Abstract
Synthetic cathinones, otherwise known as “bath salts”, have gained significant attention in the last few years as a result of increased use and abuse. One such compound, 3,4-methylenedioxypyrovalerone (MDPV), is pharmacologically and behaviorally similar to cocaine and has been shown to possess both aversive and rewarding effects. For a host of other drugs, each of these effects (and their relative balance) can be influenced by a variety of factors, including sex, which in turn impacts drug taking behavior. In this context, the present assessment sought to determine whether males and females differed in MDPV-induced CTA and CPP. Both male and female Sprague-Dawley rats underwent a combined CTA/CPP procedure, in which an injection of one of three doses of MDPV (1.0, 1.8 or 3.2 mg/kg) was paired with both a novel saccharin solution and a novel environment and changes in preferences for these stimuli were examined. Taste avoidance was evident in both sexes, although this avoidance was weaker in females compared to males. MDPV also produced place preferences in all drug-treated animals, but these preferences did not vary as a function of sex. The fact that females showed a weaker avoidance response compared to males (despite comparable preferences) suggests that females may have a heightened susceptibility to use and abuse of MDPV, paralleling results seen with cocaine and other stimulants. The present findings extend the behavioral characterization of MDPV and the factors that may alter its aversive and rewarding effects.
Keywords: place preference, taste avoidance, sex differences, MDPV
1. Introduction
In recent years, “bath salts”, or synthetic cathinones (stimulants derived from the khat plant; see Baumann, 2014), have become an increasingly visible public health concern. The rapidity with which these drugs have appeared in the general population and the magnitude of their adverse effects resulted in three of the primary parent cathinones [3,4 methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxymethcathinone (methylone) and 4-methylmethcathinone (mephedrone)] being classified as Schedule I drugs by the DEA in 2012. Since this classification, reports from poison control centers involving bath salts have decreased significantly. However, the reduced availability of these has resulted in a wide array of “replacement” compounds, in which slight chemical modifications have been made in order to circumvent legal enforcement. Given that many of these replacements still involve derivatives of the original parent compounds, it is crucial to continue the behavioral and neurochemical research of these drugs in order to make a complete abuse risk assessment (Baumann, 2014). MDPV, specifically, has been the subject of increasing research, both in our laboratory and others (see Baumann et al., 2013a, Gatch et al., 2013, King et al., 2014, Merluzzi et al., 2014) and is the most frequently found cathinone in the United States (Spiller et al., 2011).
Products containing MDPV have been reported to produce paranoid psychotic behavior, agitation, hallucinations and delirium (see Brontein et al., 2010, Penders, 2012). MDPV has been compared both anecdotally and pharmacologically to cocaine (Baumann et al., 2013b); both drugs are dopamine reuptake inhibitors, with MDPV possessing 10 times the potency as cocaine at producing locomotor activity, hypertension and tachycardia in rats. The behavioral effects of MDPV have only recently begun to be investigated. In one of the first of such assessments, Watterson et al. (2014) found that MDPV maintained self-administration in rats across a range of doses, induced escalated intake over long-access conditions and significantly lowered thresholds for brain stimulation reward. Further, Fantegrossi et al. (2012) found that both 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine (METH) reliably substituted for MDPV in a drug discrimination procedure, suggesting common interoceptive effects of these stimulants.
Given that drug self-administration is often described as the result of a balance between the aversive and rewarding effects of a drug (see Riley, 2011, Stolerman and D’Mello, 1981, Verendeev and Riley, 2013), it is important to examine each of these effects in order to determine any factors that may influence them and, thus, their impact on abuse. In one such examination of the aversive effects of MDPV, Merluzzi et al. (2014) used a range of doses (1, 1.8 and 3.2 mg/kg) to assess taste avoidance conditioning in male Sprague-Dawley rats. Rats were given successive pairings of a novel saccharin solution with an injection of MDPV or saline vehicle, and avoidance was indexed as a function of decreased saccharin intake in drug-treated animals. Adult mid and high dose groups showed aversions by Trial 2, and by Trial 4 all drug-injected groups differed significantly from vehicle with the acquisition attenuated in adolescents compared to adults (see also King et al., 2014, for a similar dose-dependent assessment with F344 and LEW rats).
In relation to the rewarding effects of MDPV, King et al. (2015) reported that the same range of doses of MDPV used in the prior assessments of MDPV-induced avoidance (1, 1.8 or 3.2 mg/kg) induced significant place preferences in adult male Sprague-Dawley rats (see also Karlsson et al., 2014, for a similar assessment in mice). Although not dose-dependent, all doses of MDPV produced significant shifts in preference for the drug-paired environment, highlighting that not only is MDPV rewarding, but it produces this reward at the same doses that produce avoidance, effects previously reported for a host of drugs of abuse (see Goudie, 1979, Riley, 2011, Wang et al., 2010, White et al., 1977).
Although these results have determined that MDPV is both pharmacologically and behaviorally similar to other abused stimulants and that it possesses both aversive and rewarding effects, much is still unknown about its abuse potential and what factors might serve to impact that potential. In this context, multiple experiential and subject variables have been shown to impact both the aversive and rewarding effects of drugs of abuse and, thus, may serve as predictive factors in determining propensity for abuse (for reviews, see Cunningham et al., 2006, Doremus-Fitzwater et al., 2010, Riley and Freeman, 2004, Tzschentke, 1998, Verendeev and Riley, 2012).
One such factor is sex. In one of the first assessments of sex differences in taste avoidance learning, Van Haaren and Hughes (1990) gave both male and female Wistar rats access to a novel saccharin solution paired with a subcutaneous injection of either 0, 5, 10 or 20 mg/kg cocaine and found that only female rats acquired cocaine-induced taste avoidance (and only at one dose − 20 mg/kg). These findings were consistent with a previous study, which reported that female rats acquired rapid cocaine-induced avoidance at both 20 and 36 mg/kg cocaine (see Goudie et al., 1978). However, other examinations with cocaine have produced mixed results. For example, Busse et al. (2005) reported that when injected subcutaneously, male Sprague-Dawley rats acquired stronger avoidance of a cocaine-paired saccharin solution than did females at 20 mg/kg. Further, Foltin and Schuster (1982) found no sex differences in rats with a conditioning dose of 24 mg/kg cocaine (given intraperitoneally). Overall, these data suggest that sex can influence a drug’s aversive effects, but that these differences are dependent on a variety of factors, including strain and route of administration.
Sex differences have also been reported in the rewarding effects of drugs. For example, Russo et al. (2003) reported that female rats acquired cocaine-induced CPP more rapidly and at lower doses than males (for similar results showing a female bias with place preference conditioning with ethanol, see Torres et al., 2014). However, the direction and magnitude of sex differences appear to be drug and strain-dependent. For example, female Wistar rats show a larger shift in preference for a nicotine-paired chamber compared to males (suggesting enhanced rewarding effects in female rats; see Torres et al., 2009), while Sprague-Dawley rats show stronger nicotine-induced preferences in males vs. females (Yararbas et al., 2010). These data suggest that, like the aversive properties of drugs, sex differences do exist in the rewarding effects, but are subject to a variety of factors.
Given these data, it is clear that sex differences can potentially alter the interoceptive effects of drugs of abuse. In order to provide a complete assessment of these aversive and rewarding effects as they relate to MDPV, these factors warrant investigation with this drug. Given the pharmacological and behavioral commonalities between MDPV and cocaine, it might be predicted that sex could shift the hedonic balance of MDPV and thus lead to more accurate predictions of its use and abuse potential when this factor is considered. Further, sex differences have not been investigated in either the aversive or rewarding drug effects of MDPV. Consequently, assessing their potential effect on MDPV’s stimulus effects is critical to predicting its overall abuse potential (see Carroll and Anker, 2010, Lynch, 2006, Schramm-Sapyta et al., 2014, Wetherington, 2010).
To that end, the present experiments will attempt to further characterize the subjective balance between the aversive and rewarding effects of MDPV. Both male and female adult Sprague-Dawley rats were run in a combined taste avoidance/place preference procedure, wherein three doses of MDPV (1, 1.8, or 3.2 mg/kg) were concurrently paired with both a novel taste and a novel place (this procedure has been previously shown with other drugs of abuse to produce both avoidance of the drug-paired taste and increased preference for the drug-paired place; see Brockwell et al., 1991, King and Riley, 2013, Simpson and Riley, 2005). Avoidance and preference, and any effect of dose, were compared between male and female rats in order to determine any effect of sex on the subjective effects of MDPV, which may provide insight into any sex-specific abuse vulnerability.
2. Materials and Methods
Sixty-four experimentally-naïve male and female Sprague-Dawley rats (n = 32/sex) were obtained from Harlan Sprague-Dawley (Indianapolis, IN) on postnatal day (PND) 21. Procedures recommended by the National Research Council (1996), the Committee on Guidelines for the Care and Use of Animals in Neuroscience and Behavioral Research (2003) and the Institutional Animal Care and Use Committee at American University were followed at all times. Upon arrival to the animal facility on PND 21, subjects were group housed (three same sex rats per OptiRat Plus polycarbonate bins; 100 cm × 99 cm × 201 cm) and maintained on ad-libitum food and water until PND 71, when experimental procedures began. Animals remained drug- and experimentally-naïve until this time.
2.1. Apparatus
The place conditioning apparatus (San Diego Instruments Place Preference System, San Diego, CA) consisted of two main conditioning chambers (28 × 21 × 34.5 cm) joined by a smaller middle chamber (14 × 21 × 34.5 cm). One of the conditioning chambers featured a white aluminum diamond plate floor with white walls; the other conditioning chamber featured a haircell-textured black plastic floor with black walls; the smaller middle chamber was outfitted with a steel rod floor and gray walls. Each individual chamber in each apparatus had its own white LED lights, and the lights were set on minimum. A total of eight identical apparatuses were used; each apparatus featured a 16 × 4 photobeam array for recording time (in seconds) spent in each chamber. The CPP room was illuminated by a 25-W red light mounted to the ceiling, and a white noise generator was used to mask background noise.
2.2. Drugs and Solutions
3,4-methylenedioxypyrovalerone hydrochloride (synthesized at the Chemical Biology Research Branch of the National Institute on Drug Abuse) was dissolved in sterile isotonic saline (0.9%) at a concentration of 1 mg/ml and was subsequently filtered through a 0.2 mm filter to remove any contaminants before being administered intraperitoneally (IP) at a dose of 1, 1.8 or 3.2 mg/kg. The drug was delivered IP to ensure consistency with the existing literature in which assessments of MDPV’s behavioral effects used this route of administration (see Fantegrossi et. al., 2013; Gatch et al., 2013; Karlsson et al., 2014; King et al., 2015; Merluzzi et al., 2013). Sterile isotonic saline was also filtered before being administered to saline controls. Injections for vehicle controls were equivolume to the highest dose of MDPV (3.2 mg/kg). Volume of the injection was manipulated in favor of concentration, given the influence that concentration has on the absorption/distribution of the drug. Sodium saccharin (0.1%; Sigma-Aldrich, St. Louis, MO) was prepared daily as 1 g/L solution in tap water.
2.3. Phase I: Habituation
Beginning on PND 71, animals were weighed and handled daily. Each subject’s daily water consumption was recorded through PND 76. On the following day, subjects had their water removed for the next 24 h to encourage consumption during training and testing. On PND 78, animals were placed in hanging stainless-steel test cages (24.3 × 19 × 18 cm) where they received 20-min access to water in graduated 50-ml Nalgene tubes. Following removal of the water tubes, animals were returned to their group-housed bins. Daily 20-min water access was repeated until consumption was stable, i.e., subjects approached and drank from the tube within 2 s of its presentation, and water consumption was within 2 ml of that from the previous day for a minimum of 4 consecutive days with no consistent increase or decrease. Once consumption was stable, Phase II began.
2.4. Phase II: Pre-Test
Following stable water consumption, each animal was given 20-min access to water in the test cage and then allowed 15-min access to the two-compartment place conditioning apparatus to obtain individual baseline times spent on each side and to assess apparatus bias (Cunningham, Tull, Rindal & Meyer, 2002; Roma & Riley, 2005). Baseline side preferences were used during conditioning, i.e., animals were injected and then placed on their initially non-preferred side (see below). Phase III began on the following day.
2.5. Phase III: Combined CTA/CPP
On the following day, animals received 20-min access to a novel saccharin solution during their daily fluid-access period after which they were immediately transported to a room adjacent to the CPP chambers. They were then assigned to one of eight groups such that consumption was comparable across groups and injected with vehicle or one of three doses of MDPV. Specifically, subjects were injected with 0 mg/kg (vehicle), 1 mg/kg, 1.8 mg/kg or 3.2 mg/kg of MDPV, yielding Groups M0, F0, M1, F1, M1.8, F1.8, M3.2 and F3.2 (n = 8 for each group). The letter in each group name denotes the sex of the animal (M for male; F for female), and the number denotes the dose of MDPV administered. After the injection, individuals were confined to their non-preferred side of the apparatus for 30 min (depending upon the initial side preference in Phase 2) and then returned to their home cages. On Day 2, the animals were given 20-min access to water, followed immediately by a saline injection and then confinement to the opposite (originally preferred) chamber of the previous day. This pattern of 20-min saccharin access, drug/vehicle injection and 30-min confinement to a CPP chamber on Day 1 followed by 20-min water access, saline injection and 30-min confinement to the other chamber on Day 2 constituted one conditioning cycle. The CTA/CPP procedure was carried out for a total of four consecutive cycles over 8 days. On Day 9, subjects were given water during the daily fluid access, followed by 15-min access to the entire place conditioning apparatus to determine any changes in time spent on the initially non-preferred side. On Day 10, subjects were given 20-min access to two Nalgene tubes (one containing tap water and the other containing saccharin solution) with placement counterbalanced to control for positioning effects, and saccharin/water consumption were measured. Immediately following this test, animals were returned to their home bins with ad libitum water access. No injections were given on the final two test days.
2.6. Statistical Analyses
2.6.1 Conditioned Taste Avoidance
Saccharin consumption throughout conditioning was analyzed with a 2 × 4 × 4 repeated measures ANOVA with between-subjects factors of Sex (Male and Female) and Dose (0, 1, 1.8 and 3.2) and a within-subjects factor of Trial (1–4). In the case of a three-way interaction, simple effects of Dose for each Sex and at each Trial (univariate analysis), Sex at each Dose and Trial (univariate analysis) and of Trial at each Dose and for each Sex (multivariate analysis) were assessed, with Bonferroni-corrected multiple comparisons as warranted.
On the two-bottle CTA test, percent saccharin of total fluid consumption was analyzed with a 2 × 4 factorial ANOVA with factors of Sex (Male or Female) and Dose (0, 1, 1.8 and 3.2). A two-way interaction was followed by univariate analyses for simple effects at each level of Sex and Dose and followed by Bonferroni-corrected pairwise comparisons as warranted.
2.6.2 Conditioned Place Preference
Percent time spent on the drug-paired side (DPS) on Pre-test and Post-test was compared with a 2 × 4 × 2 repeated measures ANOVA with between-subjects factors of Sex (Male and Female) and Dose (0, 1, 1.8 and 3.2 mg/kg MDPV) and a within-subjects factor of Test (Pre-test and Post-test). In the case of a significant interaction, simple effects of Test at each Dose and for each Sex were assessed with multivariate analyses.
2.6.3 CTA/CPP Relationship
The relationship between changes in percent time spent on the DPS (post-test percentage subtracted from pre-test percentage) and changes in saccharin consumption (Trial 4 consumption subtracted from Trial 1 consumption) was determined for individual animals within each dose group and sex, using Pearson correlation coefficient.
For all comparisons, statistical significance was set at α = 0.05.
3. Results
3.1. CTA
MDPV induced taste avoidance in all animals, an effect that was weaker in females compared to males. The 2 × 4 × 4 mixed model ANOVA on saccharin consumption revealed significant effects of Dose [F(3, 55) = 22.987] and Trial [F(3, 165) = 4.515], as well as significant Dose × Trial [F(9, 165) = 10.522] and Sex × Dose × Trial [F(9, 165) = 1.989] interactions (see Figure 1). No other main effect or significant interactions were found.
Figure 1.
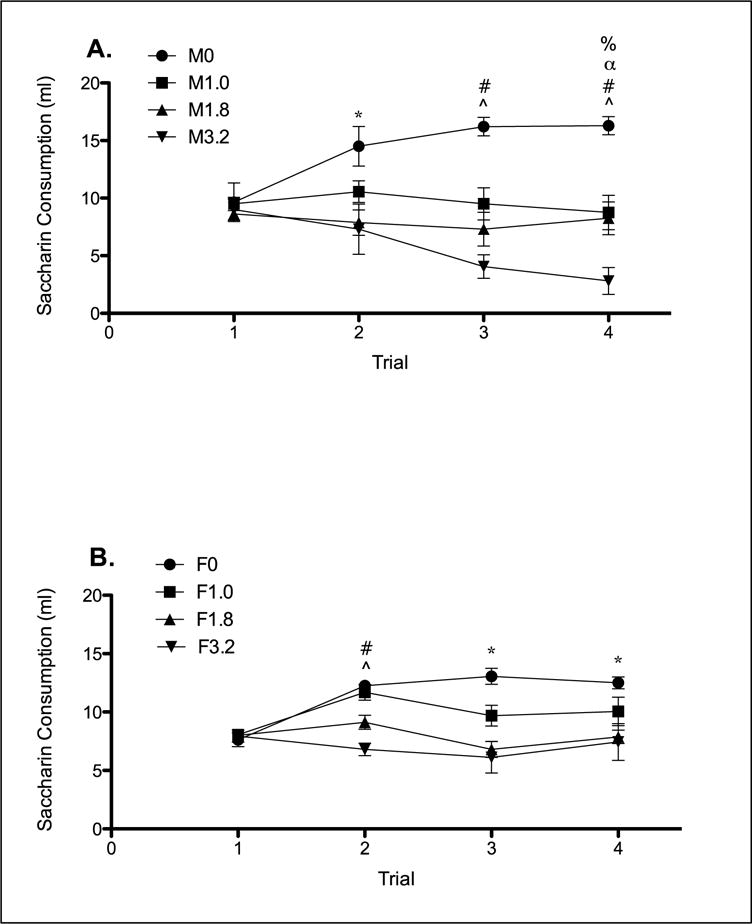
Mean (± SEM) saccharin consumption in ml over all conditioning trials. Panel A (males): *M0 significantly greater than M1.8 and M3.2; ^M0 significantly greater than all drug-treated groups; %M1.0 and M1.8 significantly greater than M3.2; #M3.2 significant decrease from Trial 1; αM3.2 significant decrease from Trial 2. Panel B (females); *F0 significantly greater than F1.8 and F3.2; ^F0 and F1.0 significantly greater than F3.2; #F1.0 significant increase from Trial 1.
Simple effects of Dose for each Sex and at each Trial were assessed with a univariate analysis which revealed significant differences on Trials 2–4 for both males [Trial 2: F(3, 55) = 7.544; Trial 3: F(3, 55) = 20.928; Trial 4: F(3, 55) = 21.026] and females [Trial 2: F(3, 55) = 4.776; Trial 3: F(3, 55) = 8.601; Trial 4: F(3, 55) = 4.0]. Corrected multiple comparisons indicated that on Trial 2, Groups M1.8 and M3.2 drank significantly less than M0. Group F3.2 drank significantly less than Groups F0 and F1. On Trial 3, all male drug groups drank significantly less than Group M0 and Group M3.2 drank significantly less than Group M1. Groups F1.8 and F3.2 drank significantly less than Group F0. On Trial 4, all male drug groups again drank significantly less than Group M.0 and Group M3.2 drank significantly less than Groups M1 and M1.8. Groups F1.8 and F3.2 again drank significantly less than Group F0.
Simple effects of Sex at each Dose and Trial were assessed with a univariate analysis which revealed significant differences at Trial 4 for Groups 3.2 [F(1,55) = 7.92] and vehicle [F(1,55): 4.952]. Corrected multiple comparisons indicated that on Trial 4, Group M3.2 drank significantly less than Group F3.2, and Group F0 drank significantly less than Group M0.
Simple effects of Trial for each Sex and at each Dose were assessed with a multivariate analysis which revealed significant differences for Groups M3.2 [F(3,53) = 8.405], F1 [F(3,53) = 5.064], M0 [F(3,53) = 12.517] and F0 [F(3,53) = 10.53] across trials. Corrected multiple comparisons indicated that Group M3.2 drank significantly less on Trials 3–4 than on Trial 1 and significantly less on Trial 4 than on Trial 2. Group F1 drank significantly more on Trial 2 than on Trial 1, but showed no differences at Trials 3–4. Both Groups M0 and F0 drank significantly more on Trials 2–4 than on Trial 1.
The 2 × 4 factorial ANOVA for percent saccharin consumption on the two-bottle test revealed a main effect of Dose [F(3,55) = 19.395], but no effect of Sex, and no Dose X Sex interaction (see Figure 3A). Collapsed across Sex, the percent saccharin consumed was significantly lower for animals treated with MDPV than for animals treated with vehicle (see Figure 3B).
Figure 3.
Mean percent time spent on the nonpreferred side (± SEM) for all groups at Pre- and Post-test, collapsed across Sex. All groups (including vehicle) showed significant increase from Pre-test to Post-test. *Significant difference from Group 0.
3.2. CPP
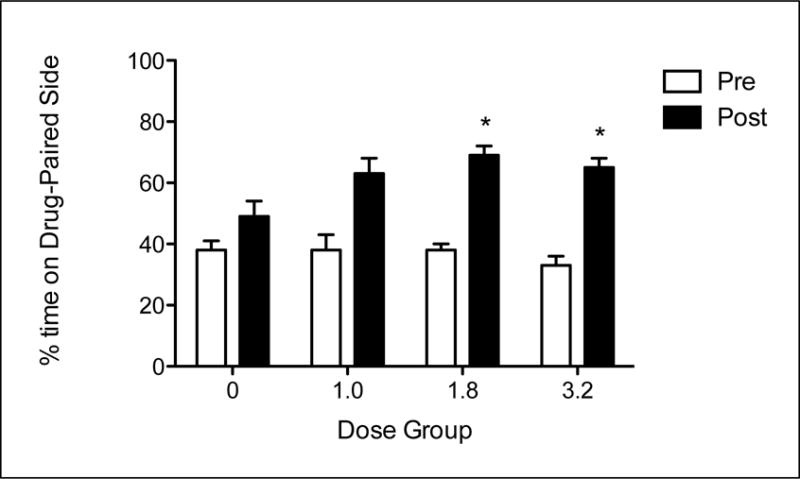
MDPV induced significant place preferences, although these were not sex-dependent. The 2 × 4 × 2 repeated measures ANOVA on percent time spent on the DPS revealed main effects of Dose [F(3,55) = 2.799] and Test [F(1,55) = 112.701] as well as a Test × Dose [F(3,55) = 4.296] interaction, but no main effect of Sex or any interactions with Sex as a factor (see Figure 3). To further explore the Test × Dose interaction, data were collapsed across Sex and a 4 × 2 repeated measures ANOVA was run with a between-subjects factor of Dose and a within-subjects factor of Test. This revealed main effects of Test [F(1,59) = 115.98], Dose [F(3,59) = 3.008] and a Test × Dose [F(3,59) = 4.447] interaction.
Simple effects of Test at each Dose were assessed with a multivariate analysis, which revealed significant differences in all four groups [0: F(1,59) = 5.245; 1.0: F(1, 59) = 30.052; 1.8: F(1, 59) = 47.051; 3.2: F(1, 59) = 5.245]. Corrected multiple comparisons indicated that all groups, including vehicle, significantly increased time spent on the DPS from Pre-Test to Post-Test. Simple effects of Dose at each Test were assessed with a univariate analysis, which revealed no significant differences at Pre-Test, but did show significant differences at Post-Test [F(3,59) = 4.564]. Corrected multiple comparisons indicated that on the Post-Test, Groups 1.8 and 3.2 spent significantly more time on the DPS than did Group 0.
3.3 CTA/CPP Relationship
Analysis of the change in the amount consumed over conditioning (Trial 4 - Trial 1) and the change in percent time on the DPS (Post-Test - Pre-Test) (within each sex and dose group) revealed minimal correlations. No significant relationship was observed for Groups M0, M1, M1.8, M3.2, F0, F1 and F3.2 (rs ≤ .575, ps > .05). The correlational analysis did reveal a significant relationship for subjects in Group F1.8, r = .873, p = .005. Specifically, animals with larger increases in percent time spent on the DPS showed larger increases in saccharin consumption (see Table 1/Figure 4).
Table 1.
The relationship between the change in the amount of saccharin consumed over conditioning (Trial 1–Trial 4) and the change in percent time on the DPS (Pre-Test to Post-Test). Each cell indicates the r and p values for the given relationship for each Sex and Dose Group.
| Male | Female | |
|---|---|---|
| Vehicle |
r= −.419 p= .350 |
r= .316 p= .446 |
| 1.0 mg/kg MDPV |
r= −.316 p= .445 |
r= −.179 p= .672 |
| 1.8 mg/kg MDPV |
r= .575 p= .136 |
r= .873 p= .005 |
| 3.2 mg/kg MDPV |
r= .457 p= .255 |
r= .527 p= .180 |
Bold font indicates significant relationship (see text for more detail).
Figure 4.
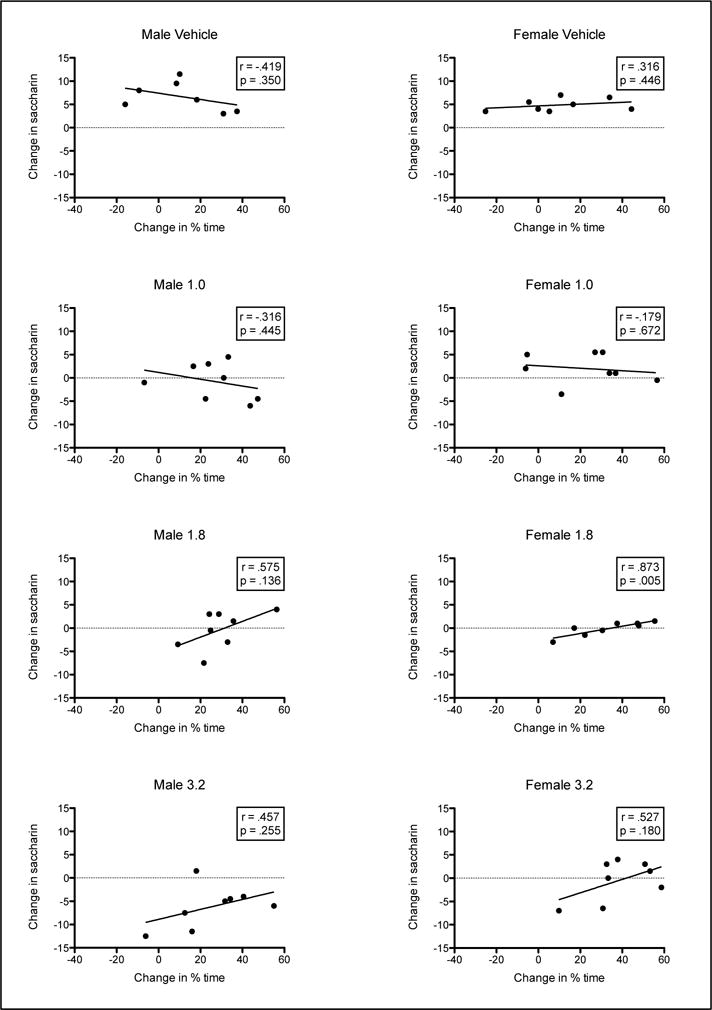
Scatterplots (with best line of fit) showing the relationship between change in the amount of saccharin consumed over conditioning (Trial 4 - Trial 1) and the change in percent time on the DPS (Post-Test - Pre-test) for each Sex and Dose Group.
4. Discussion
MDPV has been shown to have both aversive and rewarding effects (King et al., 2014, 2015, Merluzzi et al., 2014), and in order to determine how these affective properties contribute to MDPV’s relative abuse potential, it is critical to examine factors that might influence the balance between them (Gaiardi et al., 1991, Riley, 2011, Stolerman and D’Mello, 1981, Verendeev and Riley, 2013). Sex is of particular interest here, because while the data regarding the influence of sex on avoidance and reward are mixed (see above), work with a number of stimulants has demonstrated that, in general, females may be more likely to abuse these drugs (Lynch et al., 2002, Russo et al., 2003, Zakharova et al., 2009). Given the behavioral and pharmacological commonalities between MDPV and other classical stimulants, the same vulnerability might be predicted to occur with MDPV. In the present experiment, male and female rats underwent a combination taste avoidance/place preference procedure, where injections of a range of doses of MDPV (0–3.2 mg/kg) were paired with both a novel saccharin solution and a novel environment, and changes in preference for these stimuli were examined. MDPV produced reductions in saccharin consumption for all drug-treated groups that did vary with sex. MDPV also induced significant place preferences that appeared independent of sex.
As noted, MDPV induced taste avoidance, an effect consistent with prior work with this compound in the taste avoidance preparation in which male adolescent and adults were assessed (see Merluzzi et al., 2013). While MDPV induced avoidance, males and females differed in the acquisition and degree of this suppression. Specifically, males injected with MDPV displayed significant differences from control subjects at the two highest doses following only a single pairing of saccharin and MDPV. Avoidance was evident on this trial (Trial 2) for females only in the high dose group. This pattern was maintained over conditioning in that on Trials 3 and 4, all male subjects injected with MDPV drank less than controls, whereas only the two highest groups displayed avoidance. Further, at no point over conditioning did any female dose group display a significant decrease from their own baseline consumption levels (Trial 1). In fact, female subjects in the low MDPV group increase consumption form Trial 1 to Trial 2. For males, Group M3.2 displayed a significant decrease from baseline consumption on Trials 3–4 and a significant decrease from Trial 2 to Trial 4. In a direct comparison between males and females, males injected with 3.2 mg/kg MDPV drank significantly less than females on Trial 4.
Based on both the within- and between-subjects analyses, MDPV-induced taste avoidance was weaker in females compared to males, demonstrating that MDPV’s aversive effects are sex-dependent at the range of doses used. It is important to note that the fact that the differences between males and females were only evident on specific trials and with certain doses is consistent with results in other work on sex differences in taste avoidance (see Chambers and Sengstake, 1976, Roma et al., 2008, Torres et al., 2009). Given the similarities in the mechanisms of action of MDPV with cocaine, it might be expected that there would be parallels in some of their behavioral effects as well as how such effects might be impacted by factors such as sex. As noted above, work on sex differences with cocaine are somewhat mixed, subject to a host of factors including dose and route of injection (see above). As such, it is difficult to generalize the present findings to those with cocaine and other stimulants. ← WE NEED TO ADDRESS THIS.
Although there were significant sex differences in acquisition of avoidance (M > F), these differences were not evident in the two-bottle assessment in which all dose groups drank a smaller percentage of saccharin than control subjects. This is likely a reflection of the sensitivity of the two-bottle test relative to forced-choice consumption, i.e., when animals are given access to both the drug-associated taste and water in the two-bottle assessment, aversions are generally stronger (with no forced drinking) and differences among groups are not always evident in this more sensitive index of the drug’s aversive effects (for discussion, see Dragoin et al., 1971; Grote and Brown, 1971). When collapsed across sex, MDPV-injected groups drank between 10–30% saccharin compared to controls that drank approximately 80%, suggesting that any sex effects in this test may have been masked by the strong degree of suppression.
MDPV also induced significant place preferences which are comparable to the results obtained by King et al. (2015) who assessed MDPV-induced CPP in male rats at the same range of doses (0, 1.0, 1.8 and 3.2 mg/kg) used in the present assessment. In the present assessment, although all groups (independent of drug treatment) significantly increased time on the DPS from Pre-test to Post-test, subjects injected with the two highest doses of MDPV, i.e., Groups 1.8 and 3.2, spent significantly more time on the DPS at Post-test than did the vehicle animals, indicating that MDPV was rewarding in this preparation. Interestingly, these preferences did not vary as a function of sex. It might have been predicted that, given the pharmacological similarity to cocaine, MDPV might have produced stronger place preferences in females than males (see Russo et al., 2003, Zakharova et al., 2009). However, the prior studies showing larger preferences for cocaine in females compared to males utilized different strains (Russo et al., 2003) and initiated conditioning at different ages and under different experimental designs (Zakharova et al., 2009).
Given that the present assessment found that MDPV induced concurrent tastes avoidance and place preference, but that sex differences were only evident in taste avoidance, argues for a dissociation between these factors, i.e., these effects likely function independently (for a discussion, see Verendeev and Riley, 2013). This position is further supported by the correlational analyses between the MDPV-induced changes in saccharin consumed and side preferences over conditioning. As described, there was no consistent significant relationship between strength of taste avoidance and strength of place preference within sex and dose groups (Group F1.8 as the only exception; see above; see also Cunningham et al., 2002). The likelihood of obtaining one significant correlation in eight assessments is quite high by chance alone (.279) suggesting that such an effect is spurious. The fact that the significant correlation was not dose dependent supports this position. Interestingly, in a similar correlational analysis between taste avoidance and place preferences conditioned with amphetamine and morphine found only one significant relationship (out of 16 total groups) (Verendeev et al., 2011).
Recently, it has been argued that preclinical and clinical work on drug effects necessitates the inclusion of sex as a variable. While historically, male subjects have primarily been used in biomedical research, recent years have seen an increased awareness of the fact that sex can influence the direction of findings in nearly all areas of brain research, including sensitivity to drugs of abuse (see introduction; see also Cahill, 2012, Wetherington, 2007 for reviews). The fact that while MDPV produced a weakened avoidance response in females compared to males suggests that females may be more vulnerable to MDPV use and abuse (for work with other drugs, see Becker et al., 2001, Chen and Kandel, 2002, Evans and Foltin, 2010, Lynch et al., 2002) and extends the behavioral characterization of MDPV and the conditions under which the aversive and rewarding effects might be impacted. Future work investigating the sex differences in MDPV-induced avoidance would benefit from controlling for hormone levels in ovariectomized animals/and or cycle phase in intact animals, in order to determine whether gonadal hormones have the same effect on MDPV-induced behavioral effects as they do with cocaine. Additionally, there is not yet data regarding sex differences in MDPV’s behavioral or pharmacological effects in humans; the present study suggests it would be important to determine whether these differences exist and whether females may be more vulnerable to use and abuse of MDPV or other synthetic cathinones.
Figure 2.
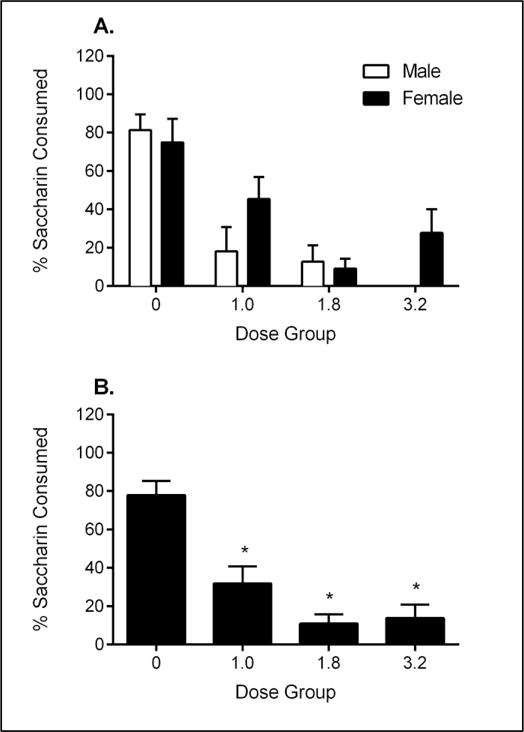
Mean percent saccharin (± SEM) consumed on two-bottle avoidance test for males and females (A) and collapsed across Sex (B). *Significant difference from Group 0.
Highlights.
-
-
MDPV induced dose-dependent conditioned taste avoidance (CTA) in all animals.
-
-
CTA was weaker in females compared to males.
-
-
MDPV induced dose-dependent conditioned place preferences (CPP).
-
-
CPP was comparable in males and females.
-
-
Females may be particularly susceptible to abuse of MDPV
Acknowledgments
This research was supported by a grant from the Mellon Foundation to ALR. A portion of this research was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism, NIH, US Department of Health and Human Services.
Footnotes
The authors have no conflicts of interest to declare.
References
- Baumann MH. Awash in a sea of ‘bath salts’: implications for biomedical research and public health. Addiction. 2014;109:1577–9. doi: 10.1111/add.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. European journal of pharmacology. 2013a;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3, 4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013b;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Annals of the New York Academy of Sciences. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Brockwell NT, Eikelboom R, Beninger RJ. Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacology Biochemistry and Behavior. 1991;38:513–7. doi: 10.1016/0091-3057(91)90006-n. [DOI] [PubMed] [Google Scholar]
- Brontein A, Spyker D, Cantilena L, Green J, Rumack B, Dart R. annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th annual report. Clin Toxicol. 2010;49:910–41. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- Busse GD, Freeman KB, Riley AL. The interaction of sex and route of drug administration in cocaine-induced conditioned taste aversions. Pharmacology Biochemistry and Behavior. 2005;81:814–20. doi: 10.1016/j.pbb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cahill L. A half-truth is a whole lie: on the necessity of investigating sex influences on the brain. Endocrinology. 2012;153:2541–3. doi: 10.1210/en.2011-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Hormones and behavior. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB. Sexually dimorphic extinction of a conditioned taste aversion in rats. Anim Learn Behav. 1976;4:181–5. doi: 10.3758/bf03214032. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug and Alcohol Dependence. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature protocols. 2006;1:1662–70. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology. 2002;160:414–24. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and cognition. 2010;72:114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Hormones and behavior. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’constituent 3, 4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2012;38:563–73. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Schuster CR. The effects of cocaine in a gustatory avoidance paradigm: A procedural analysis. Pharmacology Biochemistry and Behavior. 1982;16:347–52. doi: 10.1016/0091-3057(82)90170-8. [DOI] [PubMed] [Google Scholar]
- Gaiardi M, Bartoletti M, Bacchi A, Gubellini C, Costa M, Babbini M. Role of repeated exposure to morphine in determining its affective properties: place and taste conditioning studies in rats. Psychopharmacology. 1991;103:183–6. doi: 10.1007/BF02244201. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of’bath salt’cathinones. Behavioural pharmacology. 2013;24:437–47. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie A. Aversive stimulus properties of drugs. Neuropharmacology. 1979;18:971–9. doi: 10.1016/0028-3908(79)90161-8. [DOI] [PubMed] [Google Scholar]
- Goudie A, Dickins D, Thornton E. Cocaine-induced conditioned taste aversions in rats. Pharmacology Biochemistry and Behavior. 1978;8:757–61. doi: 10.1016/0091-3057(78)90279-4. [DOI] [PubMed] [Google Scholar]
- King HE, Riley AL. A history of morphine-induced taste aversion learning fails to affect morphine-induced place preference conditioning in rats. Learning & behavior. 2013;41:433–42. doi: 10.3758/s13420-013-0118-6. [DOI] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. 3, 4-methylenedioxypyrovalerone (MDPV)-induced conditioned taste avoidance in the F344/N and LEW rat strains. Pharmacology Biochemistry and Behavior. 2014 doi: 10.1016/j.pbb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug and alcohol dependence. 2015;146:116–9. doi: 10.1016/j.drugalcdep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Experimental and clinical psychopharmacology. 2006;14:34. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Martin GM, Bechara A, Van der Kooy D. Morphine preexposure attenuates the aversive properties of opiates without preexposure to the aversive properties. Pharmacology Biochemistry and Behavior. 1988;30:687–92. doi: 10.1016/0091-3057(88)90085-8. [DOI] [PubMed] [Google Scholar]
- Merluzzi A, Hurwitz Z, Briscione M, Cobuzzi J, Wetzell B, Rice K, et al. Differential expression of MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Developmental Psychobiology. 2014;56:943–54. doi: 10.1002/dev.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Consideration of Sex as a Biological Variable in NIH-funded Research. National Institutes of Health; 2015. [Google Scholar]
- Penders TM. How to recognize a patient who’s high on “bath salts”. The Journal of family practice. 2012;61:210–2. [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiology & behavior. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Riley AL, Freeman KB. Conditioned Flavor Aversions: Assessment of Drug-Induced Suppression of Food Intake. Current protocols in neuroscience. 2004:8.6 E. 1–8.6 E. 12. doi: 10.1002/0471142301.ns0806es29. [DOI] [PubMed] [Google Scholar]
- Roma PG, Davis CM, Kohut SJ, Huntsberry ME, Riley AL. Early maternal separation and sex differences in the aversive effects of amphetamine in adult rats. Physiol Behav. 2008;93:897–904. doi: 10.1016/j.physbeh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain research. 2003;970:214–20. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology. 2014;231:1831–9. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Baharnouri G, McQuade LE, Clarke P. Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. European Journal of Neuroscience. 2008;28:342–52. doi: 10.1111/j.1460-9568.2008.06341.x. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacology Biochemistry and Behavior. 2005;80:471–9. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs”(synthetic cathinones) in the United States. Clinical Toxicology. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Stolerman I, D’Mello G. Oral self-administration and the relevance of conditioned taste aversions. Advances in behavioral pharmacology. 1981:169–214. [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–12. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism: Clinical and Experimental Research. 2014;38:108–15. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in neurobiology. 1998;56:613–72. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Hughes CE. Cocaine-induced conditioned taste aversions in male and female Wistar rats. Pharmacology Biochemistry and Behavior. 1990;37:693–6. doi: 10.1016/0091-3057(90)90549-w. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: History and interpretation. Neuroscience & Biobehavioral Reviews. 2012;36:2193–205. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behavioural pharmacology. 2013;24:363–74. doi: 10.1097/FBP.0b013e32836413d5. [DOI] [PubMed] [Google Scholar]
- Wang Y-C, Huang ACW, Hsiao S. Paradoxical simultaneous occurrence of amphetamine-induced conditioned taste aversion and conditioned place preference with the same single drug injection: a new “pre-and post-association” experimental paradigm. Pharmacology Biochemistry and Behavior. 2010;95:80–7. doi: 10.1016/j.pbb.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV) Addiction biology. 2014;19:165–74. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: a shift in the burden of proof? Exp Clin Psychopharmacol. 2007;15:411. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Wetherington CL. Sex differences and gonadal hormone influences in drug addiction and sexual behavior: progress and possibilities. Hormones and behavior. 2010;58:2–7. doi: 10.1016/j.yhbeh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- White N, Sklar L, Amit Z. The reinforcing action of morphine and its paradoxical side effect. Psychopharmacology. 1977;52:63–6. doi: 10.1007/BF00426601. [DOI] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58:374–82. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacology Biochemistry and Behavior. 2009;92:131–4. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


