD. In this Review, we use megalencephaly to refer to brains that are +3 SD or larger. Megalencephaly has most often been classified simply as a disorder of brain size, but recent studies have shown that megalencephaly with normal cortex by imaging, megalencephaly with polymicrogyria, and dysplastic megalencephaly (including classic hemimegalencephaly) can all result from mutations of the same genes in the PI3K-AKT pathway.12,13,26 Thus, separation of megalencephaly syndromes on the basis of appearance of the cerebral cortex might not represent the underlying biology.
Table 2.
Genes and phenotypes for megalencephaly and dysplastic megalencephaly
| Gene | Cytogenetic location | Phenotypes |
|---|---|---|
| Megalencephaly-polymicrogyria and dysplastic megalencephaly | ||
| AKT3 | 1q43q44 | MPPH, DMEG12,13,26 |
| EZH2 | 7q36.1 | Weaver syndrome32–34 |
| FGFR3 | 4p16.3 | Thanatophoric dysplasia35,36 |
| PIK3CA | 3q26.32 | MCAP, CLOVES12,26,37 |
| PIK3R2 | 19p13.11 | MPPH26 |
| Focal cortical dysplasia | ||
| PTEN | 10q23.31 | FCD, BRRS, CD38 |
Key: Cyto; cytogenetic location; BRRS, Bannayan-Riley-Ruvalcaba syndrome; CD, Cowden disease; DMEG, dysplastic MEG; FCD; focal cortical dysplasia; MCAP, megalencephaly-capillary malformation syndrome; MEG, megalencephaly; MPPH, megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome.
Pathological changes
Among patients with anatomical megalencephaly – a term that excludes metabolic forms – substantial clinical heterogeneity and several different pathological types have been described. Many anatomically large brains have a normal appearance other than increased thickness of brain structures, although few recent studies have been reported.40,41
Brain imaging in other individuals with megalencephaly shows diffuse or perisylvian polymicrogyria (figure 1C), although few pathological data are available except for thanatophoric dysplasia. In this disorder, the cortical malformation consists of broad gyri and deep sulci with an atypical polymicrogyria-like cortical malformation at the depth of sulci.35,36 Heterotopic nests of neuroblasts immunoreactive for nestin and Ki-67 (also known as MKI67) occur in the intermediate and marginal zones, resembling the overproduction of intermediate progenitor cells in mice.36,42
The most severe cortical dysplasias associated with megalencephaly have been described in hemimegalencephaly. However, our experience suggests that the term hemimegalencephaly is a misnomer. In many affected individuals less than one whole hemisphere is affected (so-called hemi-hemimegalencephaly), but in others the abnormality involves more than one entire hemisphere.43 We therefore prefer the term dysplastic megalencephaly to include all forms of segmental brain overgrowth with cortical dysplasia. The pathological changes reported in dysplastic megalencephaly include atypical pachygyria that can affect one or several lobes of the brain, most of one hemisphere, and sometimes one hemisphere and part of the other. The histological changes are similar if not identical to those in FCD type 2, which is characterized by cortical dyslamination and dysmorphic neurons without (type 2a) or with (type 2b) balloon cells, blurred junctions between grey and white matter, and increased heterotopic neurons in white matter.14,44,45 In both FCD types 2a and 2b, dysmorphic neurons have enlarged cell and nucleus diameters, abnormally aggregated and peripherally displaced Nissl substance, and phosphorylated and non-phosphorylated neurofilament accumulation in the cytoplasm.14 Balloon cells present with a large cell body, glassy eosinophilic cytoplasm that lacks Nissl substance, often several nuclei, and usually no cell lineage determination into glial or neuronal cells. FCD type 2 also features substantial blurring of the junctions between grey and white matter, and reduced myelination. A recent classification consensus also identified FCD type 1, which refers to isolated lesions presenting either as radial (FCD type 1a) or tangential (FCD type 1b) dyslamination of the neocortex, microscopically identified in one or multiple lobes; FCD type 3, which occurs in combination with hippocampal sclerosis (FCD type 3a), with epilepsy associated tumours (FCD type 3b), adjacent to vascular malformations (FCD type 3c), or in association with epileptogenic lesions acquired in early life (FCD type 3d).14
The histological changes in dysplastic megalencephaly seem to be split evenly between types 2a and 2b, and also include areas of polymicrogyria.28
The highly focal and variable nature of FCD type 2b, and the pathological resemblance to tubers in tuberous sclerosis, led to the hypothesis that somatic mosaic mutations of genes that encode proteins in the mTOR pathway, which includes TSC1 and TSC2 that cause tuberous sclerosis, were implicated in FCD.46 In support of this hypothesis, several sequence variants in TSC1 were noted more often in FCD type 2b than in control brains, and loss of heterozygosity of TSC1 was reported in the brains of 11 of 24 patients with FCD type 2b.47 Furthermore, one patient with FCD type 2b had a mosaic mutation in PTEN.38
Findings from a 2012 study48 suggested that FCD type 2b but not FCD type 2a or cortical tubers in patients with tuberous sclerosis was associated with expression of human-papillomavirus 16 E6 oncoprotein in large balloon cells, but not in other dysplastic cells. However, this finding needs confirmation from other groups, because it suggests embryonic infection in the first trimester of a small number of neural progenitors, which would be a new mechanism without any experimental support. Further, the investigators reported evidence of human papillomavirus in all patients studied, which would make infection the only cause of FCD type 2b in that series. This hypothesis seems unlikely in view of emerging data about genes in the mTOR pathway.
Brain imaging
The most common cortical malformation in megalencephaly is perisylvian polymicrogyria that looks very similar to perisylvian polymicrogyria in patients with normal or small head size (figure 1C). The cortical changes in dysplastic megalencephaly are severe and consist of enlargement of part or all of one hemisphere (or less often bilateral asymmetrical involvement) with no consistent preference for which lobes of the brain are enlarged (figure 1B). Other key changes include poor differentiation between grey and white matter, enlargement of deep structures in grey matter that displace the surrounding white matter and ventricles, variable hypointense and hyperintense T2-weighted abnormalities in white matter in the central and subcortical regions, and asymmetrical enlargement of the lateral ventricle with the larger ventricle usually on the more dysplastic side.49
Patients with FCD usually have enlarged gyri with either smooth or irregular cortical surface and increased subcortical signal intensity. Some individuals with FCD type 2b have migration tracts underlined by hyperintense radially oriented white-matter bands that extend from the dysplastic cortex to the periventricular region, which has been termed transmantle dysplasia (figure 1D).50
Clinical features
The developmental and health complications of megalencephaly differ widely. The most common problems include developmental delay, intellectual disability, and seizures that can start early in life and become intractable.
Children with diffuse symmetrical or mildly asymmetrical megalencephaly, with or without associated polymicrogyria, have large head size at birth that soon exceeds +3 SD.51 Their early development is delayed, and later cognitive development varies from normal to severe intellectual disability. However, most individuals function in the upper half of this wide range, and we have met gainfully employed adults with even severe megalencephaly. Most patients with megalencephaly have mild-to-moderate hypotonia in infancy that slowly improves, especially in individuals with mild dysplasia of connective tissue. Seizures can begin at any time in childhood, but about 40% of children in our initial series had seizures with onset from the first days of life to 4 years.51 We expect the incidence to rise as our cohort ages. Only 3 of 42 children had infantile spasms and 3 others had intractable seizures, making intractable epilepsy much less frequent than in the dysplastic megalencephaly group. Some of these children also had hydrocephalus, and cerebellar tonsillar ectopia with or without Chiari malformation.
Individuals with dysplastic megalencephaly (a group that includes hemimegalencephaly) typically have early developmental delay and more severe intellectual disability than do those with diffuse symmetrical or mildly asymmetrical megalencephaly. Epilepsy usually begins in the first weeks or months of life and can include infantile spasms and other epileptic encephalopathies. Intractable epilepsy is associated with a worse developmental outcome than is controllable epilepsy.
In almost all patients with FCD type 2, the lesion is detected after onset of focal epilepsy. Early seizure onset has been associated with infantile spasms with asymmetrical or focal features.52 FCD type 2 is a frequent cause of focal status epilepticus and, together with FCD type I, is the most common pathological substrate in surgical series of epilepsy.11 Developmental delay, cognitive disability, and focal neurological deficits are only reported with extensive areas of FCD.
Syndromes and genetics
A growing number of syndromes and genes have been associated with megalencephaly, especially with more severe phenotypes. Megalencephaly without cortical malformations occurs in benign autosomal dominant macrocephaly, a poorly defined disorder. The well-established syndromes with megalencephaly include neurofibromatosis type 1 due to NF1 microdeletions that also involve the RNF135 gene and Sotos syndrome (with NSD1 mutations), Weaver syndrome (with EZH2 mutations), in addition to Bannayan-Riley-Ruvalcaba syndrome, Cowden syndrome, and severe megalencephaly with autism (all three with PTEN mutations). Several other rare disorders are associated with megalencephaly, including deletion 10q22q23 or duplication 1q21.1 or 2p24.3.
Megalencephaly with polymicrogyria occurs in megalencephaly-capillary malformation syndrome (with mutations of PIK3CA) and megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome (with mutations of PIK3R2 or AKT3),51 and may be seen in patients with Weaver syndrome as well.32 Dysplastic megalencephaly most often occurs without syndromic features, and has recently been associated with mosaic mutations of PIK3CA, AKT3, and MTOR.12 Dysplastic megalencephaly has also been associated with the linear nevus sebaceous syndrome (also known as Schimmelpenning syndrome), and also rarely with CLOVES syndrome (congenital lipomatous overgrowth with vascular, epidermal, and skeletal anomalies), tuberous sclerosis, hemi hypertrophy, and hypomelanosis of Ito.43,54
The causes of FCD types 2a and 2b are not known and remain controversial. However, these disorders are included in this Review because the pathological changes are the same as are those reported in dysplastic megalencephaly and tuberous sclerosis.
Tubulinopathies and related disorders
Classic lissencephaly and polymicrogyria have long been thought of as distinct disorders, but in recent reports lissencephaly and polymicrogyria-like cortical malformations have been associated with mutations of the same genes (tubulin or tubulin-related genes) that function during the early stages of neuronal proliferation, migration, differentiation, and axonal guidance (ie, much earlier than the genes usually associated with polymicrogyria and schizencephaly).8,55,56–58 The full range of these malformations is not yet understood, but they vary from severe lissencephaly with cerebellar hypoplasia to much less severe malformations (tables 3, 4; appendix). In view of the common pathophysiological mechanisms characterising tubulinopathies, we limit this discussion to tubulinopathies and consider classic lissencephaly, subcortical band heterotopia, and polymicrogyria in separate sections.
Table 3.
Genes and phenotypes associated with lissencephaly, subcortical band heterotopia, and polymicrogyria-like malformations
| Gene | Cytogenetic location | Phenotypes |
|---|---|---|
| LIS 3 layer with ACC | ||
| ARX | Xp22.1 | XLAG59,60 |
| LIS Reelin type with a>p gradient | ||
| RELN | 7q22.1 | LCH, Reelin-type61,62 |
| VLDLR | 9p24.2 | LCH, Reelin-type63 |
| LIS 2 and 4 layer with a>p gradient | ||
| ACTB | 7p22.1 | BWS LIS grade 4–5 a>p64 |
| ACTG1 | 17q25.3 | BWS LIS grade 4–5 a>p64 |
| DCX females | Xq23 | SBH29,65 |
| DCX males | Xq23 | ILS, LIS grades 1–445,66 |
| LIS 2 and 4 layer with p>a gradient | ||
| DYNC1H1 | 14q32.31 | ILS, LIS grade 48,67 |
| KIF2A | 5q12.1 | ILS, LIS grade 48 |
| LIS1 | 17p13.3 | ILS, LIS grade 3–466,68 |
| Deletion LIS1 and YWHAE | 17p13.3 | MDS, LIS grade 1–269 |
| TUBA1A | 12q13.12 | ILS, LIS grade3; LCH, LIS grade1–4; PMG-like55,70,71 |
| TUBB2B | 6p25.2 | ILS, LIS grade3; LCH, LIS grade1–4; PMG-like55,72 |
| TUBG1 | 17q21.2 | ILS, LIS grade 3–48 |
Key: BWS, Baraitser-Winter syndrome; Cyto; cytogenetic location; ILS, isolated lissencephaly sequence; LCH, lissencephaly with cerebellar hypoplasia; LIS, lissencephaly; MDS, Miller-Dieker syndrome; PMG, polymicrogyria; SBH, subcortical band heterotopia; XLAG, X-linked lissencephaly with abnormal genitalia. a>p, anterior more severe than posterior; p>a, posterior more severe than anterior.
Full names for genes are provided in Supplementary Table 4.
Table 4.
Associated genes, cyotogenetic rearrangements, and phenotypes in polymicrogyria
| Gene | Cytogenetic location | Syndromes/Phenotypes |
|---|---|---|
| Severe congenital microcephaly with diffuse polymicrogyria or polymicrogyria-like MCD | ||
| NDE1 | 16p13.11 | MIC FBD BDP73,74 |
| WDR62 | 19q13.12 | MIC BDP5–7 |
| Diffuse polymicrogyria plus other abnormalities | ||
| FH | 1q43 | Fumaric aciduria75 |
| KIAA1279 | 10q21.3 | Goldberg-Shprintzen syndrome76 |
| NSDHL | Xq28 | CHIME-like syndrome77 |
| OCLN | 5q13.2 | BLC, BFP78 |
| Frontal polymicrogyria plus other abnormalities | ||
| GPSM2 | 1p13.3 | Chudley-McCullough syndrome79 |
| RAB3GAP1 | 2q21.3 | Micro syndrome, BDP80 |
| RAB3GAP2 | 1q41 | Micro syndrome, BDP80 |
| RAB18 | 10p12.1 | Micro syndrome, BDP80 |
| Perisylvian polymicrogyria plus other abnormalities | ||
| CHD7 | 8q12.2 | CHARGE syndrome79 |
| Polymicrogyria with other changes of cytomegalovirus | ||
| CMV | n.a. | MIC-PMG-HHLL83 |
| Tubulinopathies with polymicrogyria-like MCD (or lissencephaly) | ||
| DYNC1H1 | 14q32.31 | ACC, microcephaly, lissencephaly, PMGL8 |
| KIF5C | 2q23.1 | ACC, microcephaly, lissencephaly, PMGL8 |
| TUBA1A | 12q13.12 | ACC, CBLH, microcephaly, lissencephaly PMGL55 |
| TUBA8 | 22q11 | ACC, CBLH, microcephaly, PMGL84 |
| TUBB2B | 6p25.2 | ACC, CBLH, microcephaly, lissencephaly, PMGL55,56,72 |
| TUBB3 | 16q24.3 | ACC, CBLH, microcephaly, PMGL85 |
| TUBB | 6p21.33 | ACC, CBLH, microcephaly, PMGL86 |
| Intermediate progenitor cell pathway defects with polymicrogyria-like MCD | ||
| TBR2/EOMES | 3p21 | ACC, microcephaly, PMGL87 |
| PAX6 | 11p13 | Aniridia88 |
| PAX6 homozygous | 11p13 | MOPH-ACC-BDP89 |
| Copy number variants associated with polymicrogyria | ||
| Deletion | 1p36.3 | BPP90 |
| Duplication | 2p13p23 | BPP90 |
| Deletion | 4q21q22 | CBLH BPP90 |
| Deletion | 6q26q27 | ACC PNH BDP90,91 |
| Deletion | 13q3 | BPP90 |
| Deletion | 18p11 | BPP90 |
| Deletion | 21q2 | BPP90 |
| Deletion | 22q11.2 | DiGeorge syndrome92 |
Key: ACC, agenesis of the corpus callosum; BDP; bilateral diffuse PMG; BFP, bilateral frontal PMG; BLC, band-like calcifications (a.k.a. pseudoTORCH); CBLH, diffuse cerebellar hypoplasia; CMV, cytomegalovirus; Cyto, cytogentic location; FBD, fetal brain disruption; homozy, homozygous mutations; MIC, microcephaly; MCD, malformation of cortical development; MOPH, microphthalmia; PMG, polymicrogyria; PMGL, polymicrogyria-like; PNH, periventricular nodular heterotopia.
Pathological changes
Available pathological studies are limited to fetal brains, with findings showing severe defects in neuronal migration resulting in absent cortical lamination, radial columnar heterotopia, ectopic neurons in white matter and the leptomeningeal spaces because of overmigration through gaps in the pial basement membrane, and defects in axonal transport.56,57 Some features, especially radial columnar heterotopia and neuronal overmigration, are not typical of classic lissencephaly or classic polymicrogyria. Findings from studies58 of homozygous mouse mutants showed thinning of the cortical epithelium, which is substantially more severe in the caudolateral portion of the telencephalon, because of a major increase in apoptosis.
Brain imaging
The malformation varies from extreme lissencephaly with completely absent gyri, total agenesis of the corpus callosum, and severe cerebellar hypoplasia (figure 1E), to less severe lissencephaly with moderate-to-severe cerebellar hypoplasia (not shown), to classic lissencephaly (figure 1G), to an atypical polymicrogyria-like cortical malformation with cerebellar hypoplasia (figure 1F). This malformation consists of a moderately thick cortex (usually 7–10 mm), variable appearance of the cortical surface and boundary between cortical and white matter that appears smooth in some areas and pebbled in others, sparse or absent intracortical microsulci typical of polymicrogyria, and a paucity of deep gyral infolding.55
Clinical features
Most children with mutations of tubulin genes have severe intellectual disability and intractable seizures. The phenotype resembles classic lissencephaly, but can be more or less severe. However, too few affected individuals and details about their phenotype have been reported to define the range of abnormalities.
Syndromes, genetics, and molecular basis
By definition, tubulinopathies are always genetic. Investigators have identified nine genes (DYNC1H1, KIF2A, KIF5C, TUBA1A, TUBA8, TUBB, TUBB2B, TUBB3, and TUBG1; tables 3, 4), but we expect additional genes to be reported in the near future. Findings from functional studies suggest that abnormal brain development in tubulinopathies results from a dominant negative effect of heterozygous missense mutations (in the absence of loss-of-function mutations) on the regulation of microtubule-dependent mitotic processes in progenitor cells, and on the trafficking activities of the microtubule-dependent molecular motors KIF2A, KIF5C, and DYNC1H1 in postmitotic neuronal cells.8 The appendix shows the range of imaging phenotypes associated with these mutations. The most severe end of the range includes lissencephaly with cerebellar hypoplasia groups c, d, and f in our earlier classification,93 and consists of severe lissencephaly grade 1, agenesis of the corpus callosum, and very severe brainstem and cerebellar hypoplasia,70 an imaging pattern that matches the two-layered form of lissencephaly. This form has been associated with mutations in TUBA1A and TUBB2B.55 The next phenotype consists of moderate lissencephaly with cerebellar hypoplasia with lissencephaly grade 4 (pachygyria) that can appear mildly asymmetrical, with variable callosal defects and moderate hypoplasia of the brainstem and cerebellum (appendix).70,94 The third phenotype has isolated lissencephaly sequence with posterior predominant lissencephaly matching the LIS1 pattern (gradient with posterior more severe than anterior; appendix).8,67,70
The fourth group includes children described as having polymicrogyria rather than lissencephaly, plus variable agenesis of the corpus callosum and constant hypoplasia of the brainstem and cerebellum. Although the cortical malformation resembles polymicrogyria, these patients have atypical features. Some patients have hypoplasia of the brainstem and cerebellum with mildly simplified gyral pattern.70
Mutations in two genes that function in early development of the dorsal (ie, pallial) cerebrum have been associated with a polymicrogyria-like cortical dysplasia that resembles the tubulinopathies: PAX6, especially in rare patients with homozygous mutations, and EOMES.87,95,98
Lissencephaly and subcortical band heterotopia
Lissencephaly or smooth brain and the associated malformation known as subcortical band heterotopia are the classic malformations associated with deficient neuronal migration.96 Lissencephaly is characterised by absent or abnormally wide gyri plus an abnormally thick cortex. Subcortical band heterotopia consists of a normal or mildly simplified gyral pattern combined with a smooth band of grey matter in the superficial and middle portions of the white matter. On the basis of findings from genetic studies, the full range of lissencephaly now extends from severe lissencephaly with cerebellar hypoplasia to classic lissencephaly to subcortical band heterotopia, and also includes a polymicrogyria-like cortical malformation that can be distinguished from both lissencephaly and typical polymicrogyria by high-resolution brain imaging (table 3).
Pathological changes
Several different types of lissencephaly have been recognised on the basis of pathological features. They are most readily distinguished on the basis of the number of cortical layers affected, and include two-layered, three-layered, and four-layered forms.97 In the most common four-layered or classic form, the cortex is 12–20 mm thick and composed of a normal marginal layer, a superficial cellular layer that corresponds to the cortical plate, a cell sparse zone, and a deep cellular layer composed of heterotopic neurons. The rare two-layered form consisting of severe lissencephaly, frequent agenesis of the corpus callosum, and severe brainstem and cerebellar hypoplasia occurs with tubulinopathies, although the causative genes are not known for more than half of cases.57,70,97
Subcortical band heterotopia consists of a normal six-layered cortex; a thin zone of white matter underlying the cortex, and a zone of dense heterotopic neurons that breaks up into nodules at the lower border, closely resembling the X-linked form of lissencephaly.97 Classic four-layered lissencephaly and subcortical band heterotopia comprise a single malformation range on the basis of reports of rare patients with areas of lissencephaly that merge into subcortical band heterotopia, and families with lissencephaly in boys and subcortical band heterotopia in girls and women.99,100
Brain imaging
For all forms of lissencephaly, the brain surface appears smooth with areas of absent (agyria) and abnormally wide (pachygyria) gyri (figure 1E, 1G). The cerebral cortex is abnormally thick – 8–15 mm compared with 2.5–4 mm for normal cortex – except when the cerebral wall is thin, as can occur with two-layered and occasionally four-layered lissencephaly. Subcortical band heterotopia represents a forme fruste of lissencephaly in which the brain surface appears normal, except for shallow sulci (figure 1H). Just beneath the cortex, often separated from it by a few mm of white matter, lies a smooth band of misplaced neurons. Thick bands (5–10 mm) occur in the subcortical white matter beneath the deepest sulci, whereas thin bands can follow the cortex up into a gyrus.17
The severity of lissencephaly and subcortical band heterotopia varies from complete or nearly complete agyria (grades 1 and 2), to mixed agyria-pachygyria (grade 3), to pachygyria only (grade 4), to mixed pachygyria-subcortical band heterotopia (grade 5), and finally to subcortical band heterotopia only (grade 6).96 The anterior to posterior gradient, sex distribution, and associated malformations are essential for recognition of the different genetic forms. Mutations of DCX, ACTB, and ACTG1 result in a gradient with anterior more severe than posterior, whereas mutations of LIS1, TUBA1A, TUBG1, DYNC1H1, and other genes produce a gradient with posterior more severe than anterior.8,55,64,96 Severe lissencephaly with agenesis of the corpus callosum in boys is strongly suggestive of ARX mutations. The most severe mutations result in a completely smooth brain surface with no apparent gradient.60,96
Clinical features
Children with the most common types of lissencephaly or subcortical band heterotopia typically seem normal as newborn babies. Most patients come to medical attention during their first year because of poor feeding, hypotonia, and abnormal arching or opisthotonus in newborn babies; delayed motor milestones later in the first year of life; or onset of seizures, which is the most common sign. The major medical problems encountered are ongoing feeding problems and gastro-oesophageal reflux, epilepsy of many different types that is often intractable, and recurrent aspiration and pneumonia due to feeding problems.
Between 35% and 85% of children with classic lissencephaly develop infantile spasms, often without classic hypsarrhythmia. The frequency of spasms is much lower in children with subcortical band heterotopia than in those with lissencephaly, but both groups of patients go on to have mixed seizure types including focal, tonic, tonic-clonic, and atypical absence seizures. The frequency and severity vary greatly, but seizures are often difficult to control. Many patients have the classic electroclinical signs of Lennox-Gastaut syndrome, with intractable tonic seizures, atypical absences, and slow spike and wave discharges.52 Among individuals with less severe developmental disabilities, cognitive development can slow after onset of seizures. Neurological outcome usually relates to lissencephaly grade or thickness of subcortical band heterotopia.17
Survival
Children with some lissencephaly syndromes (especially Miller-Dieker syndrome with deletions in 17p13.3, and severe forms of lissencephaly with cerebellar hypoplasia or the X-linked syndrome of lissencephaly with abnormal genitalia due to ARX gene mutations) have a severe course and high mortality rates. However, these data do not apply to children with less severe forms of lissencephaly, subcortical band heterotopia, or the RELN-associated type of lissencephaly with cerebellar hypoplasia, because all of these disorders are associated with better motor and cognitive function and longer survival.96
Syndromes, genetics, and molecular basis
Lissencephaly, subcortical band heterotopia, and lissencephaly with cerebellar hypoplasia are always genetic. Studies to date have identified 12 lissencephaly genes (table 3), which account for roughly 90% of patients.8,56,59,61,65,68,71,101 However, several of these genes are associated with one or more specific lissencephaly or subcortical band heterotopia syndromes with different presentations. Although deletions and mutations in LIS1 are the most common cause of lissencephaly, the contributions of these genes have been derived from separate studies over many years, making comparisons difficult.
The range of malformations associated with classic four-layered lissencephaly includes Miller-Dieker syndrome and isolated lissencephaly sequence, and extends to include subcortical band heterotopia, because a few affected individuals have both lissencephaly and subcortical band heterotopia. Miller-Dieker syndrome is a syndrome of several congenital anomalies, characterized by severe four-layered lissencephaly with diffuse agyria and no clear gradient, typical facial appearance, and other birth defects (eg, heart malformations).69 The facial features include prominent forehead, bitemporal hollowing, short nose with upturned nares, protuberant upper lip with thin vermilion border, and small jaw. All patients with Miller-Dieker syndrome have large deletions in chromo some 17p13.3 that include LIS1, YWHAE, and all intervening genes.69
Isolated lissencephaly sequence (the most common lissencephaly syndrome) consists of classic four-layered lissencephaly, normal or slightly small cerebellum, and normal facial appearance except for mild bitemporal hollowing and small jaw.102 Different patterns of lissencephaly have been reported with mutations of the known causative genes. Boys with DCX mutations have either severe lissencephaly with diffuse agyria or frontal predominant lissencephaly. Children with LIS1 deletions or other mutations have posterior predominant lissencephaly, most often with frontal pachygyria and posterior agyria (grade 3). An identical pattern occurs in children with mutations in TUBA1A codon Arg402.70
Subcortical band heterotopia is only rarely associated with other congenital anomalies. Most patients are female, because the most common cause is heterozygous mutations of the DCX gene.105 However, affected boys have also been reported.103,104 Subcortical band heterotopia associated with mutations of DCX is characterised by diffuse thick bands with no apparent gradient, or by frontal thin bands.29,106 Subcortical band heterotopia associated with mutations or deletions in LIS1 is characterised by partial posterior thin or intermediate bands with an obvious gradient, with posterior more severe than anterior heterotopia. This genotype is a rare cause of subcortical band heterotopia, and some patients have mosaic mutations in LIS128,30 or mosaic deletions in 17p13.3 (including LIS1).96
Lissencephaly with cerebellar hypoplasia was previously described as a heterogeneous range of malformations, but is now sorted into two major subtypes. The first – lissencephaly with cerebellar hypoplasia group b in an earlier classification93 – consists of mild frontal predominant lissencephaly, plus severe hippocampal and cerebellar hypoplasia and dysplasia. This pattern has been associated with mutations in RELN or VLDLR (an essential cell-surface receptor for reelin). Thus, this group is designated as reelinopathies.61,62,101,107 The second consists of more severe lissencephaly with cerebellar hypoplasia, as occurs in the tubulinopathy range.55,70 The rare Baraitser-Winter syndrome and X-linked syndrome of lissencephaly with abnormal genitalia are not discussed in this Review.60,64
Polymicrogyria with or without schizencephaly
The term polymicrogyria describes a cerebral cortex with many excessively small convolutions, which might or might not be visible on gross inspection of the brain surface.108 One specific pattern of polymicrogyria occurs in schizencephaly, and indeed several of the initial descriptions of polymicrogyria are derived from brains with schizencephaly.109,110 As part of the definition of schizencephaly, the clefts need to be lined by polymicrogyria. The pathogenesis of polymicrogyria is not understood and is probably variable in relation to its remarkable causal heterogeneity.
Pathological changes
Macroscopically, polymicrogyria appears as an irregular or pebbled cortical surface. The distribution varies greatly from bilateral symmetrical to asymmetrical to unilateral forms. The perisylvian cortex is the most frequently affected. The cortex often appears thickened to 8–12 mm, but when viewed microscopically is overfolded and not necessarily thick. Microscopically, polymicrogyria can show several different architectural patterns, although all result from abnormal development or loss of neurons in middle and deep cortical layers, variably associated with an unlayered cortical lamination, excessive folding, and fusion of adjacent gyri.111 The extent of polymicrogyria seen microscopically might be greater than that suggested by macroscopic inspection.19
When schizencephaly is present, the cortex edges can seem to fuse (closed lips) or stay at distance (open lips). The clefts of schizencephaly can be unilateral or bilateral. An area of polymicrogyria can occur in the cortex contralateral to a unilateral cleft. The cortex surrounding the cleft shows loss of laminar architecture, forming irregular heterotopic aggregates of grey matter that can be displaced into the depth of the lesion, whereas the ventricular wall is displaced upward, resulting in a seam where ependymal and malformed grey matter are adjacent.109–111
Although the term polymicrogyria as most often descriptively used in neuroimaging does not require specific histological abnormalities, neuropathologists use it to describe an abnormality of excessive gyration and a microscopic abnormality of cortical structure and lamination. In our view, such a loose definition of polymicrogyria has led to substantial confusion for both clinical care and research. Emerging data from both genetics and mouse models4,5,45,56–58 indeed show different pathogenesis for the heterogeneous group of cortical malformations all now designated as polymicrogyria, and will presumably lead to reorganisation of taxonomy in the near future.
Brain imaging
Because brain-imaging studies done with low-field strength MRI do not show microgyri well, polymicrogyria is frequently misdiagnosed as pachygyria. With use of high-field strength MRI with appropriate age-specific protocols, polymicrogyria can be reliably differentiated – especially the classic form of polymicrogyria with perisylvian location (figure 1N), schizencephaly (figure 1M), and several other forms – from other cortical malformations. In some circumstances, special techniques (eg, inversion recovery, volume averaging, focal coils, and curvilinear reformatting) might be needed.45 Ultra-high-field MRI (7T) can show polymicrogyria in brain areas where the cortex appears normal at lower field strengths and properly show the actual morphological extent of the malformation (figures 1N, 2).
Polymicrogyric cortices often appear mildly thickened (usually 6–10 mm) because of cortical overfolding (figure 2). In young children with polymicrogyria, the cortex might not appear particularly thickened because of the immature state of myelination.112 T2 signal within the cortex is usually normal. Diffusely abnormal signal in white matter should suggest in-utero infection (eg, by cytomegalovirus), peroxisomal disorders (figure 1L), or several rare polymicrogyria or polymicrogyria-like syndromes.83,113 For example, most patients with cobblestone malformations ( figure 1I, 1J, 1K; appendix) – which were once classified as polymicrogyria – have abnormal white-matter signals in infancy.
In schizencephaly, the grey matter lining the cleft has the imaging appearance of polymicrogyria with an irregular surface, deep infolding (the cleft), mildly thick cortex, and stippling of the interface between grey and white matter (figure 1M). Schizencephaly is often bilateral but frequently asymmetrical; the contralateral hemisphere should be closely assessed for milder clefts or polymicrogyria without cleft.114
Polymicrogyria has been described in several topographic patterns.20 The most common by far is bilateral perisylvian polymicrogyria (figure 1N), which varies from the posterior perisylvian region only (grade 4), to the entire perisylvian region (grade 3), to the perisylvian region with extension to other brain regions but not the poles (grade 2), to most of the brain including either or both the frontal or occipital poles (grade 1). However, the perisylvian regions are always the most severely affected. Furthermore, perisylvian polymicrogyria can be bilateral symmetrical, bilateral asymmetrical, or unilateral. Other patterns are almost always bilateral and symmetrical, and include generalised, frontal, posterior (probably), mesial parieto-occipital, and rare diffuse parasagittal polymicrogyria.21,22,115 Polymicrogyria also occurs in overlying periventricular nodular heterotopia.116
Clinical features
The clinical manifestations of polymicrogyria vary widely, and depend on several factors. The most severe outcomes occur in children with severe microcephaly (−3 SD or smaller), abnormal neurological examination (especially spasticity), widespread distribution of polymicrogyria, and additional brain malformations (especially cerebellar hypoplasia). The best outcomes are in individuals who have localised unilateral polymicrogyria without other malformations. Polymicrogyria can affect eloquent cortical areas representing language or primary motor functions, yet these functions can be retained with little or no disability.117
Bilateral perisylvian polymicrogyria with oromotor dysfunction (congenital suprabulbar palsy), intellectual disability, and epilepsy were described as the congenital bilateral perisylvian syndrome.23,24 A small group of patients have unilateral perisylvian polymicrogyria and present with mild hemiparesis or seizures. Although their developmental and neurological deficits are typically less severe than those of patients with bilateral disease, they can develop intractable seizures and epilepsy with continuous spikes and waves during sleep.25 Patients with closed-lip unilateral schizencephaly typically present with hemiparesis or motor delay, whereas patients with open-lip schizencephaly typically present with hydrocephalus or seizures.118 Outcomes are worst for patients with bilateral open-lip schizencephaly and best for those with unilateral closed-lip schizencephaly.114
Syndromes, genetics, and molecular basis
Summarising the causes of polymicrogyria is difficult because of the different pathological forms, very different distributions, evidence of extrinsic (ie, non-genetic) causes, and sometimes loose use of the term. The substantial pathological, imaging, and clinical heterogeneity suggest that polymicrogyria is not a single malformation per se, but can be grouped by diverse phenotypes.
First are phenotypes with known or presumed vascular or infectious causes. Several of the earliest descriptions of polymicrogyria came from studies of schizencephaly,119,120 which is associated with extrinsic causes such as vascular insufficiency (especially during twin pregnancies) and cytomegalovirus infections.83 Both of these causes can result in polymicrogyria without clefts. Reports linking mutations of EMX2 with schizencephaly121,122 have never been confirmed. Our experience suggests that cytomegalovirus might be a comparatively common cause of polymicrogyria.
Some copy-number variants have been associated with polymicrogyria (table 4), but only deletions in 1p36.3 and 22q11.2 are common.90,92 Indeed, when these two loci are excluded, copy number variants seem to be rare. The causal gene has not been identified for any of these loci.
All types of single-gene inheritance have been reported for polymicrogyria, including several families with autosomal dominant inheritance123,124 and some families with rare autosomal recessive forms.121,123,125,126 Several reports have described X-linked forms, although the lack of substantiation of these findings makes us skeptical of the role of these loci in polymicrogyria.123,127–129
Several syndromes with diffuse or bilateral frontal predominant polymicrogyria have been described (eg, the Warburg Micro syndrome; table 4). Pathological changes have not been defined for most of these disorders, and whether these syndromes are true polymicrogyria or polymicrogyria-like cortical malformations is usually not clear. Polymicrogyria-like cortical malformations have been reported with several metabolic diseases with severe phenotypes, including Zellweger syndrome,130,131 neonatal adreno leukodystrophy,113 fumaric aciduria,75 mitochondrial diseases,132 glutaric aciduria type 2,133 maple-syrup-urine disease,134 and histidinaemia.135 However, the histopathology differs from classic polymicrogyria for several of these disorders, especially the peroxisomal disorders and glutaric aciduria type 2.
Periventricular nodular heterotopia
Periventricular nodular heterotopia is the most frequent form of neuronal heterotopia, which is defined as groups of normal neurons in an inappropriate location. Other major types include subcortical nodular heterotopia and leptomeningeal (marginal) glioneuronal heterotopia. Periventricular nodular heterotopia, however, is the only type for which genetic and clinical associations have been extensively studied. Anatomically, periventricular nodular heterotopia consists of nodular masses of grey matter that line the ventricular walls and protrude into the lumen, resulting in an irregular outline (figure 1P). It is a fairly common malformation that can occur as a single nodule, or as many contiguous or non-contiguous nodules. When the nodules are bilateral and numerous, a genetic basis is probable and other brain malformations are often reported.18,116,136
Pathological changes
Microscopically, the heterotopic tissue contains both neurons and glial cells, and forms clusters of rounded, irregular nodules separated by layers of myelinated fibres.137 Individual nodules, or heterotopion, can lack any organisation or have rudimentary lamination.
Brain imaging
Patients with the classic X-linked form (figure 1P) typically have bilateral contiguous periventricular nodular heterotopia that spares the temporal horns, and mild cerebellar vermis hypoplasia with mega-cisterna magna.18,99 Patients with rare autosomal recessive bilateral periventricular nodular heterotopia can have severe congenital microcephaly and thin overlying cortex with abnormal gyri.138 In the fairly common posterior predominant syndromes, the periventricular nodular heterotopia is limited to the trigons, temporal, and occipital horns, and can be associated with overlying polymicrogyria (figure 1O), hippocampal and cerebellar hypoplasia, or hydrocephalus.116,136
Clinical features
The clinical presentation and course in patients with periventricular nodular heterotopia varies greatly among the recognised syndromes (table 5). In individuals with periventricular nodular heterotopia, but no other brain malformations, seizures and learning problems are common, whereas more severe developmental problems are uncommon but can occur. When microcephaly or other brain malformations are diagnosed, the likelihood of cognitive impairment increases greatly. Among all forms of periventricular nodular heterotopia, the most common and often the presenting problem is epilepsy, which has been reported in 80–90% of patients. The age at seizure onset is variable. Most patients have focal seizures, which can be easily controlled or refractory.18 Findings from studies using depth electrodes showed that the nodules are intrinsically epileptogenic, and often participate in complex epileptogenic networks, making surgical treatment of epilepsy particularly difficult.144
Table 5.
Associated genes, cyotogenetic rearrangements, and phenotypes in periventricular nodular heterotopia
| Gene | Cytogenetic location | Phenotype |
|---|---|---|
| Microcephaly with periventricular nodular heterotopia | ||
| ARFGEF2 | 20p13 | MIC PNH diffuse138 |
| Periventricular nodular heterotopia | ||
| FLNA | Xq28 | PNH diffuse18,139,149 |
| Duplication | 5p15.1 | PNH anterior140 |
| Duplication | 5p15.33 | PNH diffuse140 |
| Deletion | 5q14.3 | PNH posterior141 |
| Deletion | 6p25 | PNH scattered142 |
| Deletion-C6orf70 | 6q27 | PNH variable, PMG91 |
| Deletion | 7q11.23 | PNH anterior, Williams syndrome143 |
PNH=periventricular nodular heterotopia. PMG=polymicrogyria.
Syndromes, genetics, and molecular basis
The most common form of diffuse periventricular nodular heterotopia and the linked Ehlers-Danlos form are caused by mutations in the X–linked FLNA gene.18 This syndrome predominately affects girls, and several large pedigrees suggest increased prenatal lethality of affected boys.145 Affected girls, with rare exceptions, have normal intelligence, but might have learning problems. The phenotype in boys is much more variable, ranging from prenatal lethality to the mild female phenotype. However, cognitive impairment is more common than in females.27,139 In a large study of 120 patients with periventricular nodular heterotopia, FLNA mutations were found in 10 of 10 families with X-linked inheritance.18 Overall, mutations were reported in 49% of individuals with classic bilateral periventricular nodular heterotopia, irrespective of familial or sporadic occurrence. The sex ratio was skewed with 93% of mutations in females and 7% in males.
A rare autosomal recessive form associated with severe congenital microcephaly and diffuse bilateral periventricular nodular heterotopia has been reported in two families.138 The affected children had severe developmental handicaps. Other genetic forms of periventricular nodular heterotopia have been mapped to chromosomes 5p15, 5q14.3, 6p25, 6q27 and 7q1123 (table 5),140–143,146 but a putative causal gene has only been identified for the 6q27-related form.91
The two genes associated with periventricular nodular heterotopia (ARFGEF2 and FLNA) regulate actin binding, vesicle trafficking, cell adhesion, and function of radial glia;138,147 mutations of these genes cause abnormalities of the ventricular epithelium or neuroependyma.148 FLNA encodes a large actin-binding phosphoprotein that stabilises the cytoskeleton andcontributes to formation of focal adhesions along the ventricular epithelium.149,150 ARFGEF2 encodes a protein that phosphorylates guanine diphosphate. Guanine triphsophate activates ADP-ribosylation factors, which regulate vesicle trafficking and transport of molecules from the cell interior to cell surface for binding to other molecules or cell secretion. Thus, this protein might assist in the transport of FLNA to the cell surface.
Conclusions and future directions
The highly variable clinical presentations and burden of disability associated with MCD result from diversity in pathophysiological disruption of brain development and the consequent anatomical and functional brain impairment. Understanding of the genetic and molecular basis of cortical development is advancing rapidly, with new genes and new roles for known genes being reported, providing evidence that many cortical malformations are probably secondary to abnormalities occurring at several interdependent developmental stages. Findings from studies of human brain tissue samples that are made available after surgical resection for intractable epilepsy show that postzygotic mutations affecting a subset of neural cells can cause abnormal development and migration, changing the perception of genetic MCD as disorders affecting the whole brain. At the same time, findings from different experimental models are revealing the diversity underlying epileptogenesis in MCD, and might help to pave the way for innovative treatment strategies. For example, findings from an Arx((GCG)7/Y) knock-in mouse model showed normal development of GABAergic neurons, with normal migrations of pyramidal cells and cortical layering, but a substantial increase in the frequency of excitatory inputs associated with a remodelling of axonal arborisation of hippocampal pyramidal neurons, suggesting that epilepsy results from remodelling of the glutamate network and that secondary changes are essential for the development of disease-specific phenotypes.151 In-utero silencing of the Srpx2 gene or increased acetylation of a-tubulin causes a neuronal migration phenotype with postnatal spontaneous epileptiform activity, which can be prevented by maternal administration of the tubulin deacetylase inhibitor tubacin.152 This model provides an example of a drug based strategy to prevent epileptiform manifestations of developmental origin. Patch-clamp recordings from dysplastic neurons in acute slices of brains from patients with FCD type 2b show excitatory responses of GABA(A) receptors that are substantially attenuated by the SLC12A2 inhibitor bumetanide,153 a finding that could account for resistance of seizure activity in FCD to anticonvulsants that increase GABAergic function, and might justify add-on trials with bumetanide. These examples clearly show that addressing the diversity of mechanisms causing MCD and their secondary changes could lead to rational, targeted treatment options.
Supplementary Material
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed with the search terms “focal cortical dysplasia”, “hemimegalencephaly”, “heterotopia”, “lissencephaly”, “malformations of cortical development”, “megalencephaly”, “pachygyria”, “polymicrogyria”, and “subcortical band heterotopia”, from 1990 until July, 2013 and through quotation of classical genetic and neuropathological work. Articles were also identified through searches of the authors’ own files. With a few exceptions concerning seminal neuropathological descriptions, only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Acknowledgments
We thank Valerio Conti (Neurogenetics Laboratory, Neuroscience Department, Children’s Hospital A Meyer-University of Florence, Florence, Italy) for preparing figures 3 and 4. This work was supported by the European Union Seventh Framework Programme FP7/2007–2013 under the project DESIRE (grant agreement number 602531) to RG.
Footnotes
Contributors
Both authors contributed equally to the literature search, figures, tables, and writing.
Declaration of interests
We declare no competing interests.
References
- 1.Barkovich AJ, Kuzniecky RI, Dobyns WB, Jackson GD, Becker LE, Evrard P. A classification scheme for malformations of cortical development. Neuropediatrics. 1996;27:59–63. doi: 10.1055/s-2007-973750. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. Classification system for malformations of cortical development: update 2001. Neurology. 2001;57:2168–78. doi: 10.1212/wnl.57.12.2168. [DOI] [PubMed] [Google Scholar]
- 3.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–87. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–69. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilgüvar K, Oztürk AK, Louvi A, et al. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature. 2010;467:207–10. doi: 10.1038/nature09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholas AK, Khurshid M, Dsir J, et al. WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet. 2010;42:1010–14. doi: 10.1038/ng.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu TW, Mochida GH, Tischfield DJ, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet. 2010;42:1015–20. doi: 10.1038/ng.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirier K, Lebrun N, Broix L, et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet. 2013;45:639–47. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobyns WB, Patton MA, Stratton RF, Mastrobattista JM, Blanton SH, Northrup H. Cobblestone lissencephaly with normal eyes and muscle. Neuropediatrics. 1996;27:70–75. doi: 10.1055/s-2007-973752. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Jin Z, Koirala S, et al. GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci. 2008;28:5817–26. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden JA, Harding BN. Pathology and genetics: developmental neuropathology. Basel: Neuropath Press; 2004. Cell migration and specification disorders; pp. 34–87. [Google Scholar]
- 12.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K-AKT3-MTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–45. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poduri A, Evrony GD, Cai X, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012;74:41–48. doi: 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blümcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–74. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malinger G, Kidron D, Schreiber L, et al. Prenatal diagnosis of malformations of cortical development by dedicated neurosonography. Ultrasound Obstet Gynecol. 2007;29:178–91. doi: 10.1002/uog.3906. [DOI] [PubMed] [Google Scholar]
- 16.Righini A, Parazzini C, Doneda C, et al. Early formative stage of human focal cortical gyration anomalies: fetal MRI. AJR Am J Roentgenol. 2012;198:439–47. doi: 10.2214/AJR.11.6662. [DOI] [PubMed] [Google Scholar]
- 17.Barkovich AJ, Guerrini R, Battaglia G, et al. Band heterotopia: correlation of outcome with magnetic resonance imaging parameters. Ann Neurol. 1994;36:609–17. doi: 10.1002/ana.410360409. [DOI] [PubMed] [Google Scholar]
- 18.Parrini E, Ramazzotti A, Dobyns WB, et al. Periventricular heterotopia: phenotypic heterogeneity and correlation with Filamin A mutations. Brain. 2006;129:1892–906. doi: 10.1093/brain/awl125. [DOI] [PubMed] [Google Scholar]
- 19.Guerrini R, Dravet C, Raybaud C, et al. Epilepsy and focal gyralanomalies detected by MRI: electroclinico-morphological correlations and follow-up. Dev Med Child Neurol. 1992;34:706–18. doi: 10.1111/j.1469-8749.1992.tb11506.x. [DOI] [PubMed] [Google Scholar]
- 20.Leventer RJ, Jansen A, Pilz DT, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133:1415–27. doi: 10.1093/brain/awq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrini R, Barkovich AJ, Sztriha L, Dobyns WB. Bilateral frontal polymicrogyria: a newly recognized brain malformation syndrome. Neurology. 2000;54:909–13. doi: 10.1212/wnl.54.4.909. [DOI] [PubMed] [Google Scholar]
- 22.Guerrini R, Dubeau F, Dulac O, et al. Bilateral parasagittal parietooccipital polymicrogyria and epilepsy. Ann Neurol. 1997;41:65–73. doi: 10.1002/ana.410410112. [DOI] [PubMed] [Google Scholar]
- 23.Guerrini R, Dravet C, Raybaud C, et al. Neurological findings and seizure outcome in children with bilateral opercular macrogyric-like changes detected by MRI. Dev Med Child Neurol. 1992;34:694–705. doi: 10.1111/j.1469-8749.1992.tb11505.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuzniecky R, Andermann F, Guerrini R. Congenital bilateral perisylvian syndrome: study of 31 patients. The CBPS Multicenter Collaborative Study. Lancet. 1993;341:608–12. doi: 10.1016/0140-6736(93)90363-l. [DOI] [PubMed] [Google Scholar]
- 25.Guerrini R, Genton P, Bureau M, et al. Multilobar polymicrogyria, intractable drop attack seizures, and sleep-related electrical status epilepticus. Neurology. 1998;51:504–12. doi: 10.1212/wnl.51.2.504. [DOI] [PubMed] [Google Scholar]
- 26.Riviere JB, Mirzaa GM, O’Roak BJ, et al. the Finding of Rare Disease Genes (FORGE) Canada Consortium. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44:934–40. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrini R, Mei D, Sisodiya S, et al. Germline and mosaic mutations of FLN1 in men with periventricular heterotopia. Neurology. 2004;63:51–56. doi: 10.1212/01.wnl.0000132818.84827.4d. [DOI] [PubMed] [Google Scholar]
- 28.Sicca F, Kelemen A, Genton P, et al. Mosaic mutations of the LIS1 gene cause subcortical band heterotopia. Neurology. 2003;61:1042–46. doi: 10.1212/wnl.61.8.1042. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto N, Leventer RJ, Kuc JA, et al. Mutation analysis of the DCX gene and genotype/phenotype correlation in subcortical band heterotopia. Eur J Hum Genet. 2001;9:5–12. doi: 10.1038/sj.ejhg.5200548. [DOI] [PubMed] [Google Scholar]
- 30.Mineyko A, Doja A, Hurteau J, Dobyns WB, Das S, Boycott KM. A novel missense mutation in LIS1 in a child with subcortical band heterotopia and pachygyria inherited from his mildly affected mother with somatic mosaicism. J Child Neurol. 2010;25:738–41. doi: 10.1177/0883073809343312. [DOI] [PubMed] [Google Scholar]
- 31.Poduri A, Evrony GD, Cai X, Walsh CA. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341:1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Salem A, Alshammari MJ, Hassan H, Alazami AM, Alkuraya FS. Weaver syndrome and defective cortical development: a rare association. Am J Med Genet A. 2013;161A:225–27. doi: 10.1002/ajmg.a.35660. [DOI] [PubMed] [Google Scholar]
- 33.Gibson WT, Hood RL, Zhan SH, et al. the FORGE Canada Consortium. Mutations in EZH2 cause Weaver syndrome. Am J Hum Genet. 2012;90:110–18. doi: 10.1016/j.ajhg.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatton-Brown K, Hanks S, Ruark E, et al. the Childhood Overgrowth Collaboration. Germline mutations in the oncogene EZH2 cause Weaver syndrome and increased human height. Oncotarget. 2011;2:1127–33. doi: 10.18632/oncotarget.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hevner RF. The cerebral cortex malformation in thanatophoric dysplasia: neuropathology and pathogenesis. Acta Neuropathol. 2005;110:208–21. doi: 10.1007/s00401-005-1059-8. [DOI] [PubMed] [Google Scholar]
- 36.Itoh K, Pooh R, Kanemura Y, Yamasaki M, Fushiki S. Brain malformation with loss of normal FGFR3 expression in thanatophoric dysplasia type I. Neuropathology. 2013;33:663–66. doi: 10.1111/neup.12036. [DOI] [PubMed] [Google Scholar]
- 37.Kurek KC, Luks VL, Ayturk UM, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet. 2012;90:1108–15. doi: 10.1016/j.ajhg.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schick V, Majores M, Engels G, et al. Activation of Akt independent of PTEN and CTMP tumor-suppressor gene mutations in epilepsy-associated Taylor-type focal cortical dysplasias. Acta Neuropathol. 2006;112:715–25. doi: 10.1007/s00401-006-0128-y. [DOI] [PubMed] [Google Scholar]
- 39.DeMyer W. Megalencephaly: types, clinical syndromes, and management. Pediatr Neurol. 1986;2:321–28. doi: 10.1016/0887-8994(86)90072-x. [DOI] [PubMed] [Google Scholar]
- 40.Friede RL. Developmental Neuropathology. New York, NY: Springer-Verlag; 1989. Disturbances in bulk growth: megalencephaly, micrencephaly, atelencephaly and others; pp. 296–308. [Google Scholar]
- 41.Weidenheim KM, Escobar A, Rapin I. Brief report: life history and neuropathology of a gifted man with Asperger syndrome. J Autism Dev Disord. 2012;42:460–67. doi: 10.1007/s10803-011-1259-0. [DOI] [PubMed] [Google Scholar]
- 42.Thomson RE, Kind PC, Graham NA, et al. Fgf receptor 3 activation promotes selective growth and expansion of occipitotemporal cortex. Neural Dev. 2009;4:4. doi: 10.1186/1749-8104-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Agostino MD, Bastos A, Piras C, et al. Posterior quadrantic dysplasia or hemi-hemimegalencephaly: a characteristic brain malformation. Neurology. 2004;62:2214–20. doi: 10.1212/01.wnl.0000130459.91445.91. [DOI] [PubMed] [Google Scholar]
- 44.De Rosa MJ, Secor DL, Barsom M, Fisher RS, Vinters HV. Neuropathologic findings in surgically treated hemimegalencephaly: immunohistochemical, morphometric, and ultrastructural study. Acta Neuropathol. 1992;84:250–60. doi: 10.1007/BF00227817. [DOI] [PubMed] [Google Scholar]
- 45.Guerrini R, Dobyns WB, Barkovich AJ. Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci. 2008;31:154–62. doi: 10.1016/j.tins.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Crino PB. Focal brain malformations: seizures, signaling, sequencing. Epilepsia. 2009;50(suppl 9):3–8. doi: 10.1111/j.1528-1167.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- 47.Becker AJ, Urbach H, Scheffler B, et al. Focal cortical dysplasia of Taylor’s balloon cell type: mutational analysis of the TSC1 gene indicates a pathogenic relationship to tuberous sclerosis. Ann Neurol. 2002;52:29–37. doi: 10.1002/ana.10251. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Tsai V, Parker WE, Aronica E, Baybis M, Crino PB. Detection of human papillomavirus in human focal cortical dysplasia type IIB. Ann Neurol. 2012;72:881–92. doi: 10.1002/ana.23795. [DOI] [PubMed] [Google Scholar]
- 49.Salamon N, Andres M, Chute DJ, et al. Contralateral hemimicrencephaly and clinical-pathological correlations in children with hemimegalencephaly. Brain. 2006;129:352–65. doi: 10.1093/brain/awh681. [DOI] [PubMed] [Google Scholar]
- 50.Barkovich AJ, Kuzniecky RI, Bollen AW, Grant PE. Focal transmantle dysplasia: a specific malformation of cortical development. Neurology. 1997;49:1148–52. doi: 10.1212/wnl.49.4.1148. [DOI] [PubMed] [Google Scholar]
- 51.Mirzaa GM, Conway RL, Gripp KW, et al. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactylypolymicrogyria-hydrocephalus (MPPH) syndromes: two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am J Med Genet A. 2012;158A:269–91. doi: 10.1002/ajmg.a.34402. [DOI] [PubMed] [Google Scholar]
- 52.Guerrini R, Filippi T. Neuronal migration disorders, genetics, and epileptogenesis. J Child Neurol. 2005;20:287–99. doi: 10.1177/08830738050200040401. [DOI] [PubMed] [Google Scholar]
- 53.Mirzaa G, Ashwal S, Dobyns WB. Disorders of Brain Size. In: Swaiman KF, Ashwal S, Ferriero DF, Schor NF, editors. Swaiman’s Pediatric neurology: principles and practice. 5. Edinburgh: Elsevier Saunders; 2012. pp. 173–201. [Google Scholar]
- 54.Tinkle BT, Schorry EK, Franz DN, Crone KR, Saal HM. Epidemiology of hemimegalencephaly: a case series and review. Am J Med Genet A. 2005;139:204–11. doi: 10.1002/ajmg.a.31024. [DOI] [PubMed] [Google Scholar]
- 55.Cushion TD, Dobyns WB, Mullins JG, et al. Overlapping cortical malformations and mutations in TUBB2B and TUBA1A. Brain. 2013;136:536–48. doi: 10.1093/brain/aws338. [DOI] [PubMed] [Google Scholar]
- 56.Jaglin XH, Poirier K, Saillour Y, et al. Mutations in the β-tubulingene TUBB2B result in asymmetrical polymicrogyria. Nat Genet. 2009;41:746–52. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lecourtois M, Poirier K, Friocourt G, et al. Human lissencephaly with cerebellar hypoplasia due to mutations in TUBA1A: expansion of the foetal neuropathological phenotype. Acta Neuropathol. 2010;119:779–89. doi: 10.1007/s00401-010-0684-z. [DOI] [PubMed] [Google Scholar]
- 58.Stottmann RW, Donlin M, Hafner A, Bernard A, Sinclair DA, Beier DR. A mutation in TUBB2B, a human polymicrogyria gene, leads to lethality and abnormal cortical development in the mouse. Hum Mol Genet. 2013;22:4053–63. doi: 10.1093/hmg/ddt255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitamura K, Yanazawa M, Sugiyama N, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- 60.Kato M, Das S, Petras K, et al. Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum Mutat. 2004;23:147–59. doi: 10.1002/humu.10310. [DOI] [PubMed] [Google Scholar]
- 61.Hong SE, Shugart YY, Huang DT, et al. Autosomal recessivelissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26:93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- 62.Zaki M, Shehab M, El-Aleem AA, et al. Identification of a novel recessive RELN mutation using a homozygous balanced reciprocal translocation. Am J Med Genet A. 2007;143A:939–44. doi: 10.1002/ajmg.a.31667. [DOI] [PubMed] [Google Scholar]
- 63.Boycott KM, Bonnemann C, Herz J, et al. Mutations in VLDLR as a cause for autosomal recessive cerebellar ataxia with mental retardation (dysequilibrium syndrome) J Child Neurol. 2009;24:1310–15. doi: 10.1177/0883073809332696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riviere JB, van Bon BW, Hoischen A, et al. De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat Genet. 2012;44:440–44. S1–2. doi: 10.1038/ng.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gleeson JG, Allen KM, Fox JW, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 66.Haverfield EV, Whited AJ, Petras KS, Dobyns WB, Das S. Intragenic deletions and duplications of the LIS1 and DCX genes: a major disease-causing mechanism in lissencephaly and subcortical band heterotopia. Eur J Hum Genet. 2009;17:911–18. doi: 10.1038/ejhg.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willemsen MH, Vissers LE, Willemsen MA, et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J Med Genet. 2012;49:179–83. doi: 10.1136/jmedgenet-2011-100542. [DOI] [PubMed] [Google Scholar]
- 68.Reiner O, Carrozzo R, Shen Y, et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature. 1993;364:717–21. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 69.Cardoso C, Leventer RJ, Ward HL, et al. Refinement of a 400-kb critical region allows genotypic differentiation between isolated lissencephaly, Miller-Dieker syndrome, and other phenotypes secondary to deletions of 17p13. 3. Am J Hum Genet. 2003;72:918–30. doi: 10.1086/374320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar RA, Pilz DT, Babatz TD, et al. TUBA1A mutations causewide spectrum lissencephaly (smooth brain) and suggest that multiple neuronal migration pathways converge on a tubulins. Hum Mol Genet. 2010;19:2817–27. doi: 10.1093/hmg/ddq182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keays DA, Tian G, Poirier K, et al. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guerrini R, Mei D, Cordelli DM, Pucatti D, Franzoni E, Parrini E. Symmetric polymicrogyria and pachygyria associated with TUBB2Bgene mutations. Eur J Hum Genet. 2012;20:995–98. doi: 10.1038/ejhg.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alkuraya FS, Cai X, Emery C, et al. Human mutations in NDE1 cause extreme microcephaly with lissencephaly [corrected] Am J Hum Genet. 2011;88:536–47. doi: 10.1016/j.ajhg.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guven A, Gunduz A, Bozoglu TM, Yalcinkaya C, Tolun A. Novel NDE1 homozygous mutation resulting in microhydranencephaly and not microlyssencephaly. Neurogenetics. 2012;13:189–94. doi: 10.1007/s10048-012-0326-9. [DOI] [PubMed] [Google Scholar]
- 75.Kerrigan JF, Aleck KA, Tarby TJ, Bird CR, Heidenreich RA. Fumaric aciduria: clinical and imaging features. Ann Neurol. 2000;47:583–88. [PubMed] [Google Scholar]
- 76.Brooks AS, Bertoli-Avella AM, Burzynski GM, et al. Homozygous nonsense mutations in KIAA1279 are associated with malformations of the central and enteric nervous systems. Am J Hum Genet. 2005;77:120–26. doi: 10.1086/431244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLarren KW, Severson TM, du Souich C, et al. Hypomorphic temperature-sensitive alleles of NSDHL cause CK syndrome. Am J Hum Genet. 2010;87:905–14. doi: 10.1016/j.ajhg.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Driscoll MC, Daly SB, Urquhart JE, et al. Recessive mutations in the gene encoding the tight junction protein occludin cause bandlike calcification with simplified gyration and polymicrogyria. Am J Hum Genet. 2010;87:354–64. doi: 10.1016/j.ajhg.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doherty D, Chudley AE, Coghlan G, et al. the FORGE Canada Consortium. GPSM2 mutations cause the brain malformations and hearing loss in Chudley-McCullough syndrome. Am J Hum Genet. 2012;90:1088–93. doi: 10.1016/j.ajhg.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Handley MT, Morris-Rosendahl DJ, Brown S, et al. Mutation spectrum in RAB3GAP1, RAB3GAP2, and RAB18 and genotypephenotype correlations in warburg micro syndrome and Martsolf syndrome. Hum Mutat. 2013;34:686–96. doi: 10.1002/humu.22296. [DOI] [PubMed] [Google Scholar]
- 81.Vissers LE, van Ravenswaaij CM, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955–57. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- 82.Roll P, Rudolf G, Pereira S, et al. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet. 2006;15:1195–207. doi: 10.1093/hmg/ddl035. [DOI] [PubMed] [Google Scholar]
- 83.Barkovich AJ, Lindan CE. Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am J Neuroradiol. 1994;15:703–15. [PMC free article] [PubMed] [Google Scholar]
- 84.Abdollahi MR, Morrison E, Sirey T, et al. Mutation of the variant alpha-tubulin TUBA8 results in polymicrogyria with optic nerve hypoplasia. Am J Hum Genet. 2009;85:737–44. doi: 10.1016/j.ajhg.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poirier K, Saillour Y, Bahi-Buisson N, et al. Mutations in the neuronal β-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum Mol Genet. 2010;19:4462–73. doi: 10.1093/hmg/ddq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Breuss M, Heng JI, Poirier K, et al. Mutations in the β-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Reports. 2012;2:1554–62. doi: 10.1016/j.celrep.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baala L, Briault S, Etchevers HC, et al. Homozygous silencing of T-box transcription factor EOMES leads to microcephaly with polymicrogyria and corpus callosum agenesis. Nat Genet. 2007;39:454–56. doi: 10.1038/ng1993. [DOI] [PubMed] [Google Scholar]
- 88.Sisodiya SM, Free SL, Williamson KA, et al. PAX6 haploinsufficiency causes cerebral malformation and olfactory dysfunction in humans. Nat Genet. 2001;28:214–16. doi: 10.1038/90042. [DOI] [PubMed] [Google Scholar]
- 89.Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994;7:463–71. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- 90.Dobyns WB, Mirzaa G, Christian SL, et al. Consistent chromosome abnormalities identify novel polymicrogyria loci in 1p36.3,2p16.1-p23.1, 4q21.21-q22. 1, 6q26-q27, and 21q2. Am J Med Genet A. 2008;146A:1637–54. doi: 10.1002/ajmg.a.32293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Conti V, Carabalona A, Pallesi-Pocachard E, et al. Periventricular heterotopia in 6q terminal deletion syndrome: role of the C6orf70 gene. Brain. 2013;136:3378–94. doi: 10.1093/brain/awt249. [DOI] [PubMed] [Google Scholar]
- 92.Robin NH, Taylor CJ, McDonald-McGinn DM, et al. Polymicrogyria and deletion 22q11. 2 syndrome: window to the etiology of a common cortical malformation. Am J Med Genet A. 2006;140:2416–25. doi: 10.1002/ajmg.a.31443. [DOI] [PubMed] [Google Scholar]
- 93.Ross ME, Swanson K, Dobyns WB. Lissencephaly with cerebellar hypoplasia (LCH): a heterogeneous group of cortical malformations. Neuropediatrics. 2001;32:256–63. doi: 10.1055/s-2001-19120. [DOI] [PubMed] [Google Scholar]
- 94.Morris-Rosendahl DJ, Najm J, Lachmeijer AM, et al. Refining thephenotype of alpha-1a Tubulin (TUBA1A) mutation in patients with classical lissencephaly. Clin Genet. 2008;74:425–33. doi: 10.1111/j.1399-0004.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell TN, Free SL, Williamson KA, et al. Polymicrogyria and absence of pineal gland due to PAX6 mutation. Ann Neurol. 2003;53:658–63. doi: 10.1002/ana.10576. [DOI] [PubMed] [Google Scholar]
- 96.Dobyns WB, Guerrini R, Leventer RL. Malformations of Cortical Development. In: Swaiman KF, Ashwal S, Ferriero DM, Schor NF, editors. Swaiman’s pediatric neurology: principles and practice. 5. Edinburgh: Elsevier Saunders; 2012. pp. 202–31. [Google Scholar]
- 97.Forman MS, Squier W, Dobyns WB, Golden JA. Genotypically defined lissencephalies show distinct pathologies. J Neuropathol Exp Neurol. 2005;64:847–57. doi: 10.1097/01.jnen.0000182978.56612.41. [DOI] [PubMed] [Google Scholar]
- 98.Solomon BD, Pineda-Alvarez DE, Balog JZ, et al. Compound heterozygosity for mutations in PAX6 in a patient with complex brain anomaly, neonatal diabetes mellitus, and microophthalmia. Am J Med Genet A. 2009;149A:2543–46. doi: 10.1002/ajmg.a.33081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dobyns WB, Andermann E, Andermann F, et al. X-linked malformations of neuronal migration. Neurology. 1996;47:331–39. doi: 10.1212/wnl.47.2.331. [DOI] [PubMed] [Google Scholar]
- 100.des Portes V, Pinard JM, Smadja D, et al. Dominant X linked subcortical laminar heterotopia and lissencephaly syndrome (XSCLH/LIS): evidence for the occurrence of mutation in males and mapping of a potential locus in Xq22. J Med Genet. 1997;34:177–83. doi: 10.1136/jmg.34.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boycott KM, Flavelle S, Bureau A, et al. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am J Hum Genet. 2005;77:477–83. doi: 10.1086/444400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dobyns WB, Elias ER, Newlin AC, Pagon RA, Ledbetter DH. Causal heterogeneity in isolated lissencephaly. Neurology. 1992;42:1375–88. doi: 10.1212/wnl.42.7.1375. [DOI] [PubMed] [Google Scholar]
- 103.D’Agostino MD, Bernasconi A, Das S, et al. Subcortical band heterotopia (SBH) in males: clinical, imaging and genetic findings in comparison with females. Brain. 2002;125:2507–22. doi: 10.1093/brain/awf248. [DOI] [PubMed] [Google Scholar]
- 104.Guerrini R, Moro F, Andermann E, et al. Nonsyndromic mental retardation and cryptogenic epilepsy in women with doublecortin gene mutations. Ann Neurol. 2003;54:30–37. doi: 10.1002/ana.10588. [DOI] [PubMed] [Google Scholar]
- 105.Dobyns WB. The clinical patterns and molecular genetics of lissencephaly and subcortical band heterotopia. Epilepsia. 2010;51(suppl 1):5–9. doi: 10.1111/j.1528-1167.2009.02433.x. [DOI] [PubMed] [Google Scholar]
- 106.Gleeson JG, Luo RF, Grant PE, et al. Genetic and neuroradiological heterogeneity of double cortex syndrome. Ann Neurol. 2000;47:265–69. [PubMed] [Google Scholar]
- 107.Kolb LE, Arlier Z, Yalcinkaya C, et al. Novel VLDLR microdeletion identified in two Turkish siblings with pachygyria and pontocerebellar atrophy. Neurogenetics. 2010;11:319–25. doi: 10.1007/s10048-009-0232-y. [DOI] [PubMed] [Google Scholar]
- 108.Bielschowsky M. Uber Mikrogyrie. J Psychol Neurol. 1916;22:1–47. (in German) [Google Scholar]
- 109.Yakovlev PI, Wadsworth RC. Schizencephalies; a study of thecongenital clefts in the cerebral mantle; clefts with fused lips. J Neuropathol Exp Neurol. 1946;5:116–30. doi: 10.1097/00005072-194604000-00003. [DOI] [PubMed] [Google Scholar]
- 110.Yakovlev PI, Wadsworth RC. Schizencephalies; a study of the congenital clefts in the cerebral mantle; clefts with hydrocephalus and lips separated. J Neuropathol Exp Neurol. 1946;5:169–206. [PubMed] [Google Scholar]
- 111.Harding B, Copp AJ. Malformations. In: Graham Di, Lantos Pl., editors. Greenfield’s Neuropathology. 7. London: Arnold; 2002. pp. 357–484. [Google Scholar]
- 112.Takanashi J, Barkovich AJ. The changing MR imaging appearanceof polymicrogyria: a consequence of myelination. AJNR Am J Neuroradiol. 2003;24:788–93. [PMC free article] [PubMed] [Google Scholar]
- 113.van der Knaap MS, Valk J. The MR spectrum of peroxisomal disorders. Neuroradiology. 1991;33:30–37. doi: 10.1007/BF00593330. [DOI] [PubMed] [Google Scholar]
- 114.Barkovich AJ, Kjos BO. Schizencephaly: correlation of clinicalfindings with MR characteristics. AJNR Am J Neuroradiol. 1992;13:85–94. [PMC free article] [PubMed] [Google Scholar]
- 115.Barkovich AJ, Hevner R, Guerrini R. Syndromes of bilateralsymmetrical polymicrogyria. AJNR Am J Neuroradiol. 1999;20:1814–21. [PMC free article] [PubMed] [Google Scholar]
- 116.Wieck G, Leventer RJ, Squier WM, et al. Periventricular nodular heterotopia with overlying polymicrogyria. Brain. 2005;128:2811–21. doi: 10.1093/brain/awh658. [DOI] [PubMed] [Google Scholar]
- 117.Guerrini R, Barba C. Malformations of cortical development and aberrant cortical networks: epileptogenesis and functional organization. J Clin Neurophysiol. 2010;27:372–79. doi: 10.1097/WNP.0b013e3181fe0585. [DOI] [PubMed] [Google Scholar]
- 118.Denis D, Chateil JF, Brun M, et al. Schizencephaly: clinical and imaging features in 30 infantile cases. Brain Dev. 2000;22:475–83. doi: 10.1016/s0387-7604(00)00173-x. [DOI] [PubMed] [Google Scholar]
- 119.Dekaban A. Large defects in cerebral hemispheres associated with cortical dysgenesis. J Neuropathol Exp Neurol. 1965;24:512–30. [Google Scholar]
- 120.Levine DN, Fisher MA, Caviness VS., Jr Porencephaly with microgyria: a pathologic study. Acta Neuropathol. 1974;29:99–113. doi: 10.1007/BF00684769. [DOI] [PubMed] [Google Scholar]
- 121.Granata T, Farina L, Faiella A, et al. Familial schizencephaly associated with EMX2 mutation. Neurology. 1997;48:1403–06. doi: 10.1212/wnl.48.5.1403. [DOI] [PubMed] [Google Scholar]
- 122.Merello E, Swanson E, De Marco P, et al. No major role for the EMX2 gene in schizencephaly. Am J Med Genet A. 2008;146A:1142–50. doi: 10.1002/ajmg.a.32264. [DOI] [PubMed] [Google Scholar]
- 123.Guerreiro MM, Andermann E, Guerrini R, et al. Familial perisylvian polymicrogyria: a new familial syndrome of cortical maldevelopment. Ann Neurol. 2000;48:39–48. [PubMed] [Google Scholar]
- 124.Chang BS, Apse KA, Caraballo R, et al. A familial syndrome of unilateral polymicrogyria affecting the right hemisphere. Neurology. 2006;66:133–35. doi: 10.1212/01.wnl.0000191393.06679.e9. [DOI] [PubMed] [Google Scholar]
- 125.Hosley MA, Abroms IF, Ragland RL. Schizencephaly: case report of familial incidence. Pediatr Neurol. 1992;8:148–50. doi: 10.1016/0887-8994(92)90039-2. [DOI] [PubMed] [Google Scholar]
- 126.Hilburger AC, Willis JK, Bouldin E, Henderson-Tilton A. Familial schizencephaly. Brain Dev. 1993;15:234–36. doi: 10.1016/0387-7604(93)90072-g. [DOI] [PubMed] [Google Scholar]
- 127.Borgatti R, Triulzi F, Zucca C, et al. Bilateral perisylvian polymicrogyria in three generations. Neurology. 1999;52:1910–13. doi: 10.1212/wnl.52.9.1910. [DOI] [PubMed] [Google Scholar]
- 128.Santos NF, Secolin R, Brando-Almeida IL, et al. A new candidate locus for bilateral perisylvian polymicrogyria mapped on chromosome Xq27. Am J Med Genet A. 2008;146A:1151–57. doi: 10.1002/ajmg.a.32270. [DOI] [PubMed] [Google Scholar]
- 129.Villard L, Nguyen K, Cardoso C, et al. A locus for bilateral perisylvian polymicrogyria maps to Xq28. Am J Hum Genet. 2002;70:1003–08. doi: 10.1086/339433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wanders RJ, Schutgens RB, Barth PG. Peroxisomal disorders: a review. J Neuropathol Exp Neurol. 1995;54:726–39. doi: 10.1097/00005072-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 131.Barkovich AJ, Peck WW. MR of Zellweger syndrome. AJNR Am J Neuroradiol. 1997;18:1163–70. [PMC free article] [PubMed] [Google Scholar]
- 132.Keng WT, Pilz DT, Minns B, FitzPatrick DR. A3243G mitochondrial mutation associated with polymicrogyria. Dev Med Child Neurol. 2003;45:704–08. doi: 10.1017/s0012162203001300. [DOI] [PubMed] [Google Scholar]
- 133.Bhm N, Uy J, Kiessling M, Lehnert W. Multiple acyl-CoA dehydrogenation deficiency (glutaric aciduria type II), congenital polycystic kidneys, and symmetric warty dysplasia of the cerebral cortex in two newborn brothers. II. Morphology and pathogenesis. Eur J Pediatr. 1982;139:60–65. doi: 10.1007/BF00442082. [DOI] [PubMed] [Google Scholar]
- 134.Martin JK, Norman RM. Maple syrup urine disease in an infant with microgyria. Dev Med Child Neurol. 1967;9:152–59. doi: 10.1111/j.1469-8749.1967.tb02236.x. [DOI] [PubMed] [Google Scholar]
- 135.Corner BD, Holton JB, Norman RM, Williams PM. A case of histidinemia controlled with a low histidine diet. Pediatrics. 1968;41:1074–81. [PubMed] [Google Scholar]
- 136.Pisano T, Barkovich AJ, Leventer RJ, et al. Peritrigonal and temporo-occipital heterotopia with corpus callosum and cerebellar dysgenesis. Neurology. 2012;79:1244–51. doi: 10.1212/WNL.0b013e31826aac88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tassi L, Colombo N, Cossu M, et al. Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain. 2005;128:321–37. doi: 10.1093/brain/awh357. [DOI] [PubMed] [Google Scholar]
- 138.Sheen VL, Ganesh VS, Topcu M, et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36:69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- 139.Sheen VL, Dixon PH, Fox JW, et al. Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet. 2001;10:1775–83. doi: 10.1093/hmg/10.17.1775. [DOI] [PubMed] [Google Scholar]
- 140.Sheen VL, Wheless JW, Bodell A, et al. Periventricular heterotopia associated with chromosome 5p anomalies. Neurology. 2003;60:1033–36. doi: 10.1212/01.wnl.0000052689.03214.61. [DOI] [PubMed] [Google Scholar]
- 141.Cardoso C, Boys A, Parrini E, et al. Periventricular heterotopia, mental retardation, and epilepsy associated with 5q14. 3-q15 deletion. Neurology. 2009;72:784–92. doi: 10.1212/01.wnl.0000336339.08878.2d. [DOI] [PubMed] [Google Scholar]
- 142.Cellini E, Disciglio V, Novara F, et al. Periventricular heterotopia with white matter abnormalities associated with 6p25 deletion. Am J Med Genet A. 2012;158A:1793–97. doi: 10.1002/ajmg.a.35416. [DOI] [PubMed] [Google Scholar]
- 143.Ferland RJ, Gaitanis JN, Apse K, Tantravahi U, Walsh CA, Sheen VL. Periventricular nodular heterotopia and Williams syndrome. Am J Med Genet A. 2006;140:1305–11. doi: 10.1002/ajmg.a.31259. [DOI] [PubMed] [Google Scholar]
- 144.Kothare SV, VanLandingham K, Armon C, Luther JS, Friedman A, Radtke RA. Seizure onset from periventricular nodular heterotopias: depth-electrode study. Neurology. 1998;51:1723–27. doi: 10.1212/wnl.51.6.1723. [DOI] [PubMed] [Google Scholar]
- 145.Huttenlocher PR, Taravath S, Mojtahedi S. Periventricular heterotopia and epilepsy. Neurology. 1994;44:51–55. doi: 10.1212/wnl.44.1.51. [DOI] [PubMed] [Google Scholar]
- 146.Backx L, Fryns JP, Marcelis C, Devriendt K, Vermeesch J, Van Esch H. Haploinsufficiency of the gene Quaking (QKI) isassociated with the 6q terminal deletion syndrome. Am J Med Genet A. 2010;152A:319–26. doi: 10.1002/ajmg.a.33202. [DOI] [PubMed] [Google Scholar]
- 147.Carabalona A, Beguin S, Pallesi-Pocachard E, et al. A glial origin for periventricular nodular heterotopia caused by impaired expression of Filamin-A. Hum Mol Genet. 2012;21:1004–17. doi: 10.1093/hmg/ddr531. [DOI] [PubMed] [Google Scholar]
- 148.Ferland RJ, Batiz LF, Neal J, et al. Disruption of neural progenitorsalong the ventricular and subventricular zones in periventricular heterotopia. Hum Mol Genet. 2009;18:497–516. doi: 10.1093/hmg/ddn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fox JW, Lamperti ED, Eksioglu YZ, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–25. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- 150.Lu J, Tiao G, Folkerth R, Hecht J, Walsh C, Sheen V. Overlapping expression of ARFGEF2 and Filamin A in the neuroependymal lining of the lateral ventricles: insights into the cause of periventricular heterotopia. J Comp Neurol. 2006;494:476–84. doi: 10.1002/cne.20806. [DOI] [PubMed] [Google Scholar]
- 151.Beguin S, Crpel V, Aniksztejn L, et al. An epilepsy-related ARX polyalanine expansion modifies glutamatergic neurons excitability and morphology without affecting GABAergic neurons development. Cereb Cortex. 2013;23:1484–94. doi: 10.1093/cercor/bhs138. [DOI] [PubMed] [Google Scholar]
- 152.Salmi M, Bruneau N, Cillario J, et al. Tubacin prevents neuronal migration defects and epileptic activity caused by rat Srpx2 silencing in utero. Brain. 2013;136:2457–73. doi: 10.1093/brain/awt161. [DOI] [PubMed] [Google Scholar]
- 153.Talos DM, Sun H, Kosaras B, et al. Altered inhibition in tuberous sclerosis and type IIb cortical dysplasia. Ann Neurol. 2012;71:539–51. doi: 10.1002/ana.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.