Abstract
The aim of this study was to investigate the responses of creatine kinase (CK) and lactate dehydrogenase (LDH) after performing different resistance and aerobic exercise protocols. Twelve recreationally trained men (age, 23.2 ± 5.6 years; body mass, 84.3 ± 9.3 kg; body height, 178.9 ± 4.5 cm; and BMI, 26.3 ± 2.3 kg·m2) volunteered to participate in this study. All subjects were randomly assigned to four experimental protocols (crossover): (a) aerobic training at 60% of VO2max, (b) aerobic training at 80% of VO2max, (c) a resistance exercise (RE) session with a bi-set protocol, and (d) an RE session with a multiple sets protocol. Blood samples were collected before, immediately after and 24 hours following the experimental protocols. After 24 hours, there was a significant increase in CK for the 80% of VO2max protocol vs. the bi-set RE session (p = 0.016). Immediately after the protocols, we observed a significant increase in LDH among certain groups compared to others, as follows: multiple sets RE session vs. 60% of VO2max, bi-set RE session vs. 60% of VO2max, multiple sets RE session vs. 80% of VO2max, and bi-set RE session vs. 80% of VO2max (p = 0.008, p = 0.013; p = 0.002, p = 0.004, respectively). In conclusion, aerobic exercise performed at 80% of VO2max appears to elevate plasma CK levels more than bi-set RE sessions. However, the bi-set and multiple sets RE sessions appeared to trigger greater levels of blood LDH compared to aerobic protocols performed at 60% and 80% of VO2max.
Key words: muscle damage, resistance training, endurance training
Introduction
Creatine kinase (CK) and lactate dehydrogenase (LDH) are fragments of the myosin heavy chain (i.e., troponin I and myoglobin) and are related to muscle damage, this is because these molecules are cytoplasmatic and do not have the capacity to cross the sarcoplasmic membrane barrier (Brown et al., 1997; Willoughby et al., 2003). For this reason, increased serum concentrations of these molecules are used as an indicator of damage to muscle membrane and other tissue structures (Foschini and Prestes, 2007). Among these molecules, CK is frequently described as the best indirect marker of damage to muscle tissue, especially after resistance exercise or other exercises that require predominantly eccentric actions (Brown et al., 1997; Clarkson and Hubal, 2002; Fridén and Lieber, 1998; Koch et al., 2014).
Thus, concentric, eccentric, and static actions appear to be capable of damaging muscle (Clarkson et al., 1986; Koch et al., 2014). This damage can be specific to just a few macromolecules of tissue or result in large tears in the z-disk (Lieber and Friden, 1988), basal lamina (Koskinen et al., 2001), sarcolemma (Takekura et al., 2001), and supportive connective tissue (Stauber et al., 1990), and induce injury to contractile elements and the cytoskeleton (Roth et al., 1999, 2000).
Resistance (Rodrigues et al., 2010) and aerobic exercise (Paschalis et al., 2007) promotes CK and LDH changes that increase muscle damage after an exercise session. The magnitude of these changes appears to be related to the resistance exercise (RE) protocol (Clarkson and Hubal, 2002; Tricoli, 2001), downhill running (Clarkson and Hubal, 2002) and training status (Lieber et al., 2002).
Some studies have evaluated muscle damage by CK and/or LDH using different rest intervals between the sets (Mayhew et al., 2005; Rodrigues et al., 2010), high and low intensity (Paschalis et al., 2005), muscle contractions (Baroni et al., 2010; Fernandez-Gonzalo et al., 2014; Fridén and Lieber, 1998; Jamurtas et al., 2005), movement velocities (Chapman et al., 2006; Farthing and Chilibeck, 2003; Kleiner et al., 1996) and aerobic exercises (Bessa et al., 2016; Paschalis et al., 2007).
No data are available on the effects of different RE training methods and different aerobic exercise intensities on CK and LDH responses. However, such knowledge could help recreational or competitive athletes create effective exercise programs for muscle strength and size development. Therefore, the aim of this study was to compare CK and LDH levels after two RE training sessions (multiple sets vs. bi-sets) and two aerobic exercise sessions with different intensities (60% vs. 80% of VO2max).
Methods
Participants
Twelve recreationally trained men (age, 23.2 ± 5.6 years; body mass, 84.3 ± 9.3 kg; body height, 178.9 ± 4.5 cm; and BMI, 26.3 ± 2.3 kg·m2) volunteered to participate in this study. All subjects were randomly assigned to four experimental protocols. The sample dimension analysis was performed using G*Power 3.1 software (Faul et al., 2007). Based on a priori analysis, we adopted power of 0.80, α = 0.05, a correlation coefficient of 0.5, non-sphericity correction of 1 and an effect size of 0.37. From these values, an N of 12 subjects was calculated. The sample size was calculated based on procedures suggested by Beck (2013). This a priori statistical power analysis was conducted to reduce the likelihood of committing a type II error and to determine the minimum number of participants needed for this investigation. It was determined that the selected sample size was sufficient to provide statistical power greater than 82.8%.
Subjects were excluded if they fell in the following categories: (a) smokers, (b) individuals with some type of musculoskeletal injury in the upper or lower limbs, (c) individuals who responded positively to any of the items on the Physical Activity Readiness Questionnaire/PAR-Q (Shephard, 1988); (d) individuals who presented any medical condition that could influence the training program; and (e) individuals who did not use any nutritional supplements. All participants provided signed informed consent after being informed of the testing and training procedures to be performed during the study. The study was approved by the local Ethics Committee of Centro Universitário Metodista (protocol number 66/2012) and performed in accordance with the ethical standards of the Declaration of Helsinki.
Procedures
Data were assessed on 6 nonconsecutive days, with 72 hours between the sessions. On the first 2 days, VO2max was evaluated, anthropometric measurements and 1 repetition maximum tests (1RM) were performed. On the remaining study days, the following exercise sessions were performed to stimulate CK and LDH responses (design crossover): (a) aerobic training at 60% of VO2max, (b) aerobic training at 80% of VO2max, (c) an RE session with a bi-set protocol, and (d) an RE session with a multiple sets protocol.
VO2max Assessment
All subjects performed a session of continuous and progressive exercise to exhaustion on a treadmill ATL 10200 (Inbramed) model to determine VO2max, according to the ramp incremental method (Pollock et al., 1976).
One Repetition Maximum Test
The test protocol followed the American College of Sports Medicine recommendations (Franklin et al., 2000) using a standardized 10 min recovery period between the different test exercises. As a warm-up, each individual performed 2 sets of 5-10 repetitions at 40–60% of the individual’s perceived maximum strength. After a 1 min rest period, a second set, which consisted of 3-5 repetitions at 60–80% of perceived maximum strength, was completed. After another rest period (1 min), the strength assessments began, during which up to 5 attempts could be made; the resistance was adjusted before each new attempt. The recovery duration between the attempts was standardized at 3-5 min. The test was interrupted once the participant could not properly complete the movement, and the maximum load was recorded as the load obtained in the last complete execution. The following strategies were adopted to reduce the margin of error in the data collection procedures: (a) standardized instructions were given before the tests to ensure that the participants were aware of how to perform the entire routine; (b) the participants were instructed on proper exercise techniques; (c) all subjects received standardized verbal encouragement throughout the tests; and (d) all tests were conducted at the same time of the day for every session.
Experimental Sessions
The multiple sets RE session included the following exercises: chest press (bench Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil, with conventional bar and calibrated rings), lat pull down (Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil), knee extension (Leg Extension Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil), biceps curl (with conventional bar and calibrated rings), leg press (Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil) and triceps extension (Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil). The participants performing the multiple sets protocol completed three sets of 8-12 repetitions, with 70-80% of 1RM, 30 s of rest between the sets and 1 min of rest between the exercises.
The bi-set RE session was composed of two continuous exercises for the same muscle group, with no rest between the exercises but with 30 s of rest between the other segments. The participants performed three sets of exercises (12 repetitions total, 6 repetitions for the first exercise followed by 6 repetitions for the second exercise) in this order: chest incline + chest press (bench Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil, with conventional bar and calibrated rings), front lat pull down + behind-neck pull down (Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil), knee extension + leg press (Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil), shoulders abduction + shoulders flexion (with dumbbells), biceps curl (with conventional bar and calibrated rings) + alternated dumbbell biceps curl (with dumbbells), lying triceps extension (with conventional bar and calibrated rings) + triceps extension at machine (Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil), and standing calf raise + seated calf raise (Ajust Fitness®, Caxias do Sul, Rio Grande do Sul, Brazil). The duration of each repetition cycle for all exercises was established at 3 s (1 s for the concentric and 2 s for the eccentric muscle action).
Aerobic protocols were performed on a treadmill (ATL 10200, Inbramed) at 60% and 80% of VO2max. The treadmill speed was controlled to standardize oxygen uptake. During all exercise sessions, the participants were monitored by ergospirometry VO2000® (Aerosport Inc.) to determine caloric expenditure. The sessions were interrupted when the participant reached the 400 kcal limit, that is, not all participants reached exhaustion.
Creatine Kinase (CK) and Lactate Dehydrogenase (LDH) Assessments
CK and LDH activity was measured from serum frozen with a commercial kit, according to the manufacturer’s recommendations (Wiener Lab., Rosario, Argentina). The CK evaluation was based on the specific inhibition of a CK-M subunit with anti CK-M monoclonal antibodies. The antibodies inhibited both MM isoenzyme subunits, as M corresponded to CK-MB. The B subunits were determined using a reactive system based on an analytical technique optimized by IFCC, with N-acetylcysteine as an activator mixed with anti CK-M monoclonal antibodies. The technique was used to evaluate LDH based on the reaction of pyruvate to L-lactate. Both results (CK and LDH) were expressed as U/l. Blood samples were obtained prior to exercise, immediately post-exercise, and 24 hours after the end of the exercise protocols.
Statistical Analyses
Statistical analysis was initially performed using the Shapiro–Wilk normality test and the homoscedasticity test (Bartlett criterion).The variables showed normal distribution and homoscedasticity (p > 0.05). Analysis of variance (ANOVA) with repeated measures, followed by the Bonferroni procedure, was used to examine any possible differences in the dependent variables. Effect size (ES) was used to determine the change of magnitude between the study protocol evaluations (Rhea, 2004). The level of significance was set at p < 0.05. All statistical analyses were performed using SPSS statistical software package version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
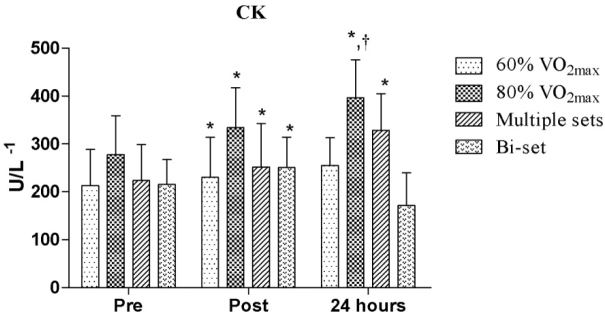
In the comparative analysis of CK, there was a significant difference between the 80% VO2max protocol and the bi-set RE session (p = 0.016) after 24 hours. In terms of the interaction time, we observed significantly increased CK activity for the 60% and 80% of VO2max protocols, as well as the multiple sets and bi-set RE sessions when comparing the pre vs. post-exercise test results (p = 0.043, ES = 0.18; p < 0.001, ES = 0.29; p = 0.001, ES = 0.29; p < 0.001; ES = 0.28, respectively). In addition, the CK activity was also greater for the 80% of VO2max protocol and multiple sets RE session at pre-exercise vs. 24 hours (p = 0.026, ES = 0.59; p = 0.043, ES = 0.71, respectively), as shown in Figure 1.
Figure 1.
Comparative analysis of creatine kinase (CK) activity between the exercise protocols
*significant difference between pre-exercise and post-exercise and between pre-exercise and 24 hours; † significant difference between 80% of VO2max 24 hours and 24 bi-set hours
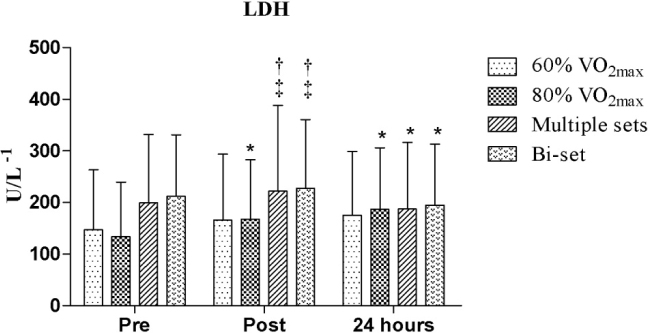
In the comparative analysis of LDH immediately after the protocols, we observed a significant increase in LDH levels among certain groups compared to others, as follows: multiple sets RE session vs. 60% of VO2max, bi-set RE session vs. 60% of VO2max, multiple sets RE session vs. 80% of VO2max, and bi-set RE session vs. 80% of VO2max (p = 0.008, p = 0.013; p = 0.002, and p = 0.004, respectively). Regarding the interaction time, we observed significantly increased LDH levels between pre and post-exercise (p = 0.022, ES = 1.54) for the 80% of VO2max protocol and (at the same intensity) between pre-exercise and 24 hours (p = 0.001, ES = 2.36), while reduced LDH levels were observed for the multiple sets and bi-set RE sessions between post-exercise and 24 hours (p = 0.001, ES = 0.22 and p = 0.002, ES = 0.18, respectively), as shown in Figure 2.
Figure 2.
Comparative analysis of lactate dehydrogenase (LDH) between the exercise protocols
*Significant difference between pre and post-exercise, pre-exercise vs. 24 hours; post-exercise vs. 24 hours; † significant difference between 60% of VO2max and multiple sets and between 60% of VO2max and bi-set; ‡ significant difference between 80% of VO2max and multiple sets and between 80% of VO2max and bi-set
Discussion
This study examined CK and LDH responses after performing different RE and aerobic protocols. To our knowledge, this study was the first to evaluate CK and LDH responses after multiple sets and bi-set RE sessions and aerobic training performed at 60% and 80% of VO2max.
Our results demonstrated significant increases in CK activity at 80% of the VO2max protocol compared to the bi-set RE session. In addition, higher CK levels were observed after 24 hours for the 80% of VO2max protocol and multiple sets RE session at pre-exercise vs. 24 hours. CK and LDH activity have been used as indicators of muscle damage after RE and aerobic exercise, in addition to indicating the degree of damage to the membranes of muscle cells (Bessa et al., 2016; Foschini and Prestes, 2007; Jamurtas et al., 2005; Kleiner et al., 1996; Paschalis et al., 2005, 2007).
The increase in CK after the 80% of VO2max protocol can be explained by the stretchshortening cycle mechanism, which generates muscle damage in the lower limbs during running (Siqueira et al., 2009). It appears that this mechanism may explain the CK elevation in the 80% of VO2max protocol compared to the bi-set RE session. These changes may take a week to return to baseline levels (Kobayashi et al., 2005). This difference may have occurred because the protocol performed at 80% of VO2máx have led the participants close to exhaustion, when compared to the 60% of 1RM protocol.
Foschini and Prestes (2007) verified the effects of the bi-set method on immune system cells, testosterone and cortisol. The authors found that the bi-set method can increase the circulating activity of total leukocytes, lymphocytes, neutrophils and monocytes (in varying proportions). These data may explain the findings of this study in terms of the CK and LDH increases observed in the bi-set method group immediately post exercise. It appears that there the immune system is restored and, consequently, the CK and LDH levels are reduced after 24 hours. That is, it is speculated that the largest increase in immune system cells can help reduce CK and LDH levels within 24 hours.
According to Rodrigues et al. (2010), RE protocols that involve moderate to high intensity exercise performed with complete maximum repetitions induce significant muscle damage to muscle fibers. This finding explains the elevated CK levels, which lasted until 24 hours after the exercises were performed in the multiple sets RE session in the present study. Paschalis et al. (2005) reported that RE, which has stress tension characteristics (high loads with 6 to 12 repetitions), promoted severe micro damage in muscle tissue at the cellular level and, therefore, resulted in a higher activity of CK. Another point that deserves attention is that lower speeds especially in the eccentric phase promote greater muscle damage (Foschini and Prestes, 2007). Considering that the speed of the eccentric and concentric phases used in this study was low (1 s concentric and 2 s eccentric), the LDH levels decreased by 24 hours at inferior levels when compared to the pretest.
Another important finding of the current study is related to the LDH response. Significant increases in LDH were observed when comparing the RE sessions (multiple sets and bi-set) with the aerobic protocols (60% and 80% of VO2max) immediately post-exercise; in addition, significant increases in LDH were observed after the 80% of VO2max protocol (up to 24 hours), and reductions were observed after the RE sessions (immediately post-exercise vs. 24 hours).
Kobayashi et al. (2005) demonstrated that aerobic exercise, such as running, may promote increased LDH activity for 12 to 24 hours. Thus, it is suggested that these immunological and hormonal changes can occur because of increased training volume and intensity, which are associated with stress tension in both types of exercise (i.e., aerobic and resistance) (Walsh et al., 2011).
This statement can justify a higher LDH activity immediately after exercise. It seems that high LDH levels last longer when individuals perform aerobic exercise at 80% of VO2max; however, this response tends to decrease in 24 hours after RE sessions.
In view of the presented results, our study has some limitations. First, as the study adopted energy expenditure of 400 kcal as a reference to standardize the end of the session, not all participants reached exhaustion. Second, this data is directed to recreationally trained participants, as elite athletes may respond in a different way given the degree of physical fitness.
Conclusions
Aerobic exercise performed at 80% of VO2max appears to elevate CK levels compared to the bi-set RE session. However, the bi-set and multiple sets RE sessions appear to lead to greater levels of LDH compared to protocols performed at 60% and 80% of VO2max. Thus, the development of new studies to verify CK and LDH acute and chronic responses is important, particularly studies involving elite athletes, various training methods, different exercises and intensities.
Acknowledgements
This work was supported by NanoSTIMA: Macro-to-Nano Human Sensing: Towards Integrated Multimodal Health Monitoring and Analytics (NORTE-01-0145-FEDER-000016), co-financed by the Fundo Europeu de Desenvolvimento Regional (FEDER) through NORTE 2020.
References
- Baroni BM, Junior ECPL, De Marchi T, Lopes AL, Salvador M, Vaz MA.. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol. 2010;110:789–796. doi: 10.1007/s00421-010-1562-z. [DOI] [PubMed] [Google Scholar]
- Beck TW.. The importance of a priori sample size estimation in strength and conditioning research. J Strength Cond Res. 2013;27:2323–2337. doi: 10.1519/JSC.0b013e318278eea0. [DOI] [PubMed] [Google Scholar]
- Bessa A, Oliveira VN, De Agostini GG, Oliveira RJS, Oliveira ACS, White G, Wells G, Teixeira DNS, Espindola FS, Mineiro U.. Exercise intensity and recovery: Biomarkers of injury, inflammation and oxidative stress. J Strength Cond Res. 2016;30:311–319. doi: 10.1519/JSC.0b013e31828f1ee9. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Child RB, Day SH, Donnelly AE.. Exercise-induced skeletal muscle damage and adaptation following repeated bouts of eccentric muscle contractions. J Sports Sci. 1997;15:215–222. doi: 10.1080/026404197367498. [DOI] [PubMed] [Google Scholar]
- Chapman D, Newton M, Sacco P, Nosaka K.. Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int J Sports Med. 2006;27:591–598. doi: 10.1055/s-2005-865920. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Byrnes WC, McCormick KM, Turcotte LP, White JS.. Muscle soreness and serum creatine kinase activity following isometric, eccentric, and concentric exercise. Int J Sports Med. 1986;7:152–155. doi: 10.1055/s-2008-1025753. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Hubal MJ.. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- Farthing JP, Chilibeck PD.. The effects of eccentric and concentric training at different velocities on muscle hypertrophy. Eur J Appl Physiol. 2003;89:578–586. doi: 10.1007/s00421-003-0842-2. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A.. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalo R, Lundberg TR, Alvarez-Alvarez L, Paz JA.. Muscle damage responses and adaptations to eccentric-overload resistance exercise in men and women. Eur J Appl Physiol. 2014;114:1075–1084. doi: 10.1007/s00421-014-2836-7. [DOI] [PubMed] [Google Scholar]
- Foschini D, Prestes J.. Acute hormonal and immune responses after a bi-set strength training. Fit Perform J. 2007;6:38–44. [Google Scholar]
- Franklin BA, Whaley MH, Howley ET. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- Fridén J, Lieber RL.. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell Tissue Res. 1998;293:165–171. doi: 10.1007/s004410051108. [DOI] [PubMed] [Google Scholar]
- Jamurtas AZ, Theocharis V, Tofas T, Tsiokanos A, Yfanti C, Paschalis V, Koutedakis Y, Nosaka K.. Comparison between leg and arm eccentric exercises of the same relative intensity on indices of muscle damage. Eur J Appl Physiol. 2005;95:179–185. doi: 10.1007/s00421-005-1345-0. [DOI] [PubMed] [Google Scholar]
- Kleiner DM, Worley ME, Blessing DL.. Creatine kinase response to various protocols of resistance exercise. J Strength Cond Res. 1996;10:15–19. [Google Scholar]
- Kobayashi Y, Takeuchi T, Hosoi T, Yoshizaki H, Loeppky JA.. Effect of a marathon run on serum lipoproteins, creatine kinase, and lactate dehydrogenase in recreational runners. Res Q Exerc Sport. 2005;76:450–455. doi: 10.1080/02701367.2005.10599318. [DOI] [PubMed] [Google Scholar]
- Koch AJ, Pereira R, Machado M.. The creatine kinase response to resistance exercise. J Musculoskelet Neuronal Interact. 2014;14:68–77. [PubMed] [Google Scholar]
- Koskinen SOA, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TES.. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1292–R1300. doi: 10.1152/ajpregu.2001.280.5.R1292. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J.. Selective damage of fast glycolytic muscle fibres with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand. 1988;133:587–588. doi: 10.1111/j.1748-1716.1988.tb08446.x. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Shah S, Fridén J.. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin Orthop Relat Res. 2002;403:S90–S99. doi: 10.1097/00003086-200210001-00011. [DOI] [PubMed] [Google Scholar]
- Mayhew DL, Thyfault JP, Koch AJ.. Rest-interval length affects leukocyte levels during heavy resistance exercise. J Strength Cond Res. 2005;19:16–22. doi: 10.1519/R-14113.1. [DOI] [PubMed] [Google Scholar]
- Paschalis V, Giakas G, Baltzopoulos V, Jamurtas AZ, Theoharis V, Kotzamanidis C, Koutedakis Y.. The effects of muscle damage following eccentric exercise on gait biomechanics. Gait Posture. 2007;25:236242. doi: 10.1016/j.gaitpost.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Paschalis V, Koutedakis Y, Jamurtas AZ, Mougios V, Baltzopoulos V.. Equal volumes of high and low intensity of eccentric exercise in relation to muscle damage and performance. J Strength Cond Res. 2005;19:184–188. doi: 10.1519/R-14763.1. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC.. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- Rhea MR.. Determining the magnitude of treatment effects in strength training research through the use the effect size. J Strength Cond Res. 2004;18:918–920. doi: 10.1519/14403.1. [DOI] [PubMed] [Google Scholar]
- Rodrigues BM, Dantas EHM, De Salles BF, Miranda HL, Koch AJ, Willardson JM, Simão R.. Creatine kinase and lactate dehydrogenase responses after upper-body resistance exercise with different rest intervals. J Strength Cond Res. 2010;24:1657–1662. doi: 10.1519/JSC.0b013e3181d8e6b1. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA.. High-volume, heavy-resistance strength training and muscle damage in young and older women. J Appl Physio. 2000;88:1112–1118. doi: 10.1152/jappl.2000.88.3.1112. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA.. Ultrastructural muscle damage in young vs. older men after high-volume, heavy-resistance strength training. J Appl Physio. 1999;86:1833–1840. doi: 10.1152/jappl.1999.86.6.1833. [DOI] [PubMed] [Google Scholar]
- Shephard RJ.. PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5:185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- Siqueira LO, Muccini T, Dall Agnol I, Filla L, Tibbola P, Luvison A, Costa L, Moreira JCF.. Serum chemistry test and urinalysis parameter analysis in half marathon athletes. Arq Bras Endocrinol Metab. 2009;53:844–852. doi: 10.1590/s0004-27302009000700008. [DOI] [PubMed] [Google Scholar]
- Stauber WT, Clarkson PM, Fritz VK, Evans WJ.. Extracellular matrix disruption and pain after eccentric muscle action. J Appl Physio. 1990;69:868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- Takekura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N.. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation–contraction coupling in rat skeletal muscle. J Physiol. 2001;533:571–583. doi: 10.1111/j.1469-7793.2001.0571a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoli V.. Mechanisms involved in delayed onset muscle soreness etiology. Rev Bras Ciên Mov. 2001;9:39–44. [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop N, Fleshner M, Green C, Pedersen BK, Hoffman-Goete L.. Position statement part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Willoughby DS, McFarlin B, Bois C.. Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med. 2003;24:15–21. doi: 10.1055/s-2003-37197. [DOI] [PubMed] [Google Scholar]