Abstract
Objective
To identify predictors of the receipt of medical care, including the receipt of pre-drug screening, for diagnostically targeted fungal or mycobacterial infections among patients prescribed a tumor necrosis factor inhibitor (TNFi).
Methods
We conducted a case–control study using deidentified patient health claims information from a data set representing a commercially insured US population of 15 million patients annually from January 1, 2007 to December 31, 2009. Descriptive statistics as well as a 2-sample t-test, chi-square test of association, Fisher’s exact test, and multivariate logistic regression were used for data analysis.
Results
A total of 30,772 patients received a TNFi during the study period. Of these, 158 patients (0.51%) developed targeted fungal and/or mycobacterial infections (cases). The median number of infections per case was 1.0 (interquartile range 1.0–2.0). Tuberculosis was diagnosed in 61% of cases, followed by histoplasmosis in 60%, nontuberculous mycobacterial infections in 11%, coccidioidomycosis in 10%, unspecified fungal infection in 8%, blastomycosis in 4%, cryptococcal infection in 3%, and pneumocystosis in 2%. Compared to controls (n = 474), a higher proportion of cases were prescribed prednisone (55% versus 37%; P < 0.001). Patients who were prescribed prednisone during the study period were twice as likely as those not taking prednisone to seek medical care attributable to a targeted fungal or mycobacterial infection (odds ratio 2.03; P < 0.001).
Conclusion
Development of a targeted fungal or mycobacterial infection among patients taking a TNFi is rare. Concomitant use of prednisone predicted development of such infections.
Tumor necrosis factor inhibitors (TNFi) are biologic medications that exert their effect by inhibiting TNF, a proinflammatory cytokine (1). Currently, there are 5 TNFi (infliximab [Remicade; Janssen], adalimumab [Humira; AbbVie], etanercept [Enbrel; Amgen], certolizumab [Cimzia; UCB], and golimumab [Simponi; Janssen]) approved by the US Food and Drug Administration for the treatment of conditions including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and ulcerative colitis (2).
A multitude of bacterial and fungal infections have been associated with TNFi use, including tuberculosis, histoplasmosis, blastomycosis, coccidioidomycosis, invasive candidiasis, aspergillosis, legionellosis, listeriosis, and pneumocystosis (2). Despite the health risks associated with these infections, screening for these infectious processes prior to initiating TNFi has not been standardized in practice.
Currently, it is recommended that providers prescribing a TNFi screen for tuberculosis prior to initiating the medication and then yearly for the duration of medication use (3). Guidelines for tuberculosis screening prior to implementing treatment include either a tuberculin skin test (TST) or serum interferon-γ–release assays (IGRAs) and possibly a chest radiograph if results are positive or if active disease is strongly suspected (3). It is estimated that one-third of the world population is infected with tuberculosis, with the US reporting >9,500 new cases in 2013 (4). In 2014, van der Have and colleagues reported the cost of a TST and chest radiograph to be the equivalent of $87 and the cost of an IGRA to be the equivalent of $63 (5). Furthermore, the World Health Organization reported that the US budget for tuberculosis control was $137 million in 2013, indicating the impact of this disease (6).
Although there is limited evidence to guide screenings for other bacterial and fungal infections prior to initiating or during the use of TNFi, the American Academy of Dermatology has provided recommendations suggesting that screenings for histoplasmosis and coccidioidomycosis should be considered prior to initiating TNFi therapy (3). Histoplasmosis is endemic in the Ohio and Mississippi River Valleys, and the incidence rates for this infection range from 0.55 to 12.30 cases per 100,000 person-years in the US, indicating a large number of exposures (7,8). The majority of people with histoplasmosis infections remain asymptomatic or, at most, may have minor and nonspecific ailments, making knowledge of exposure extremely difficult (9). A urinary antigen analysis or blood test is a first step for determining exposure to histoplasmosis, with a chest radiograph, biopsies, and other more extensive testing performed as needed. Coccidioidomycosis is endemic in Southwest areas of the US. This infectious condition affected 42.6 of 100,000 persons in the US in 2011 (10). Screening recommendations include a chest radiograph and serum blood tests for the presence of fungi (3). Guidelines also recommend obtaining a history of residing in or traveling to an area where the disease is endemic prior to the initiation of TNFi treatment in an effort to guide the necessity for screening (3).
Although blastomycosis and pneumocystosis are 2 additional fungal infections associated with the use of TNFi, screening for these infections prior to initiating TNFi has not been a clinical practice recommendation set forth by professional medical organizations. Blastomycosis, a fungal infection caused by Blastomyces dermatitidis, occurs most frequently in persons residing in the Midwest, South Central, and Southeastern portions of the US (11). There are estimated to be 1–2 cases per 100,000 persons in the US (11,12). Pneumocystis pneumonia, a fungal infection caused by Pneumocystis jiroveci, is diagnosed in immunocompromised patients and is associated with high mortality rates if untreated (13). Nontuberculous mycobacterial infections are spread through contact with soil, water, food, and animals and can cause severe illnesses in immunocompromised patients. These infections are not surveilled in the US; thus, their incidence is not well known (14).
There are few studies describing the incidence of and risk factors for developing bacterial and fungal infections in patients who have taken TNFi. Thus, we used a case–control study design to identify factors predicting the likelihood of receivingmedical care, including receiving predrug screening, for diagnostically targeted fungal or mycobacterial infections among patients prescribed a TNFi.
PATIENTS AND METHODS
Procedures
Deidentified patient health claims information was extracted from a data set representing a commercially insured US population of 15 million patients annually from January 1, 2007 to December 31, 2009. Patient-level data included administrative data (e.g., age and sex), pharmacy claims data (e.g., national drug code), physician and facility claims (e.g., procedure codes, diagnosis codes), and laboratory test results (e.g., logical observation identifiers names and codes, name of laboratory test).
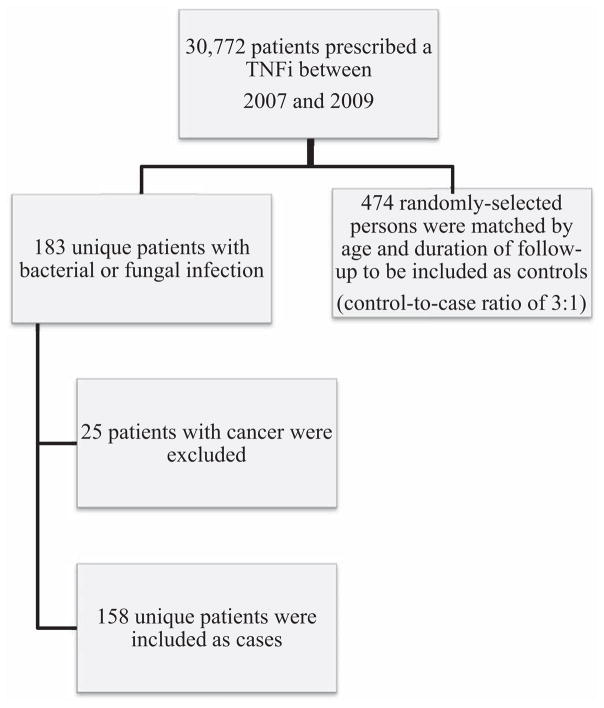
At the beginning, all patients who obtained a TNFi for any indication were extracted from the data set (Figure 1), which resulted in 30,772 unique patients. These patients were then followed forward in time and defined as cases if they received medical care (inpatient or outpatient) for one (or more) targeted fungal or mycobacterial infections with corresponding International Classification of Diseases, Ninth Revision (ICD-9) codes, as follows: mycobacterial infection (031.0–031.9), blastomycosis (116.0), coccidioidomycosis (114.0– 114.9), cryptococcal infection (117.5), histoplasmosis (115.90– 115.99; 115.10–115.19; 115.00–115.09; 136.9), pneumocystosis (136.6), tuberculosis disease (010.00–018.96), and unspecified fungal infection (unspecified mycosis; 117.9). A total of 183 unique patients were identified in this group. Although an increased risk of developing viral infections while taking TNFi has been reported in the literature, this was outside the scope of the proposed study (3,4).
Figure 1.
Flow chart showing derivation of the cases and controls for the study. TNFi = tumor necrosis factor inhibitor.
Of the 183 patients who received medical care for a targeted fungal or mycobacterial infection, 25 had also been diagnosed as having cancer. Because this diagnosis significantly complicated the clinical interpretation of a retrospective analysis, we excluded patients with cancer. The final analysis included 158 patients with a targeted fungal or mycobacterial infection. These patients were defined as the cases in the data set. Individuals who did not seek medical care attributable to targeted fungal or mycobacterial infections were defined as potential controls. The minimum duration of followup (i.e., from the TNFi index date through the end of the study) for the cases was 97 days, so only controls with at least 97 days of followup were eligible for inclusion in the study. Controls were randomly selected to frequency-match cases for the duration of followup (based on quartiles) and age (based on 10-year increments). While persons who obtained any TNFi were considered for case inclusion criteria, the 158 patients who met these criteria were found to have been prescribed adalimumab, etanercept, infliximab, or certolizumab. Therefore, all potential controls prescribed a different TNFi were not included in the sampling frame prior to random selection. The control-to-case sampling ratio was 3:1; therefore, 474 controls were included in the analysis, yielding a total sample size of 632.
Demographic and clinical information was abstracted from the commercial insurance database. Demographic information included date of birth, sex, race, educational level, and state of residence. Race was dichotomized as “white” versus “other,” and education was categorized as “some college” versus “high school or less.” State of residence was categorized into region as “Northeast,” “Midwest,” “West,” or “South” (15).
Clinical information abstracted included an indicator for diagnosis of cancer, HIV, or diabetes mellitus and concomitant prescription drug use (trimethoprim/sulfamethoxazole [TMP/SMX], prednisone, or other medications potentially affecting risk of infection). TMP/SMX prescription was reported as yes or no for each individual. Prednisone was abstracted as dose (mg) and supply (days) for each individual prescription (allowing for the possibility of multiple prescriptions per person). Because more than half (58%) of the patients in the study were not prescribed prednisone, a binary variable for prednisone use was created for each patient to indicate whether they were prescribed prednisone at any time during the study period. A list of medications that could potentially increase risk of infection was compiled, and then an expert panel, which included a dermatologist, gastroenterologist, rheumatologist, infectious disease subspecialist, pulmonologist, and pharmacist rated each medication as conferring a “high” or “low” risk of infection (Table 1). Based on prescription records, a binary variable was created for each patient to indicate whether the patient was receiving a medication rated as conferring a “high” risk of infection (yes/no). Additional information extracted from the records included the generic and trade name of the prescribed TNFi and the index date (i.e., date of this prescription). Data were also extracted on the use of isoniazid and/or rifampin prior to obtaining a TNFi.
Table 1.
Medication name, risk conferred, and frequency and percentage of patients in the sample*
| Medication name | Risk | Frequency | Percent |
|---|---|---|---|
| Azathioprine | High | 34 | 5.38 |
| Budesonide | Low | 34 | 5.38 |
| Budesonide inhaled formulations | Low | 23 | 3.64 |
| Chloroquine | Low | 1 | 0.16 |
| Cyclosporine | High | 9 | 1.42 |
| Hydroxychloroquine | Low | 65 | 10.28 |
| Leflunomide | High | 46 | 7.27 |
| Mercaptopurine | High | 28 | 4.43 |
| Mesalamine | Low | 81 | 12.81 |
| Methotrexate | High | 245 | 38.77 |
| Penicillamine | Low | 1 | 0.16 |
| Prednisone† | – | 264 | 41.77 |
| Sulfasalazine | Low | 42 | 6.65 |
| Ustekinumab | High | 1 | 0.16 |
A total of 203 patients were not taking any medications.
During the analysis, prednisone was not classified as “high” or “low” risk but was analyzed separately.
Data for screening procedures for targeted infections of interest for the 6-month period prior to initiating TNFi were abstracted using Current Procedural Terminology (CPT) codes (Table 2). The codes extracted for screening were based on expert consensus (the expert panel included a dermatologist, gastroenterologist, rheumatologist, infectious disease subspecialist, pulmonologist, and pharmacist).
Table 2.
Screening tests and CPT codes*
| Test | CPT code |
|---|---|
| Tuberculin skin test | 86580 |
| Interferon-γ release assay | 86480 |
| T-Spot.TB | 86481 |
| Chest radiograph | 71020 |
| Fungal cultures | 87101; 87220; 87106; 87107; 87140; 87143; 87149; 87158 |
| Histoplasmosis fungal culture detection and serologic test–specific antibodies | 87385 |
| Bronchoalveolar lavage | 32997; 31624 |
| Histoplasmosis polymerase chain reaction | 90249 |
| Computed tomography of the chest | 71260; 71250; 71270 |
| Coccidioidal serologic test IgG and IgM | 86635 |
| Cryptococcal serum antigen | 87327 |
CPT = Current Procedural Terminology.
Outcome data were used to identify patients receiving an inpatient or outpatient medical treatment attributed to targeted fungal or mycobacterial infections. For each patient, a binary variable for receipt of medical care (yes/no) was created, and the number of infections was assessed. Any event occurring within 2 weeks of the baseline event (i.e., targeted fungal or mycobacterial infection) was considered the same event. An infection occurring more than 2 weeks after the previous infection was considered a separate infection.
Statistical analysis
Study variables were summarized by cases (receipt of medical care attributed to a targeted fungal or mycobacterial infection) and controls using means and SDs or frequency distributions and compared using a 2-sample t-test, chi-square test of association, or Fisher’s exact test. To compare the likelihood of receiving medical care due to fungal or mycobacterial infections between pre-drug screened and nonscreened patients, we used multivariate logistic regression. Covariates included in this model were race, education, geographic region, TNFi prescribed, diabetes mellitus, prednisone prescription, prescription of other medication conferring “high” risk of infection, TMP/SMX prescription, and pre-drug screening test. HIV status was considered, but no patients in the study sample had a history of HIV diagnosis. Odds ratios (ORs), 95% confidence intervals, and P values were determined. The Hosmer-Lemeshow goodness-of-fit test assessed model fit. Variance inflation factors were used to check for multicollinearity. All data analysis was conducted using SAS software version 9.3. An alpha level of 0.05 was used in all statistical tests to determine significance.
RESULTS
This age- and followup time frequency–matched case–control study included 632 patients (158 cases and 474 controls). The mean ± SD age of the patients was 46.7 ± 13.0 years (Table 3). More than half (60%) were women, and approximately three-fourths (77%) were white. The majority had at least some college education. Patients prescribed a TNFi were predominantly from the South (52%) and Midwest (23%). Etanercept was the most commonly prescribed TNFi (42%), followed by adalimumab (37%) and infliximab (21%). Less than 1% of patients used certolizumab. Almost 15% of patients had diabetes mellitus and none had HIV. Approximately half of patients were prescribed another medication that conferred a “high” risk of infection concomitant with the TNFi. Less than 10% were prescribed TMP/SMX. Less than half (42%) were prescribed prednisone at any time during the study period. Among those who were prescribed prednisone, the mean ± SD daily dose was low (4.6 ± 6.3 mg). Patients prescribed prednisone who were diagnosed as having inflammatory bowel disease took the highest average daily dose (mean ± SD 6.7 ± 9.0 mg), followed by patients with rheumatoid arthritis (4.4 ± 5.2 mg), psoriasis (4.3 ± 8.9 mg), and ankylosing spondylitis (3.3 ± 3.3 mg). Almost half of patients (49%) received pre-drug screening (TST, chest radiograph, etc.). Among patients who received pre-drug screening, the most commonly used tests were the TST (85%) and chest radiograph (31%).
Table 3.
Comparison of demographic and clinical characteristics between the cases and controls*
| Total sample (n = 632) | Cases (n = 158) | Controls (n = 474) | P† | |
|---|---|---|---|---|
| Age, mean ± SD years | 46.7 ± 13.0 | 46.9 ± 13.2 | 46.6 ± 13.0 | 0.80 |
| Sex | 0.81 | |||
| Male | 255 (40.3) | 65 (41.1) | 190 (40.1) | |
| Female | 377 (59.7) | 93 (58.9) | 284 (59.9) | |
| Race | 0.96 | |||
| White | 487 (77.1) | 122 (77.2) | 365 (77.0) | |
| Other | 145 (22.9) | 36 (22.8) | 109 (23.0) | |
| Education | 0.13 | |||
| Some college | 386 (62.9) | 106 (68.0) | 280 (61.1) | |
| High school or less | 228 (37.1) | 50 (32.0) | 178 (38.9) | |
| Region | 0.034 | |||
| Northeast | 69 (10.9) | 19 (12.0) | 50 (10.6) | |
| Midwest | 143 (22.6) | 29 (18.4) | 119 (24.0) | |
| South | 326 (51.6) | 76 (48.1) | 250 (52.7) | |
| West | 94 (14.9) | 34 (21.5) | 60 (12.7) | |
| Diagnosis‡ | – | |||
| Psoriasis | 183 (27.6) | 51 (27.1) | 132 (19.9) | |
| Rheumatoid arthritis | 319 (48.0) | 92 (48.9) | 227 (47.7) | |
| Inflammatory bowel disease | 105 (15.8) | 29 (15.4) | 76 (16.0) | |
| Ankylosing spondylitis | 57 (8.6) | 16 (8.5) | 41 (8.6) | |
| TNF inhibitor prescribed | 0.84 | |||
| Adalimumab | 231 (36.5) | 60 (38.0) | 171 (36.1) | |
| Etanercept | 262 (41.5) | 61 (38.6) | 201 (42.4) | |
| Infliximab | 135 (21.4) | 36 (22.8) | 99 (20.9) | |
| Certolizumab | 4 (0.6) | 1 (0.6) | 3 (0.6) | |
| Diabetes mellitus | 0.058 | |||
| Yes | 91 (14.4) | 30 (19.0) | 61 (12.9) | |
| No | 541 (85.6) | 128 (81.0) | 413 (87.1) | |
| Prescribed trimethoprim/sulfamethoxazole | 0.077 | |||
| Yes | 51 (8.1) | 18 (11.4) | 33 (7.0) | |
| No | 281 (91.9) | 140 (88.6) | 441 (93.0) | |
| Prescribed prednisone | <0.001 | |||
| Yes | 264 (41.8) | 87 (55.1) | 177 (37.3) | |
| No | 368 (58.2) | 71 (44.9) | 297 (62.7) | |
| Prescribed medication conferring high risk of infection | 0.17 | |||
| Yes | 318 (50.3) | 87 (55.1) | 231 (48.7) | |
| No | 314 (49.7) | 71 (44.9) | 243 (51.3) | |
| Pre-drug screening test | 0.33 | |||
| Yes | 311 (49.2) | 83 (52.5) | 228 (48.1) | |
| No | 321 (50.8) | 75 (47.5) | 246 (51.9) | |
| Followup, mean ± SD days | 618.2 ± 235.4 | 621.8 ± 239.3 | 617.0 ± 234.3 | 0.83 |
Except where indicated otherwise, values are the number (%) of subjects. TNF = tumor necrosis factor.
By 2-sample t-test, chi-square test of association, or Fisher’s exact test.
P values are not reported because patients may have had >1 diagnosis, and therefore, the groups are not mutually exclusive.
There were no differences in age, sex, race, or education between patients who received treatment for a targeted fungal or mycobacterial infection (cases) and those who did not (controls). A higher proportion of controls than cases were from the Midwest (24% versus 18%) or the South (53% versus 48%). Although not statistically significant compared to controls, a higher proportion of cases had diabetes mellitus (19% versus 13%; P = 0.06) and were prescribed TMP/SMX (11% versus 7%; P = 0.08). A higher proportion of cases were prescribed prednisone (55% versus 37%; P < 0.001). There were no differences in the percentage of cases and controls based on specific TNFi, use of other medication conferring a “high” risk of infection, or duration of followup time. We did not expect differences in age or duration of followup time, since those were used as matching variables for selection of controls.
Twelve patients who were prescribed a TNFi obtained a prescription for rifampin. Seven of these 12 patients did not receive a documented screening. A mycobacterial infection was diagnosed following the administration of a TNFi in 4 of the 12 patients; histoplasmosis was diagnosed following TNFi administration in 3 of the 12 patients, and tuberculosis was diagnosed following TNFi administration in 1 of the 12 patients and prior to administration in 2 of the 12 patients. Two of the 12 patients did not develop an infection, and 1 of those 2 patients was known to have had a screening test prior to TNFi administration.
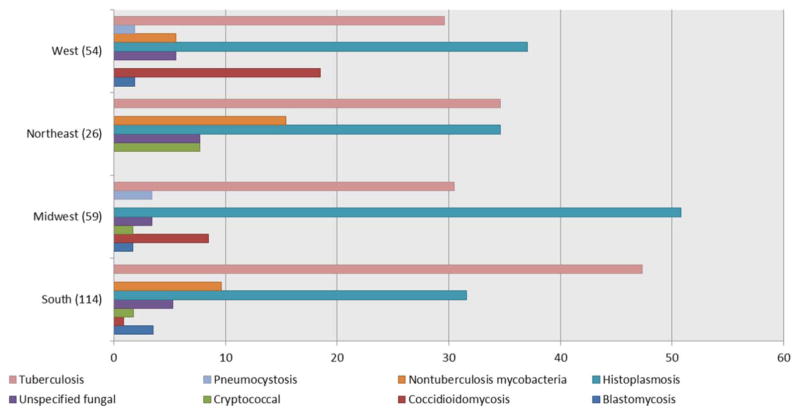
Among the 158 cases, the median number of targeted fungal and mycobacterial infections per case was 1.0 (interquartile range 1.0–2.0). The most common infection was tuberculosis, which was diagnosed in 97 cases (61%), followed by histoplasmosis, which affected 95 cases (60%). Less commonly diagnosed infections were nontuberculous mycobacterial infections (11%), coccidioidomycosis (10%), unspecified fungal infection (8%), blastomycosis (4%), cryptococcal infection (3%), and pneumocystosis (2%). Among the 158 cases, there was a total of 253 infections (113 patients with 1 infection, 21 patients with 2 infections, 12 patients with 3 infections, 5 patients with 4 infections, 2 patients with 5 infections, 4 patients with 6 infections, and 1 patient with 8 infections) (Figure 2). Of the 45 patients who developed multiple infections, only 4 were diagnosed using 2 codes for the targeted fungal and mycobacterial infections. The distribution of infections differed for each geographic region (Figure 2). There was no difference in the percentage of patients receiving prednisone, TMP/SMX, or other medications conferring a “high” risk of infection between those with 1 infection and those with multiple infections. The mean ± SD duration from the TNFi index prescription date to the first receipt of medical care for one of these infections was 281 ± 203 days.
Figure 2.
Percentages of different types of infections in each US Census region. Some of the 158 persons in our sample had >1 infection. Therefore, a total of 253 infections were distributed among the West (n = 54), Northeast (n = 26), Midwest (n = 59), and South (n = 114) regions.
Table 4 displays results of the multivariate logistic regression model that describe the likelihood of receiving medical care due to a targeted fungal or mycobacterial infection. The overall model was significant (χ2 = 29.6, P = 0.005). Pre-drug screening was not associated with the likelihood of developing a targeted fungal or mycobacterial infection requiring medical care (P = 0.70), nor was race, education, type of TNFi, diabetes mellitus, or concomitant prescription use. The significant covariates in the model were region (West versus South comparison) and prednisone prescription. Patients residing in the West were 77% more likely to seek medical care due to an infection, compared to those residing in the South (OR 1.77; P = 0.03). Patients who were prescribed prednisone during the study period were 103% more likely to seek medical care attributable to a targeted fungal or mycobacterial infection, compared to those not taking prednisone (OR 2.03; P < 0.001). The Hosmer-Lemeshow goodness-of- fit test was not significant (χ2 = 8.3, P = 0.40), suggesting that the model fit the data well. All variance inflation factors were <4, suggesting that multicollinearity was not distorting parameter estimates.
Table 4.
Multivariate logistic regression modeling the likelihood of receiving medical care for a targeted fungal or mycobacterial infection (n = 614)*
| Estimated OR (95% CI) | P | |
|---|---|---|
| Race | ||
| White vs. other | 0.89 (0.57–1.40) | 0.61 |
| Education | 0.19 | |
| Some college vs. high school or less | 1.31 (0.87–1.95) | |
| Region | ||
| Midwest vs. South | 0.86 (0.53–1.41) | 0.56 |
| Northeast vs. South | 1.17 (0.64–2.14) | 0.62 |
| West vs. South | 1.77 (1.05–2.98) | 0.032 |
| TNF inhibitor prescribed | ||
| Adalimumab vs. etanercept | 1.05 (0.69–1.62) | 0.81 |
| Infliximab vs. etanercept | 1.08 (0.66–1.77) | 0.77 |
| Certolizumab vs. etanercept | 0.78 (0.07–8.21) | 0.83 |
| Diabetes mellitus | ||
| Yes vs. no | 1.58 (0.96–2.63) | 0.075 |
| Prescribed prednisone | ||
| Yes vs. no | 2.03 (1.37–3.00) | <0.001 |
| Prescribed other medication conferring high risk of infection | ||
| Yes vs. no | 1.02 (0.69–1.52) | 0.92 |
| Prescribed trimethoprim/sulfamethoxazole | ||
| Yes vs. no | 1.45 (0.77–2.71) | 0.25 |
| Pre-drug screening test | ||
| Yes vs. no | 1.08 (0.74–1.59) | 0.69 |
A total of 18 patients were omitted due to having missing data for ≥1 variable in the model. OR = odds ratio; 95% CI = 95% confidence interval; TNF = tumor necrosis factor.
DISCUSSION
We report a number of clinically important findings in this study. First, of the 30,772 persons who were prescribed a TNFi from 2007 to 2009 in this data set, excluding those with a previous cancer diagnosis, 158 (0.51%) received medical care attributable to a targeted fungal or mycobacterial infection. Second, of the cases, 61% had tuberculosis, 60% had histoplasmosis, 11% had nontuberculous mycobacterial infections, 10% had coccidioidomycosis, 8% had unspecified fungal infection, 4% had blastomycosis, 3% had cryptococcal infection, and 2% had pneumocystosis.
Our finding of a low incidence of developing these targeted fungal and mycobacterial infections while taking a TNFi is supported by prior research. Baddley and colleagues conducted a large retrospective study of 33,342 new TNFi users and found that 80 had developed a nonviral opportunistic infection (0.24%) (8). Of those 80 cases, the most prevalent nonviral opportunistic infections included pneumocystosis (20%), nocardiosis (13%), tuberculosis (12.5%), and histoplasmosis (11.3%). Baddley and colleagues obtained data from 4 data sets, including the National Medicaid and dual Medicaid- Medicare database, TennCare, New Jersey’s Pharmaceutical Control for the Elderly programs linked to Medicare data, and Kaiser Permanente Northern California. Although Baddley and colleagues did not report the distribution of the sample in each geographic region in the US, given the databases that they used, it is possible that the different distribution of specific infections reported in that study as compared to ours is a result of the different proportions of geographic locations represented; this in turn would affect the reported patient exposures to infectious agents (8,15). Although the overall population from which the data were obtained is not distributed equally in each geographic region (16% from the West, 27% from the Midwest, 44.6% from the South, and 12.4% from the Northeast), we have described the distribution of specific infections in each geographic region in Figure 2.
In the multivariate analysis, the most significant predictor of developing a targeted infection in our study was the use of prednisone. The use of other medications commonly considered high risk and prescreening for targeted infections did not predict the development of a targeted infection when included in the multivariate model. In the bivariate comparisons of cases versus controls, taking prednisone (P < 0.001) was significantly associated with developing a targeted infection. Baddley and colleagues reported an adjusted hazard ratio of 2.5 for developing a nonviral opportunistic infection with the use of any glucocorticoids in new TNFi users (8). This supports the potential risk of the use of this medication reported in the present study. Neither study reported the effect of disease activity on developing a targeted nonviral infection. Thus, the question remains whether increased infection rates are related solely to the use of glucocorticoids or also to the active disease for which the medication is being prescribed. Interestingly, a higher percentage of patients who developed a targeted fungal or mycobacterial infection in the current study were taking TMP/SMX compared with controls. It is possible that providers recognized the risk of infection in this population and made attempts at controlling infectious processes among those most vulnerable.
This study has a number of limitations. First, this was a retrospective review of a commercial insurance database; therefore, data were available for those medications and treatments that were charged for by health care providers and paid for using insurance coverage. It is possible that additional health screenings and medications were obtained and paid for with other resources. There may also have been inaccuracies in providing ICD-9 and CPT codes. Given the retrospective nature of the data collection, we were also unable to assess the number of patients who were screened and then not given a TNFi.
In conclusion, findings from this study suggest that the development of a targeted fungal or mycobacterial infection among patients taking a TNFi is rare (0.51%). The use of prednisone predicted the development of targeted fungal or mycobacterial infection. While other personal and clinical variables were marginally associated with infection status in the bivariate analysis, they were not significant predictors of infection in the multivariate logistic model.
Acknowledgments
Drs. Salt and Huaman’s work was supported in part by the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences, NIH (grant UL1-TR- 000117). Access to the large commercially insured data set was made available with funding from the Clinical and Translational Science Award Consortium, NCRR, NIH (grant UL1-TR-000117).
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Salt had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Salt, Wiggins, Rayens, Huaman, Schwieterman, Merkley, Jones.
Acquisition of data. Salt, Wiggins, Rayens, Huaman.
Analysis and interpretation of data. Salt, Wiggins, Rayens, Huaman, Mannino, Crofford.
References
- 1.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, MacDonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;16:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Rheumatology. Anti-TNF. 2012 https://www.rheumatology.org/Practice/Clinical/Patients/Medications/Anti-TNF/
- 3.Chirch LM, Cataline PR, Dieckhaus KD, Grant-Kels JM. Proactive infectious disease approach to dermatologic patients who are taking tumor necrosis factor–alfa antagonists: Part II. Screening for patients on tumor necrosis factor–alfa antagonists. J Am Acad Dermatol. 2014;71:11.e1–7. doi: 10.1016/j.jaad.2014.01.879. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. TB: data and statistics. 2014 http://www.cdc.gov/tb/statistics/default.htm.
- 5.Van der Have M, Oldenburg B, Fidder HH, Belderbos TD, Siersema PD, van Oijen MG. Optimizing screening for tuberculosis and hepatitis B prior to starting tumor necrosis factor-α inhibitors in Crohn’s disease. Dig Dis Sci. 2014;59:554–63. doi: 10.1007/s10620-013-2820-9. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. United States of America: tuberculosis profile. 2014 http://www.who.int/tb/country/data/profiles/en/
- 7.Centers for Disease Control and Prevention. Histoplasmosis. 2014 http://www.cdc.gov/fungal/diseases/histoplasmosis/index.html.
- 8.Baddley JW, Winthrop KL, Patkar NM, Delzell E, Beukelman T, Xie F, et al. Geographic distribution of endemic fungal infections among older persons, United States. Emerg Infect Dis. 2011;17:1664–9. doi: 10.3201/eid1709.101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauffman CA. Histoplasmosis. Clin Chest Med. 2009;30:217–25. v. doi: 10.1016/j.ccm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Definition of valley fever (coccidioidomycosis) 2014 http://www.cdc.gov/fungal/diseases/coccidioidomycosis/definition.html.
- 11.Chapman SW, Dismukes WE, Proia CA, Bradshaw RW, Pappas PG, Threlkeld MG, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801–12. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Fungal infections: blastomycosis. 2015 http://www.cdc.gov/fungal/diseases/blastomycosis/statistics.html.
- 13.Centers for Disease Control and Prevention. Fungal infections: pneumocystis pneumonia. 2015 http://www.cdc.gov/fungal/diseases/pneumocystis-pneumonia/statistics.html.
- 14.Centers for Disease Control and Prevention. Other mycobacterial infections. 2014 http://www.cdc.gov/nczved/divisions/dfbmd/diseases/nontb_mycobacterium/technical.html.
- 15.U.S. Census Bureau. Geographic terms and concepts—census divisions and census regions. 2015 http://www.census.gov/geo/reference/gtc/gtc_census_divreg.html.