Abstract
We investigated the effects of fluid ingestion during exercise in different environments on the serum brain-derived neurotrophic factor and cognition among athletes. Ten collegiate male athletes (soccer, n = 5; rugby, n = 5) were enrolled, and they completed running tests in the following four conditions (60 min each): 1) thermoneutral temperature at 18°C (group 18); 2) high ambient temperature at 32°C without fluid ingestion (group 32); 3) high ambient temperature at 32°C with water ingestion (group 32+W); and 4) high ambient temperature at 32°C with sports drink ingestion (group 32+S). Serum brain-derived neurotrophic factor levels significantly increased in group 18 immediately after exercise when compared with those at rest and were significantly higher than those in group 32 immediately and 60 min after exercise (p < 0.05). In the Stroop Color and Word Test, significantly increased Word, Color, and Color-Word scores were observed in group 18 immediately after exercise compared to those at rest (p < 0.05). However, the Color-Word score appeared to be significantly lower in group 32 immediately after exercise compared to the other groups (p < 0.05) and at 60 min post-exercise compared to group 18 (p < 0.05). We found that the exercise performed in a thermoneutral environment improved cognitive function, but the exercise performed in a hot environment did not. The differences according to the exercise environment would be largely affected by brain-derived neurotrophic factor, and fluid ingestion regardless of the type of drink (water or sports beverage) was assumed to have contributed to the improvement in cognitive function caused by exercising in a hot environment.
Key words: acute exercise, heat stress, fluid replacement, cognitive function
Introduction
Major international sporting events such as the Summer Olympics and FIFA World Cup are sometimes held during the summer season in the northern hemisphere, which exposes players to high ambient conditions for a long time (Racinais et al., 2015). Hot environments can induce impairment of endurance performance, unlike environments with milder/lower temperatures (Maughan, 2010). Moreover, it can induce hyperthermia by excess dehydration (Crandall and González-Alonso, 2010), which affects cognitive function by decreased hydration status and body temperature (Bandelow et al., 2010). In particular, a rise in body temperature due to prolonged exercise in a high intensity or at a high ambient temperature generally results in dehydration through perspiration for homeostasis of body temperature (Wilmore and Costill, 2004). Plasma volume decreases by up to 37% after high-intensity exercise at a high ambient temperature (Nadel et al., 1990). The consequences of body fluid loss >2% of one’s body weight by high heat and exercise include impaired cognitive functions (Grandjean and Grandjean, 2007). Participation in soccer games by men and boys younger than 19 years were respectively the first and fourth reasons of exertional heat-related injuries according to the large epidemiological survey by Nelson et al. (2011). Hence, alternative strategies to reduce thermal stress in these sports events are required.
In contrast, other studies reported that exercising in the absence of intense thermal stress and dehydration enhances memory and cognitive functions by increasing secretions of proteins associated with neurogenesis such as serotonin (5-HT) and insulin-like growth factor-1 (IGF-1) (Mattson et al., 2007). IGF-1 and 5-HT play an important role in the expression of neurotrophic factors such as the brain-derived neurotrophic factor (BDNF) (Carro et al., 2000; Mattson et al., 2004). This was evident after the artificial injection of IGF-1 into the peripheral blood vessel, which increased the expression of BDNF in the hippocampus of the brain after exercise (Carro et al., 2000), and 5-HT was crucially involved, along with noradrenaline, in the regulatory mechanism that governed BDNF expression in the brain (Mattson et al., 2004). BDNF is an important neurotrophic protein expressed in various regions of the brain, and it enhances neural growth and maturation, synaptogenesis memory formation, and consolidation (Adachi et al., 2014; Ding et al., 2006; Mattson et al., 2004). BDNF reportedly increased in response to acute exercise (Ferris et al., 2007). Additionally, a previous study showed a high coefficient (r = 0.81) between the serum BDNF level and cortical BDNF level among rats (Karege et al., 2002); therefore, it is assumed that serum BDNF is a surrogate measure of cortical BDNF and brain functions in mammals.
As discussed, previous studies generally reported impaired cognitive functions after dehydration; however, the results from the studies examining the effects of fluid replacement after dehydration on cognitive functions are very limited. It was also reported that exercise at a high ambient temperature caused hyperthermia (Mora-Rodriguez, 2012), and a high level of hyperthermia impaired cognitive function (Racinais et al., 2008); yet, mild heat strain improved it (Caldwell et al., 2012). In addition, water intake during exercise at a high ambient temperature can alleviate the rise in core temperature (Wilmore and Costill, 2004), and especially the ingestion of sports drinks containing carbohydrates and electrolytes was more effective than ingesting water (Bergeron et al., 2006). Therefore, there is a possibility that the type of drink used for fluid ingestion during exercise at a high ambient temperature also affects BDNF, which plays an important role in cognitive function. Thus, this study aimed to investigate the effects of different fluid supplements (water vs. sports beverage) on the serum BDNF levels and cognitive functions of collegiate male rugby and soccer athletes frequently exposed to heat stress and dehydration due to prolonged exposure to hot environments, in a thermoneutral environment (18°C) vs. a hot environment (32°C).
Methods
Participants
Ten collegiate male athletes (soccer, n = 5; rugby, n = 5) who volunteered for the current study were students of the Yonsei University, Seoul, Republic of Korea. All participants were non-smokers and had no medical complications. To minimize the error of variables according to the fitness level, only subjects with aerobic capacities within the upper 10% of the maximal aerobic capacity, which is higher than 51.4 mL/kg/min of VO2max (ACSM, 2009), were included. The physical characteristics of the participants are shown in Table 1. The study protocol was approved by the ethics committee of the Physical Education department at the Yonsei University, Seoul, Republic of Korea. All participants provided written informed consent, and the study conformed to the standards set by the latest revision of the Declaration of Helsinki.
Table 1.
The physical characteristics of the participants
| Variables | Groups |
|||
|---|---|---|---|---|
| 18 | 32 | 32+W | 32+S | |
| N | 10 | |||
| Age (years) | 18.8 ± 0.2 | |||
| Body height (cm) | 175.6 ± 2.2 | |||
| Body mass (kg) | 70.7 ± 2.2 | |||
| Body fat (%) | 12.1 ± 0.7 | |||
| VO2max (mL/kg/min) | 56.6 ± 1.6 | |||
| Hemoglobin (g/dL) | 15.6 ± 0.2 | 15.4 ± 0.3 | 15.4 ± 0.2 | 15.5 ± 0.2 |
| Hematocrit (%) | 48.7 ± 0.9 | 48.8 ± 0.8 | 48.4 ± 0.7 | 48.8 ± 0.5 |
Data are presented as mean ± standard error.
Screening tests
All participants who volunteered for the current study underwent preliminary tests and medical examinations before the exercise trials in order to meet the aforementioned selection criteria (athletic careers: more than 6 years; VO2max: higher than 51.4 mL/kg/min; resting heart rate: less than 80 beats/min; systolic blood pressure: lower than 120 mmHg; diastolic blood pressure: lower than 80 mmHg; body fat: lower than 20%).
Screening tests included measurements of body height, body composition (i.e., body mass, fat mass, and fat free mass), and maximal exercising capacities (i.e., VO2max and maximal heart rate [HRmax]). Body height was measured using a stadiometer (HD, STDK Co., Japan), and body composition was assessed with a bioimpedance body composition analyzer (Inbody 220, Biospace, Seoul, Korea). For accurate analyses, all participants were asked to urinate prior to the tests and to wear shorts during the test procedures. Food and beverages were restricted to all participants 2 h prior to testing.
The VO2max measurement was performed on a treadmill (Q65, Quinton Inc., Chicago, IL, USA), according to the Bruce protocol, with increases of 2% in the incline and 1.29-1.45 km/h in the speed every 3 min from the initial incline level and speed (10% and 2.74 km/h, respectively). Gas analyses were performed using a portable ergospirometer (MetaMax 3B, Cortex, Leipzig, Germany) at every 10 s for volumes of single ventilation, oxygen uptake, carbon dioxide emission, a respiratory exchange ratio, and a breathing rate per minute. The HRmax was determined during VO2max measurements using a heart rate monitor (Polar a5, Polar, Finland).
Exercise trials
The exercise intensity for the trials was set at 75% of the heart rate reserve (HRR) for every participant, which was based on data obtained from the screening tests. According to the advanced study (Sécher and Ritz, 2012) that reported that 2% of dehydration with regard to exercise intensity and duration could affect cognitive function, exercise duration should be set to 60 minutes in order to induce more than 2% of dehydration in a hot environment. All participants completed running tests in the following four conditions: 1) a thermoneutral temperature at 18°C (group 18), 2) a high ambient temperature at 32°C without fluid ingestion (group 32), 3) a high ambient temperature at 32°C with water ingestion (group 32+W), and 4) a high ambient temperature at 32°C with sports drink ingestion (group 32+S). The exercise intensity and humidity (50%) in all trials were identical. The trials were performed in the order of groups 18, 32, 32+W, and 32+S, and each trial was separated by 7 days to avoid any transient effects on the physiological and psychological conditions of the participants. The experimental condition used a cross-over design to minimize the adaption from the repetitive exercise trials, as follows: 1) participant numbers ① ~ ⑤: Group 18 → Group 32 → Group 32+W → Group 32+S, and 2) participant numbers ⑥ ~ ⑩: Group 32 → Group 32+S → Group 18 → Group 32+W.
The experimental condition at 18°C and 50% relative humidity was considered thermoneutral for physical activity, so it was referred to as the comfort zone where a low heat stress index is reported (McArdle et al., 2005). In contrast, the condition at 32°C and 50% relative humidity was considered adverse, as it frequently results in heat cramps (ACSM, 1984). Both experimental conditions – a thermoneutral environment and a high ambient temperature – were set up using a walk-in type temperature and humidity chamber (E Series; ESPEC, Tokyo, Japan). During exercise, all participants were asked to wear only shorts to minimize the differences in body temperature and dehydration levels due to clothing.
Tympanic temperature was measured using infrared ear thermometry (Thermoscan IRT-4520, Braun, Kronberg, Germany) eight times (during rest, at every 10 min during the exercise trial, immediately after the exercise trial, and 60 min post-exercise trial) as the surrogates for core temperature.
Levels of dehydration were calculated using the difference in body weight before and immediately after the trial in group 32. The same amount of fluid (water or sports drink), according to body weight loss after group 32 completed the trials, was given to the participants for fluid ingestion with multiple breaks during exercise.
The water and sports drink (6% carbohydrate, 20.9 mEq/l Na+, 6.1 mEq/l K+, and 9.5 mEq/l CI-) were prepared at 15°C, which is the temperature at which the absorption rate and sensation of a refreshment is the best (ACSM et al., 2007b).
Blood sampling and analyses
Blood samples were collected at three occasions: during rest (Rest), immediately after the exercise (IAE), and at 60 min post-exercise (60MPE) from the volunteers’ antecubital veins. Blood samples were collected for analyses of plasma free tryptophan (f-Trp), IGF-1, and BDNF levels. An automatic haematology analyser (Sysmex XE-2100; Sysmex, Kobe, Japan) was used to calculate the changes in the plasma level (ΔPV) of haemoglobin (Hb) and haematocrit (Hct). ΔPV at IAE and 60MPE was calculated by using the formula of Dill and Costill (1974).
Plasma f-Trp levels were measured using liquid chromatography-tandem mass spectrometry. Sulfosalicylic acid (50 mg) was added to 1 µL of prepared samples for deproteinization, and then 20 µL of deproteinized samples were injected into the sample capsules. The absorbance was measured at 570 nm for detecting reacted f-Trp using a commercially available amino acid analyzer (Biochrom 20; Pharmacia Biotech, Buckinghamshire, UK).
Serum IGF-1 concentrations were determined using an IGF-1 Quantikine sandwich enzyme immunoassay (#DG100, R&D Systems, St. Louis, MO, USA). Serum samples were pretreated to release IGF-1 from the binding proteins and were diluted 100-fold with a pretreatment constituent prior to the assay. To determine well the optical density of each sample, they were read within 30 min of adding 50 µL of stop solution. The samples were read using a micro-plate reader (EMax Presion, Molecular Devices, Menlo Park, CA, USA) at 450 nm and 540 nm.
Serum BDNF concentrations were determined by the sandwich enzyme immunoassay method using a commercially available BDNF human enzyme-linked immunoassay kit (#DBD00, R&D Systems, St. Louis, MO, USA), and the measurement was performed based on the manufacturer’s instructions. The absorbance was measured at 450 nm and was corrected at 570 nm by a micro-plate reader (EMax Presion).
Cognitive function tests
For measuring cognitive functions, the Stroop Color and Word Test was performed three times: at Rest, IAE, and 60MPE, as previously described (Golden and Freshwater, 2002). The tests were comprised of Word reading (W), Color reading (C), and Color-Word reading (CW) parts. Each part contained 100 items arranged in 5 columns and 20 rows. For W, the participants were asked to read aloud three randomly arranged words (i.e., red, blue and green) written in black ink; for C, the participants were asked to read aloud the colors of red, blue and green, which appeared in three different inks; for CW, the participants were asked to read aloud the colors of the words that appeared in random order. All participants were given 45 s for each part, and the number of correct performances was counted. In addition, all participants took the pre-test more than 3 times to minimize the learning effect that occurs following repetitive testing.
Statistical analyses
Data are presented as means ± standard of error (SE). Statistical significance in tympanic temperature, blood parameters (i.e., f-Trp, IGF-1, and BDNF), and the Stroop Color and Word Test scores for the groups and measuring periods were determined using a two-way repeated analysis of variance (ANOVA) for comparisons. The simple main effects on measured variables were determined by using one-way ANOVA. The Tukey’s post hoc test was followed to conservatively locate significant differences. Statistical significance was set at p < 0.05.
Results
Tympanic temperatures
The tympanic temperature was significantly increased from Rest to IAE in all groups (p < 0.05). In addition, the tympanic temperature was significantly higher at 10 min and 20 min of exercise in group 32 than in group 18 (p < 0.05), while it was significantly higher at 30 min of exercise, IAE, and 60MPE in group 32 compared to all other groups (p < 0.05). At 40 min and 50 min of exercise, it was significantly higher in group 32 compared to groups 18 and 32+S (p < 0.05) (Figure 1).
Figure 1.
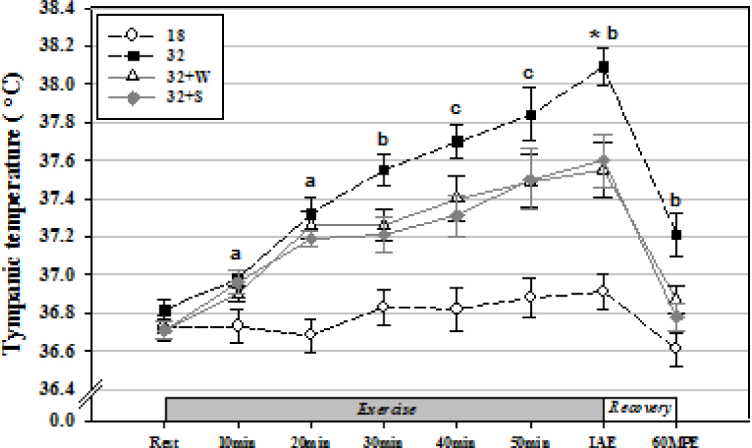
Changes in tympanic temperature by fluid ingestion with water or a sports drink during treadmill exercise in a thermoneutral environment and at a high ambient temperature. Values are mean ± standard error. IAE, immediately after exercise; 60MPE, 60 min post-exercise. *Significantly different from Rest in all groups (p < 0.05); a Significantly higher in Group 32 compared to Group 18 (p < 0.05);b Significantly higher in Group 32 compared to Groups 18, 32+W, and 32+S (p < 0.05); andc Significantly higher in Group 32 compared to Groups 18 and 32+S (p < 0.05).
Plasma free tryptophan levels
The plasma f-Trp levels were significantly increased at IAE when compared with those at rest in group 18 (p < 0.05). In addition, the plasma f-Trp levels at IAE were significantly higher in group 18 than in group 32 (p < 0.05; Figure 2A).
Figure 2.
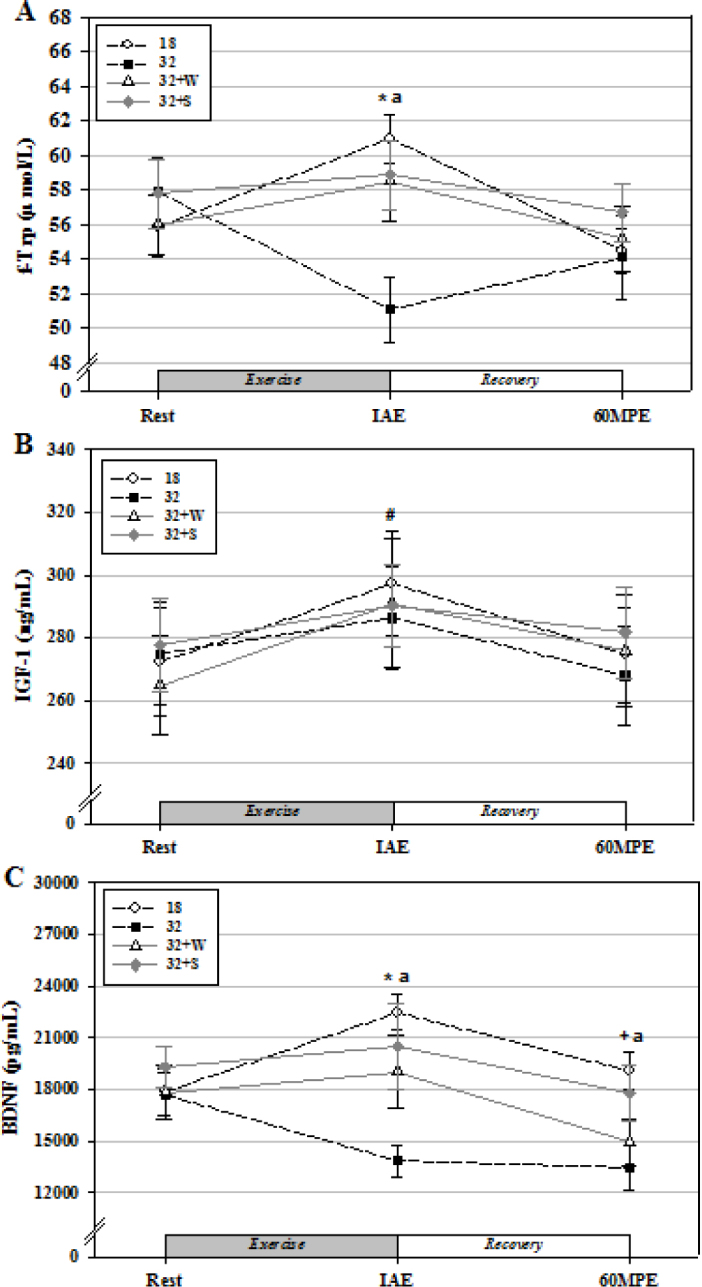
Changes in plasma f-Trp (A), serum IGF-1 (B), and BDNF (C) levels from rest to 60MPE by fluid intake of water or a sports drink during treadmill running in a thermoneutral environment and in an environment with a high ambient temperature.
Values are mean ± standard error. * Significantly different from Rest in group 18 (p < 0.05); # Significantly different from Rest in all groups (p < 0.05); + Significantly different from IAE in group 18 (p < 0.05); anda Significantly higher in group 18 compared to group 32 (p < 0.05).
Serum insulin-like growth factor-1 levels
In all the groups, the serum IGF-1 levels were significantly increased at IAE when compared with those at rest (p < 0.05). No significant differences were observed between the groups (Figure 2B).
Serum brain-derived neurotrophic factor levels
The serum BDNF levels were significantly increased at IAE when compared with those at rest, as well as at 60MPE when compared with those at IAE in group 18 (p < 0.05). In addition, the serum BDNF levels at IAE and 60MPE were significantly higher in group 18 than in group 32 (p < 0.05; Figure 2C).
Stroop Color and Word Test scores
The Word scores were significantly increased at IAE in group 18 compared to those at Rest (p < 0.05), while no significant change was observed in the other groups (Figure 3A).
Figure 3.

Changes in the Stroop Color and Word Test scores from Rest to 60MPE by fluid ingestion of water or a sports drink during treadmill running in a thermoneutral environment and at a high ambient temperature: Word (A), Color (B), and Color-Word (C). Values are mean ± standard error.
* significantly different from Rest in group 18 (p < 0.05); + significantly different from Rest in groups 18, 32+W, and 32+S (p < 0.05); # significantly different from Rest in groups 18 and 32+W (p < 0.05);asignificantly lower in group 32 compared to groups 18, 32+W, and 32+S (p < 0.05); and b significantly lower in group 32 compared to group 18 (p < 0.05).
The Color scores were significantly increased at IAE and 60MPE in group 18 compared to those at Rest (p < 0.05), while no significant change was observed in the other groups (Figure 3B). The Color-Word scores were significantly increased at IAE and 60MPE in groups 18 and 32+W compared to those at Rest (p < 0.05), while this was observed in group 32+S only at IAE (p < 0.05). In addition, the CW score was significantly lower at IAE in group 32 than that in the other groups (p < 0.05). At 60MPE, the CW score was significantly lower in group 32 than in group 18 (p < 0.05) (Figure 3C).
Discussion
Tympanic temperature is usually used to measure changes in body temperature during exercise (Lim et al., 2008), as it is a good indicator of the core temperature (Brinnel and Cabanac, 1989). The results of the current study showed that tympanic temperature significantly increased after the exercise; group 32 presented significantly higher values than group 18. These findings are consistent with those of previous studies, which reported that exercise at a high ambient temperature and humidity compared to thermoneutral conditions resulted in impaired heat control capacity, thereby inducing a higher core temperature and body fluid loss (ACSM et al., 2007a). It is assumed that the high ambient temperature condition (32°C, 50%) set in the current study impaired the heat control capacity by disturbing the convection and evaporation process, resulting in disturbed homeostasis for body temperature.
In addition, tympanic temperature from 30 minutes of exercise to IAE appeared to be significantly lower in groups 32+W and 32+S than in group 32. The exercise in a hot environment induced excessive dehydration that reduced plasma volume and a blood flow to the skin, resulting in the increase in core temperature by thermodiffusional interruption (Wilmore and Costill, 2004), but water supplementation during exercise could alleviate this increase (Grandjean and Grandjean, 2007). This study also showed that water and sports drink supplementation during exercise alleviated the increase in core temperature, which resulted in the low tympanic temperature.
It has been previously reported that glucose administered with fluid during exercise spares the liver glycogen storage and reduces the rate of gluconeogenesis, which results in an approximate 6% reduction in heat production within the liver (Hamilton et al., 1991). Moreover, increased osmotic pressure and an unbalanced sodium level together result in a rise in body temperature, and a drink containing glucose and electrolytes such as sodium attenuates an increase in osmotic pressure and maintains body fluid volume by balancing electrolyte levels (Shi and Gisolfi, 1998). Similarly, the reason why group 32+S showed significantly lower tympanic temperature than group 32 at the 40th and 50th min of exercise is that resupplementation of glucose and electrolytes through intake of sports drink lowered the core temperature. The finding of a previous study (Bergeron et al., 2006) that intake of sports drink rather than water significantly lowered core temperature during exercise supports this phenomenon.
A previous study suggested that changes in hydration status by exercise may influence cognitive functions (Maughan et al., 2007). Accordingly, the current study investigated the effects of dehydration and fluid ingestions in a high ambient temperature on cognitive functions by examining the f-Trp, which was the precursor to 5-HT, IGF-1, BDNF, and the Stroop Color and Word Test.
The results of the present study showed that in group 18, the plasma f-Trp levels were significantly increased at IAE when compared with those at rest. In general, the plasma f-Trp level is known to increase after exercise. During exercise, blood catecholamine increases while insulin and blood glucose decrease, and these phenomena stimulate lipolysis, which results in elevated non-esterified fatty acids (NEFA) levels (Badawy, 2010). Albumin has a low affinity for tryptophan while having a high affinity for NEFA, so when the concentration of NEFA increases, albumin dissociates from tryptophan and then binds to NEFA. Accordingly, plasma f-Trp becomes elevated. In the current study, plasma f-Trp increased at IAE compared to that at Rest, which may be attributed to the increased elevated NEFA levels. Of note, the plasma f-Trp levels in group 32 were significantly lower than those in group 18 at IAE. Secretion of vasopressin from the hypothalamus and posterior pituitary gland increases during exercise for homeostasis of fluid. It was previously reported that secretion increased up to 8-folds (Wade, 1984), depending on various factors, such as ambient temperature, hydration status, exercise intensity, and duration (Duncker and Bache, 2008). For example, Roy et al. (2001) reported elevated vasopressin after a 90 min exercise on a cycle ergometer at 60% of VO2max, and Melin et al. (1980) reported a 4.8–5-fold increase in vasopressin levels after exercise at an intensity higher than 80% of the VO2max. Fluid levels also affect the vasopressin level. Paik (2006) reported that no significant increase in the vasopressin level was observed when sufficient fluid was administered during a 15-min exercise at 70% of VO2max and a 4 h exercise at low intensity. Thus, changes in the vasopressin level largely depend on hydration status rather than exercise intensity or duration (Brandenberger et al., 1986). In the current study, weight loss achieved by dehydration during exercise at 75% HRR without any fluid ingestion in group 32 was approximately 3% (2.9 ± 0.1%) of body weight. This loss was more than 2-folds compared to group 18 (1.3 ± 0.2%). Although it was not examined, it is expected that vasopressin levels in group 32 would be significantly higher than these in group 18, if ΔPV (6.2 ± 1.7%) between the groups and other reported findings were considered. Increased vasopressin is associated with decreased NEFA, and consequently, plasma f-Trp (Badawy, 2010). Rofe and Williamson (1983) reported a significant reduction in NEFA levels after administering vasopressin in rats after 24 h fasting. Therefore, decreased plasma f-Trp in group 32 is presumed to be the result of increased vasopressin secretion.
In all the groups, the levels of IGF-1, which plays a critical role in BDNF expression along with 5-HT, were significantly increased at IAE when compared with those at rest. This supports the previous finding that exercise increases circulating IGF-1 levels, which is associated with neurogenesis and memory functions (Ding et al., 2006). Meanwhile, Judelson et al. (2008) conducted research on the effects of dehydration on serum IGF-1 levels with three experimental settings of euhydration, mild dehydration (2.5% of body weight), and severe dehydration (5% of body weight), and they reported the highest serum IGF-1 in the severe dehydration group after exercise. In addition, Monnier et al. (2000) reported a positive coefficient (r = 0.546) between blood viscosity and serum IGF-1. However, when considering the reports that regulation of the hippocampal BDNF depends not only on the neurotransmitter system and the neuroendocrine system (Cotman and Berchtold, 2002), but also on IGF-1 (Ding et al., 2006), there was no association between free IGF-1 and cognitive functions (Kalmijn et al., 2000). More thorough research on the relationship between IGF-1 and cognitive functions is necessary.
The current study also examined serum BDNF levels and the association with serum IGF-1 and plasma f-Trp levels; the results showed that serum BDNF levels significantly increased at IAE compared to those at Rest, and the values were restored to the level that was similar to that at Rest in group 18. This finding is consistent with the results of previous studies that reported increased serum BDNF levels after acute exercise in human participants (Ferris et al., 2007; Winter et al., 2007; Zoladz et al., 2008). Furthermore, Ferris et al. (2007) reported that serum BDNF levels in 15 healthy adults significantly increased after a graded exercise test (GXT); Winter et al. (2007) reported that serum BDNF levels among 27 healthy men significantly increased after high-intensity sprint exercise well above the lactate threshold; and Zoladz et al. (2008) reported that serum BDNF among 13 healthy men significantly increased after a VO2max test. Although BDNF of which molecular weight is 27 kDa can bypass the blood-brain barrier (BBB) (Serra-Millàs, 2016; Zoladz et al., 2008), serum BDNF can be used as a parameter for cortical BDNF levels, as serum BDNF and cortical BDNF levels showed a high coefficient (r = 0.81) in an animal study (Karege et al., 2002). Considering the reports by Zoladz et al. (2008) and Karege et al. (2002), it is assumed that increased peripheral BDNF induced by exercise in a thermoneutral environment (group 18) that circulates into the brain potentially affects brain health and cognitive functions. Serum BDNF levels in group 32 appeared to be lowest at IAE and significantly lower than those in group 18. To explain this phenomenon with respect to decreased plasma f-Trp levels, low hydration status such as dehydration increases the secretion of vasopressin, which, in turn, lowers plasma f-Trp levels. Exercise accelerates lipolysis to increase plasma f-Trp levels (Badawy, 2010). Increased plasma f-Trp is circulated into the brain by bypassing BBB (Fernstrom, 1994) to increase 5-HT, which, in turn, increases BDNF expression (Mattson et al., 2004). However, our study showed the lowest plasma f-Trp levels in group 32 among the four conditions, indicating that exercise-induced dehydration does not increase BDNF expression. Yet, it was difficult to determine whether the change in serum BDNF concentrations resulted from f-TRP, because BDNF is expressed in several non-neuronal tissues such as muscle, thymus, heart, liver, lung, and spleen as well as vascular smooth muscle cells. Furthermore, a recent study suggested that platelet activation was important in the control of BDNF release in tissues and controled peripheral BDNF levels (Serra-Millàs, 2016). In addition, increased peripheral BDNF levels caused by exercise induce the release of cerebral BDNF, and this phenomenon can be accelerated by exercising at high environmental temperatures (Goekint et al., 2011). Goekint et al. (2011) reported that serum BDNF levels were significantly increased after use of a cycle ergometer at 30°C compared with the 18°C condition, due to an increase in BBB permeability. Interestingly, the current study found the opposite result in BDNF levels compared to the study by Goekint et al. (2011). This might be because the stress levels from environmental temperature and exercise were insufficient to cause BBB leakage. The core temperature was increased to over 39°C after exercising in a 30°C environment in the study by Goekint et al. (2011), whereas the tympanic temperature (38.1 ± 0.3°C) of group 32 at IAE in this study was lower than the temperature (about 38.5°C) after exercising at 18°C in the study by Goekint et al. (2011). Taken together with the results of another study (Watson et al., 2005), these findings indicate that an increase in core temperature is important in the increase of BBB permeability.
The Stroop Color and Word Test is a popular tool for examining brain functions such as creativity, automatization, autonomic nervous system, and cognition (Golden and Freshwater, 2002), and it has been used for examining the alternation in brain functions after acute exercise in previous studies (Ferris et al., 2007; Yanagisawa et al., 2010). In the current study, the Stroop Color and Word Test was used to examine the effects of dehydration by exercise and fluid ingestion of water or sports drink during exercise in a thermoneutral environment and at a high ambient temperature on cognitive functions in accordance with changes in serum BDNF levels.
The findings showed that group 18 appeared to have significantly improved Word and Color-Word scores at IAE and a significantly improved Color score at 60MPE compared to those at Rest. Yanagisawa et al. (2010) reported that enhanced cognitive functions proven by significantly improved Stroop test scores after acute cycle ergometer exercise at 50% VO2peak were due to enhanced activation of the dorsolateral prefrontal cortex elicited by exercise. Significantly improved Stroop test scores in group 18 of the current study may be attributed to the increased cognitive functions induced by increased serum BDNF levels after an acute exercise as suggested by Ferris et al. (2007). Of note, group 32 had a significantly lower Color-Word score at IAE than the other groups, and this may be due to dehydration induced by exercise that resulted in relatively lower serum BDNF levels, which directly influenced cognitive functions. In other words, excessive dehydration increased the secretion of vasopressin, and subsequently, decreased plasma f-Trp levels did not induce a sufficient increase in BDNF expression to positively affect cognitive functions.
In contrast, it is assumed that an excessive secretion of vasopressin was prevented in two fluid replaced groups (32+W and 32+S), contributing to the relatively higher expression of BDNF to positively affect cognitive functions. In addition, dehydration induced by exercise at a high ambient temperature increases permeability of BBB and impairs normal functions of BBB (Maughan et al., 2007). These increases of BBB permeability can lower cognitive functions, but because fluid ingestion is effective at preserving BBB integrity when exercising in a high temperature environment (Tomporowski et al., 2007; Watson et al., 2006), it is assumed that the alleviation of dehydration through supplementation with water is linked to protection of the BBB, resulting in attenuation of the cognitive function decrease. Future studies should investigate BDNF and S100 calcium protein β, which are blood markers of BBB disruption to study this phenomenon in more detail. Moreover, dehydration by 2.2% of body weight is reported to be associated with decreased left lateral ventricle volume, which negatively affects brain morphology (Kempton et al., 2009). Thus, dehydration does not only suppress BDNF expression and brain functions, but it also disturbs the maintenance of normal brain morphology. These negative consequences can be attenuated by sufficient fluid ingestion. Consequently, we found that the exercise in a thermoneutral environment improved cognitive function, but the exercise in a hot environment did not. The difference in cognitive function according to exercise environment is assumed to be largely affected by BDNF, and fluid ingestion regardless of the type of drink (water or sports drink) during exercise in a hot environment can help improve cognitive function.
Conclusion
The current study investigated whether prolonged exercise at a high ambient temperature affects athletes’ cognition negatively and if fluid replacement could reduce that negative effect. It is expected that these findings will be used to provide information on the importance of fluid replacement during a competition and during training for both athletes and sport coaches experiencing excessive heat stress and dehydration resulting from prolonged exercise (e.g., soccer and/or rugby) at a high ambient temperature. For the limitations of the current study, the amount of fluid replaced during exercise (240–330 mL every 10 min) was equivalent to the amount of weight loss after exercise at a high ambient temperature and is not applicable in actual sports events. Accordingly, more research is warranted to define the optimal amount of fluid to be replaced to maintain homeostasis and the maximal exercising capacity for elite athletes.
Acknowledgements
This work was supported by the Dong-A University research fund.
References
- Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H.. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J Biol Chem. 2014;5:409–428. doi: 10.4331/wjbc.v5.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACSM’s Health-Related Physical Fitness Assessment Manual. 3rd. Baltimore: Lippincott Williams & Wilkins; 2009. American College of Sports Medicine. [Google Scholar]
- Prevention of thermal injuries during distance running. Position stand. Med J Aust. 1984;141:876–879. American College of Sports Medicine. [PubMed] [Google Scholar]
- Armstrong LE, Casa DJ, Millard-Stafford M, Moran DS, Pyne SW, Roberts WO.. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007a;39:556–572. doi: 10.1249/MSS.0b013e31802fa199. American College of Sports Medicine. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS.. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007b;39:377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- Badawy AA.. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2010;24:809–815. doi: 10.1177/0269881108098965. [DOI] [PubMed] [Google Scholar]
- Bandelow S, Maughan R, Shirreffs S, Ozgünen K, Kurdak S, Ersöz G, Binnet M, Dvorak J.. The effects of exercise, heat, cooling and rehydration strategies on cognitive function in football players. Scand J Med Sci Sports. 2010;3:148–160. doi: 10.1111/j.1600-0838.2010.01220.x. [DOI] [PubMed] [Google Scholar]
- Bergeron MF, Waller JL, Marinik EL.. Voluntary fluid intake and core temperature responses in adolescent tennis players: sports beverage versus water. Br J Sports Med. 2006;40:406–410. doi: 10.1136/bjsm.2005.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger G, Candas V, Follenius M, Libert JP, Kahn JM.. Vascular fluid shifts and endocrine responses to exercise in the heat. Effect of rehydration. Eur J Appl Physiol Occup Physiol. 1986;55:123–129. doi: 10.1007/BF00714993. [DOI] [PubMed] [Google Scholar]
- Brinnel H, Cabanac M.. Tympanic temperature is a core temperature in humans. J Therm Biol. 1989;14:47–53. [Google Scholar]
- Caldwell JN, Patterson MJ, Taylor NA.. Exertional thermal strain, protective clothing and auxiliary cooling in dry heat: evidence for physiological but not cognitive impairment. Eur J Appl Physiol. 2012;112:3597–3606. doi: 10.1007/s00421-012-2340-x. [DOI] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I.. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC.. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Crandall CG, González-Alonso J.. Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf) 2010;199:407–423. doi: 10.1111/j.1748-1716.2010.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill DB., Costill DL.. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F.. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Bache RJ.. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD.. Dietary amino acids and brain function. J Am Diet Assoc. 1994;94:71–77. doi: 10.1016/0002-8223(94)92045-1. [DOI] [PubMed] [Google Scholar]
- Ferris LT, Williams JS, Shen CL.. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- Goekint M, Roelands B, Heyman E, Njemini R, Meeusen R.. Influence of citalopram and environmental temperature on exercise-induced changes in BDNF. Neurosci Lett. 2011;494:150–154. doi: 10.1016/j.neulet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Golden C, Freshwater S. A manual for the adult stroop color and word test. Chicago, IL: Stoelting; 2002. [Google Scholar]
- Grandjean AC, Grandjean NR.. Dehydration and cognitive performance. J Am Coll Nutr. 2007;26:549–554. doi: 10.1080/07315724.2007.10719657. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Gonzalez-Alonso J, Montain SJ, Coyle EF.. Fluid replacement and glucose infusion during exercise prevent cardiovascular drift. J Appl Physiol. 1991;71:871–877. doi: 10.1152/jappl.1991.71.3.871. [DOI] [PubMed] [Google Scholar]
- Judelson DA, Maresh CM, Yamamoto LM, Farrell MJ, Armstrong LE, Kraemer WJ, Anderson JM.. Effect of hydration state on resistance exercise-induced endocrine markers of anabolism, catabolism, and metabolism. J Appl Physiol. 2008;105:816–824. doi: 10.1152/japplphysiol.01010.2007. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Janssen JA, Pols HA, Lamberts SW, Breteler MM.. A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J Clin Endocrinol Metab. 2000;85:4551–4555. doi: 10.1210/jcem.85.12.7033. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M.. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Ettinger U, Schmechtig A, Winter EM, Smith L, McMorris T, Wilkinson ID, Williams SC, Smith MS.. Effects of acute dehydration on brain morphology in healthy humans. Hum Brain Mapp. 2009;30:291–298. doi: 10.1002/hbm.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CL, Byrne C, Lee JK.. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann Acad Med Singapore. 2008;37:347–353. [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B.. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Maughan RJ.. Distance running in hot environments: a thermal challenge to the elite runner. Scand J Med Sci Sports. 2010;3:95–102. doi: 10.1111/j.1600-0838.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- Maughan RJ, Shirreffs SM, Watson P.. Exercise, heat, hydration and the brain. J Am Coll Nutr. 2007;26:604–612. doi: 10.1080/07315724.2007.10719666. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Essentials of exercise physiology. 3rd. Philadelphia: PA, Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Melin B, Eclache JP, Geelen G, Annat G, Allevard AM, Jarsaillon E, Zebidi A, Legros JJ, Gharib C., Plasma AVP. neurophysin, renin activity, and aldosterone during submaximal exercise performed until exhaustion in trained and untrained men. Eur J Appl Physiol Occup Physiol. 1980;44:141–151. doi: 10.1007/BF00421092. [DOI] [PubMed] [Google Scholar]
- Monnier JF, Benhaddad AA, Micallef JP, Mercier J, Brun JF.. Relationships between blood viscosity and insulin-like growth factor I status in athletes. Clin Hemorheol Microcirc. 2000;22:277–286. [PubMed] [Google Scholar]
- Mora-Rodriguez R.. Influence of aerobic fitness on thermoregulation during exercise in the heat. Exerc Sport Sci Rev. 2012;40:79–87. doi: 10.1097/JES.0b013e318246ee56. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Mack GW, Nose H. Influence of fluid replacement beverages on body fluid homeostasis during exercise and recovery. Carmel: Cooper Publishing; 1990. [Google Scholar]
- Nelson NG, Collins CL, Comstock RD, McKenzie LB.. Exertional heat-related injuries treated in emergency departments in the U.S., 1997-2006. Am J Prev Med. 2011;40:54–60. doi: 10.1016/j.amepre.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Paik IY. Exercise and energy metabolism. Seoul, Republic of Korea: Daehanmedia; 2006. [Google Scholar]
- Racinais S, Alonso JM, Coutts AJ, Flouris AD, Girard O, González-Alonso J, Hausswirth C, Jay O, Lee JK, Mitchell N, Nassis GP, Nybo L, Pluim BM, Roelands B, Sawka MN, Wingo J, Périard JD.. Consensus Recommendations on Training and Competing in the Heat. Sports Med. 2015;45:925–938. doi: 10.1007/s40279-015-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racinais S, Gaoua N, Grantham J.. Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol. 2008;586:4751–4762. doi: 10.1113/jphysiol.2008.157420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofe AM, Williamson DH.. Mechanism for the ‘anti-lipolytic’ action of vasopressin in the starved rat. Biochem J. 1983;212:899–902. doi: 10.1042/bj2120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy BD, Green HJ, Grant SM, Tarnopolsky MA.. Acute plasma volume expansion in the untrained alters the hormonal response to prolonged moderate-intensity exercise. Horm Metab Res. 2001;33:238–245. doi: 10.1055/s-2001-14943. [DOI] [PubMed] [Google Scholar]
- Sécher M, Ritz P.. Hydration and cognitive performance. J Nutr Health Aging. 2012;16:325–329. doi: 10.1007/s12603-012-0033-0. [DOI] [PubMed] [Google Scholar]
- Serra-Millàs M.. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J Psychiatry. 2016;6:84–101. doi: 10.5498/wjp.v6.i1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Gisolfi CV.. Fluid and carbohydrate replacement during intermittent exercise. Sports Med. 1998;25:157–172. doi: 10.2165/00007256-199825030-00003. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, Beasman K, Ganio MS, Cureton K.. Effects of dehydration and fluid ingestion on cognition. Int J Sports Med. 2007;28:891–896. doi: 10.1055/s-2007-965004. [DOI] [PubMed] [Google Scholar]
- Wade CE.. Response, regulation, and actions of vasopressin during exercise: a review. Med Sci Sports Exerc. 1984;16:506–511. doi: 10.1249/00005768-198410000-00015. [DOI] [PubMed] [Google Scholar]
- Watson P, Shirreffs SM, Maughan RJ.. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1689–1694. doi: 10.1152/ajpregu.00676.2004. [DOI] [PubMed] [Google Scholar]
- Wilmore JH, Costill DL. Physiology of sport and exercise. (3rd ed.). Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S.. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Dan I, Tsuzuki D, Kato M, Okamoto M, Kyutoku Y, Soya H.. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K.. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59:119–132. [PubMed] [Google Scholar]


