Abstract
Extracellular vesicles (EVs) are widely studied as a system of intercellular communication, as markers of various diseases, as well as a vehicle for delivery of various bioactive molecules to various cells. Investigation of EVs’ structure and function requires their isolation and precise quantification. However, in the current literature, there are significant discrepancies in the estimated numbers of EVs in different body fluids. In part, this discrepancy is due to the difference in EVs isolation protocols used by different investigators. A common protocol that includes ExoQuick™ is often used to isolate EVs from body fluids and culture medium. Here, we show that in the case of isolation of EVs from blood, thrombin should be omitted from the protocol as clots formed due to the thrombin-triggered coagulation may entrap many EVs thus leading to the underestimation of their numbers.
Keywords: Extracellular vesicles, quantification, thrombin, nanoparticles
Introduction
Small extracellular vesicles (EVs) are released by cells of almost all types both in vivo and in vitro.1 It is currently understood that EVs are important biological factors constituting a third system of intercellular communication after contact interaction and communication via soluble factors. Populations of EVs are heterogeneous, carrying large varieties of proteins and different types of small RNAs that both may alter the physiology of the interacting cells.2 Over the last several years, there is a growing interest in studying EVs as biological messengers and exploring their role as promising candidates for new potential therapeutic and diagnostic tools.3 In particular, blood EVs are now investigated as a factor in cardiovascular diseases.2
Detailed analysis of EVs and their relevance to various pathologies requires EVs isolation and precise quantification. However, in the current literature, there are significant discrepancies in the estimated numbers of EVs in different body fluids. In part, this discrepancy is due to the difference in EVs isolation protocols used by different investigators.
Various methods of EVs isolation have been described.2,4 The demonstration of the presence of exosomes in plasma by Caby et al. in 20055 already relied on two major methods of isolation: differential centrifugation followed by sucrose gradient floatation and immunoaffinity purification on large latex beads coated with anti-CD63 antibodies. In this early work, aside from a longer initial centrifugation to accommodate the high viscosity of plasma, no other modifications were introduced in the sample treatment. Later, new methods were introduced, including volume excluding polymers used for viral preparations,6–8 which are the basis for commercial products such as ExoQuick. Size exclusion chromatography (SEC) has also become a method of choice for EV isolation from plasma.9,10 When compared to other methods, SEC seems to provide a preparation devoid of protein contamination as determined by mass spectrophotometry11 and results in the identification of new exosome markers in plasma. More recently, a new method based on plasma protein organic solvent precipitation has been developed for the purification of plasma microvesicles.12 The considerations linked to the choice of the source, blood versus plasma, and the method of purification have been discussed in a position article from the International Society of Extracellular Vesicles,4 which concludes that because of clot-induced platelet vesiculation, the number of EVs is higher in serum than in plasma, making plasma the most physiological milieu to study blood EVs. The direct purification of microvesicles using nano-sized magnetic particles has been reported for the detection of CD34+ exosomes in plasma of acute myeloid leukemia patient13 or Epithelial cell adhesion molecule (EpCAM)+ EVs,14 although such studies did not compare the effect of coagulation on the isolation of vesicles.
In particular, fractions of small (<300 nm) EVs carrying specific surface antigens can be isolated by capturing them with magnetic particles coupled to antibodies against EV surface proteins. Many of the EV isolation methods are used in combination with a commercial product, ExoQuick™, a volume excluding polymer that precipitates EVs. ExoQuick is widely and successfully used to purify EVs from various biological fluids such as ascites, urine as well as from cell culture media.15,16 However, for various aims, in particular, those related to the study of cardiovascular diseases, EVs need to be isolated from blood plasma. For blood plasma, thrombin is added to the ExoQuick isolation protocol. Here, we report on the comparison of individual EV isolation from blood plasma by EV capturing with magnetic particles through major histocompatibility complex class-I (MHC-I) or CD31, a technique we recently developed that allows analysis of antigenic composition of individual EVs.17 We compared direct EVs isolation from blood plasma with: (i) a commercially available kit “ExoQuick 5™” which includes thrombin, (ii) same protocol but without thrombin treatment, and (iii) without ExoQuick but with thrombin treatment. We found that in the case of thrombin, the yield of EVs was significantly lower compared to direct isolation or isolation with ExoQuick but without thrombin. Thrombin-induced clotting may entrap a significant number of vesicles thus leading to the underestimation of EVs quantities.
Materials and methods
Normal blood plasma was obtained from the NIH blood bank. Blood was collected in several 8-ml tubes with sodium citrate (3.2%); the first tube was discarded to avoid collecting EVs released by platelets activated by venipuncture. Collection tubes were centrifuged at 3000 × g for 15 min to obtain platelet-poor plasma (PPP) that was aliquoted and frozen at −80°C. From one of the PPP-containing tubes, after thawing, we isolated EVs directly using carboxyl-terminated 15-nm iron oxide magnetic nanoparticles (MNPs; 1 mg; Ocean NanoTech, Springdale, Arkansas, USA) coupled to mouse–anti-human monoclonal antibodies (either against MHC-I or against CD31); (BioLegend, San Diego, California, USA) or with isotype control antibodies (Figure 1). We stained captured vesicles with fluorescent anti-CD9-Phycoerythrin (PE) (BioLegend) and anti-CD41-Allophycocyanin (APC) (BD, Pharmingen, San Diego, California, USA) antibodies or with isotype control antibodies17 (Figure 1). Alternatively, in combination with the MNP capture, we used ExoQuick either with thrombin according to the manufacturer instruction or without thrombin. The MNP–EV-detection antibodies complexes were separated in a strong magnetic field from free antibodies and EVs that do not carry the capture antigen and their numbers were evaluated with a flow cytometer. EVs captured by nanoparticles were analyzed with an LSRII (BD Biosciences, San Jose, California, USA) flow cytometer equipped with 355-, 407-, 488-, 532-, and 638-nm laser lines. For volumetric control, 123count eBeads™ (eBioscience, San Diego, California, USA) were used. A known number of beads were added to the sample to be analyzed and by taking into account the dilution factor of the original bead solution, the volume of sample analyzed was estimated based on the number of 123 count eBeads acquired in each sample. On the basis of this volumetric measurement, the numbers of recorded events were recalculated into EV concentrations. Also, to estimate EVs’ size, a Megamix-Plus SSC (Biocytex, Marseille, France) was used. Compensation beads (BD) were used to perform compensation controls. Alexa-Fluor 488 5C Maleimide (Carlsbad, California, USA)-labeled EVs were a generous gift of Dr Lifson (NCI-Frederick, Frederick, Maryland, USA). The arbitrary fluorescent units (AFU) of the spiked samples were measured with a Safire2 microplate reader (Tecan, Männedorf, Switzerland) with the following settings: excitation at 490 nm (5 nm bandwidth) and emission at 540 nm (20 nm bandwidth, 10 flashes). EV concentration measurements were performed on NanoSight NS 300 (Salisbury, UK) according to the manufacturer instruction.
Figure 1.
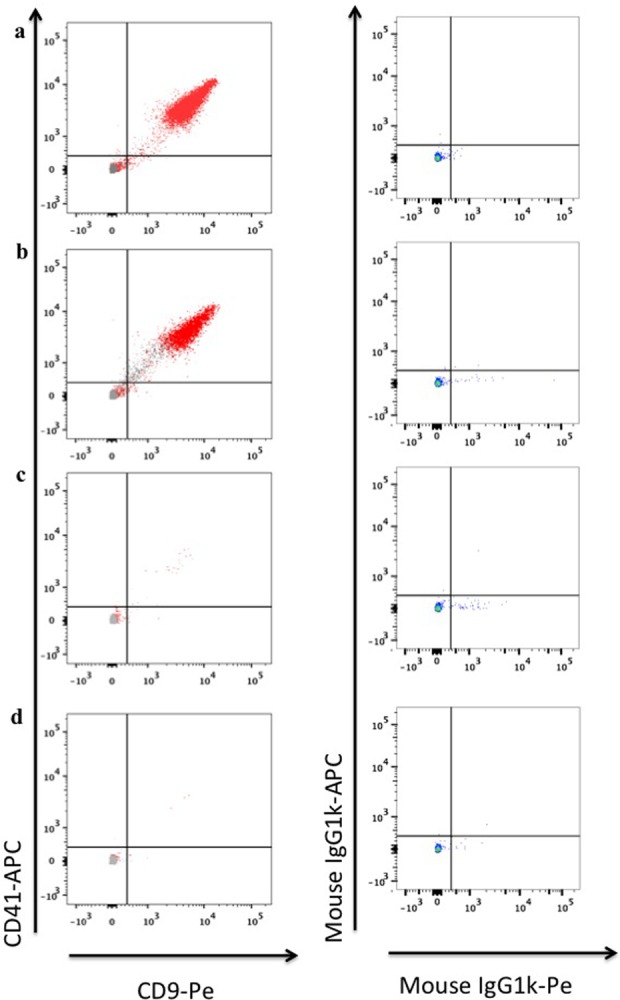
Specificity of EV capture and staining. (a) EVs were captured from untreated PPP either with MHC-I-MNPs (red) or with isotype control mouse immunoglobulin G (MsIgG)-MNPs (gray) and stained with anti-CD41-APC and anti-CD9-PE antibodies (left panel); EVs were captured from untreated PPP with MHC-I-MNPs and stained for isotype control antibodies mouseIgG1k-APC and mouseIgG1k-PE (right panel). (b) EVs were captured from PPP treated with ExoQuick either with MHC-I-MNPs (red) or with isotype control MsIgG-MNPs (gray) and stained with anti-CD41-APC and anti-CD9-PE (left panel); EVs were captured from PPP treated with ExoQuick with MHC-I-MNPs and stained for isotype control antibodies mouseIgG1k-APC and mouseIgG1k-PE (right panel). (c) EVs were captured from PPP treated with thrombin and ExoQuick, either with MHC-I-MNPs (red) or with isotype control MsIgG-MNPs (gray) and stained with anti-CD41-APC and anti-CD9-PE antibodies (left panel); EVs were captured from PPP treated with thrombin and ExoQuick with MHC-I-MNPs and stained for isotype control antibodies mouseIgG1k-APC and mouseIgG1k-PE (right panel). (d) EVs were captured from PPP treated with thrombin either with MHC-I-MNPs (red) or with isotype control MsIgG-MNPs (gray) and stained with anti-CD41-APC and anti-CD9-PE (left panel); EVs captured from PPP treated with thrombin with MHC-I-MNPs and stained for isotype control antibodies mouseIgG1k-APC and mouseIgG1k-PE (right panel). EV: extracellular vesicle; PPP: platelet-poor plasma; MHC-I: major histocompatibility complex class-I; MNP: magnetic iron oxide nanoparticle; CD: cluster of differentiation.
Results and discussion
In this study, we compared protocols for EVs isolation from blood PPP using as a test system MHC-I+/CD9+/CD41+ EVs. After purification of plasma with ExoQuick or without such purification, we captured EVs from the PPP with MNPs coupled to anti-MHC-I antibodies.17 We stained these vesicles for CD41 and CD9 and quantified them with flow cytometry.17 Also, we isolated EVs with MNPs coupled to CD31 and stained these vesicles for CD9 and for CD41, an antigen that attributes these vesicles to platelets. The vesicles we isolated were not necessarily exosomes but may be membrane-derived vesicles as well.
As we showed earlier and confirmed here, this can be used either directly or after purification of the EV-containing fluid with ExoQuick.17 Capturing with MNPs coupled to specific antibodies against EVs surface antigens and isolation of these EVs on a magnetic column resulted in obtaining an antigen-specific fraction of individual EVs17 (Figure 1). Specifically, to compare different protocols of EVs isolation, PPP sample from each donor was divided into several aliquots. One aliquot was subjected to the ExoQuick purification according to the manufacturer’s protocol with thrombin, another aliquot was purified with ExoQuick but the thrombin step was omitted, the third aliquot of EVs was treated with thrombin only, and the fourth aliquot was left untreated. Then, all four samples were subjected to EV isolation by capturing with anti-MHC-I MNPs coupled to specific antibodies, stained with fluorescent anti-CD9-PE and anti-CD41-APC antibodies, and separated the MNP-captured EVs on a magnetic column.
The majority of MHC-I captured EVs from untreated PPP were double positive for CD41 and CD9 and therefore we compared their numbers isolated with three other protocols. On average, upon direct isolation, there were 3276 ± 1875 (n = 4) of CD41+ CD9+ MHC-I MNP-captured EVs per microliter of plasma. As expected, the number of EVs directly isolated from PPP varied from donor to donor. To exclude the effects of this variability, for plasma from each donor, we normalized the number of isolated EVs with the ExoQuick and/or thrombin by the number of EVs isolated directly from untreated PPP. Also, we characterized the size of the vesicles using MegaMix sizing beads. The size of EVs isolated directly from untreated PPP and with ExoQuick without thrombin was similar (Figure 2).
Figure 2.
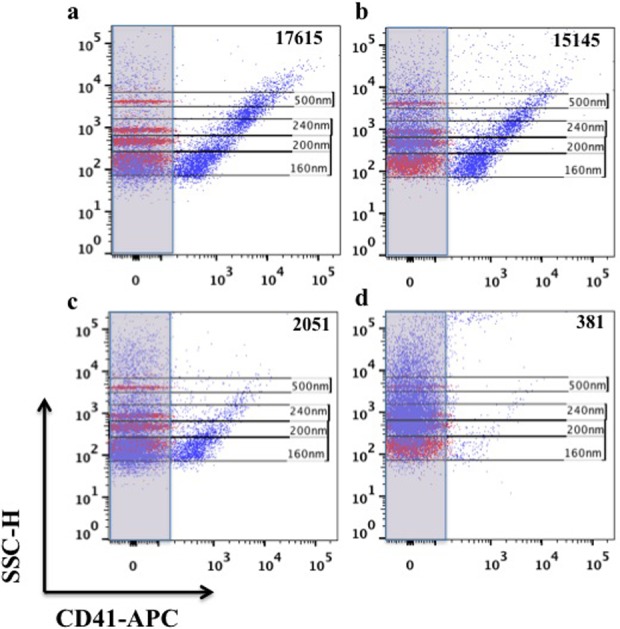
Size evaluation of captured EVs. EVs were captured with anti-MHC-I-MNPs from: (a) untreated PPP, (b) PPP treated with ExoQuick, (c) PPP treated with thrombin, and (d) PPP treated with thrombin and ExoQuick and stained for CD41 (purple). Size of EVs was estimated by MegaMix beads of different sizes (160 nm, 200 nm, 240 nm, and 500 nm) shown on each dot plot in red. EV: extracellular vesicle; MHC-I: major histocompatibility complex class-I; MNP: magnetic iron oxide nanoparticle; PPP: platelet-poor plasma; CD: cluster of differentiation.
However, when we used the full ExoQuick protocol (with thrombin), we isolated with MNPs fewer EVs by about one order of magnitude compared to direct isolation (Figure 3). A similar decrease of MNP-isolated vesicles was observed when PPP was treated with thrombin only (Figure 3). Sizing of EVs with flow cytometry indicated that the number of larger vesicles decreased predominantly (Figure 2).
Figure 3.
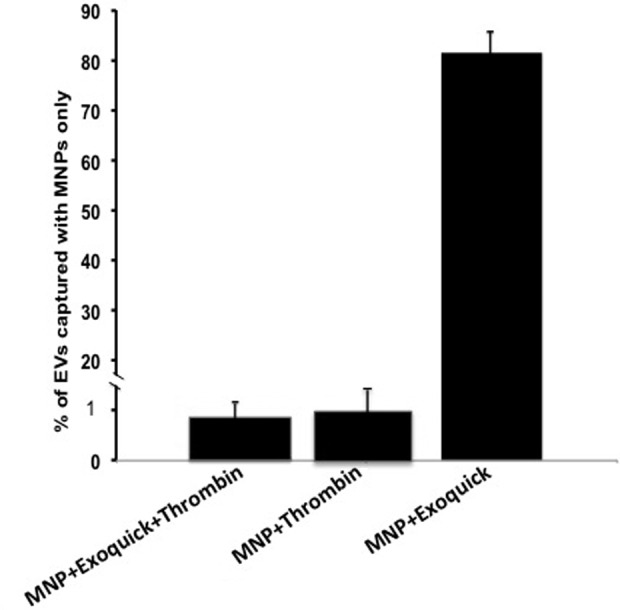
Comparison of different protocols for EV isolation. CD9+/CD41+ EVs were isolated from PPP by capture with MNPs coupled to anti-MHC-I antibodies and the numbers of captured vesicles were evaluated. For each sample, isolation was performed under four conditions: EVs were captured with MNPs (i) from untreated PPP, (ii) from PPP treated with thrombin, (iii) from PPP treated with thrombin and ExoQuick, and (iv) from PPP treated with ExoQuick. To exclude the effects of the donor-to-donor variability, for plasma from each donor, the number of captured EVs was normalized by the number of EVs captured from untreated PPP. Presented are means ± SEM. EV: extracellular vesicle; CD: cluster of differentiation; PPP: platelet-poor plasma; MNP: magnetic iron oxide nanoparticle; MHC-I: major histocompatibility complex class-I; SEM: standard error of the mean.
Unlike ascites, urine, or cell culture media, addition of thrombin to blood induces coagulation and formation of clot that is stabilized with fibrin.18 In order to test whether the decrease of EV counts was due to formation of the clot that may trap EVs, we omitted the thrombin step from our protocol and added only the ExoQuick polymer alone. In this case, the numbers (2757 ± 1608, n = 4) of EVs captured with anti-MHC-I antibodies and positive for CD41 and CD9 were not significantly different from the corresponding numbers of EVs isolated from the untreated PPP. To confirm these results with other antigens, from a sample of plasma, we isolated EVs with anti-CD31 MNPs and again stained them with fluorescent antibodies against CD41 and CD9. In agreement with the above-described results with the anti-MHC-I MNPs, the number of EVs in the preparation subjected to ExoQuick (with the thrombin step) was close to an order of magnitude lower than that isolated from the untreated PPP obtained from the same donor (4.7 × 102 vs. 3.3 × 103/µl). If the thrombin was omitted from the ExoQuick protocol, the number of EVs was similar to that obtained from untreated PPP (3.47 × 103/µl).
These results were confirmed with two other techniques: to monitor the distribution of EVs between different fractions in the course of their isolation, we spiked plasma with Alexa-Fluor 488 5C Maleimide-labeled EVs.17 The initial fluorescence of this spiked plasma was on average 5954 AFU. We divided this preparation of EV-spiked plasma into two parts: to one part, we added ExoQuick and to the second part, we added thrombin and then ExoQuick as described in the original protocol. Measurements of fluorescence showed that with Exoquick only, we recovered on average 4268 AFU (72% of the spiked EVs). In contrast, when we added thrombin resulting in clot formation, only 1620 AFU of the fluorescent EVs remain in the solution (about 25%) for further isolation with Exoquick, while the rest was entrapped in the clot. Furthermore, we evaluated the loss of EVs in these spiked preparations with NanoSight (Salisbury, UK). According to this evaluation, due to thrombin-induced clotting, we lost about 65% of particles (1.4 × 108/µl with thrombin + Exoquick vs. 4 × 108/µl with ExoQuick only).
In summary, we captured EVs with magnetic nanoparticles coupled to MHC-I antibodies as this antigen is presented on most of the cells and by this way we captured EVs generated by a broad spectra of cells. Also, in this article, we captured EVs through CD31 and stained them for CD9 and for CD41, which links them to platelets. Whatever the biogenesis of the captured EVs, when isolating them from blood samples either a direct capture with magnetic particles should be applied or if the ExoQuick protocol is to be used, the thrombin step should be omitted since it seems that the formed clot traps a significant number of EVs leading to the underestimation of the amount of EVs in the original plasma. In addition to this quantitative effect, induction of clotting by thrombin may selectively trap those vesicles that express receptors for clotting proteins such as tissue factor.19 This may explain the numerical difference in the amount of antigen-specific versus non-specific EVs trapped in the clot in the above-described experiment. Isolation of EVs from plasma is different from EVs isolation from other body fluids or from culture medium when thrombin is not recommended to be used with ExoQuick. Even if thrombin is added into these media, there will be no coagulation and therefore the original ExoQuick protocol results in an adequate isolation of EVs. Differences in the protocols for EVs isolation may contribute to various discrepancies in the reported estimation of the number of different vesicles in body fluids.
Acknowledgement
Author Jean-Charles Grivel is currently affiliated with Sidra Medical and Research Center, Doha, Qatar.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study of AA, WF, J-C.G, and LM was supported by the NICHD Intramural Program. The work of MV and EV was supported by the Russian Federation Government grant #14.B25.31.0016.
References
- 1. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14(3): 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015; 4: 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015; 523(7559): 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013; 2: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol 2005; 17(7): 879–887. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto KR, Alberts BM, Benzinger R, et al. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 1970; 40(3): 734–744. [DOI] [PubMed] [Google Scholar]
- 7. Lewis GD, Metcalf TG. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol 1988; 54(8): 1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol 1973; 20(3): 391–394. [DOI] [PubMed] [Google Scholar]
- 9. Taylor DD, Gerçel-Taylor C, Lyons KS, et al. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res 2003; 9(14): 5113–5119. [PubMed] [Google Scholar]
- 10. de Menezes-Neto A, Sáez MJ, Lozano-Ramos I, et al. Size-exclusion chromatography as a stand-alone methodology identifies novel markers in mass spectrometry analyses of plasma-derived vesicles from healthy individuals. J Extracell Vesicles 2015; 4: 27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baranyai T, Herczeg K, Onódi Z, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One 2015; 10(12): e0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallart-Palau X, Serra A, Wong AS, et al. Extracellular vesicles are rapidly purified from human plasma by PRotein Organic Solvent PRecipitation (PROSPR). Sci Rep 2015; 5: 14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong CS, Muller L, Boyiadzis M, et al. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One 2014; 9(8): e103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalra H, Adda CG, Liem M, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013; 13(22): 3354–3364. [DOI] [PubMed] [Google Scholar]
- 15. Sohel MM, Hoelker M, Noferesti SS, et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One 2013; 8(11): e78505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chugh PE, Sin SH, Ozgur S, et al. Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLoS Pathog 2013; 9(7): e1003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arakelyan A, Ivanova O, Vasilieva E, et al. Antigenic composition of single nano-sized extracellular blood vesicles. Nanomedicine 2015; 11(3): 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ariens RA, La TS, Weise JW, et al. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood 2002; 100(3): 743–754. [DOI] [PubMed] [Google Scholar]
- 19. Bastida E, Ordinas A, Escolar G, et al. Tissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesis. Blood 1984; 64(1): 177–184. [PubMed] [Google Scholar]


