Abstract
The skeletal muscle L-type Ca2+ channel (CaV1.1), which is responsible for initiating muscle contraction, is regulated by phosphorylation by cAMP-dependent protein kinase (PKA) in a voltage-dependent manner that requires direct physical association between the channel and the kinase mediated through A-kinase anchoring proteins (AKAPs). The role of the actin cytoskeleton in channel regulation was investigated in skeletal myocytes cultured from wild-type mice, mdx mice that lack the cytoskeletal linkage protein dystrophin, and a skeletal muscle cell line, 129 CB3. Voltage dependence of channel activation was shifted positively, and potentiation was greatly diminished in mdx myocytes and in 129 CB3 cells treated with the microfilament stabilizer phalloidin. Voltage-dependent potentiation by strong depolarizing prepulses was reduced in mdx myocytes but could be restored by positively shifting the stimulus potentials to compensate for the positive shift in the voltage dependence of gating. Inclusion of PKA in the pipette caused a negative shift in the voltage dependence of activation and restored voltage-dependent potentiation in mdx myocytes. These results show that skeletal muscle Ca2+ channel activity and voltage-dependent potentiation are controlled by PKA and microfilaments in a convergent manner. Regulation of Ca2+ channel activity by hormones and neurotransmitters that use the PKA signal transduction pathway may interact in a critical way with the cytoskeleton and may be impaired by deletion of dystrophin, contributing to abnormal regulation of intracellular calcium concentrations in dystrophic muscle.
Keywords: calcium channel, protein phosphorylation, muscular dystrophy
Voltage-gated Ca2+ channels present in most cells play a critical role in converting cellular electrical activity into an intracellular Ca2+ signal. Neurotransmitter and hormone release from neurons and endocrine cells, gene expression in many cell types, and contraction of cardiac, smooth, and skeletal muscle are all dependent on the activity of voltage-gated Ca2+ channels. In skeletal muscle cells, the CaV1.1 channels in transverse tubules have two distinct functional roles. They serve as the voltage sensor for excitation–contraction coupling and transduce a voltage-dependent conformational change into activation of the ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum (1, 2). Ca2+ release from the sarcoplasmic reticulum rapidly initiates contraction. In addition, on a slower time scale, the L-type Ca2+ current conducted by these channels is activated, and the resulting Ca2+ influx increases contractile force in subsequent contractions (3, 4) and regulates gene expression and other muscle activities (5).
Activation of skeletal muscle Ca2+ channels is increased by cAMP-dependent phosphorylation in response to adrenergic stimulation (3, 6) and is further potentiated by strong depolarization (7–9), which requires cAMP-dependent protein kinase (PKA) phosphorylation (7). PKA is often anchored near substrates by A-kinase anchoring proteins (AKAPs), which bind the kinase through interaction with its regulatory subunits (10, 11). Voltage-dependent potentiation of Ca2+ channel activity requires anchored PKA (12, 13) interacting with AKAP-15, a Ca2+ channel-associated AKAP (14, 15). Direct binding of AKAP-15 to skeletal muscle Ca2+ channels via a leucine zipper motif is required for regulation of Ca2+ channel function (16).
The actin cytoskeleton affects multiple ion channels, including voltage-gated Na+ channels (17–19), Ca2+ channels (19–22), K+ channels (23, 24), Cl– channels (25–28), amiloride-sensitive epithelial Na+ channels (26, 29, 30), mechanosensitive Na+ channels (31), GABAA receptors (32), and glutamate receptors (33, 34). The cytoskeleton is composed of the following three primary types of filaments: microfilaments composed of actin monomers, intermediate filaments composed of intermediate filament proteins, and microtubules composed of α- and β-tubulin dimers. In the experiments reported here, we examined the effects on Ca2+ channel function of mutation of the submembrane microfilament cytoskeletal linker protein dystrophin, treatment with compounds that alter the structure of actin microfilaments, and treatment with compounds that influence PKA phosphorylation. Our results suggest that actin microfilaments regulate the activity of the skeletal muscle L-type Ca2+ channel, allowing the contractility and morphology of the cell to influence Ca2+ channel function. Failure of this regulation of Ca2+ channel activity may contribute to degeneration of muscle fibers in muscular dystrophy.
Materials and Methods
Cell Culture. Mouse skeletal muscle myotubes were prepared from either primary cultures of neonatal mouse muscle (C57bl/10SnJ control mice, mdx mice; The Jackson Laboratory) or the immortalized mouse skeletal muscle cell line, 129 CB3 (35). Primary cultures were prepared from 1- to 3-day-old mice by using the Worthington Neonatal Cardiomyocyte Isolation System. Briefly, skeletal muscle was removed from forelimbs, minced, then incubated overnight in 50 μg/ml trypsin at 5°C in Ca- and Mg-free Hanks' balanced salt solution. Then, 0.2 mg/ml trypsin inhibitor was added, and the tissue was warmed to 37°C. Next, 1,500 units of collagenase in 5 ml of Leibovitz L-15 medium was added, and the tissue was incubated at 37°C for 35 min. After incubation, tissue was triturated, and dissociated myoblasts were filtered through a Falcon cell strainer, sedimented at 100 × g, then plated in medium containing 10% heat-inactivated horse serum and 5% FBS. Myoblasts differentiated in this medium after 4–6 days in culture in a 5% CO2 incubator at 37°C. We grew 129 CB3 myoblasts in DMEM supplemented with 10% FBS (HyClone) in a 5% CO2 incubator at 37°C. When cells reached confluence, medium containing FBS was replaced with medium containing 2% heat-inactivated horse serum (GIBCO/BRL) to promote differentiation into myotubes. Nearly spherical myotubes <50 μm were chosen for recording.
Electrophysiology. Barium currents through skeletal muscle Ca2+ channels were recorded by using the whole-cell configuration of the patch-clamp technique. Patch electrodes were pulled from VWR Scientific micropipettes and fire-polished to produce an inner tip diameter of 4–6 μm. Currents were recorded by using a List EPC-7 patch clamp amplifier (List Electronics, Darmstadt, Germany) and filtered at 2 kHz (8-pole Bessel filter, –3 dB). Data were acquired by using fastlab software (Indec Systems, Santa Cruz, CA). Voltage-dependent currents have been corrected for leak by using an on-line P/4 subtraction paradigm. The extracellular bath saline contained 150 mM Tris, 2 mM MgCl2, and 10 mM BaCl2, pH-adjusted to 7.3 with methanesulfonic acid. The intracellular patch electrode saline contained 130 mM N-methyl-d-glucamine, 10 mM EGTA, 60 mM Hepes, 2 mM MgATP, and 1 mM MgCl2, pH-adjusted to 7.3 with methanesulfonic acid. All experiments were performed at room temperature (20–23°C). No nonlinear outward currents were detected under these conditions.
Reagents. Protein kinase inhibitor (PKI) (5–24) amide, calyculin A, and cytochalasin D were obtained from LC Laboratories (Woburn, MA), and phalloidin and protein phosphatase inhibitor 2 were obtained from Calbiochem. The catalytic subunit of PKA was purified from bovine heart as described in ref. 36.
Results and Discussion
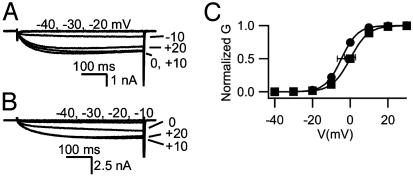
Altered Ca2+ Channel Activation and Potentiation in Myocytes Lacking Dystrophin. Skeletal muscle myocytes were dissociated from WT and mdx mice and analyzed by whole-cell voltage-clamp recording. Barium currents activated by depolarization were similar in WT and mutant mice (Fig. 1 A and B). However, these ion currents did not activate until more positive potentials in the mdx mouse myotubes, resulting in a mean conductance–voltage relationship that was shifted 6 mV in the depolarizing direction (Fig. 1C).
Fig. 1.
Voltage dependence of Ca2+ channel activation in mdx skeletal muscle. (A) Currents recorded from a control mouse myotube in response to 500-ms-long pulses to the indicated potentials. (B) Current traces from an mdx myotube. (C) Mean current–voltage relationships from control (•) and mdx (▪) mouse myotubes. The mean peak Ba2+ currents for control and mdx myocytes were not significantly different in this sample of cells (954 pA for control and 737 pA for mdx), minimizing possible contributions of series resistance to the voltage shifts measured.
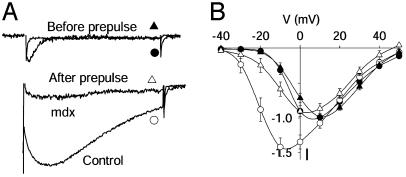
Ca channels in skeletal muscle are subject to marked voltage-dependent potentiation after a depolarizing prepulse (Fig. 2A). For myotubes from control mice, the current measured after a conditioning prepulse to +80 mV for 200 ms was 22-fold larger than before the prepulse, in contrast to potentiation in myotubes from mdx mice, which was only 6-fold larger (Fig. 2 A). This striking reduction in voltage-dependent potentiation suggests a fundamental alteration in Ca2+ channel regulation in dystrophic mice.
Fig. 2.
Reduced voltage-dependent potentiation in mdx skeletal muscle. Ca2+ channel current was recorded in the whole-cell patch clamp mode from control and mdx mouse skeletal muscle myocytes. (A) Examples of Ca2+ channel potentiation after a conditioning prepulse in control and mdx muscle. From a holding potential of –80 mV, cells were depolarized to –20 mV for 300 ms in test pulse 1 and returned to the holding potential. A prepulse to +80 mV for 200 ms was applied, the cells were briefly repolarized to –60 mV, and Ba2+ currents were recorded during a second identical test pulse. Currents in test pulse 1 were normalized to the same amplitudes (control, •; mdx, ▴). To allow direct comparison of potentiation after the prepulse the currents in test pulse 2 were normalized by the same factor (control, ○; mdx, ▵). The rapidly activated and inactivated current in the “Before prepulse” trace is T-type current, which was observed in a subset of the cells. Data are representative of 10 and 12 experiments, respectively. (B) Ca2+ channel current–voltage relations measured before (control, •; mdx, ▴) and after (control, ○; mdx, ▵) conditioning prepulses are shown to compare channel potentiation at the indicated test potentials. Data are mean ± SEM (n = 9 for each).
Voltage-dependent potentiation is a result of strong channel activation during the conditioning prepulse, from which the channel does not completely recover before the second test pulse (7, 8). Therefore, potentiation is greatest at potentials where the channel is only weakly activated under control conditions (–30 to 0 mV) and does not occur at potentials where the channel is fully activated (+10 to +50 mV). This effect can be seen by plotting current–voltage relations for control myotubes before and after a conditioning prepulse as in Fig. 2B (filled circles, before; open circles, after). The conditioning prepulse causes a negative shift in the current-voltage relationship, but the same level of current was observed at potentials more positive than +10 mV. The same protocol applied to mdx myotubes (Fig. 2B, triangles) produced little potentiation in the sensitive range of potentials (–30 to 0 mV) and a small reduction in Ca2+ channel current at positive potentials (+10 to +50 mV), presumably resulting from channel inactivation. These results show that potentiation of Ca2+ channel activity in mdx myotubes is decreased for all test pulse potentials.
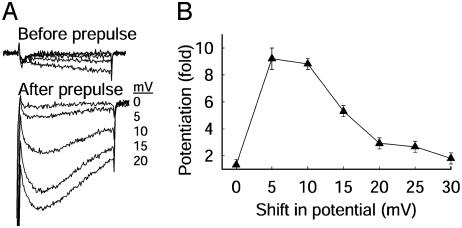
Two other membrane potentials are relevant for potentiation, the potential at which potentiation is induced (the prepulse potential) and the repolarized potential between the prepulse and the test pulse. To examine the contribution of overall membrane potential to the loss of potentiation, we tested whether potentiation could be restored by shifting all potentials in the protocol positively by 5 to 30 mV in 5-mV increments to compensate for the positive shift of the voltage dependence of activation. Under these conditions, current during the first test pulse increased little, whereas current measured after the conditioning prepulse increased dramatically (Fig. 3A). Shifting membrane potential positively in 5-mV increments restored potentiation for shifts of +5 and +10 mV (Fig. 3B). This result indicated that the shift in the voltage dependence of Ca2+ channel gating in mdx myocytes was sufficient to cause the reduction in voltage-dependent potentiation.
Fig. 3.
Recovery of voltage-dependent potentiation in mdx myocytes by compensation for the positive shift of the voltage dependence of activation. (A) Ca2+ channel potentiation in mdx myocytes measured with the same protocol as in Fig. 2 but with all potentials shifted by the indicated amounts. (B) Mean potentiation (± SEM, n = 5) in mdx muscle vs. shift in membrane potential is shown.
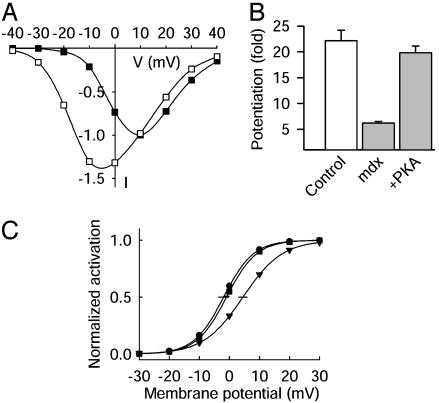
Restoration of Normal Voltage Dependence by PKA in mdx Myocytes. The level of intracellular PKA activity controls voltage-dependent potentiation. When PKA localization was disrupted by intracellular application of a peptide that inhibited anchoring of PKA (12), the addition of 2 μM PKA catalytic subunit restored potentiation to control levels. When 2-μM PKA catalytic subunit was perfused into mdx myotubes from the patch pipette, voltage-dependent potentiation was restored to control level over the sensitive range of membrane potentials (Fig. 4 A and B). Thus, PKA can restore voltage-dependent potentiation of Ca2+ channel activity in mdx myotubes with disrupted cytoskeletal function.
Fig. 4.
Restoration of voltage-dependent potentiation in mdx skeletal muscle by PKA. (A) Ca2+ channel current–voltage relations measured before (▪) and after (□) conditioning prepulses are shown for mdx muscle to which additional catalytic subunit of PKA (2 μM) was applied through the patch pipette. (B) Mean potentiation (± SEM, n = 5–12) measured at –20 mV for control muscle, mdx muscle, and mdx muscle with 2 μM added PKA are shown. (C) Voltage dependence of Ca2+ channel activation is shown for control muscle (•, n = 14), mdx muscle (▾, n = 12), and mdx muscle with 2 μM added PKA (▪, n = 6).
PKA shifts the voltage dependence of activation of skeletal muscle Ca2+ channels to more negative membrane potentials (13, 37). A similar negative shift in the voltage dependence of activation was observed in mdx myotubes perfused with 2 μM PKA (Fig. 4C). This negative shift of the voltage dependence of activation may contribute to restoration of voltage-dependent potentiation by PKA in mdx myotubes, because potentiation also was substantially restored by a comparable depolarization (Fig. 3B). These results show that disruption of cytoskeletal interaction with the Ca2+ channel by the mdx mutation alters the voltage dependence of activation and therefore blocks normal voltage-dependent potentiation. The Ca2+ channel in the mutant mice still can be modulated by activation of PKA, which shifts the voltage dependence of activation toward negative potentials and restores voltage-dependent potentiation.
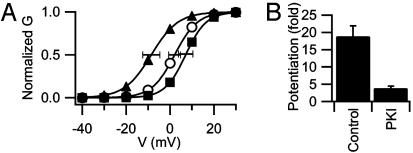
Effects of PKA on Voltage Dependence of Ca2+ Channel Activity in Wild-Type (WT) Myocytes. Our experiments with mdx mice showed that permanent loss of dystrophin in mdx mutant mice and acute activation of PKA had opposing functional effects on activation of the Ca2+ channel. To test whether acute inhibition of cytoskeletal function has similar effects, we examined functional interactions between the actin cytoskeleton and PKA in WT 129 CB3 myocytes (35). Perfusion of PKA into untreated 129 CB3 myotubes under whole-cell voltage clamp shifted the conductance–voltage relationship 10 mV in the negative direction in comparison with control (Fig. 5A), confirming that PKA can modulate the voltage dependence of Ca2+ channel activation. To assess the full range of PKA modulation on Ca2+ channel activity, the effect of PKA inhibition on voltage dependence and potentiation was studied in experiments where the inhibitor peptide PKI (5–24) amide was included in the recording pipette. The mean voltage dependence of Ca channel activation was shifted toward positive potentials by PKI, but the change did not reach statistical significance (Fig. 5A). Thus, there is little basal modulation of Ca2+ channel activity by PKA at the resting membrane potential of 129 CB3 myocytes under our experimental conditions. Inhibition of PKA with PKI substantially reduced voltage-dependent potentiation (Fig. 5B). Thus, the results of these experiments are consistent with our previous findings that activation of PKA shifts the voltage dependence of activation of skeletal muscle Ca2+ channels toward more negative potentials and is required for voltage-dependent potentiation (13, 37).
Fig. 5.
Effect of PKA activity on voltage dependence of Ca2+ channel activation. (A) Mean conductance–voltage relationships in control mouse 129 CB3 myotubes (○), myotubes dialyzed intracellularly with PKA (▴), and myocytes dialyzed intracellularly with the PKA inhibitor peptide, PKI (▪). (B) Mean potentiation of Ca2+ channels in 129 CB3 cells (± SEM, n = 20, 9) measured by using the voltage-clamp protocol of Fig. 2.
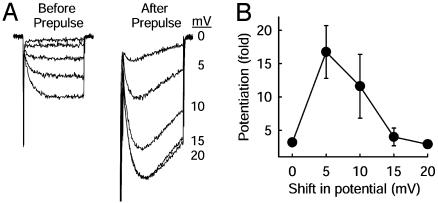
As for the mdx myocytes, voltage-dependent potentiation could be restored in the presence of PKI by imposing a positive shift in all of the potentials applied during the potentiation protocol (Fig. 6). Potentiation was fully restored by a 5-mV positive shift. In this respect, inhibition of PKA and disruption of the dystrophin gene have the same effect on the voltage dependence of Ca2+ channel activation and on voltage-dependent potentiation.
Fig. 6.
Recovery of potentiation in the presence of PKI by compensation for the shift in the voltage dependence of activation. (A) Examples of Ca2+ channel potentiation measured in PKI-treated muscle cells with increasing shift in membrane potential. Ca2+ channel potentiation was first measured by using the voltage-clamp protocol of Fig. 2, then all membrane potentials were shifted as indicated on the abscissa, and potentiation was measured again. (B) Mean potentiation (± SEM) vs. shift in membrane potential in the potentiation protocol (n = 13) is shown.
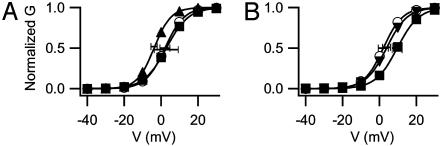
Effect of Disruption of the Actin Cytoskeleton. Loss of dystrophin by mutation in mdx mice breaks the connection of the actin cytoskeleton to the plasma membrane and causes marked rearrangement of the protein components of the cytoskeleton throughout the life of the mdx myocytes (38). Does acute impairment of the organization of the actin cytoskeleton also impair Ca2+ channel regulation? To examine this question, we tested whether direct pharmacological alteration of the actin cytoskeleton in WT myocytes could cause similar effects. Intracellular application of 20 μM cytochalasin D, a filamentous actin disrupter, had no effect on the voltage dependence of Ca2+ channel activation (Fig. 7A), even though concentrations as low as 2 μM disrupt the cytoskeleton of cultured myotubes (39). Intracellular application of 2 μM PKA in the presence of cytochalasin D caused a normal negative shift of 5.9 mV in the voltage dependence of activation (Fig. 7A). Thus, acute disruption of the actin cytoskeleton with cytochalasin D has little effect on basal Ca2+ channel activity and does not prevent regulation by PKA.
Fig. 7.
Effect of actin filament stabilization and destabilization. (A) Destabilization of actin filaments with cytochalasin D. Shown are mean conductance–voltage relationships as in Fig. 5 for control myocytes (○), myocytes treated with 20 μM cytochalasin D (▪), and myocytes treated with cytochalasin D and intracellularly dialyzed with PKA during recording (▴). (B) Stabilization of actin filaments with phalloidin. Shown are mean conductance–voltage relationships in control myocytes (○), myocytes treated with phalloidin (▪), and myocytes treated with phalloidin plus intracellular PKA (▴).
The function of the cytoskeleton is dynamic and can be impaired by compounds that cause either stabilization or destabilization (40). To test the effects of cytoskeletal stabilization, voltage-dependent potentiation was examined in myotubes treated with the microfilament stabilizer phalloidin. Phalloidin shifted Ca2+ channel conductance–voltage relationships toward more positive potentials by 7.6 mV (Fig. 7B). When PKA also was included in the pipette in the presence of phalloidin, the voltage dependence of activation was shifted 5.8 mV toward negative potentials, similar to the negative shift in the presence of cytochalasin D. Thus, although stabilization of the actin cytoskeleton by phalloidin inhibits Ca2+ channel activation, regulation of the channel by PKA remains intact. These results support the conclusion that the dynamic activity of the cytoskeleton, which is inhibited by phalloidin, is essential for normal Ca2+ channel activity. Stabilization of the actin cytoskeleton by phalloidin may result in a mechanical force that stabilizes the Ca2+ channel in the closed conformation.
Convergent Regulation of Skeletal Muscle Ca2+ Channels by PKA and the Actin Cytoskeleton. The cytoskeleton has been shown previously to regulate neuronal (20, 22) and cardiac (21) CaV1.2 channels, and regulation of the CaV1 family of channels by PKA has been demonstrated in many different systems (41, 42). Our results extend these findings by demonstrating functional effects of the cytoskeleton on the skeletal muscle Ca2+ channel and interaction of those functional effects with regulation by PKA. By using multiple approaches to perturb the actin cytoskeleton (dystrophin mutation, destabilization by cytochalasin, and stabilization by phalloidin), we found that perturbation of the membrane interaction and dynamic function of the cytoskeleton impairs the normal voltage dependence of gating and normal voltage-dependent potentiation of the skeletal muscle Ca2+ channel. Deletion of dystrophin prevents normal attachment of the actin cytoskeleton to the plasma membrane and causes reorganization of the proteins of the submembrane cytoskeleton but does not alter the localization of CaV1.1 channels at the transverse tubule–sarcoplasmic reticulum junction (38). Our results indicate that this detachment of functional actin filaments shifts the voltage dependence of Ca2+ channel activation positively and impairs voltage-dependent potentiation in mdx myocytes. Depolymerization of the actin cytoskeleton with cytochalasin does not affect Ca2+ channel function, indicating that normal function of the cytoskeleton is not required for the function and regulation of CaV1.1 channels in myocytes. However, stabilization of the filamentous actin cytoskeleton in a polymerized state with phalloidin shifts the voltage dependence of activation positively and impairs voltage-dependent potentiation. Thus, stabilization of the actin cytoskeleton in a polymerized state and release of the actin cytoskeleton from its membrane attachment sites, with consequent reorganization of the cytoskeleton, both stabilize the closed state of Ca2+ channels and prevent potentiation. We speculate that detachment of the filamentous actin cytoskeleton from its membrane attachment with dystrophin favors the polymerized state of those microfilaments that modulate CaV1.1 channels, resulting in a similar functional state as stabilization of polymerized microfilaments with phalloidin. These effects might be mediated by direct interactions between Ca2+ channels and polymerized filamentous actin microfilaments or through intermediary proteins that bridge them.
The positive shift in the voltage dependence of channel activation caused by mutation of dystrophin or stabilization of the cytoskeleton by phalloidin can be reversed by protein phosphorylation by PKA. These results demonstrate a complex, interactive regulation of Ca2+ channel gating by the cytoskeleton and cAMP-dependent protein phosphorylation. PKA enhances Ca2+ channel activity by increasing peak Ca2+ current, shifting the voltage dependence of activation to more negative potentials and increasing the probability and duration of openings of the skeletal muscle Ca2+ channel, as recorded in skeletal muscle cells (3, 6, 7), purified and reconstituted Ca2+ channel preparations (37, 43, 44), and transfected cells expressing cloned Ca2+ channels (13). Both the α1 and β subunits are phosphorylated by PKA (44–50), but the sites responsible for regulation are not yet known.
Voltage-dependent potentiation of the skeletal muscle L-type Ca2+ channel by brief depolarizing prepulses also requires protein phosphorylation (7, 12, 13), but our current results indicate that PKA plays a modulatory rather than a direct role in the potentiation process. We found that voltage-dependent potentiation did not absolutely require PKA activity because it could be restored in the presence of the PKA inhibitor peptide PKI by appropriate adjustment of the voltage in the stimulation protocol. PKA was found to restore normal voltage dependence of activation after it was shifted to more positive potentials by manipulations that disrupted microfilament function, and this effect also was correlated with restoration of voltage-dependent potentiation. Thus, the role of PKA in voltage-dependent potentiation is to maintain the voltage dependence and kinetics of activation in an appropriate voltage range. We propose that kinase anchoring is required for effective phosphorylation of the Ca2+ channel by PKA catalytic subunit stochastically released from nearby holoenzyme/AKAP15 complexes and that this steady-state phosphorylation of the Ca2+ channel is required for normal voltage dependence of gating and normal voltage-dependent potentiation.
Skeletal myocytes in cell culture do not have the complex architecture of mature muscle fibers. In mature muscle fibers, voltage-gated Ca2+ channels in transverse tubules are segregated from most of dystrophin, which is primarily located in the costamers in the sarcolemma. Nevertheless, loss of organization or dimensional stability of the actin cytoskeleton by deletion of dystrophin may have long-range effects on the interactions of the actin cytoskeleton with Ca2+ channels in transverse tubules, which then may impair Ca2+ channel regulation and may potentially contribute to cell damage and death in muscular dystrophy.
Significance for Muscular Dystrophy. Duchenne and Becker muscular dystrophies are X-linked disorders caused by mutation or deletion of dystrophin (51). We have found that deletion of dystrophin in the mdx mouse causes a shift in Ca2+ channel voltage dependence toward more positive potentials and reduces channel potentiation. A similar positive shift of 9 mV was reported by Hocherman and Bezanilla (23) for the skeletal muscle delayed rectifier K+ channel in mdx muscle. If similar disruption of Ca2+ channel activity occurs in individuals with muscular dystrophy, it would lead to a disruption in Ca2+-regulated gene expression. In addition to being the voltage sensor for excitation-contraction coupling, the skeletal muscle Ca2+ channel has a privileged connection to regulation of gene expression that bypasses the Ca2+ signal generated by the sarcoplasmic reticulum and ryanodine-sensitive Ca2+ release channels (5). Reduction in this Ca2+ current may be partially responsible for the disruptions in gene expression observed in the mdx mouse and in individuals with Duchenne and Becker muscular dystrophies (52–56). Because trophic effects on gene expression are important in maintenance of muscle fiber function and structural integrity, alteration in the regulation of gene expression by voltage-gated L-type Ca2+ currents may contribute significantly to the progressive muscle dysfunction and cell death associated with these diseases.
In addition to these effects on gene regulation, deletion of the dystrophin gene and the resulting alteration in Ca2+ channel regulation may alter Ca2+ homeostasis in dystrophic muscle fibers. Muscle fibers in mdx mice have an unusually high resting Ca2+ level and impaired Ca2+ homeostasis. Subsarcolemmal Ca2+ concentration is increased, as assessed in patch clamp studies, the activity of Ca leak channels is increased; and there is increased Ca2+ in the sarcoplasmic reticulum (57–59). The molecular basis for this impairment in Ca2+ homeostasis is unknown, but it may be a consequence of or a response to the impaired regulation of voltage-gated Ca2+ channel.
Acknowledgments
We thank Dr. Stanley C. Froehner (Department of Physiology and Biophysics, University of Washington) for critical comments on a draft of the manuscript. This work was supported by a research grant from the Muscular Dystrophy Association (to W.A.C.) and by a postdoctoral research fellowship from the Muscular Dystrophy Association (to B.D.J.).
Author contributions: B.D.J., T.S., and W.A.C. designed research; B.D.J. and T.S. performed research; B.D.J., T.S., and W.A.C. analyzed data; and B.D.J., T.S., and W.A.C. wrote the paper.
Abbreviations: PKA, cAMP-dependent protein kinase; PKI, protein kinase inhibitor; AKAP, A-kinase anchoring protein.
References
- 1.Tanabe, T., Beam, K. G., Powell, J. A. & Numa, S. (1988) Nature 336, 134–139. [DOI] [PubMed] [Google Scholar]
- 2.Rios, E. & Pizarro, G. (1991) Physiol. Rev. 71, 849–908. [DOI] [PubMed] [Google Scholar]
- 3.Arreola, J., Calvo, J., Garcia, M. C. & Sánchez, J. A. (1987) J. Physiol. (London) 393, 307–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huerta, M., Muniz, J., Trujillo, X. & Lomeli, J. (1991) Jpn. J. Physiol. 41, 851–860. [DOI] [PubMed] [Google Scholar]
- 5.Huang, C.-F., Flucher, B. E., Schmidt, M. M., Stroud, S. K. & Schmidt, J. (1994) Neuron 13, 167–177. [DOI] [PubMed] [Google Scholar]
- 6.Schmid, A., Renaud, J. & Lazdunski, M. (1985) J. Biol. Chem. 260, 13041–13046. [PubMed] [Google Scholar]
- 7.Sculptoreanu, A., Scheuer, T. & Catterall, W. A. (1993) Nature 364, 240–243. [DOI] [PubMed] [Google Scholar]
- 8.Fleig, A. & Penner, R. (1996) J. Physiol. (London) 494, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmeyer, D., Melzer, W., Pohl, B. & Zollner, P. (1992) J. Physiol. (London) 457, 639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin, C. S. (1994) Biochim. Biophys. Acta 1224, 467–479. [PubMed] [Google Scholar]
- 11.Murphy, B. J. & Scott, J. D. (1998) Trends. Cardiovasc. Med. 8, 89–95. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, B. D., Scheuer, T. & Catterall, W. A. (1994) Proc. Natl. Acad. Sci. USA 91, 11492–11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, B. D., Brousal, J. P., Peterson, B. Z., Gallombardo, P. A., Hockerman, G. H., Lai, Y., Scheuer, T. & Catterall, W. A. (1997) J. Neurosci. 17, 1243–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray, P. C., Tibbs, V. C., Catterall, W. A. & Murphy, B. J. (1997) J. Biol. Chem. 272, 6297–6302. [DOI] [PubMed] [Google Scholar]
- 15.Gray, P. C., Johnson, B. D., Westenbroek, R. E., Hays, L. G., Yates, I. J., Scheuer, T., Catterall, W. A. & Murphy, B. J. (1998) Neuron 20, 1017–1026. [DOI] [PubMed] [Google Scholar]
- 16.Hulme, J. T., Ahn, M., Hauschka, S. D., Scheuer, T. & Catterall, W. A. (2002) J. Biol. Chem. 277, 4079–4087. [DOI] [PubMed] [Google Scholar]
- 17.Undrovinas, A. I., Shander, G. S. & Makielski, J. C. (1995) Am. J. Physiol. 269, H203–H214. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto, G. & Sakai, H. (1979) J. Membr. Biol. 50, 1–14. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda, J., Kameyama, M. & Yamaguchi, K. (1981) Nature 294, 82–85. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, B. D. & Byerly, L. (1993) Neuron 10, 797–804. [DOI] [PubMed] [Google Scholar]
- 21.Galli, A. & DeFelice, L. J. (1994) Biophys. J. 67, 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, B. D. & Byerly, L. (1994) Pflügers Arch. 429, 14–21. [DOI] [PubMed] [Google Scholar]
- 23.Hocherman, S. D. & Bezanilla, F. (1996) J. Physiol. 493, 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, W. H., Cassola, A. & Giebisch, G. (1994) Am. J. Physiol. 267, F592–F598. [DOI] [PubMed] [Google Scholar]
- 25.Fischer, H., Illek, B. & Machen, T. E. (1995) J. Physiol. 489, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prat, A. G., Holtzman, E. J., Brown, D., Cunningham, C. C., Reisin, I. L., Kleyman, T. R., McLaughlin, M., Jackson, G. R., Jr., Lydon, J. & Cantiello, H. F. (1996) J. Biol. Chem. 271, 18045–18053. [DOI] [PubMed] [Google Scholar]
- 27.Schwiebert, E. M., Mills, J. W. & Stanton, B. A. (1994) J. Biol. Chem. 269, 7081–7089. [PubMed] [Google Scholar]
- 28.Suzuki, M., Miyazaki, K., Ikeda, M., Kawaguchi, Y. & Sakai, O. (1993) J. Membr. Biol. 134, 31–39. [DOI] [PubMed] [Google Scholar]
- 29.Berdiev, B. K., Prat, A. G., Cantiello, H. F., Ausiello, D. A., Fuller, C. M., Jovov, B., Benos, D. J. & Ismailov, I. I. (1996) J. Biol. Chem. 271, 17704–17710. [DOI] [PubMed] [Google Scholar]
- 30.Cantiello, H. F., Stow, J. L., Prat, A. G. & Ausiello, D. A. (1991) Am. J. Physiol. 261, C882–C888. [DOI] [PubMed] [Google Scholar]
- 31.Corey, D. P. & Garcia-Anoveros, J. (1996) Science 273, 323–324. [DOI] [PubMed] [Google Scholar]
- 32.Whatley, V. J., Mihic, S. J., Allan, A. M., McQuilkin, S. J. & Harris, R. A. (1994) J. Biol. Chem. 269, 19546–19552. [PubMed] [Google Scholar]
- 33.Paoletti, P. & Ascher, P. (1994) Neuron 13, 645–655. [DOI] [PubMed] [Google Scholar]
- 34.Rosenmund, C. & Westbrook, G. L. (1993) Neuron 10, 805–814. [DOI] [PubMed] [Google Scholar]
- 35.Pinçon-Raymond, M., Vicart, P., Bois, P., Chassande, O., Romey, G., Varadi, G., Li, Z. L., Lazdunski, M., Rieger, F. & Paulin, D. (1991) Dev. Biol. 148, 517–528. [DOI] [PubMed] [Google Scholar]
- 36.Kaczmarek, L. K., Jennings, K. R., Strumwasser, F., Nairn, A. C., Walter, U., Wilson, F. D. & Greengard, P. (1980) Proc. Natl. Acad. Sci. USA 77, 7487–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mundiña-Weilenmann, C., Ma, J., Rios, E. & Hosey, M. M. (1991) Biophys. J. 60, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams, M. W. & Bloch, R. J. (1999) J. Cell Biol. 144, 1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson, P. A., Brown, S., McGrath, M. J., Coghill, I. D., Gurung, R. & Mitchell, C. A. (2003) Am. J. Physiol. 284, C681–C695. [DOI] [PubMed] [Google Scholar]
- 40.Stracke, M. L., Soroush, M., Liotta, L. A. & Schiffmann, E. (1993) Kidney Int. 43, 151–157. [DOI] [PubMed] [Google Scholar]
- 41.McDonald, T. F., Pelzer, S., Trautwein, W. & Pelzer, D. J. (1994) Physiol. Rev. 74, 365–507. [DOI] [PubMed] [Google Scholar]
- 42.Catterall, W. A. (2000) Annu. Rev. Cell Dev. Bio. 16, 521–555. [DOI] [PubMed] [Google Scholar]
- 43.Nunoki, K., Florio, V. & Catterall, W. A. (1989) Proc. Natl. Acad. Sci. USA 86, 6816–6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flockerzi, V., Oeken, H. J., Hofmann, F., Pelzer, D., Cavalie, A. & Trautwein, W. (1986) Nature 323, 66–68. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi, M., Seagar, M. J., Jones, J. F., Reber, B. F. & Catterall, W. A. (1987) Proc. Natl. Acad. Sci. USA 84, 5478–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis, B. M. & Catterall, W. A. (1985) Proc. Natl. Acad. Sci. USA 82, 2528–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Röhrkasten, A., Meyer, H. E., Nastainczyk, W., Sieber, M. & Hofmann, F. (1988) J. Biol. Chem. 263, 15325–15329. [PubMed] [Google Scholar]
- 48.O'Callahan, C. M. & Hosey, M. M. (1988) Biochemistry 27, 6071–6077. [DOI] [PubMed] [Google Scholar]
- 49.Rotman, E. I., De Jongh, K. S., Florio, V., Lai, Y. & Catterall, W. A. (1992) J. Biol. Chem. 267, 16100–16105. [PubMed] [Google Scholar]
- 50.Rotman, E. I., Murphy, B. J. & Catterall, W. A. (1995) J. Biol. Chem. 270, 16371–16377. [DOI] [PubMed] [Google Scholar]
- 51.Sunada, Y. & Campbell, K. P. (1995) Curr. Opin. Neurol. 8, 379–384. [PubMed] [Google Scholar]
- 52.Matsumura, K. & Campbell, K. P. (1993) Neuromuscul. Disord. 3, 109–118. [DOI] [PubMed] [Google Scholar]
- 53.Grozdanovic, Z., Christova, T., Gosztonyi, G., Mellerowicz, H., Blottner, D. & Gossrau, R. (1997) Histochem. J. 29, 97–104. [DOI] [PubMed] [Google Scholar]
- 54.Niebroj-Dobosz, I. & Lukasiuk, M. (1995) J. Neurol. 242, 82–86. [DOI] [PubMed] [Google Scholar]
- 55.Olichon-Berthe, C., Gautier, N., Van Obberghen, E. & Le Marchand-Brustel, Y. (1993) Biochem. J. 291, 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minetti, C., Tanji, K. & Bonilla, E. (1992) Neurology 42, 1751–1754. [DOI] [PubMed] [Google Scholar]
- 57.Robert, V., Massimino, M. L., Tosello, V., Marsault, R., Cantini, M., Sorrentino, V. & Pozzan, T. (2001) J. Biol. Chem. 276, 4647–4651. [DOI] [PubMed] [Google Scholar]
- 58.McCarter, G. C. & Steinhardt, R. A. (2000) J. Membr. Biol. 176, 169–174. [DOI] [PubMed] [Google Scholar]
- 59.Mallouk, N., Jacquemond, V. & Allard, B. (2000) Proc. Natl. Acad. Sci. USA 97, 4950–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]