Abstract
Background
Drug use is thought to be a balance of the rewarding and aversive effects of drugs. Understanding how various factors impact these properties and their relative balance may provide insight into their abuse potential. In this context, the present study attempted to evaluate the effects of drug history on the aversive effects of 3,4-methylenedioxypyrovalerone (MDPV), one of a variety of synthetic cathinones (collectively known as “bath salts”).
Methods
Different groups of male Sprague-Dawley rats were exposed to either vehicle or MDPV (1.8 mg/kg) once every fourth day for five total injections prior to taste avoidance conditioning in which a novel saccharin solution was repeatedly paired with either vehicle, MDPV (1.8 mg/kg), the related psychostimulant cocaine (18 mg/kg) or the emetic lithium chloride (LiCl) (13.65 mg/kg).
Results
In animals pre-exposed to vehicle, all three drugs induced significant and comparable taste avoidance relative to animals injected with vehicle during conditioning. MDPV pre-exposure attenuated the avoidance induced by both MDPV and cocaine (greater attenuation for MDPV than cocaine), but had no effect on that induced by LiCl.
Conclusions
These findings suggest that a history of MDPV use may reduce or attenuate MDPV and cocaine’s (but not LiCl’s) aversive effects. The implications for such changes in MDPV’s aversive effects to its potential use and abuse were discussed.
Keywords: MDPV, Cocaine, LiCl, Conditioned Taste Avoidance, Drug Pre-exposure
1. Introduction
Drugs of abuse have both rewarding and aversive effects, and it is the balance of these two affective properties that impact the use and abuse of these compounds (Colechio et al., 2014; Riley et al. 2009; Stolerman, 1985; Wise et al., 1976). Understanding this relative balance and the factors that influence it may be critical in predicting vulnerability to the drug’s use and abuse (Cunningham et al., 2009; Riley, 2011). One class of drugs only recently investigated in this context is the synthetic cathinones, e.g., 3,4-methylenedioxypyrovalerone (MDPV), mephedrone (4-methylmethcathinone) and methylone. These drugs, known as “bath salts”, initially appeared in reports from the CDC and emergency rooms across the United States in 2010, and at the outset these products could easily be purchased over the internet or in small retail locations such as head shops and gas stations (Ross et al., 2011; Spiller et al., 2011). Often causing severe hallucinations, paranoia, violent behaviors, tachycardia and even death (Baumann et al., 2014; Spiller et al., 2011), they pose a significant threat, and yet they are relatively unknown compared to other abused compounds.
Although only recently introduced, these compounds have been examined for their aversive and rewarding effects in a number of preclinical models (see King and Riley, 2016), and similar to a host of other drugs of abuse, they produce both effects. For example, King and her colleagues (2015 colleagues (2014a) reported that MDPV supported conditioned place preferences (CPP) in male and female Sprague-Dawley rats (for CPP with other synthetic cathinones, see Karlsson et al., 2014; Lisek et al., 2012). In related work, Watterson et al. (2012b) reported that MDPV reduced ICSS thresholds (for assessments with other bath salts, see Banono et al., 2014; Robinson et al., 2012; Watterson et al., 2012a), a reduction indicative of the drug’s rewarding effects (Carlezon and Chartoff, 2007; Fouriezos and Nawiesniak, 1987; Reid, 1987). Conversely, Merluzzi and his colleagues (2013) reported that both adolescent and adult male Sprague-Dawley rats acquired dose-dependent MDPV-induced conditioned taste avoidance (CTA; adults > adolescents) (see also King and Riley, 2013). In relation to the relative balance of the rewarding and aversive effects of the cathinones, Aarde and his colleagues (2015 colleagues (2013) demonstrated that MDPV supported intravenous self-administration (see also Schindler et al., 2015; Watterson et al., 2014b; for related findings with mephedrone and methylone, see Aarde et al., 2015; Creehan et al., 2015; Schindler et al., 2015; Vandewater et al., 2015; Watterson et al., 2014b, 2012a). That these rewarding, aversive and reinforcing effects are evident across a number of conditions and with a variety of bath salt constituents suggests the generalizability of the abuse potential of this class of compounds.
What is less well characterized is how these effects (and their balance) are impacted by factors reported to affect other drugs of abuse. Such factors include sex, age and genetics, among others (see Cunningham et al., 2009; Riley, 2011). One that has received increasing attention in this context is drug history. For example, rats with prior exposure to cocaine display sensitized conditioned place preferences (Lett, 1985) and attenuated taste avoidance (Riley and Diamond, 1998), indicative of changes in the rewarding and aversive effects of the drug, respectively. Such sensitizing (Koob and LeMoal, 2005) and attenuating (Randich and LoLordo, 1979; Riley and Simpson, 2001) effects of drug history have been reported for a variety of drugs. In this context, Verendeev and Riley (2012) argue that drugs of abuse should be seen as complex compounds with multiple stimulus effects, some of which are aversive and some of which are rewarding. With drug history, these aversive effects decrease and the rewarding effects increase, thereby changing the affective balance of the compound and potentially its likelihood for use and abuse (for such demonstrations with ethanol, see Camarini and Hodge, 2004; Sanders and Spear, 2007; for cocaine, see Schenk and Partridge, 2000; 1997; for related discussion, see Ettenberg et al., 2015). Although the cathinones have not been examined in this context, Gregg and his colleagues (2013) have recently reported that pre-exposure to mephedrone sensitized mephedrone- and cocaine-, but not methamphetamine-, induced locomotor activity in rats. Further, prior exposure to MDPV sensitizes motor activity to itself and methamphetamine (Watterson et al., 2016) and prior exposure to MDPV and 4-methylmethcathinone (4-MMC) (alone and in combination) sensitizes motor activity to itself as well as to cocaine (Berquist et al., 2016). Interestingly, these interactions are not necessarily symmetrical in that at the specific doses tested exposure to cocaine does not impact mephedrone-induced locomotor activity (Gregg et al., 2013) and exposure to methamphetamine does not sensitize MDPV-induced locomotion (Watterson et al., 2016).
To date, there is no work assessing how exposure to the cathinones impacts their affective properties or those of other drugs, although a recent case report (Cesar, 2015) noted that bath salt users overall are likely to co-use other illicit compounds, increasing the likelihood of such interactions. The present experiment began this examination by assessing the effects of pre-exposure to MDPV on MDPV-, cocaine- and lithium chloride-induced taste avoidance, a behavioral index of the aversive effects of drugs (see Riley and Tuck, 1985; see www.CTAlearning.com). The effects of drug pre-exposure on taste avoidance learning are well characterized (see Randich and LoLordo, 1979; Riley and Simpson, 2001) and evident with both cocaine (Riley and Diamond, 1998; Simpson and Riley, 2005) and LiCl (Davis et al., 2007; Riley et al., 1976), indicating that the aversive effects of these two drugs are sensitive to drug history. The effects of MDPV pre-exposure on MDPV-induced avoidance was examined given that the basic question was how MDPV history might impact its own aversive effects. Cocaine-induced taste avoidance was examined in these assessments given its shared mechanisms of action with MDPV, i.e., DA and NE reuptake inhibition (as opposed to monoamine release by mephedrone and methylone; see Baumann et al., 2014; Simmler et al., 2013). Lithium chloride (LiCl) was used as a negative control given that LiCl produces strong taste avoidance (see Gore-Langton et al., 2015; Nachman and Ashe, 1973), but not via similar neurochemical actions to bath salts (see Meyer and Quenzer, 2013; Stahl, 2009).
2. General Methods
2.1. Subjects
The subjects were 64 experimentally naïve male Sprague-Dawley rats from Harlan Sprague-Dawley, Indianapolis, IN. Rats entered the animal research facility at American University on postnatal day (PND) 24 and were allowed to mature undisturbed with the exception of weekly weight assessments from PND 24 though PND 77. Beginning on PND 78, animals were weighed daily as an index of health status and to reduce handling stress during the experimental procedures. Subjects were 90 days old and weighed between 328 and 451 grams at the beginning of the study. Procedures recommended by the National Research Council (1996), the Committee on Guidelines for the Care and Use of Animals in Neuroscience and Behavioral Research (2003) and the Institutional Animal Care and Use Committee at American University were followed at all times.
2.2. Drugs and solutions
MDPV (synthesized at the Molecular Targets and Medications Discovery Branch, NIDA) was dissolved in isotonic saline (0.9%) at a concentration of 1 mg/ml before being injected intraperitoneally (IP) at a dose of 1.8 mg/kg. Cocaine (generously provided by NIDA) was dissolved in isotonic saline at a concentration of 10 mg/ml and injected subcutaneously (SC; 18 mg/kg). LiCl was dissolved in isotonic saline at a concentration of 6.4 mg/ml and injected IP (13.65 mg/kg). The doses and routes of administration chosen produce comparable avoidance among the three compounds and allow for comparisons for the effects of MDPV history (see Ferrari et al., 1991; Gore-Langton et al., 2015; Merluzzi et al., 2013). To control for the different routes of administration for MDPV and LiCl vs. cocaine, equivolume isotonic saline (vehicle) was injected either IP or SC to control subjects. Each drug (and vehicle) solution was prepared daily and passed through a 0.2 um filter prior to injection. Saccharin (sodium saccharin, Sigma) was prepared as a 1g/l (0.1%) solution in tap water.
2.3. Apparatus
Subjects were housed two per home-cage in OptiRat Plus cages (38.9 × 56.9 × 26.2 cm; 1181 cm2) throughout the study. The room was maintained on a 12-h light/dark cycle (0800 – 2000h) at 23 °C. Unless stated otherwise, food and water were available ad libitum. During habituation, training and testing (see below), animals were transferred to individual hanging, stainless-steel wire mesh cages (24.3 × 19 × 18 cm) on the front of which graduated Nalgene tubes could be placed for fluid presentation.
2.4. Procedure
2.4.1. Phase I: Habituation
On PND 90, subjects were deprived of water for 23 h and 40 min and on the following day were given 20-min access to tap water in the stainless-steel cages. Following this exposure, the animals were returned to their home cages. This procedure was repeated for 6 days to allow water consumption to stabilize. Fluid was presented in graduated 50-ml Nalgene tubes and indexed by the difference between the pre- and post-consumption volumes.
2.4.2. Phase II: Pre-exposure
On PND 97, the subjects were presented with 20-min access to tap water. They were matched on water consumption and assigned to two groups such that water consumption was comparable. Five hours later, they were given an injection of either MDPV (1.8 mg/kg) or equivolume saline (n = 32 per group). The dose was based on previous research in which 1.8 mg/kg MDPV induced intermediate taste avoidance (King et al., 2015). Following the injection, subjects were returned to their home cages. On each of the following 3 days, subjects were given 20-min water access but were not injected. This cycle of one injection day followed by 3 water-recovery days was repeated until all subjects received a total of five complete cycles over the course of 20 days.
2.4.3. Phase III: Conditioning
On Day 1 of this phase (PND 117), subjects were given 20-min access to a novel saccharin solution during their fluid-access period. Immediately following saccharin consumption, animals in each pre-exposure group were assigned to one of four drug groups such that consumption was comparable. This resulted in a total of eight groups, i.e., VV, VM, VC, VL, MV, MM, MC and ML (n = 8 per group). The first letter in each group name denotes the pre-exposure condition (vehicle or MDPV), and the second letter indicates the injection given during conditioning (vehicle, MDPV, cocaine or LiCl). Immediately after saccharin consumption and group assignments, animals were transported to an adjacent room where they were given their corresponding injections. Once injections were completed, subjects were returned to their home cages. For the next 3 days, rats were given access to tap water for 20 min but not injected. This cycle of 20-min saccharin access paired with a drug/vehicle injection (Day 1) followed by 20-min water access on the subsequent 3 days (Day 2–4) constituted one conditioning cycle. This procedure was repeated for four additional cycles.
2.5. Statistical Analysis
To assess whether MDPV pre-exposure affected fluid consumption or growth, water intake and body weight over pre-exposure were analyzed using a 2 × 23 mixed model ANOVA with a between-subjects factor of Pre-exposure Drug (vehicle, MDPV) and a within-subjects factor of Pre-exposure Day (PND 94–116). In the event of a Pre-exposure Drug × Pre-exposure Day interaction, differences between groups were tested with one-way ANOVAs with Bonferroni corrections. During CTA conditioning, differences in saccharin consumption were analyzed using a 2 × 4 × 5 mixed-model ANOVA with between-subject factors of Pre-exposure Drug (vehicle, MDPV) and Conditioning Drug (vehicle, MDPV, cocaine, LiCl) and a within-subjects factor of Trial (1–5). In the case of a three-way interaction, simple effects of Trial at each Pre-exposure Drug and Conditioning Drug (multivariate analysis) and the effects of Pre-exposure Drug at each Conditioning Drug and Trial (univariate analysis) were assessed and followed with Bonferonni-corrected multiple comparisons as warranted. Significance levels were set at p ≤ 0.05.
3. Results
3.1 Pre-exposure
The 2 × 23 mixed model ANOVA on water consumption over pre-exposure revealed a significant effect of Pre-exposure Day [F(22,1320) = 10.503, p ≤ 0.05], but not of Pre-exposure Drug. There was no significant Pre-exposure Drug × Pre-exposure Day interaction. Although there was a significant effect of Pre-exposure Day, a repeated measures one-way ANOVA with Bonferroni-corrected contrasts revealed that there were no significant changes in consumption over days compared to the baseline consumption on Day 94 (see Figure 1).
Figure 1.
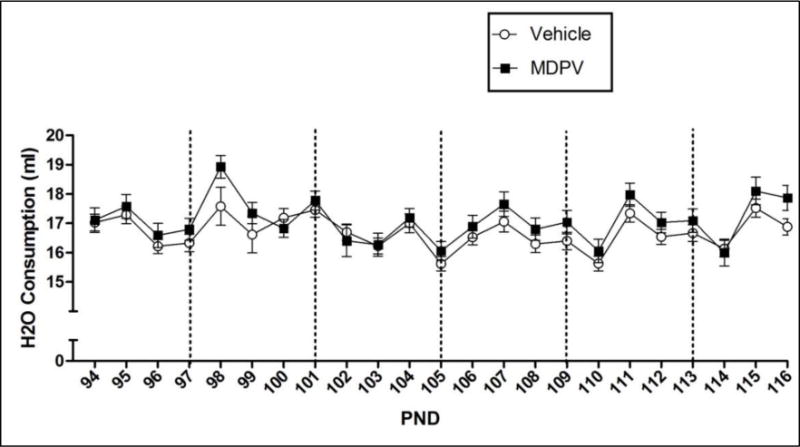
Mean (+/− SEM) water consumption (ml) of groups pre-exposed to water or MDPV during the pre-exposure period (drug/vehicle pre-exposure days are designated by vertical dotted lines). There was no effect of Pre-exposure Drug nor a significant interaction of Pre-exposure Drug and Pre-exposure Day. Significance was p ≤ 0.05; n = 32 per group.
Similarly, the 2 × 23 mixed model ANOVA on body weight over pre-exposure revealed a significant effect of Pre-exposure Day [F(22,1320) =13.873, p ≤ 0.05], but not Pre-exposure Drug or a Pre-exposure Drug × Pre-exposure Day interaction. There was no significant Pre-exposure Drug × Pre-exposure Day interaction. Although there was a significant effect of Pre-exposure Day, a repeated measures one-way ANOVA with Bonferroni-corrected contrasts revealed that there were no significant changes in body weights over days compared to the baseline weight on Day 94 (see Figure 2).
Figure 2.
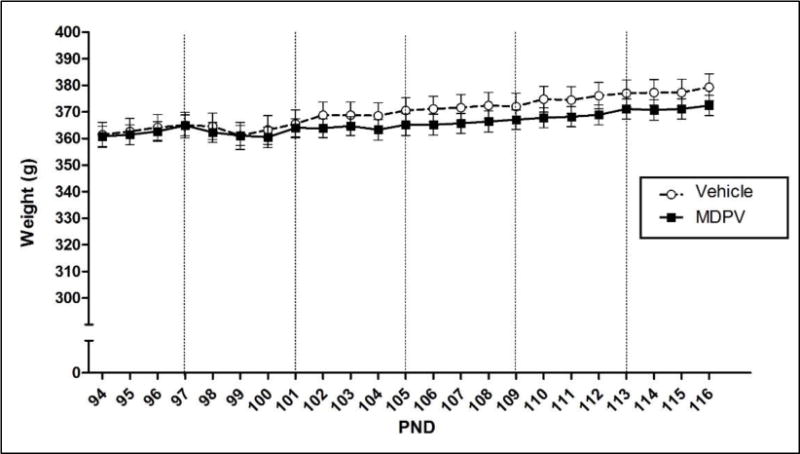
Mean (+/− SEM) body weight (g) of groups pre-exposed to water or MDPV during the pre-exposure period (drug/vehicle pre-exposure days are designated by vertical dotted lines). There was no effect of Pre-exposure Drug nor a significant interaction of Pre-exposure Drug and Pre-exposure Day. Significance was p ≤ 0.05; n = 32 per group.
3.2 Conditioned Taste Avoidance
MDPV pre-exposure attenuated the avoidance induced by both MDPV and cocaine, but had no effect on avoidance induced by LiCl. The 2 × 4 × 5 mixed model ANOVA on saccharin consumption over conditioning revealed a significant main effect of Trial [F(4,216) = 100.281, p ≤ 0.05] and Conditioning Drug [F(12,216) = 22.597, p ≤ 0.05], as well as significant Pre-exposure Drug × Conditioning Drug [F(3,54) = 4.134, p ≤ .05], Trial × Conditioning Drug [F(3,54) = 66.971, p ≤ 0.05] and Trial × Pre-exposure Drug × Conditioning Drug [F(3,54) = 3.536, p ≤ 0.05] interactions. There was no significant interaction of Trial × Pre-exposure Drug. Given the significant three-way interaction, univariate analysis and Bonferonni-corrected multiple comparisons examined the differences between groups on individual trials.
Vehicle pre-exposed groups
Animals pre-exposed to the MDPV vehicle and injected with vehicle following saccharin consumption (Group VV) consumed approximately 12 ml on the initial access to saccharin (see Figure 3; open circles, top left panel). These subjects significantly increased saccharin consumption over conditioning, drinking approximately 18 ml on each remaining trial (all ps ≤ 0.05). Animals injected with MDPV (Group VM; open circles, top right panel), cocaine (Group VC; open circles, bottom left panel) and LiCl (Group VL; open circles, bottom right panel) also drank approximately 12 ml on the initial exposure to saccharin. Each of these groups significantly decreased saccharin consumption by Trial 2, indicative of the acquisition of a conditioned taste avoidance (all ps ≤ 0.05). Although there were no differences in saccharin consumption among the vehicle pre-exposed groups on Trial 1, all drug-injected subjects under the vehicle-pre-exposed condition (Groups VM, VC and VL) drank less saccharin than Group VV on Trials 2–5 (all ps ≤ 0.05). There were no differences among the drug-injected groups.
Figure 3.
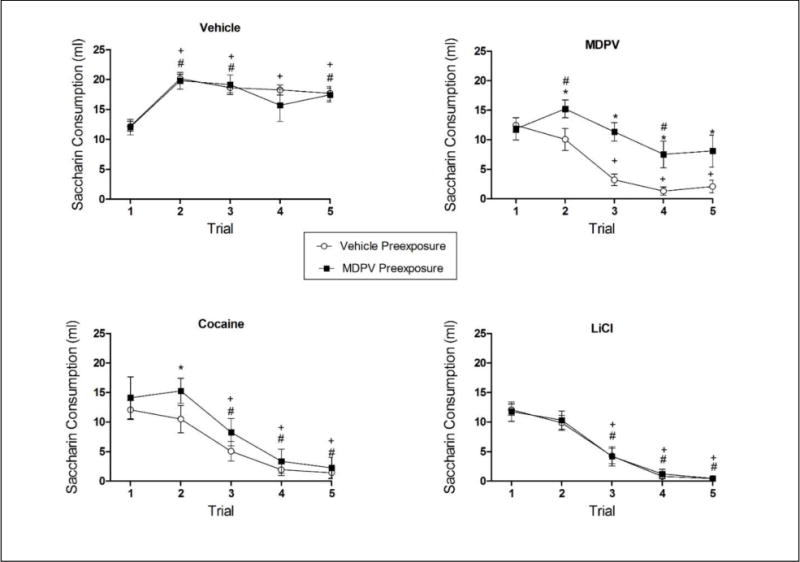
Mean (+/− SEM) saccharin consumption over taste avoidance conditioning for animals pre-exposed to vehicle (open circle) and MDPV (closed squares) and injected during conditioning with vehicle (top left panel), MDPV (top right panel), cocaine (bottom left panel) and LiCl (bottom right panel). +significantly different from Trial 1 (vehicle-pre-exposed); #significantly different from Trial 1 (MDPV pre-exposed); *significant difference between vehicle and MDPV pre-exposed groups. Significance was p ≤ 0.05; n = 8 per group.
MDPV pre-exposed groups
Animals pre-exposed to MDPV and injected with vehicle following saccharin consumption (Group MV) consumed approximately 12 ml on the initial access to saccharin and significantly increased saccharin consumption on the remaining trials (all ps ≤ 0.05 except Trial 4), indicating that MDPV pre-exposure had no effect on saccharin consumption in animals for which saccharin was paired with a vehicle injection (see Figure 3; closed squares, top left panel). Animals pre-exposed to MDPV and injected with MDPV (Group MM; closed square, top right panel), cocaine (Group MC; closed square, bottom left panel) and LiCl (Group ML; closed square, bottom right panel) during conditioning also drank approximately 12 ml on the initial exposure to saccharin. Each of these groups eventually decreased saccharin consumption over trials, although they differed in the degree and rate at which this occurred. For example, subjects in Group MM increased saccharin consumption on Trial 2 and decreased from their own baseline only on Trial 4, whereas subjects in Groups MC and ML displayed significant decreases in saccharin consumption from their own baseline on Trials 3–5 (all ps ≤ 0.05). Further, Group ML drank less saccharin than Group MV on the second conditioning trial, although Groups MM and MC did not differ from Group MV on this exposure. With repeated conditioning, all drug-conditioned groups displayed a taste avoidance, drinking significantly less than Group MV (all ps ≤ 0.05).
MDPV vs. vehicle pre-exposure
The fact that animals pre-exposed to MDPV and conditioned with MDPV and cocaine did not differ from their vehicle-conditioned controls on Conditioning Trial 2, while vehicle-pre-exposed animals conditioned with these drugs did differ (see above), suggests that avoidance was retarded in these MDPV pre-exposed groups. This was also evident when direct comparisons were made between the two pre-exposure conditions. Specifically, vehicle and MDPV pre-exposed animals injected with vehicle during conditioning (Groups VV and MV, respectively) did not differ at any point (see Figure 3; top left panel,). On the other hand, subjects pre-exposed to MDPV and conditioned with MDPV (Group MM) displayed weaker taste avoidance on Trials 2–5 relative to vehicle pre-exposed subjects conditioned with MDPV (Group VM; see Figure 3; top right panel). Subjects pre-exposed to MDPV and conditioned with cocaine (Group MC) displayed retarded acquisition relative to their vehicle-pre-exposed controls conditioned with cocaine (Group VC), drinking significantly more saccharin on Trial 2 (p ≤ 0.05), although showing no differences on the subsequent conditioning trials (see Figure 3; bottom left panel). On the other hand, animals conditioned with LiCl drank comparable amounts of saccharin independent of MDPV (Group ML) or vehicle (Group VL) pre-exposure, i.e., there was no evidence of MDPV pre-exposure on LiCl-induced taste avoidance (see Figure 3; bottom right panel).
4. Discussion
Given that the balance of the aversive and rewarding effects of drugs has been suggested to contribute to drug taking, it is important to determine which factors may influence the strength of each of these and, thus, their overall balance. Accordingly, the present study examined the impact of MDPV history on its own aversive effects as well as those of a related psychostimulant, cocaine, and a typical emetic, LiCl. As described, although all three compounds induced significant taste avoidance, the avoidance induced by both MDPV and cocaine was significantly attenuated by MDPV pre-exposure, but not that induced by LiCl.
The fact that MDPV pre-exposure attenuated its own ability to induce a taste avoidance parallels similar findings with a host of other drugs of abuse assessed in this basic design (for reviews, see Randich and LoLordo, 1979; Riley and Simpson, 2001). Under such conditions, the acquisition of taste avoidance and/or its asymptotic level is significantly affected, indicative of a reduction in the drug’s aversive effects. Although generally attenuated, with continued pairings of saccharin with MDPV, taste avoidance was eventually acquired indicating that while the aversive effects of the drug had been weakened with pre-exposure there remained sufficient aversive effects to induce a taste avoidance (for a related comparison, see Simpson and Riley, 2005). That pre-exposure to MDPV also attenuated cocaine-induced taste avoidance is interesting in light of the fact that the neurochemical mechanism(s) mediating cocaine’s actions are not identical to those of MDPV. Specifically, although both MDPV and cocaine inhibit the reuptake of the monoamines at their respective transporters, i.e., DAT, NET and SERT, MDPV is more potent than cocaine at inhibiting the reuptake of dopamine (MDPV: IC50 4.1 ± 0.6 nM, COC: IC50 211 ± 19 nM) and norepinephrine (MDPV: IC50 25.9 ± 5.6 nM, COC: IC50 292 ± 34 nM), and less potent at inhibiting the reuptake of serotonin (MDPV: IC50 3305 ± 485 nM, COC: IC50 313 ± 17 nM) with DAT/SERT ratios of 806:1 (MDPV) compared to 1.5:1 (cocaine) (Glennon and Young, 2016; Karch 2015; King et al., 2014b; Marusich et al., 2014; Simmler et al., 2013). Given their shared (but not identical) mechanisms of action, it is not surprising that the effects of MDPV pre-exposure on MDPV avoidance were greater than that on cocaine (for a discussion of cross-drug pre-exposure, see Serafine and Riley, 2013). The fact that with repeated conditioning trials, consumption for the MDPV pre-exposed, cocaine-injected subjects eventually decreased is similar to other work assessing the effects of cocaine history on cocaine-induced avoidance, again suggesting that the remaining aversive effects of the drug are sufficient to reduce consumption (for comparison, see Riley and Diamond, 1998; Simpson and Riley, 2005). As noted, LiCl was used as a negative control given that although it produces strong taste avoidance (see Gore-Langton et al., 2015; Nachman and Ashe, 1973), there is little evidence that it impacts the monoamines (see Jope, 1999; Stahl, 2009). In fact, it has been reported to increase catecholamine reuptake and facilitate the release of serotonin, effects quite different from that of MDPV (Meyer and Quenzer, 2013). As such, there was no expectation of any effects of MDPV pre-exposure on taste avoidance induced by LiCl. The failure of MDPV to impact LiCl-induced taste avoidance is not a function of the inability of LiCl to be affected by drug history in general. Animals pre-exposed to LiCl display significantly attenuated LiCl-induced avoidance that parallels that seen with other drugs (both classical emetics and drugs of abuse; see Riley and Simpson, 2001). The lack of attenuation with LiCl suggests that MDPV’s effects on itself and cocaine were likely due to its actions on monoamine reuptake. This discussion of the mechanism of action of MDPV relative to those of cocaine and LiCl to account for the likelihood or failure of any effects of MDPV pre-exposure must be qualified in that little is truly known about the biochemical bases of the aversive effects of any of these compounds. That is, the effects of each of these compounds in general do not necessarily indicate which of these effects mediates their ability to induce taste avoidance. Of the three compounds examined, the only one for which the aversive effects have been systematically examined is cocaine, and even here there is no consensus as to its mediation (see Serafine and Riley, 2013 for a discussion). Consequently, it remains speculative as to why MDPV attenuates the effects of cocaine but not LiCl.
Independent of the specific biochemical mechanism(s) involved, the fact that MDPV pre-exposure significantly attenuated taste avoidance induced by both MDPV and cocaine suggests a history of MDPV use may reduce or attenuate their aversive effects. Based on work with several other compounds, the attenuation of a drug’s aversive effects appears to shift the balance of their affective properties more to reward, impacting their potential use and abuse (for examples, see Camarini and Hodge, 2004; Sanders and Spear, 2007). Although it is possible that the reported changes in MDPV’s aversive effects may affect its abuse potential, there are several caveats to this position. First, given the recently reported motor sensitizing effects of cathinone pre-exposure (see Berquist et al., 2016; Gregg et al., 2013; Watterson et al., 2015), such a history could also impact MDPV’s rewarding effects (e.g., changes in MDPV-induced place preference conditioning) and until these assessments are made it is impossible to predict the direction of change in the overall balance and how the change might impact the drug’s self-administration. This is especially relevant given that the aversive and rewarding effects of drugs appear to be dissociable, i.e., changes in one effect is not necessarily paralleled by changes in the other (see Simpson and Riley, 2005; Verendeev and Riley, 2011; for a review, see Verendeev and Riley, 2012). Secondly, independent assessments of changes in drug intake need to be directly made, i.e., the effects of drug history on MDPV self-administration must be assessed to determine the potential for increased and/or escalated use. Also, given the effects of MDPV history on cocaine-induced taste avoidance, similar work needs to be extended to cocaine’s rewarding (CPP) and reinforcing (SA) effects. Further, given that the effects of drug history have been reported to be asymmetrical for some compounds, i.e., Drug A affects Drug B, but not vice versa (for a discussion, see Grakalic and Riley, 2002; for a review, see Riley and Simpson, 2001), the impact of cocaine on MDPV needs further assessment as well. It is interesting to note that several studies assessing the serial interactions between cathinones and psychostimulants (see Gregg, 2013; Watterson et al., 2016), the interaction was asymmetrical. For example, mephedrone exposure sensitizes cocaine’s motoric effects, but cocaine has no effect on motor activity induced by mephedrone (Gregg, 2013). Similarly, exposure to MDPV sensitizes methamphetamine-induced motor activity, but not vice versa (Watterson et al., 2016). Finally, it will be important to determine how general the effects of a history with bath salts are, i.e., whether the work reported here with MDPV will generalize to other bath salts which have been reported to have different effects in other behavioral designs (e.g., see Bonano et al., 2014; Creehan et al., 2015; Gregg and Rawls, 2014; Karlsson et al., 2014; Schindler et al., 2015; Watterson et al., 2012a, 2014a; for a discussion of differences between enantiomers of MDPV, see Gannon et al., 2016). By examining the effects of various factors such as drug history on the rewarding and aversive effects of the bath salts, insights into their abuse potential (and thereby procedures to reduce this vulnerability) might be gained.
Acknowledgments
This work was supported by a grant from the Mellon Foundation to Anthony L. Riley.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology. 2015;232:3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde S, Huang P, Creehan K, Dickerson TJ, Taffe M. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotostimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Solis E, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL. Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci. 2014;34(46):15150–15158. doi: 10.1523/JNEUROSCI.3223-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Traxler HK, Mahler AM, Baker LE. Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinine (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug Alcohol Depend. 2016;164:128–134. doi: 10.1016/j.drugalcdep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Hodge CW. Ethanol preexposure increases ethanol self-administration in C57and DBA/2J mice. Pharmacol Biochem Behav. 2004;79:623–632. doi: 10.1016/j.pbb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoco. 2007;2(11):2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Cesar A weekly FAX from the center for substance abuse research. 2015;24(9):2004–2005. [Google Scholar]
- Colechio EM, Imperio CG, Grigson PS. Once is too much: conditioned aversion develops immediately and predicts future cocaine self-administration behavior in rats. Behav Neurosci. 2014;19(2):161–169. doi: 10.1037/a0036264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–97. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 387–421. [Google Scholar]
- Davis CM, Riley AL. The effects of cocaine preexposure on cocaine-induced taste aversion learning in Fischer and Lewis rat strains. Pharmacol Biochem Behav. 2007;87:198–202. doi: 10.1016/j.pbb.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Fomenko V, Kaganovsky K, Shelton K, Wenzel JM. On the positive and negative affective responses to cocaine and their relation to drug self-administration in rats. Psychopharmacology. 2015;232:2363–2375. doi: 10.1007/s00213-015-3873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari CM, O’Connor DA, Riley AL. Cocaine-induced taste aversions: effect of route of administration. Pharmacol Biochem Behav. 1991;38:267–271. doi: 10.1016/0091-3057(91)90277-9. [DOI] [PubMed] [Google Scholar]
- Fouriezos G, Nawiensniak E. A comparison of two methods designed to rapidly estimate thresholds of rewarding brain stimulation. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; New York: 1987. pp. 447–462. [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused “bath salt” constituent 3, 4-methylenedioxypyrovalerone in mice: drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther. 2016;356:615–623. doi: 10.1124/jpet.115.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP) Brain Res. 2016 doi: 10.1016/j.brainresbull.2016.04.011. http://doi.org/10.1016/j.brainresbull.2016.04.011. [DOI] [PMC free article] [PubMed]
- Gore-Langton JK, Flax SM, Pomfrey RL, Wetzell BB, Riley AL. Measures of the aversive effects of drugs: a comparison of conditioned taste and place aversions. Pharmacol Biochem Behav. 2015;134:99–105. doi: 10.1016/j.pbb.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Grakalic I, Riley AL. Asymmetric serial interactions between ethanol and cocaine in taste aversion learning. Pharmacol Biochem Behav. 2002;73(4):787–795. doi: 10.1016/s0091-3057(02)00905-x. [DOI] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz AB, Rawls SM. Mephedrone interactions with cocaine: prior exposure to “bath salt” constituent enhances cocaine-induced locomotor activation in rats. Behav Pharmacol. 2013;24(8):684–688. doi: 10.1097/FBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Rawls SM. Behavioral pharmacology of designer cathinones: a review of the preclinical literature. Life Sci. 2014;97:27–30. doi: 10.1016/j.lfs.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry. 1999;4:117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- Karch SB. Cathinone neurotoxicity (The “3M’s”) Curr Neuropharmacol. 2015;13:21–25. doi: 10.2174/1570159X13666141210225009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol. 2014;115(5):411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- King HE, Riley AL. The affective properties of synthetic cathinones: the role of reward and aversion in drug abuse. In: Bauman M, editor. Neuropharmacology of New Psychoactive Substances: Current Topics in Behavioral Neuroscience. Springer-Verlag; New York: 2016. pp. 1–17. [Google Scholar]
- King HE, Riley AL. A history of morphine-induced taste aversion learning fails to affect morphine-induced place preference conditioning in rats. Learn Behav. 2013;41(4):433–442. doi: 10.3758/s13420-013-0118-6. [DOI] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL. Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol Biochem Behav. 2015;137:16–22. doi: 10.1016/j.pbb.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depend. 2014a;146(1):116–119. doi: 10.1016/j.drugalcdep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. 3,4-methylenedioxypyrovalerone (MDPV)-induced conditioned taste avoidance in the F344/N and LEW rat strains. Pharmacol Biochem Behav. 2014b;126:163–169. doi: 10.1016/j.pbb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. Academic Press; London: 2005. pp. 71–108. [Google Scholar]
- Lett BT. The painlike effect of gallamine and naloxone differs from sickness induced by lithium chloride. Behav Neurosci. 1985;99(1):145–150. doi: 10.1037//0735-7044.99.1.145. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depen. 2012;126(1):257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, Riley AL. Age-dependent MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Dev Psychobiol. 2013;56(5):943–954. doi: 10.1002/dev.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Quenzer LF. Psychopharmacology: Drugs, the Brain and Behavior. second. Sinauer Associates, Inc; Sunderland: 2013. pp. 275–302. [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav. 1973;10:73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- Randich A, LoLordo VM. Associative and nonassociative theories of the UCS preexposure phenomenon: implications for Pavlovian conditioning. Psychol Bull. 1979;86(3):523–548. [PubMed] [Google Scholar]
- Reid LD. Tests involving pressing for intracranial stimulation as an early procedure for screening likelihood of addiction of opioids and other drugs. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; New York: 1987. pp. 447–462. [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Riley AL, Davis CM, Roma PG. Strain differences in taste aversion learning: implications for animal models of drug abuse. In: Reilly SS, Todd R, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 247–275. [Google Scholar]
- Riley AL, Diamond HF. The effects of cocaine preexposure on the acquisition of cocaine-induced taste aversions. Pharmacol Biochem Behav. 1998;63(2):193–199. doi: 10.1016/s0091-3057(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Riley AL, Jacobs WJ, LoLordo VM. Drug exposure and the acquisition and retention of a conditioned taste aversion. J Comp Physiol Psych. 1976;90(8):799–807. doi: 10.1037/h0077251. [DOI] [PubMed] [Google Scholar]
- Riley AL, Simpson GR. The attenuating effects of drug preexposure on taste aversion conditioning: generality, experimental parameters, underlying mechanisms and implications for drug use and abuse. In: Mowrer RR, Klein SB, editors. Contemporary Learning Theory. 2nd. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 2001. pp. 505–559. [Google Scholar]
- Riley AL, Tuck DL. Conditioned taste aversions: a behavioral index of toxicity. Ann N Y Acad Sci. 1985;443:272–292. doi: 10.1111/j.1749-6632.1985.tb27079.x. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga C. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res. 2012;234(1):76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967–968. doi: 10.1056/NEJMc1107097. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcoholism: Clin Exper Res. 2007;31(7):1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav. 1997;57(3):543–550. doi: 10.1016/s0091-3057(96)00447-9. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization to cocaine’s reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav. 2000;66(4):765–770. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinon (methylone) in male rats. Psychopharmacology. 2015;233(10):1981–1990. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine K, Riley AL. Cocaine-induced conditioned taste aversions: role of monoamine reuptake inhibition. In: Hall S, Uhl G, editors. Serotonin: Biosynthesis, Regulation and Health Implications. Nova Science; New York: 2013. pp. 257–292. [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–479. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Essential Psychopharmacology. third. Cambridge University Press; New York: 2009. pp. 668–672. [Google Scholar]
- Stolerman IP. Motivational effects of opioids: evidence on the role of endorphins in mediating reward or aversion. Pharmacol Biochem Behav. 1985;23:877–881. doi: 10.1016/0091-3057(85)90086-3. [DOI] [PubMed] [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology. 2015;99:538–545. doi: 10.1016/j.neuropharm.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Relationship between the rewarding and aversive effects of morphine and amphetamine in individual subjects. Learn Behav. 2011;39(4):399–408. doi: 10.3758/s13420-011-0035-5. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: history and interpretation. Neurosci Biobehav Rev. 2012:2193–2205. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Burrows BT, Hernandez RD, Moore KN, Grabenauer M, Marusich JA, Olive MF. Effects of alpha-pyrrolidinopentiophenone and 4-methyl-N-ethylcathinone, two synthetic cathinones commonly found in second-generation “bath salts,” on intracranial self-stimulation thresholds in rats. Int J Neuropsychopharmacol. 2014a;18(1):1–7. doi: 10.1093/ijnp/pyu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Olive MF. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J Addict Res Ther. 2012a;(Suppl 9) doi: 10.4172/2155-6105.S9-002. http://doi.org/10.4172/2155–6105.S9–002. [DOI] [PMC free article] [PubMed]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2012b;19(2):165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, Olive MF. Sensitization to the motor stimulant effects of 3, 4-methylenedioxypyrovalerone (MDPV) and cross-sensitization to methamphetamine rats. J Drug Alcohol Res. 2016 doi: 10.4303/jdar/235967. http://doi10.4303/jdar/235967. [DOI] [PMC free article] [PubMed]
- Watterson LR, Olive MF. Synthetic cathinones and their rewarding and reinforcing effects in rodents. Adv Neurosci. 2014b doi: 10.1155/2014/209875. http://doi:10.1155/2014/209875. [DOI] [PMC free article] [PubMed]
- Wise RA. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191(4233):1273–1276. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]


