HISTORICAL BACKGROUND
Over the course of the 20th century, concepts evolved as evidence accumulated concerning the existence and delineation of intrinsic neural systems controlling waking and sleeping. Waking was once thought to be maintained by sensory inputs and sleep to result from the cessation of sensory inputs to the brain (Bremer, 1929; Kleitman, 1939). Yet, from variable alterations of waking and sleeping that occurred with cerebral lesions clinically in humans or experimentally in animals, both waking and sleeping were found to be generated actively by intrinsic neural systems. Based upon analyses of human brains following death from encephalitis lethargica, von Economo (1930) was among the first to propose that waking and sleeping systems were localized in different regions of the forebrain since hypersomnolence was associated with lesions of the posterior hypothalamus whereas insomnia was associated with lesions of the anterior hypothalamus and preoptic area (Figure 9.1). Moruzzi and Magoun (1949) went on to show that the brainstem reticular formation together with the posterior hypothalamus were both necessary and sufficient for the maintenance of a waking state (Figure 9.1).
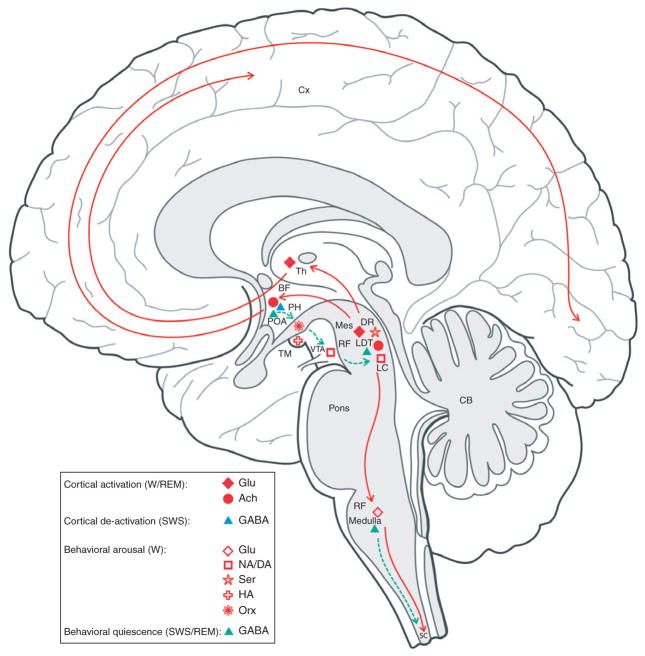
Fig. 9.1.
Sleep–wake state substrates. Sagittal schematic view of the human brain depicting neurons with their chemical neurotransmitters and pathways by which they influence cortical activity or behavior across the sleep–wake cycle. Neurons which are active during waking (red symbols) include cells with ascending projections toward the cortex, which stimulate cortical activation, and cells with descending projections toward the spinal cord, which stimulate behavioral arousal with postural muscle tone. Those with predominantly ascending projections discharge in association with fast, gamma electroencephalogram (EEG) activity and cease firing with slow, delta activity to be active during both wakefulness and rapid eye movement sleep (W/REM, filled red symbols); they include neurons which release glutamate (Glu, diamonds) or acetylcholine (ACh, circles) (see Figures 9.2 and 9.3). Those with more diffuse or descending projections discharge in association with behavioral arousal and electromyogram (EMG) activity and cease firing with muscle atonia to be active during W and silent during REM (W, empty red symbols); they include neurons which release glutamate (Glu, diamonds), noradrenaline (norepinephrine) (NA, square), serotonin (Ser, star), histamine (HA, cross) or orexin (Orx, asterisk) (see Figure 9.4). Neurons which are active during sleep (blue or aqua symbols) include cells with ascending projections toward the cortex, which dampen fast cortical activity, and those with descending projections toward the hypothalamus, brainstem, or spinal cord, which diminish behavioral arousal and muscle tone. Those with projections to the cortex or local area discharge in association with slow EEG activity during slow-wave sleep (SWS, blue triangle; see Figure 9.3); those with descending projections discharge in association with decreasing muscle tone and EMG (SWS/REM, aqua triangles; see Figure 9.3). They include particular GABAergic neurons in the basal forebrain and preoptic area that bear α2-adrenergic receptors and are thereby inhibited by NA. Also shown are GABAergic neurons in the pontomesencephalic tegmentum, which can inhibit local reticular or monoaminergic neurons, and GABAergic neurons (together with glycinergic neurons, not shown) in the ventral medullary reticular formation that project directly to the spinal cord where they can inhibit neck and other motor neurons during sleep. BF, basal forebrain; CB, cerebellum; Cx, cortex; DR, dorsal raphe; GABA, gamma-aminobutyric acid; LC, locus coeruleus nucleus; LDT, laterodorsal tegmental nucleus; Mes, mesencephalon; PH, posterior hypothalamus; POA, preoptic area; RF, reticular formation; SC, spinal cord; Th, thalamus; TM, tuberomammillary nucleus; VTA, ventral tegmental area. (Adapted from Jones (2005).)
Cortical activation and deactivation
In the early studies, it was established that lesions in the rostral pontine and mesencephalic reticular formation, extending into the posterior hypothalamus, resulted in a loss of fast electroencephalographic (EEG) activity typical of cortical activation of the wake state (Lindsley et al., 1950). In contrast, lesions of the ascending sensory pathways or even complete sensory deafferentation did not diminish the amount of cortical activation (Vital-Durand and Michel, 1969). The absence of waking signs in the experimental animals was similar to that in humans diagnosed as comatose and found to have lesions of the rostral brainstem and posterior diencephalon (Plum and Posner, 1980).
Electrical stimulation of the brainstem reticular formation in an anesthetized animal evoked cortical activation which was conducted along two major pathways into the forebrain to reach the cortex (Starzl et al., 1951) (Figure 9.1). The first route was into the thalamus from where impulses were in turn conveyed in a relatively wide-spread manner to the cerebral cortex, particularly the frontal regions. The second route was ventral to the thalamus extending through the hypothalamus up to the basal forebrain from where impulses were also conveyed in a widespread manner to the cortex. This ventral extrathalamic route was found to be sufficient, since cortical activation could still be attained following total ablation of the thalamus.
Neuroanatomical studies revealed that the neurons within the netlike, or reticular, core of the brainstem were characterized by long radiating dendrites which received collateral inputs from multiple sensory modalities and by long branching axons which ascended from the rostral brainstem into the thalamus and/or into the hypothalamus and up to the basal forebrain (Nauta and Kuypers, 1958; Scheibel and Scheibel, 1958). The ascending reticular activating system thus had the capacity to respond to multiple sensory inputs and to transmit in turn impulses to widely distributed areas of the cortex, through neurons in the diffuse thalamocortical projection system and a basalocortical projection system.
Electrical stimulation of the thalamus could elicit widespread cortical activation. However, the effect of the stimulation depended upon its frequency. Whereas high-frequency stimulation elicited fast cortical activity, low-frequency stimulation recruited spindle-like or slow wave-like activity on the cerebral cortex, which resembled the EEG activity of sleep (Akert et al., 1952). Similarly, stimulation in the preoptic area and basal forebrain could activate the cortex, yet depending upon the precise location and frequency, could also elicit slow-wave activity and a state of sleep (Hess, 1957; Sterman and Clemente, 1962a, b). It thus appeared that, within the same regions of the forebrain, different patterns of discharge by the same or different neurons could stimulate either cortical activation and waking or cortical slow-wave activity and sleeping.
Behavioral arousal and quiescence
Neurons of the reticular formation were also seen to send descending projections into the spinal cord (Scheibel and Scheibel, 1958) (Figure 9.1). As evident from electrical stimulation, reticulospinal neurons could stimulate movement and enhance postural muscle tone, recorded on the electromyogram (EMG), as typical of behavioral arousal (Sprague and Chambers, 1954). Yet, depending upon the condition of the animal, such stimulation in the medulla could also inhibit muscle tone, reflexes, and movement (Magoun and Rhines, 1946). Thus presumably different neurons in the brainstem evoked behavioral arousal with postural muscle tone or behavioral quiescence with muscle atonia.
THE RETICULAR ACTIVATING SYSTEM
Forebrain projecting reticular neurons
The neurons of the reticular formation with ascending projections are concentrated in the oral pontine and mesencephalic reticular formation, although they are present in smaller numbers in the caudal pontine and medullary reticular formation (Jones and Yang, 1985). They project rostrally to the midline and intralaminar thalamic nuclei which form the nonspecific thalamocortical projection system that project in turn in a widespread manner to the cerebral cortex (Figure 9.1). The reticular neurons also project through the hypothalamus up to the level of the basal forebrain. In the mesencephalon, they discharge at their highest rate in association with fast cortical activity that occurs during both wakefulness and rapid eye movement (REM) sleep (Steriade et al., 1982). Considerable evidence indicates that neurons of the ascending reticular activating system utilize the neurotransmitter glutamate (Glu) and thus excite through multiple Glu receptors (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), kainate, N-methyl-D-aspartic acid (NMDA) or metabotropic) their target neurons in the thalamus, hypothalamus, and/or basal forebrain (Kaneko et al., 1989, 2002; McCormick, 1992; Jones, 1995).
There are also a small number of GABAergic neurons distributed through the reticular formation which would have the capacity to inhibit other neurons in the region (Figure 9.1). These could correspond to a small number of neurons which actually increase their rate of firing during sleep (Steriade et al., 1982). In studies using c-Fos expression as a reflection of neural activity, a number of GABAergic neurons in the reticular formation did appear to be active during sleep (Maloney et al., 1999, 2000).
Spinal projecting reticular neurons
Neurons through the reticular formation project to the spinal cord, though in greatest numbers from the caudal pontine and medullary fields, and terminate variably in the dorsal horn, intermediate zone or ventral horn (Jones and Yang, 1985). The vast majority of pontine and medullary reticular neurons discharge at their highest rate during waking in association with movements (Siegel et al., 1977, 1979). They decrease or cease firing with slow-wave sleep (SWS). Many fire in association with phasic activity during REM sleep. Considerable evidence indicates that the large, thus presumably reticulospinal neurons of the pontine and medullary reticular formation utilize the neurotransmitter Glu (Kaneko et al., 1989, 2002; Jones, 1995) (Figure 9.1).
A large number of smaller neurons through the reticular formation and a small number of spinally projecting medium-sized neurons in the medullary reticular formation synthesize gamma-aminobutyric acid (GABA) (Jones et al., 1991). In addition, these or other medium-sized reticulospinal neurons utilize the inhibitory neurotransmitter glycine (Fort et al., 1993). Such GABAergic or glycinergic reticular or reticulospinal neurons could exert an inhibitory influence upon other excitatory reticulospinal neurons or brainstem and spinal motor neurons (Holstege and Bongers, 1991). They could represent the small percentage of reticular neurons that increase their discharge rate with quiet waking and sleep, relative to active waking and discharge maximally with muscle atonia during REM sleep (Sakai et al., 1981). They could also correspond to the small number of medullary reticular neurons that discharge in association with loss of muscle tone which occurs in narcolepsy with cataplexy (Siegel et al., 1991). Indeed, many medullary reticular neurons which are GABAergic express c-Fos with REM sleep, as evident during rebound following deprivation (Maloney et al., 1999, 2000). In any event, both excitatory and inhibitory reticulospinal neurons can influence movement and muscle tone such as to stimulate behavioral arousal or reciprocally promote behavioral quiescence and different states (Figure 9.1).
The cholinergic pontomesencephalic neurons
Neurons which utilize acetylcholine (ACh) as a neurotransmitter were proposed to form a major contingent of the reticular formation based upon histochemical staining for its catabolic enzyme, acetylcholinesterase (AChE), by Shute and Lewis (1967) (Figure 9.1). The cholinergic contingent of the activating system was considered by Shute & Lewis to be preeminent, thus leading them to designate the entire system as the “cholinergic reticular activating system.” With application of immunohistochemical staining for the synthetic enzyme choline acetyltransferase (ChAT), the cholinergic neurons were later found to be more limited in their distribution and localized to two major cell groups in the brainstem, the laterodorsal tegmental nucleus and pedunculopontine tegmental nucleus (Mesulam et al., 1983b). Like other neurons of the reticular formation, nonetheless, these cholinergic neurons project forward into the forebrain. They project prominently to the thalamus, including most densely to themedial and lateral geniculate nuclei and the midline and intralaminar nuclei. They can thus excite both specific and non-specific thalamocortical projection systems. Acting through both nicotinic and muscarinic receptors, indeed, ACh depolarizes and excites the thalamic projection neurons and evokes tonic firing by them to stimulate thalamocortical activation and prevent slow-wave activity (McCormick, 1992). The pontomesencephalic cholinergic neurons also project through the extrathalamic ventral ascending pathway into the posterior hypothalamus where they influence wake-promoting neurons (see below) and to a lesser degree up to the basal forebrain (Jones and Cuello, 1989; Ford et al., 1995).
Although cholinergic neurons have not yet been unequivocally identified in the pontomesencephalic tegmentum in recording studies, neurons considered to be “possibly” cholinergic were recorded in the region of those cells in the cat and shown to discharge in association with cortical activation during both wake (W) and REM sleep (W/REM) (El Mansari et al., 1989) (Figure 9.1). In addition, however, some “possibly” cholinergic neurons were found to discharge only during REM sleep and in association with the muscle atonia of that state (El Mansari et al., 1989; Kayama et al., 1992). It is thus possible that particular cholinergic neurons, which project to the pontomedullary reticular formation (Mitani et al., 1988; Jones, 1990; Semba et al., 1990), might generate REM sleep with muscle atonia. Injection of the cholinergic agonist, carbachol, into the pontomesencephalic tegmentum induces cortical activation with muscle atonia and other signs of REM sleep (George et al., 1964; Baghdoyan et al., 1984). Such action might be possible through different effects of ACh upon different neurons mediated by muscarinic type 1 (M1) and 2 (M2) receptors. ACh could inhibit (through M2 receptors) glutamatergic reticular neurons involved in facilitating activity and muscle tonus and excite (through M1 receptors) particular GABAergic neurons involved in inhibiting activity and muscle tone (Figure 9.1).
FOREBRAIN RELAYS OF THE ACTIVATING SYSTEM
The nonspecific thalamocortical projection system
The midline and intralaminar nuclei of the thalamus, unlike the sensory and motor relay nuclei, project to multiple regions of the cerebral cortex in a thus nonspecific manner, often in highest density to frontal regions, though for some nuclei in a truly diffuse manner in high density to all regions (Herkenham, 1986) (Figure 9.1). They discharge at their highest rate in association with cortical activation during waking and REM sleep. Their discharge is generally tonic and relatively fast during these states (Glenn and Steriade, 1982). Indeed, they can attain frequencies in the gamma range (~40 Hz) in association with similar gamma EEG activity, which they may accordingly stimulate (Steriade et al., 1993a). They utilize Glu as a neurotransmitter, as proven recently by their content of vesicular Glu transporter 2 (VGluT2) (Fremeau et al., 2001; Kaneko et al., 2002; Hur and Zaborszky, 2005).
Like other thalamocortical projection neurons, those of the midline and intralaminar nuclei change both their rate and mode of discharge during SWS (Steriade et al., 1993a). Due to intrinsic properties, all thalamic projection neurons have two modes of firing, tonic and bursting, the latter mediated by a calcium low-threshold spike (LTS), which is activated when the neurons are hyperpolarized (Steriade and Llinas, 1988). This hyperpolarization occurs when the thalamic neurons are released from excitatory influences from the brainstem-activating systems. Moreover, the reticular thalamic neurons which surround and innervate the thalamic relay neurons begin first to discharge in bursts when removed from this depolarizing influence. The reticular thalamic neurons utilize GABA as a neurotransmitter and thereby further hyperpolarize the thalamocortical projection neurons in an active and punctual manner. They accordingly entrain the projection neurons in rhythmic bursting, which occurs first at a spindle frequency (12–14 Hz) and then at a delta frequency (1–4 Hz), as well as a slower oscillation (0.1–1 Hz). These patterns of SWS are thus transmitted through thalamo-cortico-thalamic loops as a product of the intrinsic properties of the neurons within those circuits (Steriade et al., 1993b). During these slow patterns, thalamocortical transmission of sensory inputs is virtually blocked and consciousness is lost.
The basalocortical projection system
First identified by immunohistochemical staining for AChE, the innervation of the cerebral cortex by cholinergic fibers from the basal forebrain was originally proposed by Shute and Lewis (1967) to represent the important relay of the brain reticular activating system to the cerebral cortex within the “cholinergic reticular activating system” (Figure 9.1). Moreover, a potent excitatory effect of ACh upon cortical neurons was demonstrated by Krnjevic and Phillis (1963) and proposed to underlie the fast cortical activity that characterized activation. Indeed, pharmacological enhancement of ACh with physostigmine, the AChE inhibitor, or administration of muscarinic or nicotinic agonists stimulated cortical activation with waking (Domino et al., 1968). Blocking muscarinic receptors with atropine led to deactivation of the cortex with predominant slow-wave activity, despite continued behavioral arousal, and thus disassociation between cortical activity and behavior (Longo, 1966). It was also found by Jasper and Tessier (1971) that ACh release from the cerebral cortex was maximal in association with cortical activation during both waking and REM sleep (Celesia and Jasper, 1966). These early studies thus indicated that ACh and cholinergic neurons played an important, if not critical, role in cortical activation which occurs during waking and REM sleep and thus in cortical activation, irrespective of behavioral arousal.
As identified and delineated by ChAT immunohistochemistry, the cholinergic neurons are distributed across the basal forebrain from rostral to caudal in the medial septum (MS), nuclei of the diagonal band of Broca (DBB), magnocellular preoptic nucleus (MCPO), substantia innominata (SI) and globus pallidus (GP), as described in the rat brain (Mesulam et al., 1983b) and corresponding largely to what was originally called the nucleus basalis magnocellularis of Meynert in primates (Mesulam et al., 1983a). Collectively these cell groups provide a rich cholinergic innervation to the hippocampus and paleocortex (predominantly from MS-DBB) and to the entire neocortex (predominantly from MCPO-SI-GP). In the cortex, cholinergic fibers innervate both interneurons and pyramidal cells across all layers (Beaulieu and Somogyi, 1991). ACh exerts excitatory influences upon both cell types, predominantly through muscarinic (M1) receptors and also inhibitory influences upon some interneurons (through M2 receptors) (McCormick, 1993). The influence of the basal forebrain cholinergic neurons upon cortical neurons prevents slow-wave cortical activity and promotes fast cortical activity, particularly in a gamma range (30–60 Hz) (Metherate et al., 1992; Cape and Jones, 2000).
As recently determined by juxtacellular labeling and immunohistochemical identification of recorded neurons in rats, the cholinergic basal forebrain neurons discharge maximally in association with cortical activation during waking and REM sleep or, as it is more appropriately called in rats, paradoxical sleep (PS) (Lee et al., 2005b) (Figure 9.2). Moreover, they fire in high-frequency spike bursts with gamma and theta activity during active, attentive waking and during PS (Figure 9.2, expanded traces). As typical of W/PSactive (or W/REM, as represented in the human brain in Figure 9.1), their discharge is positively correlated with high-frequency gamma EEG activity and negatively correlated with slow delta EEG activity, and it is not correlated with EMG amplitude (Figure 9.3). In contrast to thalamocortical neurons of the nonspecific thalamocortical projection system, the cholinergic cells cease firing prior to and during SWS. Since ACh stimulates cortical activation, the cessation of discharge by the cholinergic cells is likely a determinant in the natural onset of SWS, including spindle, delta, and slow oscillations in the cortex.
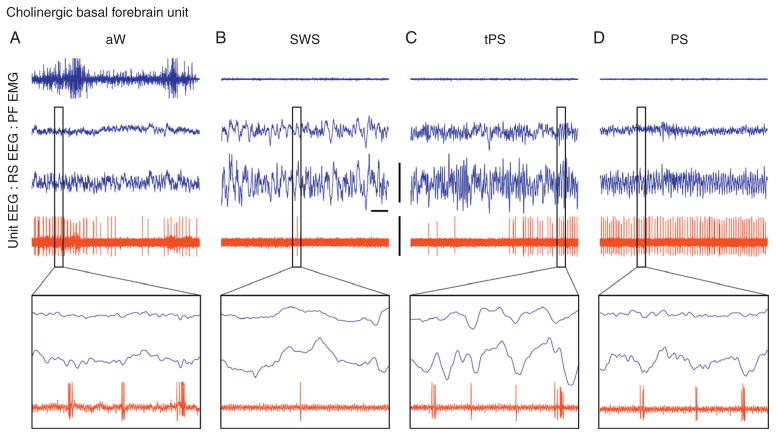
Fig. 9.2.
Discharge of a cholinergic basal forebrain neuron across sleep–wake states. Record of a neuron labeled by juxtacellular technique with Neurobiotin (Nb) and identified by immunohistochemistry for choline acetyltransferase (ChAT) as cholinergic in the magnocellular preoptic nucleus (MCPO) of the rat. As evident in 10-second traces (above), the unit fired during aW, virtually ceased firing during SWS, resumed firing during tPS, and discharged maximally during PS. As evident in expanded 0.5-second traces (below), the unit discharged in rhythmic bursts of spikes with theta EEG activity that was present intermittently during periods of aW, toward the end of tPS, and continuously during PS. aW, active wake; EMG, electromyogram; EEG, electroencephalogram; PF, prefrontal cortex; RS, retrosplenial cortex; SWS, slow-wave sleep; tPS, transition to paradoxical sleep; PS, paradoxical sleep. Bar for horizontal scale: 1 second. Bar for vertical scales: 1 mV for EEG/EMG and 1.5 mV for unit. (Reprinted with permission from Lee et al. (2005b).)
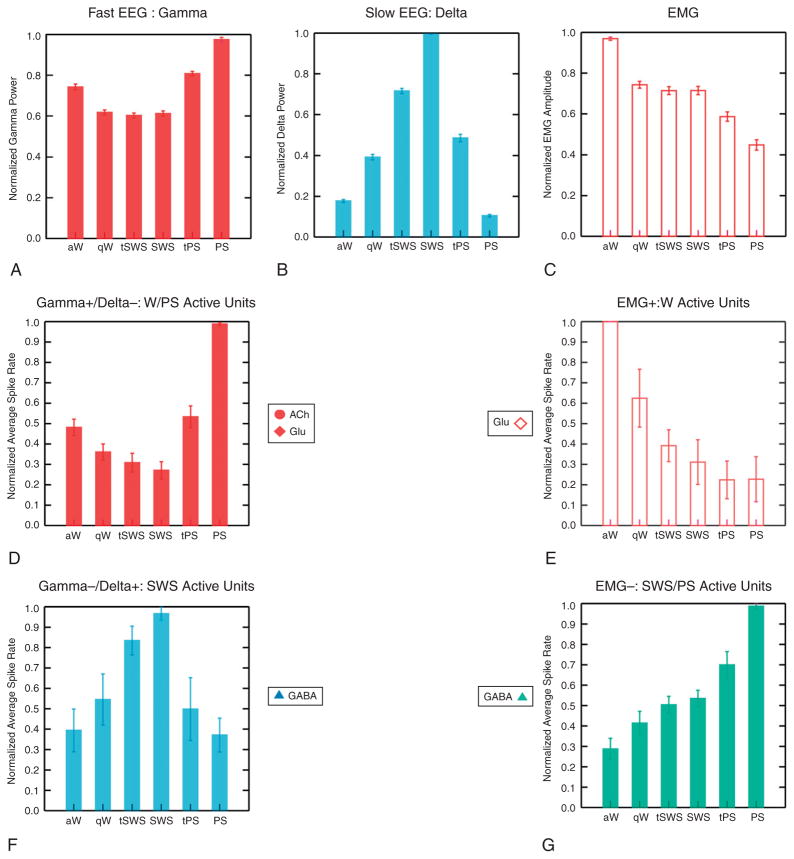
Fig. 9.3.
Sleep–wake-related electroencephalogram (EEG)/electromyogram (EMG) and unit activity of basal forebrain neurons in the rat. Normalized average gamma power (A), delta power (B), and EMG amplitude (C) across all sleep–wake stages in the rat. (D–G) Normalized average unit spike rate for basal forebrain cell groups. In D, waking (W)/paradoxical sleep (PS)-active cells, whose discharge is positively correlated with gamma EEG activity and negatively correlated with delta EEG activity (including putative cholinergic cells represented in Figure 9.1 in the human brain as W/REM cells, circles). E shows W-active cells whose discharge is positively correlated with EMG amplitude and which fire maximally during W (including putative glutamatergic cells represented in Figure 9.1, diamonds). F shows SWS-active cells whose discharge is negatively correlated with gamma and positively correlated with delta EEG activity and which fire maximally during SWS (including putative GABAergic neurons represented in Figure 9.1, triangles). G shows SWS/PS-active cells whose discharge is negatively correlated with EMG amplitude and which fire at progressively higher rates during SWS through PS (including putative GABAergic neurons represented in Figure 9.1 as SWS/REM cells, triangles). aW, active wake; qW, quiet wake; tSWS, transition to slow-wave sleep; SWS, slow-wave sleep; tPS, transition to paradoxical sleep; PS, paradoxical sleep. (Reprinted with permission from Jones, (2005).)
In addition to cholinergic neurons, other neurons, including glutamatergic and GABAergic neurons, are distributed through the basal forebrain and give rise to cortical, local, or descending projections (Jones, 2004, 2005; Henny and Jones, 2008). These noncholinergic cells are heterogeneous in their response to different neurotransmitters, in their activity profile across sleep–wake states, and in their role in modulating cortical activity and sleep–wake states, as will be elaborated below. Some presumed glutamatergic neurons, likely having cortical projections, discharge like the cholinergic basalocortical projection neurons, maximally in association with cortical activation during waking and REM sleep (Figure 9.3). Other presumed glutamatergic neurons, likely having descending projections, discharge maximally with behavioral arousal during waking. Some GABAergic neurons discharge also in parallel with the cholinergic cells; yet another important contingent discharges in an inverse manner to the cholinergic and presumed glutamatergic neurons and could thus promote sleep (see below) (Figure 9.3).
DIFFUSELY PROJECTING AROUSAL SYSTEMS
The reticular formation and cholinergic pontomesencephalic tegmental neurons have the capacity to influence widespread areas of the forebrain and cortex through their projections to the major subcortical relay stations. Some also give rise to branching axons with descending as well as ascending projections of some distance, thus allowing simultaneous influence upon forebrain and spinal cord systems (Jones and Yang, 1985). It is thus likely that some neurons of the reticular formation can simultaneously stimulate cortical activation and behavioral arousal with enhanced muscle tone and/or motor activity. In the case of the cholinergic neurons, some may actually stimulate cortical activation while dampening behavioral arousal and diminishing muscle tone in the generation of REM sleep. Such widespread influence can thus determine the state of the brain and organism.
Following the development of histofluorescent techniques in the 1960s, other cell groups were revealed within the brainstem which contained monoamines and which gave rise to highly diffuse projections through the entire central nervous system (Dahlstrom and Fuxe, 1964; Ungerstedt, 1971b). Moreover, they acted as neuromodulators able to influence the activity of other neurons or actions of other neurotransmitters on those neurons in a relatively subtle, slow, and prolonged manner. As proposed by Jouvet (1969), the monoamines and their neural systems appeared ideally suited to influence – if not determine – sleep–wake states. Most notable of these, the locus coeruleus nucleus neurons were found to contain noradrenaline (NA) (norepinephrine) and to give rise to varicose axons which branched and sent collaterals through the entire nervous system, such as potentially to permit from one neuron the simultaneous release of NA throughout the brain and spinal cord (Jones and Moore, 1977; Jones and Yang, 1985). Indeed, this small cluster of neurons in the brain resembles a central sympathetic ganglion, sending fibers to broad regions and releasing NA from the varicosities along its axons to influence its multiple target cells in a nonsynaptic manner (Descarries et al., 1977). The locus coeruleus noradrenergic neurons thus appeared to represent an ideal substrate stimulating arousal.
Early pharmacological studies had indicated a potent influence of the catecholamines, NA and dopamine (DA), in stimulating waking with behavioral arousal (Jouvet, 1972). Amphetamine, which releases NA and DA, evoked a prolonged waking state characterized by fast cortical activity and pronounced behavioral arousal. Depletion of NA and DA by inhibition of catecholamine synthesis (with α-methyl-para-tyrosine, AMPT) resulted in decreases in waking and increases in sleep.
Noradrenergic locus coeruleus neurons
The locus coeruleus neurons project along the same ascending pathways as the neurons of the reticular formation; however, while innervating the relay stations in the thalamus, hypothalamus and basal forebrain, they send axons further along to innervate the entire cerebral cortex directly (Jones and Yang, 1985) (Figure 9.1). Other neurons send axons through the brainstem to innervate neurons therein, yet extend their fibers into the entire spinal cord, and a certain number innervate through bifurcating axons both the forebrain and the spinal cord. Through these regions, NA exerts different effects upon different neurons through different receptors. In the thalamus, NA serves mainly to depolarize and excite both specific and nonspecific thalamocortical projection neurons by acting primarily upon α1-adrenergic receptors and thus stimulating fast tonic discharge and preventing slow bursting discharge of the thalamic neurons to promote cortical activation (McCormick, 1992). In the posterior hypothalamus, NA also excites wake-promoting neurons (Bayer et al., 2005) (see below). In the basal forebrain, the cholinergic neurons are similarly excited by NA through α1-adrenergic receptors (Fort et al., 1995). NA also excites motor systems and exerts a direct excitatory influence upon motor neurons in the spinal cord (Sqalli-Houssaini and Cazalets, 2000). Indeed, the excitatory influence of NA upon motor neurons and their activity is also evident in brainstem motor neurons as an important tonic influence that determines their activity and tonus during waking (Fenik et al., 2005). It is notable that NA inhibits certain neurons through α2-adrenergic receptors; indeed, sleep-promoting neurons in the forebrain appear to be inhibited by NA (see below). According to their projections and the effects of NA released by their diffusely projecting fibers, the locus coeruleus noradrenergic neurons thus have the capacity simultaneously to stimulate cortical activation and behavioral arousal of waking and to prevent sleep.
As established many years ago without the need to identify recorded neurons as NA-containing in the locus coeruleus, given the very compact and homogeneous aggregation of these cells in the rat brain, locus coeruleus noradrenergic neurons discharge selectively during waking, diminish firing during SWS, and cease firing altogether during PS (Aston-Jones and Bloom, 1981) as W-active cells (W, Figure. 9.1). Their discharge during waking is maximal in response to sensory stimuli and situations that are associated with high behavioral arousal, stress, and activation of the peripheral sympathetic nervous system (Jacobs et al., 1991). Their discharge would thus be associated with behavioral arousal and incompatible with SWS and PS. Indeed, as was formally proposed by McCarley and Hobson (1975) many years ago, locus coeruleus noradrenergic neurons could prevent the occurrence of PS through an inhibitory influence upon cholinergic PS promoting neurons in the pontomesencephalic tegmentum. They can also prevent the muscle atonia of PS by their excitatory influence upon motor neurons (Fenik et al., 2005).
Dopaminergic mesencephalic neurons
The DA-containing neurons are located in the mesencephalic tegmentum concentrated within the substantia nigra and ventral tegmental area. Although the dopaminergic neurons do not project in the diffuse manner of the noradrenergic neurons, they nonetheless reach broad areas of the forebrain, particularly the dorsal striatum from the substantia nigra and the ventral striatum and cortex from the ventral tegmental area (Moore and Bloom, 1979). They also project to the thalamus (Sanchez-Gonzalez et al., 2005) and on to cholinergic basal forebrain neurons (Jones and Cuello, 1989; Gaykema and Zaborszky, 1996), similar to noradrenergic neurons. They influence target neurons in differing manners through D1 or D2 receptors. From early lesion studies, DA neurons appeared to influence behavioral arousal more than cortical activity, since their destruction in animals resulted in akinesia with little change in cortical activation, as in Parkinson patients (Ungerstedt, 1971a; Jones et al., 1973). Yet, evidence subsequently indicated that these neurons can also facilitate cortical activation by enhancing gamma EEG activity along with attentive behavior (Montaron et al., 1982).
Recordings from identified DA-containing neurons have not yet been realized in naturally sleeping–waking animals. Early studies described the activity of possibly dopaminergic neurons, which particularly in the ventral tegmental area are intermingled with a vast majority of nondopaminergic neurons, across the sleep–waking cycle, and concluded that they did not change their average firing rate across this cycle (Miller et al., 1983), a very surprising finding. On the other hand, studies employing c-Fos as an indicator of activity presented evidence that dopaminergic neurons of the ventral tegmental area are more active during waking and REM sleep (W/REM) than SWS (Maloney et al., 2002). A study employing electrophysiological properties as a marker for dopaminergic neurons found that possibly DA-containing neurons of the ventral tegmental area discharged in bursts of spikes during aroused waking and during PS (Dahan et al., 2007). It is thus possible that dopaminergic neurons are more similar to cholinergic neurons, from which they receive input and to which they project, and thereby discharge maximally during both W and PS. Considering the important role of DA in the limbic system, such activity could mediate the emotive aspects of particular waking and dream states.
Given, however, that the drugs employed in the prevention of hypersomnolence including narcolepsy with cataplexy act prominently upon both NA and DA release (Wisor et al., 2001), it is currently not clear whether dopaminergic neurons in the ventral mesencephalon (or perhaps diencephalon) function to promote behavioral arousal and prevent sleep-like noradrenergic neurons (Figure 9.1) or might be active during REM sleep to stimulate cortical activation without stimulating behavioral arousal. In any event, it is likely that the enhanced release of both NA and DA is important for the antinarcoleptic action of amphetamines and modafinil (Lin et al., 1992).
Serotonergic raphe neurons
The influence of serotonin (Ser, also 5-hydroxytryptamine, 5-HT) upon EEG activity and sleep–wake states is different from that of the catecholamines. Indeed, it is so different that the early pharmacological and lesion studies indicated to Jouvet (1972) that the serotonergic raphe neurons generated SWS. Inhibition of Ser synthesis (with para-chlorophenylalanine, PCPA) and lesions of serotonergic raphe neurons both produced insomnia. Yet, upon recording from possibly serotonergic raphe neurons, it was surprisingly discovered that the presumed serotonergic cells discharged during waking, diminished firing during SWS, and ceased firing during REM sleep (McGinty and Harper, 1976). They are, thus, like noradrenergic neurons, W-active cells (W, Figure 9.1). In contrast to noradrenergic locus coeruleus neurons, however, the presumed serotonergic raphe neurons do not discharge during response and orientation to sensory stimuli and do not fire under conditions of physiological stress when the sympathetic nervous system is activated (Jacobs and Fornal, 1999). They do fire during motor activity and particularly during rhythmic motor patterns, such as grooming or locomotion. Ser is known to facilitate locomotor activity and directly excite motor neurons, particularly through 5-HT2 receptors (Barbeau and Rossignol, 1990; Kjaerulff and Kiehn, 2001). In that serotonergic raphe neurons can also attenuate sensory inputs (Fields and Basbaum, 1978), it is possible that their activity prevents sensory inputs from disrupting rhythmic motor activity during locomotion or grooming (Jacobs and Fornal, 1999). The major serotonergic projections into the spinal cord dorsal and ventral horns derive from medullary raphe nuclei (magnus, pallidus, and obscurus). Serotonergic neurons also project into the forebrain (particularly from the dorsal and central superior raphe nuclei) along the major ascending brainstem pathways, and like the noradrenergic neurons, also beyond to reach directly the cerebral cortex. Ser can inhibit many thalamic neurons, including the intralaminar nuclei, through 5-HT1 receptors, though exciting others through 5-HT2 receptors (Monckton and McCormick, 2002). Ser inhibits cholinergic basal forebrain neurons through 5-HT1 receptors and thereby diminishes gamma EEG activity (Khateb et al., 1993; Cape and Jones, 1998). Serotonergic raphe neurons would thus promote waking and behavioral arousal along with rhythmic motor activity but not cortical activation with sensory responsiveness, which would be attenuated. Promotion of such rhythmic pattern generation that would underlie behaviors such as grooming might favor a more relaxed waking state from which sleep would follow more easily than from a highly attentive state. Ser does nonetheless facilitate muscle tone and antagonize the hyperpolarization of motor neurons that occurs with the muscle atonia of REM sleep (Kubin et al., 1992; Fenik et al., 2005). It can also prevent REM sleep initiation by inhibiting cholinergic pontomesencephalic neurons (Luebke et al., 1992).
Histaminergic tuberomammilary neurons
Histamine (HA) was long thought to have a wakepromoting influence since antihistaminergic drugs, used for the treatment of allergies, were associated with somnolence (Lin et al., 1988; Schwartz et al., 1991). It was subsequently discovered that, like the noradrenergic locus coeruleus neurons, the histaminergic neurons give rise to a highly diffuse innervation of the brain and spinal cord. The histaminergic neurons are also relatively tightly clustered in the posterior hypothalamus concentrated in the tuberomammillary nucleus (Figure 9.1). They excite target neurons in the brain, including thalamocortical projection neurons, cholinergic basal forebrain neurons, and cortical neurons predominantly through H1 receptors, by which HA also appears to stimulate fast cortical activity (McCormick, 1992; Reiner and Kamondi, 1994; Khateb et al., 1995).
Recently identified histaminergic neurons have been recorded in the tuberomammillary nucleus of the mouse across natural sleep–waking states (Takahashi et al., 2006). Like other monoaminergic neurons, these cells discharge during waking and cease firing during sleep as W-active, or even wake-specific, cells supposedly not discharging at all during SWS or PS (W, Figure 9.1). Their discharge was particularly elevated during attentive waking, more so than during waking with movement. They would appear to differ in this way from both the noradrenergic and serotonergic neurons and have been postulated to play a particularly important role in attention. Such a role was supported by a diminished arousal response to novel stimuli seen in mice with knockout of the gene for histidine decarboxylase, the synthetic enzyme for HA (Parmentier et al., 2002). It is also noteworthy that in narcoleptic dogs, presumed histaminergic neurons continued to fire, in contrast to noradrenergic locus coeruleus neurons, during episodes of cataplexy (John et al., 2004). Such discharge seemingly did not affect the immobility or muscle atonia of the abnormal state and could be partly responsible for the state of alertness and conscious awareness that can persist during cataplectic episodes in dogs and humans. Histaminergic neurons are nonetheless normally active during attentive and aroused waking when, as W-active cells, they would promote cortical activation and attention.
Early lesion studies, employing particularly large electrolytic or thermolytic lesions, of each of the activating or arousal systems, including the reticular formation with the posterior hypothalamus (Lindsley et al., 1950) and the catecholaminergic neurons (Jones et al., 1973), revealed major deficits or elimination of cortical activation, behavioral arousal and the waking state in experimental animals, corroborating observations in human cases of coma following large brainstem lesions (Plum and Posner, 1980; Parvizi and Damasio, 2003). Yet, when using more refined techniques for performing lesions and particularly using neurotoxins selective for cell bodies and neurons containing particular neurotransmitters or bearing particular receptors, no long-lasting deficits in waking or cortical activation were apparent (Jones et al., 1977; Webster and Jones, 1988; Denoyer et al., 1991; Holmes and Jones, 1994; Blanco-Centurion et al., 2004, 2006, 2007). These results, emerging over many years now, have indicated that no one neural system or neurotransmitter is critical for generating a waking state, although any one might be sufficient. The activating and arousal systems are thus multiple, parallel, and partially redundant; although, as discussed above, each plays a slightly different role and is invoked by a slightly different condition. On the other hand, it was quite surprising to learn in recent years that one particular peptide, its receptors and the neurons that release it, appeared to be critical for the maintenance of waking and behavioral arousal, since in its absence narcolepsy with cataplexy occurs (Chemelli et al., 1999; Lin et al., 1999; Peyron et al., 2000; Thannickal et al., 2000). This peptide is orexin (Orx, also called hypocretin).
Orexinergic posterior hypothalamic neurons
In the 1990s, two groups simultaneously discovered a new set of peptides in the hypothalamus, Sakurai and his colleagues (1998) called them orexins (Orx A and B), meaning peptides that would stimulate appetite and eating; de Lecea and his colleagues (1998) called them hypocretins (Hcrt 1 and 2), meaning peptides that are contained in hypothalamic neurons and have similarities with the gut hormone, secretin. Just 1 year later, it was discovered by Yanagisawa and his collaborators that knockout of the gene for Orx in mice resulted in narcolepsy and cataplexy (Chemelli et al., 1999) and by Mignot and his collaborators (2002) that it was the gene for the Hrct 2 (Orx 2) receptor that was lacking in dogs with narcolepsy-cataplexy (Lin et al., 1999). Indeed, humans having suffered from narcolepsy with cataplexy were subsequently found to have mutations in Orx (Hcrt) genes, low levels of Orx (Hcrt) in cerebrospinal fluid and/or loss of Orx-containing neurons in the hypothalamus (Peyron et al., 2000; Thannickal et al., 2000). Clearly, orexinergic hypothalamic neurons play a critical role in maintaining waking.
The Orx neurons are located in the posterior portion of the hypothalamus, where they are distributed across the lateral hypothalamus, perifornical area, and dorsomedial nucleus (Peyron et al., 1998) (Figure 9.1). Like the noradrenergic locus coeruleus neurons, they give rise to highly diffuse projections extending through the forebrain to reach the subcortical relays of the activating systems in the thalamus and basal forebrain and to continue up to the cerebral cortex. They also project through the hypothalamus, the brainstem, and into the spinal cord. According to orexin’s effects following intracerebroventricular administration and to the effects of elimination of Orx or Orx neurons in knockout mice, the orexinergic system facilitates cortical activation and arousal, stimulates the hypothalamopituitary-adrenal and hypothalamo-pituitary-thyroid axis and excites both sympathetic and motor systems (Lubkin and Stricker-Krongrad, 1998; Shirasaka et al., 1999; Hara et al., 2001; Espana et al., 2002; Yamanaka et al., 2003). Orx neurons thus stimulate arousal while activating neuroendocrine, sympathetic, and motor systems to support and sustain activity through the physiological changes associated with increased energy metabolism. This influence occurs through the excitatory action of Orx on Orx-1 or Orx-2 receptors upon multiple neurons, including cortical neurons, midline thalamocortical projection neurons, cholinergic basal forebrain neurons, histaminergic neurons, cholinergic pontomesencephalic neurons, noradrenergic locus coeruleus neurons, and motor neurons (Horvath et al., 1999; Bayer et al., 2001, 2004; Eggermann et al., 2001; Burlet et al., 2002; Yamuy et al., 2004). Interestingly, no inhibitory actions of Orx have been found, even upon the sleep-promoting neurons which are inhibited by NA (see below) (Bayer et al., 2002). The Orx neurons can thus play a central role in stimulating arousal by exciting all other arousal systems while activating neuroendocrine, sympathetic, and motor systems that support arousal and activity.
Studies utilizing c-Fos expression or release of Orx indicated that Orx neurons are active and release their peptide in association with waking and arousal during the active period of the day (Kiyashchenko et al., 2002; Zeitzer et al., 2003). Yet, it remained uncertain whether they became silent during sleep and particularly REM sleep until recording from identified Orx neurons was achieved by juxtacellular labeling in the rat (Lee et al., 2005a; Mileykovskiy et al., 2005). Orx neurons were thus found to discharge during waking and virtually cease firing during SWS and PS (Figure 9.4). Their discharge occurred during active waking and was correlated with postural muscle tone recorded on the nuccal EMG (Figure 9.4, expanded traces). The Orx neurons were thus like other neurons whose discharge was positively correlated with EMG (Figure 9.3) and whose profile could be typified as W-on and PS-off (W, Figure 9.1). In their case, however, in contrast to other known cell groups, including the noradrenergic locus coeruleus neurons, their discharge is necessary to maintain active waking, since it is in their absence that narcolepsy with cataplexy occurs.
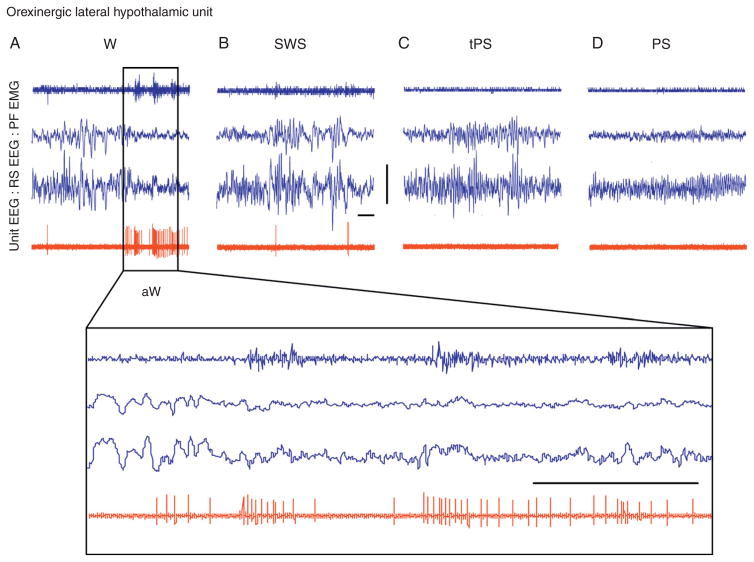
Fig. 9.4.
Discharge of an Orx neuron across sleep–wake states. Record of a neuron labeled by juxtacellular technique with Neurobiotin (Nb) and identified by immunohistochemistry for Orx in the rat. As evident in 10-second traces (above), the unit fired during wakefulness (A) and was virtually silent during slow-wave sleep (B), transition to paradoxical sleep (C), and paradoxical sleep (D). As evident in an expanded trace (of approximately 4 seconds, below), the unit discharged during active wake (aW) and increased firing phasically in association with increases in muscle tone seen on the EMG. aW, active wake; EEG, electroencephalogram; EMG. electromyogram; PF, prefrontal cortex; PS, paradoxical sleep; RS, retrosplenial cortex; SWS, slow-wave sleep; tPS, transition to paradoxical sleep; W, wake.
Horizontal scale bars: 1 second. Vertical scale bar: 1 mV for EEG, 0.5 mV for EMG, and 2 mV for unit. (Reprinted with permission from Lee et al. (2005a).
Since cataplexy is often elicited by an emotional stimulus or also in animals by food, it appears to be triggered by activation of systems which act in an opposite manner to the Orx neurons during those conditions. The cholinergic neurons, which are active during both waking and REM sleep, and ACh or its agonists, which can evoke cortical activation with muscle atonia or a REM sleep-like state, could exert this opposing influence. Indeed, this influence could be exerted from both the basal forebrain and brainstem to result in cortical activation associated with a loss of muscle tonus (Reid et al., 1994a, b; Nishino et al., 1995; Cape et al., 2000) during conditions when orexin release is absent, such as during natural REM sleep or narcoleptic attacks occurring in the absence of orexinergic transmission, which would otherwise override the cholinergic influence to excite motor and sympathetic systems.
SLEEP-PROMOTING SYSTEMS
Although it is clear that thalamic neurons play an important role in shaping the activity of the cortex across waking and sleeping, their influence depends upon their pattern of discharge, which is tonic and fast during waking and becomes bursting and slow during sleep. In contrast, there are neurons in the forebrain and brainstem which are selectively active during sleep and thus appear to play a specific role in promoting sleep.
Preoptic region and basal forebrain
From early studies involving lesions or stimulation, the preoptic region and basal forebrain were known to have the capacity to exert a sleep-promoting influence. Early lesions produced insomnia (McGinty and Sterman, 1968). Stimulation produced a predominance of parasympathetic responses, including decreased heart rate, blood pressure, respiration, and temperature along with decreased activity (Hess, 1957) and sleep (Sterman and Clemente, 1962a, b).
Single-unit recording studies revealed neurons in the preoptic area and basal forebrain that discharged maximally during sleep (Szymusiak and McGinty, 1986; Alam et al., 1996; Szymusiak et al., 1998). In both these regions, however, such cells are intermingled with cells which discharge maximally during waking and PS in association with cortical activation (above) or less commonly during waking alone (above) (Koyama and Hayaishi, 1994; Lee et al., 2005b). Sleepactive neurons are of two types, one which discharges in association with cortical slow-wave, delta activity during SWS and another which discharges in association with progressively decreasing muscle tonus during SWS and PS (Figure 9.3). The SWS cell group could influence cortical deactivation or slow-wave activity by ascending projections to the cortex or local projections on to basalocortical cholinergic neurons. The SWS/PS cell group could influence muscle tone and behavioral quiescence by descending projections to the posterior hypothalamus and brainstem (Figure 9.1).
GABAergic neurons
Sleep-active neurons have also been revealed by c-Fos expression during sleep recovery following deprivation (Sherin et al., 1996). By this technique, the majority of sleep-active cells in the preoptic area and basal forebrain have been found to contain the synthetic enzyme for GABA (glutamic acid decarboxylase, GAD) (Gong et al., 2004; Modirrousta et al., 2004). Yet, many GABAergic neurons are active during waking and cortical activation, as evident from both c-Fos and juxtacellular recording studies (Manns et al., 2000; Modirrousta et al., 2004). The sleep-active GABAergic cells must then be different in other ways from the Wactive or W/PS-active GABAergic cells.
From in vitro pharmacological studies performed first in the basal forebrain and then in the ventrolateral preoptic area (VLPO), it was discovered that, whereas cholinergic neurons were depolarized and excited by NA through α1-adrenergic receptors, a small contingent of cells, which were identified as GABAergic in the VLPO, were hyperpolarized and inhibited by NA through α2-adrenergic receptors (Fort et al., 1995, 1998; Gallopin et al., 2000). Moreover, following juxtacellular recording and labeling of neurons that discharge maximally with slow-wave activity, it was found that a large proportion of these were GABAergic and that these particular GABAergic cells bear α2-adrenergic receptors (Manns et al., 2003).
An important contingent of sleep-promoting neurons would thus be composed of GABAergic neurons in the basal forebrain and preoptic area which are inhibited by NA and would thus be disinhibited when NA release declines as locus coeruleus neurons cease discharge with decreasing arousal (Jones, 2005). Reciprocally, by releasing GABA, the sleep-promoting neurons can inhibit cortical or subcortical systems promoting cortical activation or behavioral arousal, including noradrenergic, histaminergic, and orexinergic neurons (Sherin et al., 1998; Steininger et al., 2001; Henny and Jones, 2006).
It should also be mentioned that there are GABAergic neurons through the hypothalamus and brainstem, which can also function to inhibit arousal-promoting neurons. Notably, GABAergic neurons in the pontomesencephalic tegmentum and medulla appear to be active with sleep and PS recovery during which they may inhibit local monoaminergic cells, other reticular neurons, or motor neurons (Maloney et al., 1999, 2000; Fenik et al., 2005) (see above) (Figure 9.1). Glycinergic neurons also participate in this process (Chase et al., 1989; Boissard et al., 2002), and both GABA and glycine are released in high concentrations in the region of brainstem and spinal cord motor neurons during muscle atonia (Kodama et al., 2003).
In addition, the neurons of the thalamic reticular nucleus which shape spindling activity utilize GABA through which they hyperpolarize and pace the relay neurons to induce bursting in thalamo-cortico-thalamic circuits (Steriade et al., 1994) (see above).
GABA and hypnotic drugs
Given the important role of GABA in inhibiting neurons of the arousal systems and thus in promoting sleep, it is not surprising that many hypnotic drugs as well as anesthetics act as GABA agonists (Lancel, 1999; Gottesmann, 2002; Mignot et al., 2002; Rudolph and Antkowiak, 2004; Mendelson, 2005). Such drugs act upon the benzodiazepine site of the GABAA receptor (linked to chloride channels) to enhance and prolong GABA’s action or directly upon the GABAA receptor to mimic its action and promote respectively spindling activity or slow-wave activity along with sleep. Some drugs act upon GABAB receptors (linked to potassium channels) and promote slow-wave activity along with minimal muscle tone with sleep.
SUMMARY
Waking and sleeping are actively generated by neuronal systems distributed through the brainstem and forebrain with different projections, discharge patterns, neurotransmitters, and receptors. Specific ascending systems stimulate cortical activation, characterized by fast, particularly gamma, activity which occurs during waking and REM sleep. In addition to glutamatergic neurons of the reticular formation and thalamus, cholinergic pontomesencephalic and basal forebrain neurons are integral components of the ascending activating system. Discharging during W and REM sleep, cholinergic neurons stimulate cortical activation in the presence or absence of postural muscle tone and behavioral arousal. Comprised by glutamatergic reticulospinal and other neurons, specific descending systems stimulate behavioral arousal, characterized by postural muscle tone along with motor activity during waking.
Diffusely projecting systems give rise to both ascending and descending projections and thus simultaneously facilitate both cortical activation and behavioral arousal. These include the neurons containing the modulatory neurotransmitters NA, DA, Ser, HA, and Orx. Commonly discharging during waking and ceasing to discharge during SWS and REM sleep, these systems excite through particular receptors other neurons of the activating and arousal systems to promote waking and prevent sleep. Largely parallel in their projections and actions, they are partially redundant and thus not individually necessary for the generation of waking and arousal. On the other hand, Orx is necessary for the maintenance of waking since in its absence narcolepsy with cataplexy occurs in humans and animals. These neurons excite all other activating and arousal systems along with neuroendocrine, sympathetic, and motor systems to support activity, arousal, and muscle tone.
Sleeping is initiated by inhibition of the activating and arousal systems. This inhibition is effected at multiple levels through particular GABAergic neurons which become active during sleep. Neurons in the preoptic area and basal forebrain play a particularly important role in this process. Some become active during SWS, promoting deactivation and slow-wave activity in the cerebral cortex. Others discharge at progressively increasing rates during SWS and REM sleep, promoting behavioral quiescence and diminishing muscle tone. Through their projections and inhibitory neurotransmitter, they have the capacity to inhibit the monoaminergic neurons and Orx neurons in the brainstem and hypothalamus. They are in turn inhibited by NA through α2-adrenergic receptors. During REM sleep, cholinergic systems become active and stimulate cortical activation, while the monoaminergic and orexinergic systems are inhibited, leaving motor and other neurons devoid of their excitatory influence. The selective inhibition of these systems and additional direct inhibition of motor neurons by GABA (and glycine) produces a loss of behavioral responsiveness and postural muscle tone, despite maintained activation of the cerebral cortex, which characterizes this “paradoxical” state of sleep as well as its pathological manifestation in narcolepsy with cataplexy.
Acknowledgments
Most of the recent research of the author presented in this article was funded by grants from the Canadian Institutes of Health Research and US National Institutes of Health and performed at the Montreal Neurological Institute by Maan Gee Lee, Ian Manns, Oum Hassani, Mandana Modirrousta, Pablo Henny, Frederic Brischoux, and Lynda Mainville, to whom I am most grateful. I am also thankful to my collaborators Michel Muhlethaler and colleagues at the Centre Médicale Universitaire in Geneva, whose work is also mentioned. I also thank Napoleon Soberanis for his assistance with the schematic figures.
References
- Akert K, Koella WP, Hess RJ. Sleep produced by electrical stimulation of the thalamus. Am J Physiol. 1952;168:260–267. doi: 10.1152/ajplegacy.1951.168.1.260. [DOI] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in wakefulness and non rapid eye movement sleep. Brain Res. 1996;718(1–2):76–82. doi: 10.1016/0006-8993(96)00035-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, et al. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514(1):55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, et al. Orexins (hypocretins) directly excite tuberomammillary neurones. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Saint-Mleux B, et al. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22(18):7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer L, Serafin M, Eggermann E, et al. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J Neurosci. 2004;24(30):6760–6764. doi: 10.1523/JNEUROSCI.1783-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, et al. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130(4):807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Somogyi P. Enrichment of cholinergic synaptic terminals on GABAergic neurons and coexistence of immunoreactive GABA and choline acetyltransferase in the same synaptic terminals in the striate cortex of the cat. J Comp Neurol. 1991;304:666–680. doi: 10.1002/cne.903040412. [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, et al. Effects of hypocretin2-saporin and antidopamine-beta-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone. Eur J Neurosci. 2004;19(10):2741–2752. doi: 10.1111/j.1460-9568.2004.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C, Xu M, Murillo-Rodriguez E, et al. Adenosine and sleep homeostasis in the basal forebrain. J Neurosci. 2006;26(31):8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27(51):14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, et al. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16(10):1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Bremer F. Cerveau ‘isolé’ et physiologie du sommeil. C R Soc Biol (Paris) 1929;102:1235–1241. [Google Scholar]
- Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22(7):2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Differential modulation of high frequency gamma electroencephalogram activity and sleep–wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep–wake state. Eur J Neurosci. 2000;12:2166–2184. doi: 10.1046/j.1460-9568.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- Cape EG, Manns ID, Alonso A, et al. Neurotensininduced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. J Neurosci. 2000;20:8452–8461. doi: 10.1523/JNEUROSCI.20-22-08452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16:1053–1064. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, et al. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32(6):1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62(Suppl 232):1–55. [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyer M, Sallanon M, Buda C, et al. Neurotoxic lesion of the mesencephalic reticular formation and/or the posterior hypothalamus does not alter waking in the cat. Brain Res. 1991;539:287–303. doi: 10.1016/0006-8993(91)91633-c. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Lapierre Y. Noradrenergic axon terminals in the cerebral cortex of rat. III. Topometric ultrastructural analysis. Brain Res. 1977;133:197–222. doi: 10.1016/0006-8993(77)90759-4. [DOI] [PubMed] [Google Scholar]
- Domino EF, Yamamoto K, Dren AT. Role of cholinergic mechanisms in states of wakefulness and sleep. Progr Brain Res. 1968;28:113–133. doi: 10.1016/S0079-6123(08)64547-1. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sakai M, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep–waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- Espana RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943(2):224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005;14(4):419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Brain stem control of spinal pain transmission neurons. Ann Rev Physiol. 1978;40:193–221. doi: 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes C, Mainville L, et al. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Fort P, Luppi PH, Jouvet M. Glycine-immunoreactive neurones in the cat brain stem reticular formation. Neuroreport. 1993;4(9):1123–1126. [PubMed] [Google Scholar]
- Fort P, Khateb A, Pegna A, et al. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea pig brain. Eur J Neurosci. 1995;7:1502–1511. doi: 10.1111/j.1460-9568.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Fort P, Khateb A, Serafin M, et al. Pharmacological characterization and differentiation of non-cholinergic nucleus basalis neurons in vitro. Neuroreport. 1998;9:1–5. doi: 10.1097/00001756-199801050-00013. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Fort P, Eggermann E, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Zaborszky L. Direct catecholaminergiccholinergic interactions in the basal forebrain. II. Substantia nigra-ventral tegmental area projections to cholinergic neurons. J Comp Neurol. 1996;374(4):555–577. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- George R, Haslett W, Jenden D. A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- Glenn LL, Steriade M. Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci. 1982;2:1387–1404. doi: 10.1523/JNEUROSCI.02-10-01387.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, et al. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556(Pt 3):935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111(2):231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Innervation of orexin/hypocretin neurons by GABAergic, glutamatergic or cholinergic basal forebrain terminals evidenced by immunostaining for presynaptic vesicular transporter and postsynaptic scaffolding proteins. J Comp Neurol. 2006;499:645–661. doi: 10.1002/cne.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27(3):654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. New perspectives on the organization and evolution of nonspecific thalamocortical projections. In: Jones EG, Peters A, editors. Cerebral Cortex. Vol. 5. Plenum; New York: 1986. pp. 403–445. [Google Scholar]
- Hess WR. The Functional Organization of the Diencephalon. Grune & Stratton; New York: 1957. [Google Scholar]
- Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medullary reticular formation for sleep–wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1179–1200. doi: 10.1016/0306-4522(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Bongers CMH. A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat. Brain Res. 1991;566:308–315. doi: 10.1016/0006-8993(91)91715-d. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–159. [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483(3):351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2 Suppl):9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Abercrombie ED, Fornal CA, et al. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Progr Brain Res. 1991;88:159–165. doi: 10.1016/s0079-6123(08)63805-4. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172:601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- John J, Wu MF, Boehmer LN, et al. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42(4):619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Immunohistochemical study of choline acetyl transferase-immunoreactive processes and cells innervating the pontomedullary reticular formation. J Comp Neurol. 1990;295:485–514. doi: 10.1002/cne.902950311. [DOI] [PubMed] [Google Scholar]
- Jones BE. Reticular formation. Cytoarchitecture, transmitters and projections. In: Paxinos G, editor. The Rat Nervous System. Academic Press; Sydney, Australia: 1995. pp. 155–171. [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Progr Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic – catecholamine, serotonin, and acetylcholine – neurons. Neuroscience. 1989;31:37–61. doi: 10.1016/0306-4522(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:23–53. [PubMed] [Google Scholar]
- Jones BE, Yang T-Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Jones BE, Bobillier P, Pin C, et al. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58:157–177. doi: 10.1016/0006-8993(73)90830-5. [DOI] [PubMed] [Google Scholar]
- Jones BE, Harper ST, Halaris AE. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep–wakefulness states and the response to amphetamine. Brain Res. 1977;124:473–496. doi: 10.1016/0006-8993(77)90948-9. [DOI] [PubMed] [Google Scholar]
- Jones BE, Holmes CJ, Rodriguez-Veiga E, et al. GABA-synthesizing neurons in the medulla: their relationship to serotonin-containing and spinally projecting neurons in the rat. J Comp Neurol. 1991;312:1–19. doi: 10.1002/cne.903130210. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- Jouvet M. The role of monoamines and acetylcholinecontaining neurons in the regulation of the sleep–waking cycle. Ergeb Physiol. 1972;64:165–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Itoh K, Shigemoto R, et al. Glutaminaselike immunoreactivity in the lower brainstem and cerebellum of the adult rat. Neuroscience. 1989;32:79–98. doi: 10.1016/0306-4522(89)90109-7. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444(1):39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ohta M, Jodo E. Firing of “possibly” cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- Khateb A, Fort P, Alonso A, et al. Pharmacological and immunohistochemical evidence for a serotonergic input to cholinergic nucleus basalis neurons. Eur J Neurosci. 1993;5:541–547. doi: 10.1111/j.1460-9568.1993.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Khateb A, Fort P, Pegna A, et al. Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience. 1995;69:495–506. doi: 10.1016/0306-4522(95)00264-j. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22(13):5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. 5-HT modulation of multiple inward rectifiers in motoneurons in intact preparations of the neonatal rat spinal cord. J Neurophysiol. 2001;85(2):580–593. doi: 10.1152/jn.2001.85.2.580. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Sleep and Wakefulness. University of Chicago Press; Chicago: 1939. [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci. 2003;23(4):1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Hayaishi O. Firing of neurons in the preoptic/anterior hypothalamic areas in rat: its possible involvement in slow wave sleep and paradoxical sleep. Neurosci Res. 1994;19:31–38. doi: 10.1016/0168-0102(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Phillis JW. Pharmacological properties of acetylcholine-sensitive cells in the cerebral cortex. J Physiol (Lond) 1963;166:328–350. doi: 10.1113/jphysiol.1963.sp007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, et al. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22(1):33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani O, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005a;25(28):6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Alonso A, et al. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005b;25(17):4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cats. Neuropharmacol. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- Lin JS, Roussel B, Akaoka H, et al. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–326. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lindsley DB, Schreiner LH, Knowles WB, et al. Behavioral and EEG changes following chronic brain stem lesions. Electroencephalogr Clin Neurophysiol. 1950;2:483–498. doi: 10.1016/0013-4694(50)90086-1. [DOI] [PubMed] [Google Scholar]
- Longo VG. Behavioral and electroencephalographic effects of atropine and related compounds. Pharmacol Rev. 1966;18:965–996. [PubMed] [Google Scholar]
- Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun. 1998;253(2):241–245. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Greene RW, Semba K, et al. Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc Natl Acad Sci. 1992;89:743–747. doi: 10.1073/pnas.89.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189:58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Actions of acetylcholine in the cerebral cortex and thalamus and implications for function. Prog Brain Res. 1993;98:303–308. doi: 10.1016/s0079-6123(08)62412-7. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- McGinty D, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Magoun HW, Rhines R. An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol. 1946;9:165–171. doi: 10.1152/jn.1946.9.3.165. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. C-Fos expression in GABAergic, serotonergic and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney K, Mainville L, Jones BE. C-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci. 2002;15:1–6. doi: 10.1046/j.1460-9568.2002.01907.x. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20:9252–9263. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Lee MG, Modirrousta M, et al. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. Eur J Neurosci. 2003;18(3):723–727. doi: 10.1046/j.1460-9568.2003.02788.x. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. Hypnotic medications: mechanisms of action and pharmacologic effects. In: Kryger MH, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. Elsevier Saunders; Philadelphia: 2005. pp. 444–451. [Google Scholar]
- Mesulam M-M, Mufson EJ, Levey AI, et al. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ, Wainer BH, et al. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983b;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5(Suppl):1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, et al. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and waking in the rat. Brain Res. 1983;273(1):133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Hallanger AE, et al. Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat. Brain Res. 1988;451:397–402. doi: 10.1016/0006-8993(88)90792-5. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. GABAergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience. 2004;129(3):803–810. doi: 10.1016/j.neuroscience.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Monckton JE, McCormick DA. Neuromodulatory role of serotonin in the ferret thalamus. J Neurophysiol. 2002;87(4):2124–2136. doi: 10.1152/jn.00650.2001. [DOI] [PubMed] [Google Scholar]
- Montaron M-F, Bouyer J-J, Rougeul A, et al. Ventral mesencephalic tegmentum (VMT) controls electrocortical beta rhythms and associated attentive behaviour in the cat. Behav Brain Res. 1982;6:129–145. doi: 10.1016/0166-4328(82)90010-9. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Ann Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Nauta WJH, Kuypers HGJM. Some ascending pathways in the brain stem reticular formation. In: Jasper HH, Proctor LD, Knighton RS, et al., editors. Reticular Formation of the Brain. Little, Brown; Boston: 1958. pp. 3–30. [Google Scholar]
- Nishino S, Tafti M, Reid MS, et al. Muscle atonia is triggered by cholinergic stimulation of the basal forebrain: implication for the pathophysiology of canine narcolepsy. J Neurosci. 1995;15:4806–4814. doi: 10.1523/JNEUROSCI.15-07-04806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, et al. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep–wake control. J Neurosci. 2002;22(17):7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126(Pt 7):1524–1536. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Plum F, Posner JB. The Diagnosis of Stupor and Coma. Davis; Philadelphia: 1980. [Google Scholar]
- Reid MS, Siegel JM, Dement WC, et al. Cholinergic mechanisms in canine narcolepsy II. Acetylcholine release in the pontine reticular formation is enhanced during cataplexy. Neuroscience. 1994a;59(3):523–530. doi: 10.1016/0306-4522(94)90174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Tafti M, Nishino S, et al. Cholinergic regulation of cataplexy in canine narcolepsy in the pontine reticular formation is mediated by M2 muscarinic receptors. Sleep. 1994b;17:424–435. [PMC free article] [PubMed] [Google Scholar]
- Reiner PB, Kamondi A. Mechanisms of antihistamineinduced sedation in the human brain: H1 receptor activation reduces a background leakage potassium current. Neuroscience. 1994;59:579–588. doi: 10.1016/0306-4522(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5(9):709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Sakai K, Sastre J-P, Kanamori N, et al. State-specific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In: Pompeiano O, Ajmone-Marsan C, editors. Brain Mechanisms and Perceptual Awareness. Raven Press; New York: 1981. pp. 405–429. [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, et al. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25(26):6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Structural substrates for integrative patterns in the brain stem reticular core. In: Jasper HH, Proctor LD, Knighton RS, et al., editors. Reticular Formation of the Brain. Little, Brown; Boston: 1958. pp. 31–55. [Google Scholar]
- Schwartz JC, Arrang JM, Garbarg M, et al. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991;71(1):1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- Semba K, Reiner PB, Fibiger HC. Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience. 1990;38(3):643–654. doi: 10.1016/0306-4522(90)90058-c. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, et al. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, et al. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18(12):4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, et al. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277(6 Pt 2):R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- Shute CCD, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967;90:497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Siegel JM, McGinty DJ, Breedlove SM. Sleep and waking activity of pontine gigantocellular field neurons. Exp Neurol. 1977;56:553–573. doi: 10.1016/0014-4886(77)90321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Wheeler RL, McGinty DJ. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. 1979;179:49–60. doi: 10.1016/0006-8993(79)90488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, et al. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JM, Chambers WW. Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized and in the decerebrated cat. Am J Physiol. 1954;176:52–64. doi: 10.1152/ajplegacy.1953.176.1.52. [DOI] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR. Noradrenergic control of locomotor networks in the in vitro spinal cord of the neonatal rat. Brain Res. 2000;852(1):100–109. doi: 10.1016/s0006-8993(99)02219-2. [DOI] [PubMed] [Google Scholar]
- Starzl TE, Taylor CW, Magoun HW. Ascending conduction in reticular activating system, with special reference to the diencephalon. J Neurophysiol. 1951;14:461–477. doi: 10.1152/jn.1951.14.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Gong H, McGinty D, et al. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429(4):638–653. [PubMed] [Google Scholar]
- Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Steriade M, Oakson G, Ropert N. Firing rates and patterns of midbrain reticular neurons during steady and transitional states of the sleep–waking cycle. Exp Brain Res. 1982;46:37–51. doi: 10.1007/BF00238096. [DOI] [PubMed] [Google Scholar]
- Steriade M, Curro Dossi R, Contreras D. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (approximately 40 Hz) spike-bursts at approximately 1000 Hz during waking and rapid eye movement sleep. Neuroscience. 1993a;56(1):1–9. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993b;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 1994;17:199–208. doi: 10.1016/0166-2236(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Clemente CD. Forebrain inhibitory mechanisms: cortical synchronization induced by basal forebrain stimulation. Exp Neurol. 1962a;6:91–102. doi: 10.1016/0014-4886(62)90080-8. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Clemente CD. Forebrain inhibitory mechanisms: sleep patterns induced by basal forebrain stimulation in the behaving cat. Exp Neurol. 1962b;6:103–117. doi: 10.1016/0014-4886(62)90081-x. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, et al. Sleep– waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26(40):10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971a;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971b;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Vital-Durand F, Michel F. Effects of sensory deafferentation on the wakefulness-sleep cycle. J Physiol (Paris) 1969;61(Suppl 1):186. [PubMed] [Google Scholar]
- von Economo C. Sleep as a problem of localization. Journal of Nervous and Mental Disease. 1930;71(3):249–259. [Google Scholar]
- Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep–waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, et al. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21(5):1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, et al. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24(23):5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, et al. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23(8):3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]