Abstract
Active suppression of tumor-specific T lymphocytes can limit the immune-mediated destruction of cancer cells. Of the various strategies used by tumors to counteract immune attacks, myeloid suppressors recruited by growing cancers are particularly efficient, often resulting in the induction of systemic T lymphocyte dysfunction. We have previously shown that the mechanism by which myeloid cells from tumor-bearing hosts block immune defense strategies involves two enzymes that metabolize l-arginine: arginase and nitric oxide (NO) synthase. NO-releasing aspirin is a classic aspirin molecule covalently linked to a NO donor group. NO aspirin does not possess direct antitumor activity. However, by interfering with the inhibitory enzymatic activities of myeloid cells, orally administered NO aspirin normalized the immune status of tumor-bearing hosts, increased the number and function of tumor-antigen-specific T lymphocytes, and enhanced the preventive and therapeutic effectiveness of the antitumor immunity elicited by cancer vaccination. Because cancer vaccines and NO aspirin are currently being investigated in independent phase I/II clinical trials, these findings offer a rationale to combine these treatments in subjects with advanced neoplastic diseases.
Keywords: arginase, immunosuppression, myeloid cells, nitric oxide, immunotherapy
Identifying effective treatments for cancer is a clinical priority. Despite the plethora of evidence in preclinical models, the most advanced immunotherapy, either active or passive, has had limited success in human clinical trials (1). Basis for this conclusion appears to involve at least in part the progressive accumulation of myeloid cells, which exert a powerful inhibitory activity on antitumor lymphocytes as a function of tumor growth. In tumor-bearing hosts, for example, tumor progression is often associated with altered hematopoiesis, which leads to the accumulation of myeloid cells at the tumor site and in blood, secondary lymphoid organs, and bone marrow (2–4).
Mouse myeloid cells express the CD11b and Gr-1 markers, have a mixed mature–immature myeloid phenotype, and are responsible for the induction of tumor-specific and nonspecific T cell dysfunctions, which are frequently observed not only in transplantable tumors but also in tumors spontaneously arising with transgenic expression of tissue-restricted oncogenes (2, 5). These cells have been termed myeloid suppressor cells (MSCs) and arise from bone marrow and other hematopoietic organs exposed to systemically released factors acting on myelomonocytic precursors (reviewed in refs. 2, 3, and 6). Moreover, MSCs are the final effectors of a circuit that negatively affects tumor immunity and that requires participation of natural killer T cells. Cytotoxic T lymphocyte (CTL)-mediated tumor immunosurveillance was recently shown to be down-regulated by TGF-β released by CD11b+/Gr-1+ cells, an event driven by cytokine IL-13 release from CD1d-restricted natural killer T cells (7). This circuit is activated very early in tumor progression and does not require MSC accumulation in secondary lymphoid organs.
Elimination of MSCs, either in vitro or in vivo, completely reverses the suppression of CD8+ T cell responses and prevents tumor recurrence in a number of experimental models, suggesting that tumor-specific immunity is not damaged but rather temporarily impaired in tumor-bearing hosts (7–10). Despite this initial evidence, definitive proof that blocking MSC-dependent suppression restores immune reactivity of tumor-specific T lymphocytes in tumor-bearing hosts is still lacking. Moreover, anti-MSC treatments are not selective, in part because of the lack of unique MSC markers. An alternative approach involves interference with the inhibitory processes of MSCs by bioactive molecules. By using cloned myeloid suppressor lines and freshly isolated MSCs as a tool to define the inhibitory pathway used to limit T lymphocyte activation, we found that MSCs inhibit antigen-activated but not quiescent T cell responses by a mechanism requiring key enzymes of l-arginine metabolism: the inducible forms of nitric oxide (NO) synthase (NOS)2 and arginase (ARG1) (11, 12). NOS2 and ARG1 can act separately or synergistically. NOS2 generates NO that blocks the signal cascade from the IL-2 receptor (13), whereas l-arginine depletion induced by ARG1 activity causes loss of the zeta chain of the T cell receptor, with consequent impairment of the its signaling properties (14, 15). Induction of both enzymes generates peroxynitrites by NOS2 under conditions of limited l-arginine availability, causing activated T cells to undergo apoptosis (3, 11, 12).
Selective antagonists of these enzymes or molecules interfering with reactive oxygen species generation have proven beneficial in controlling myeloid-dependent suppression in vitro in mouse and human tumors (3, 16, 17) but are often endowed with severe side effects that preclude their systemic administration. Coupling of a NO-releasing moiety to conventional nonsteroidal antiinflammatory drugs or other antiinflammatory drugs was attempted to reduce the gastrointestinal toxicity of these compounds (18). These new molecules have interesting properties, such as antioxidant activity, suppression of reactive oxygen species generation (19), inhibition of proinflammatory cytokine release by mononuclear cells (20), feedback inhibition of NOS2 catalytic activity (21), and suppression of cancer cell proliferation (22), all of which make them attractive and safe candidates for alleviating MSC-induced alterations of the immune system in tumor-bearing hosts.
Materials and Methods
Cell Lines. CT26 (H-2d), a BALB/c carcinogen-induced colon carcinoma; MBL-2 (H-2b), a Moloney virus-induced lymphoma; P815 (H-2d) mastocytoma; C26-GM, a cell line derived from the C26 colon carcinoma (H-2d) genetically modified to release granulocyte/macrophage colony-stimulating factor; and line N2C (H-2d), a primary mammary carcinoma cell line, have been described in refs. 11, 13, 23, and 24. The 293-Ld cell line is a human embryonic kidney cell line stably transfected with pLd-444 plasmid expressing the H-2 Ld class-I molecule. The MSC-2 line (H-2d) was immortalized and cloned as described in ref. 13. Cells were grown in DMEM (Invitrogen) or in RPMI medium 1640 (Euroclone, Wetherby, U.K.) supplemented with 2 mM l-glutamine/10 mM Hepes/20 μM 2-mercaptoethanol/150 units/ml streptomycin/200 units/ml penicillin/10% heat-inactivated FBS (Invitrogen).
Plasmid Construction and Mice. The env insert was excised from plasmid pcDL-Srd296-H52 with AccI, subcloned in pBluescript, and then cloned in pcDNA3 (Invitrogen) between EcoRI and XhoI (23). For plasmid p185, the portion encoding the extracellular and transmembrane domains of mutated rat p185neu was cloned in pcDNA3, as described in ref. 25. BALB/c (H-2d) and C57BL/6 (H-2d) mice (8 weeks old) were purchased from Harlan (SanPietro al Natisone, Udine, Italy). Mice, inoculated s.c. on the left flank with tumor cells, were treated with drugs twice a day and examined every other day to follow tumor growth by caliper measurement. The animals were killed after 9 days or when the tumor became ulcerated or its area was greater than either 1 or 2 cm2 (prevention and therapy experiments, respectively). Animal care and procedures conformed to institutional guidelines in compliance with national and international regulations.
Chemicals. NO-donating aspirins [NCX 4060, 2-(acetyloxy)benzoic acid 2-(nitrooxymethyl)phenyl ester; NCX 4016, 2-(acetyloxy)benzoic acid 3-(nitrooxymethyl)phenyl ester], the denitrated analog of NCX 4016 [NCX 4017, 2-(acetyloxy)benzoic acid 3-(hydroxymethyl)phenyl ester], and aspirin were provided by NicOx. NO aspirins were dissolved in DMSO (Sigma–Aldrich) and then diluted to final concentration in 2.5% CM-cellulose (Sigma–Aldrich). NG-monomethyl-l-arginine (l-NMMA; Calbiochem) was used at 500 μM, and l-norvaline (l-norv; Calbiochem) was used at 5 mM. Guanidinoethyldisulfide bicarbonate (Sigma–Aldrich), S-nitroso-N-acetylpenicillamine (Molecular Probes), manganese-(III)-tetrakis-(4-benzoic-acid)-porphyrin (Calbiochem), and isosorbide mononitrate (Sigma–Aldrich) were used at the concentrations reported in Fig. 1.
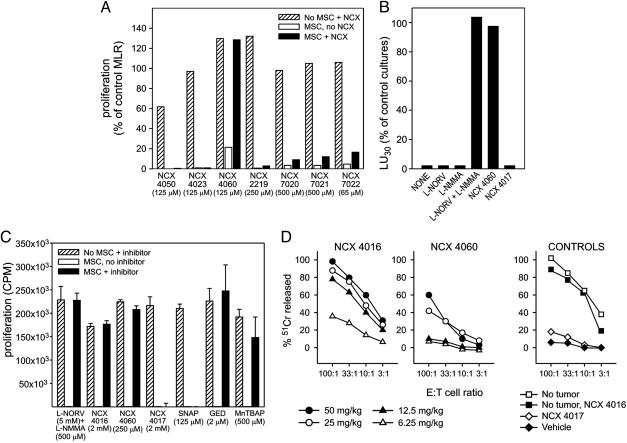
Fig. 1.
NO aspirin restores T lymphocyte function inhibited by myeloid suppressors. (A) Normal splenocytes stimulated in MLR by alloreactive cells are inhibited by MSCs. NCX 4016 restores the proliferative response. Data are expressed as the percentage of proliferation detected in control MLR without MSCs and drugs. The lowest drug concentration that did not negatively affect the proliferation of T lymphocytes in control cultures is reported. (B) Pooled splenocytes from BALB/c tumor-bearing mice were stimulated with alloreactive cells. Results are shown as the fraction of lytic units30 measured in the MLR from tumor-free mice in the presence of the various inhibitors. (C) Splenocyte proliferation induced by anti-CD3 and anti-CD28 is inhibited by MSCs and restored by different NO aspirin isomers. Data are expressed as the cpm means (± SE) of triplicates. SNAP, S-nitroso-N-acetylpenicillamine; GED, guanidinoethyldisulfide bicarbonate; MnTBAP, manganese-(III)-tetrakis-(4-benzoic-acid)-porphyrin. (D) Alloreactivity is restored in tumor-bearing mice by oral or i.p. administration of NCX 4016 or NCX 4060, respectively. Cytotoxicity by MLR-derived lymphocytes from tumor-free mice (no tumor) or control mice treated with NCX 4017 by gavage is shown for comparison.
DNA Immunization and Evaluation of CTL Response. Plasmid DNA, resuspended in nuclease-free water at a concentration of 1 mg/ml, was mixed with 6 mg/ml poly-l-glutamate and 150 nM NaCl. BALB/c mice were anesthetized with an i.p. injection of Avertin (Sigma–Aldrich) and injected bilaterally into tibial muscles with 50 μg of p185 or 100 μg of pcDNA3-env. A two-stainless-steel-parallel-plate caliper electrode was connected to a T820 Electro Square Porator (BTX, San Diego) and applied to the muscle immediately after DNA delivery for electroporation (26). Splenocytes (3 × 106) from BALB/c mice, some previously treated with NO aspirin, were incubated with 3 × 106 γ-irradiated C57BL/6 splenocytes. After five days, cultures were tested for ability to lyse 2 × 104 allogenic (MBL-2) or control (P815) target cells in a 5-h 51Cr-release assay as described in ref. 11.
Proliferation Assay. BALB/c splenocytes (7.5 × 105 cells per well) were stimulated with 7.5 × 105 γ-irradiated C57BL/6 splenocytes in 96-well, flat-bottom plates (BD Falcon labware, Becton Dickinson) in the presence of γ-irradiated MSC-2 cells (2.25 × 104) previously treated with IL-4 (23), with or without NO aspirins. Alternatively, splenocytes were stimulated in wells that had been coated with 3 μg/ml anti-CD3 (2C11, ATCC) and 2 μg/ml anti-CD28 (clone 37.5, ATCC). After 3 days of incubation, cultures were pulsed with 1 μCi (1 Ci = 37 GBq) of 3H-TdR (PerkinElmer) per well for 18 h, and 3H-TdR incorporation was measured by scintillation counting.
Synthesis of MHC/Peptide Tetrameric Complexes. The MHC class-I Ld-restricted peptide corresponding to amino acids 423–431 of gp70 (AH1 peptide) was synthesized by automatic solid-phase procedures (23). Peptide corresponding to amino acids 876–884 of β-Gal protein was synthesized and purified by Technogen (Naples). Phycoerythrin-labeled H-2-peptide tetramers were produced by mixing the biotinylated complexes with extravidin–phycoerythrin (Sigma–Aldrich) and were validated by staining CTL clones with the appropriate specificity (11).
Mixed Leukocyte Peptide Cultures, ELISA, and Determination of NO Production and ARG Activity. Splenocytes (2.5 × 107) were stimulated with gp70Ld peptides (1 μM) for 5 days in 10 ml of DMEM 10% FBS in 25-cm2 tissue culture flasks (BD Falcon labware, Becton Dickinson) at 37°C in 5% CO2. Splenocytes (106 cells) from mixed leukocyte peptide cultures were coincubated for 24 h with 105 target cells; the supernatant was then harvested and tested for released IFN-γ in a sandwich ELISA assay (Endogen, Boston) by following the manufacturer's instructions. NO released in the cultures was measured by Griess reaction as the amount of NO3– and NO2– produced by using a nitrate/nitrite assay kit (Cayman Chemical, Ann Arbor, MI). ARG activity was measured in cell lysates as described, and 1 unit of ARG was defined as the amount that catalyzes the formation of 1 μg of urea per min.
Histology and Immunohistochemistry. Acetone-fixed cryostat sections were immunostained with anti-NOS2 (Transduction Laboratories, Lexington, KY) or anti-nitrotyrosine (Chemicon). For the double CD11b/Gr-1 immunostaining, cryostat sections were incubated with a rat IgG2b monoclonal antibody against CD11b (Harlan Sera-Lab, Leicestershire, U.K.) and a FITC-conjugated Fab fragment of goat anti-rat IgG (Jackson ImmunoResearch). After being rinsed, sections were incubated with unlabeled blocking Fab fragments of goat anti-rat IgG (Jackson ImmunoResearch). Sections were then incubated with a rat IgG2b monoclonal antibody anti-Gr-1 (clone RB6-8C5, ATCC) and an Alexa Fluor 546-conjugated goat anti-rat IgG (Molecular Probes).
Results
NO Aspirin Corrects T Lymphocyte Dysfunction in Vitro. We developed a simple in vitro assay to rapidly test NO nonsteroidal antiinflammatory drugs in search of molecules that interfere with the immunosuppressive activity of MSCs. A mixed leukocyte reaction (MLR) assay was established with and without γ-irradiated MSC clones at concentrations sufficient to completely inhibit the proliferation of allostimulated T lymphocytes. Different NO donor molecules were tested at various doses to compare their relative efficacy. Only NCX 4060, a NO aspirin, restored in vitro T lymphocyte proliferation in response to alloantigens in the presence of MSC (Fig. 1A). Aspirin had no effect (data not shown).
Generation of alloreactive CTL was impaired in BALB/c mice inoculated with the adenocarcinoma C26 transduced to express granulocyte/macrophage colony-stimulating factor, one of the factors known to induce MSC accumulation and immune unresponsiveness (Fig. 1B) (9, 11). As previously shown (11), T lymphocyte suppression depended on l-arginine metabolism and was reversed by a mixture of NOS and ARG1 inhibitors, l-NMMA and l-norv, respectively (Fig. 1B). NCX 4060 alone was effective in abolishing MSC-induced T cell unresponsiveness and recovering alloreactive CTL in tumor-bearing mice (Fig. 1B). NCX 4017, a nitroaspirin spacer molecule devoid of the NO-donating group and used as a control in these studies, was without effect, indicating that NO release was essential for the in vitro activity of NO aspirin (Fig. 1B).
NCX 4060 is the prototype of NO-donating aspirins characterized by an aromatic spacer. Different stereoisomers of NO aspirin are available, including NCX 4016. Both compounds were able to restore proliferation of T lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 in the presence of MSC (Fig. 1C). Conversely, the NO donor (S-nitroso-N-acetylpenicillamine) and NCX 4017 were ineffective at all of the tested concentrations, indicating that neither NO release alone nor salycilates are sufficient to reverse suppression. Peroxynitrites produced by MSCs under conditions of l-arginine deprivation, as in the case of ARG1 activation (11, 16), are known to be extremely toxic to T lymphocytes (17) and are involved in the MSC inhibitory pathways (3, 11). Accordingly, either a combination of ARG and NOS inhibitors or peroxynitrites scavangers [guanidinoethyldisulfide bicarbonate and manganese-(III)-tetrakis-(4-benzoic-acid)-porphyrin] restored the proliferative response impaired by MSCs, indicating that a relevant contribution to MSC suppressive activity was given by peroxynitrite production from combined ARG and NOS enzyme activity.
NO Aspirin Corrects Systemic T Lymphocyte Dysfunction in Vivo. To evaluate their in vivo activity, we tested both NCX 4016 and NCX 4060 in tumor-bearing mice. NCX 4016 was previously shown to be very effective when given orally in animals and humans (18). Indeed, whereas NCX 4060 was active only when given i.p., NCX 4016 restored the compromised alloreactive CTL response when given orally by gavage for 9 consecutive days at concentrations ranging between 12.5 and 50 mg·kg–1·day–1 in mice bearing the colon carcinoma C26-GM (Fig. 1D). Because MLR was performed in the absence of any drug, we can conclude that MSCs from NCX 4016-treated tumor-bearing mice had lost their immunosuppressive activity and NCX 4016 was effective in vivo. NCX 4016 did not alter the number and percentage of MSCs in the spleen (11 ± 3.2% vs. 12.9 ± 6.5% CD11b+/Gr-1+ in tumor-bearing mice either treated with vehicle or NCX 4016, respectively), suggesting that it influenced events downstream of MSC accumulation (see below). The same doses that restored the immune reactivity to alloantigens caused only a slight retardation of tumor growth (Fig. 5, which is published as supporting information on the PNAS web site). When the NCX 4016 gavage was terminated, the tumor progressed and no statistically different survival rates among groups emerged (data not shown). Administration of either NCX 4017 or a NO donor had no effect on T lymphocyte responsiveness and tumor growth, thus confirming the peculiar biological activity of NO aspirin (Figs. 1D and 5).
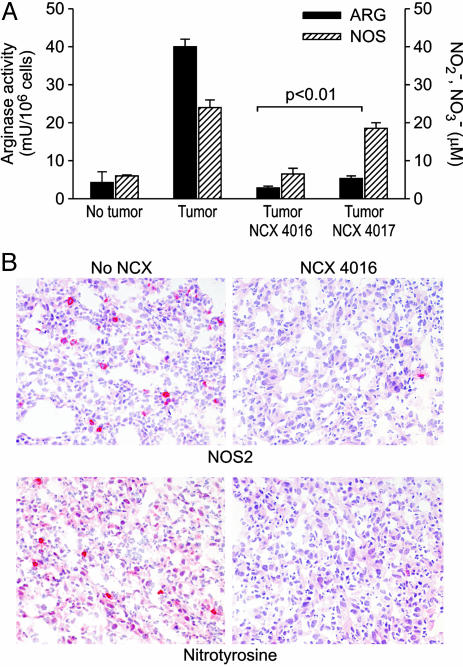
NO Aspirin Affects ARG Activity and NOS Activity in Tumor-Bearing Mice. Because ARG1 and NOS2 are central to the inhibitory activity of MSC on T lymphocytes (11, 12), we evaluated the in vivo effects of NO aspirin on these enzymes. As previously described (11), CD11b+ splenocytes derived from tumor-bearing mice had a significant increase in ARG and NOS activity compared with the same cell fraction isolated from tumor-free mice (t test, P < 0.01 (Fig. 2A). NCX 4016 given orally reduced both enzyme activities in CD11b+ splenocytes from tumor-bearing mice to the levels of CD11b+ cells isolated from tumor-free mice. Interestingly, NCX 4017 did not affect the NOS activity of tumor-bearing CD11b+ splenocytes, but significantly reduced urea production by ARG in the same cells, suggesting that different portions of the NO aspirins had different pharmacological activities: NO release was essential for NOS inhibition, whereas the aspirin spacer portion was responsible for the ARG-dependent effect. NOS2 was also absent in cell infiltrates of s.c. C26-GM tumor specimens taken from mice treated with NCX 4016 (Fig. 2B). For the lack of reagents, we were not able to detect changes in ARG1 protein in tumor infiltrates by immunohistochemistry; however, the elevated ARG enzyme activity detected in CD11b+ cells infiltrating the tumor was reduced by oral NCX 4016 administration (Fig. 6, which is published as supporting information on the PNAS web site). At physiological pH, peroxynitrite undergoes heterolytic cleavage to form hydroxyl anion and nitronium ion that nitrates protein tyrosine residues (27). Thus, the presence of nitrotyrosine on proteins can be used as a marker of in vivo peroxynitrite formation. Orally administered NCX 4016 completely abrogated intratumoral peroxynitrite production, as detected by the almost complete disappearance of nitrotyrosines in tissue sections (Fig. 2B). Finally, tumors treated with NCX 4016 had a lower content of CD11b+ and Gr-1+ cells (34.57 ± 11.67 vs. 58.5 ± 17.17, ×400 field, 0.180 mm2 per field; P < 0.01), suggesting either a decreased recruitment or decreased survival of intratumoral MSCs (Fig. 6).
Fig. 2.
NO aspirin treatment inhibits NOS and ARG activity in vivo.(A) Effect of oral administration of NCX 4016 and NCX 4017 on ARG and NOS activity in CD11b+ splenocytes isolated from tumor-bearing mice. Data are from three separate experiments and given as mean ± SE. (B) The immunohistochemical findings on C26-GM tumors taken from NCX-4016-treated and untreated (no NCX) mice were in line with the loss of NOS2 and ARG activity shown in A. NOS2 and nitrotyrosine (in red), largely present in untreated mice tumors, were almost absent in treated mice.
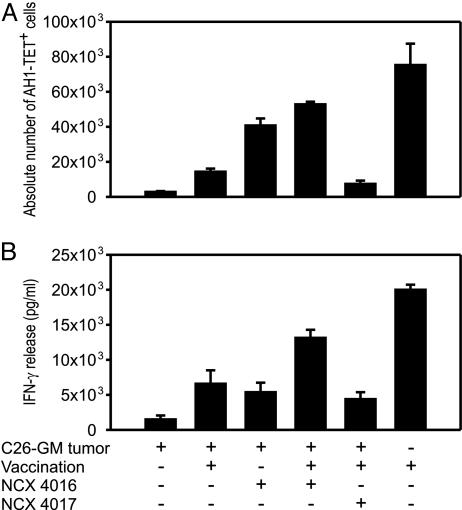
NO Aspirin Alleviates Tumor-Specific Unresponsiveness. To define the antitumor response in mice receiving NO aspirin, we monitored the specific immune response to endogenous retroviral gp70 env protein and its immunogenic AH1 peptide shared by several murine tumors (23). Splenocytes isolated from C26-GM tumor-bearing mice were stimulated with AH1 Ld-restricted peptide derived from gp70 antigen and, after 5 days of culture, stained with AH1-Ld H-2 tetramers (AH1-TET) to enumerate tumor-specific T lymphocytes and test for IFN-γ production in response to AH1, as described in ref. 23.
Tumor-specific CD8+ T lymphocytes were not detected in tumor-bearing mice receiving either the vehicle alone or the control NCX 4017 (data not shown), whereas an expanded population of AH1-TET+/CD8+ lymphocytes was promptly recovered from tumor-bearing mice treated with the NO-releasing NCX 4016 for 9 consecutive days (Fig. 3A). Vaccination with γ-irradiated CT26, a gp70-positive colon carcinoma, induced the appearance of AH1-TET+ lymphocytes in tumor-free mice but not in tumor-bearing mice. Interestingly, the γ-irradiated cell-based vaccine did not substantially increase the number of AH1-TET+ cells in NCX 4016-treated mice (Fig. 3A), but it did enhance the effects of NCX 4016 (and not those of NCX 4017), improving the overall functional response to AH1 antigen compared with tumor-bearing mice that had received either NCX 4016 or vaccine alone (Fig. 3B).
Fig. 3.
Oral administration of NO aspirin recovers the number and function of tumor-specific CD8+ T cells induced by cancer vaccine. (A) The absolute number of AH1-TET-positive cells was calculated among gated CD3/CD8-positive cells after subtraction of the background staining obtained by negative control β-Gal876–884-Ld/TET. (B) Function of peptide-stimulated T lymphocytes was evaluated by IFN-γ release. The values of IFN-γ release are reported as the difference between the values obtained in the presence of peptide-pulsed and unpulsed 293-Ld cells used as stimulators in a 24-h assay. Data are represented as mean ± SE (n = 5).
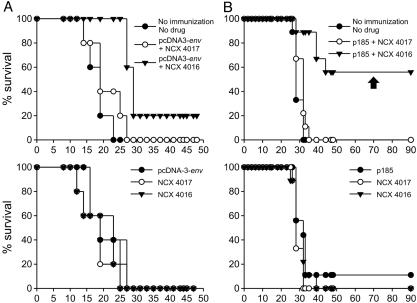
NO Aspirin Potentiates the Effects of Recombinant Cancer Vaccines. Based on these findings, we tested whether combining the vaccination against gp70 with NCX 4016 treatment might result in enhanced antitumor activity. We have previously shown that DNA immunization with gp70 antigen is very weak in the absence of adjuvants (23) and does not result in a significant antitumor effect despite the induction of AH1-specific T lymphocytes. The CT26 cell line is an unmanipulated colon carcinoma closely related to C26 that was previously shown to exert immunosuppressive activity during its progression (9, 28). In this experiment, mice were vaccinated with the empty pcDNA3 plasmid (control) or pcDNA3-env (encoding the full-length env gene), challenged with CT26, and then treated by gavage with either NCX 4016 or NCX 4017 from day 1 to 18 after challenge. Only mice that received pcDNA3-env and were orally fed NCX 4016 showed a significant prolongation of survival; moreover, 20% remained tumor-free for up to 120 days, when the experiment was terminated (P < 0.01) (Fig. 4A).
Fig. 4.
Adjuvant effect of oral NO aspirin on recombinant cancer vaccines. (A) Oral NO aspirin enhances the DNA vaccine coding for an endogenous retrovirus (pcDNA3-env) to prevent challenge with a colon carcinoma. Only the survival rates of mice vaccinated with pcDNA3-env and treated with NCX 4016 differed significantly (Mantel–Haenszel test, P < 0.01) from untreated controls. (B) Oral NO aspirin enhances the therapeutic effectiveness of DNA vaccination on established carcinomas. Only mice treated with the combination NCX 4016 and p185-encoding DNA vaccine showed a significant prolongation of survival (Mantel–Haenszel test, P < 0.01). Control age-matched mice and mice that had rejected the tumor were challenged in the contralateral flank and omolateral superior limb with N2C or CT26 cells, respectively, 70 days after the DNA vaccination (arrow).
These experiments demonstrated that NO aspirin enhanced the antitumor response directed at weak antigens, at least during the initial phases of tumor development. To investigate the effect of NCX 4016 and vaccination on established tumors, we used a different tumor model previously shown to induce high numbers of MSCs. The primary mammary carcinoma cell line N2C was derived from female BALB-neuT mice that spontaneously developed mammary tumors for the transgenic expression of the activated rat HER-2/neu p185 oncogene (24). s.c. N2C tumors were cured by immunization with a plasmid DNA encoding the extracellular and transmembrane portion of HER-2/neu (p185) when the vaccination was performed with a mean tumor area of <20 ± 0.9 mm2 (data not shown). However, when the tumor reached a mean size of 48 mm2 (Fig. 4B and Fig. 7, which is published as supporting information on the PNAS web site), MSCs accumulated in the spleen and blood (where they comprised 22.1 ± 6.5% and 60 ± 2.5% of total cells, respectively) and DNA vaccination was no longer effective. As in the previous model, oral administration of NCX 4016 (and not NCX 4017) restored vaccine efficacy, prolonging the survival of vaccinated mice, 56% of which were cured of their tumors (Fig. 4B). These cured mice rejected a second challenge of N2C cells, but not of unrelated CT26 cells, given 70 days after the initial DNA vaccination, indicating the existence of a tumor-specific memory response (Figs. 4B and 7).
Discussion
Different strategies have been used to correct the immune dysfunction induced in T lymphocytes by myeloid suppressors (7, 29, 30), but none of them was shown to restore the immune reactivity against tumor-associated antigens in tumor-bearing hosts. In this study, we demonstrated that by pharmacologically interfering with pathways used by MSCs to suppress T lymphocyte function, immune response against tumor antigens was unmasked and the therapeutic effectiveness of active immunotherapy was enhanced. NO aspirin is the prototype of a class of immune modulators that do not operate by favoring T lymphocyte priming, as in the case of traditional adjuvants, but rather by neutralizing negative signals that suppress T lymphocyte expansion in tumor-bearing hosts. To the best of our knowledge, there is no other single molecule that possesses such properties in vivo.
Two derivatives of NO-donating aspirin were used in our experiments: NCX 4060 and NCX 4016, positional isomers with the NO-donating group on the spacer. The activity of these compounds was compared with that of control drugs, including NO donors and NCX 4017, an analogue of NCX 4016 that is devoid of the NO-donating group. The main activity of NO aspirin is to restrain ARG and NOS activities in spleen and tumor-associated myeloid cells. Feedback inhibition of NOS activity and expression by NO aspirin was previously demonstrated (21) and, as expected, depended entirely on the NO donation given that it was not observed after treatment with the control NCX 4017. ARG inhibition, conversely, was carried out by the NO-lacking moiety NCX 4017, which is composed of aspirin and an aromatic spacer. Both molecules, NCX 4016 and 4017, reduced ARG activity when added to cell lysates at an IC50 of >10-fold the dose administered to tumor-bearing mice (data not shown), a dose too high to hypothesize an in vivo direct activity of the salicylic portion of NO aspirin. It is more likely that salicylates operate indirectly by inhibiting the intracellular signal transducer and activator of transcription 6-mediated signals triggered by cytokines, such as IL-4 and IL-13, which are potent ARG inducers in myeloid cells (7, 31–34).
Our results indicate that inhibition of both enzymes is crucial for the NO aspirin pharmacological effect. Activation of ARG1 or NOS2 in MSC was shown to exert inhibitory effects in different tumor models: ARG1 depletes l-arginine in local microenvironment leading to loss of the CD3 zeta chain in T lymphocytes and their functional paralysis after antigen recognition (15), whereas NO produced by NOS2 blocks the signaling through the IL-2 receptors of T lymphocytes by impeding phosphorylation of the intracellular signaling proteins signal transducer and activator of transcription 5, Akt, and extracellular signal-regulated kinase (12). However, these enzymes also have a synergistic effect. Depletion of the cytosolic l-arginine content by ARG1 triggers the generation of superoxide from the NOS2 reductase domain, eventually leading to formation of peroxynitrites, highly reactive oxidizing agents that nitrate protein-associate tyrosines and damage different biological targets, including T lymphocytes (17, 35). NCX 4016 but not NCX 4017 was shown to reduce the intratumoral content of nitrotyrosine, further supporting the idea that peroxynitrites are largely generated by the combined ARG1 and NOS2 activity in tumor-infiltrating myeloid cells (Fig. 2B).
Given the extremely pleiotropic effects of nitroso compounds, other mechanisms of action can be postulated. For example, NO nonsteroidal antiinflammatory drugs are known to suppress neutrophil adherence to the vascular endothelium in response to chemotaxins (36); this might account for the reduction in the flow of myeloid cells at the tumor site observed in mice orally treated with NO aspirin (Fig. 6). Nonsteroidal antiinflammatory drugs and NO aspirin in particular possess also an interesting antiproliferative/proapoptitic activity in several cancer cell lines (22, 37), and in some models of in vivo carcinogenesis, either chemically induced (38) or spontaneous, as in the case of transgenic APCMin/+ mice (39). NO aspirin is a very potent inhibitor of vascular smooth muscle cell proliferation (40) so, in theory, might reduce the blood supply to the growing tumors. It must be pointed out, however, that, in our experiments, orally administered NO aspirin had a very marginal effect on the growth of all of the transplantable tumors we used (Figs. 4 and 5), whereas it completely relieved tumor-induced immune dysfunctions. Moreover, staining with CD31 to enumerate the endothelial cells in mice treated with NCX 4016 did not show any difference from the untreated mice (87 ± 13 vs. 80 ± 6 cells for no treatment and NCX 4016, respectively; ×400 field; 0.180 mm2 per field), suggesting that neoangiogenesis dependent on factors released by the growing tumors was not altered. Finally, although we observed a reduction of tumor-infiltrating MSCs, we did not detect any change in the number of splenic MSCs, which are responsible for the systemic immunosuppression. All these findings clearly indicate that NO aspirin adjuvant effect was independent of direct antitumor activity.
In the present study, we have demonstrated the adjuvant activity of NO aspirin in two tumor models selected to prove its activity in treating small or large tumors, with or without systemic MSC expansion. In the first model (CT26 colon carcinoma), the DNA vaccine induced a weak response not sufficient to cure established tumors but potentially useful for limited tumor burdens (Fig. 4A). In the second model, the N2C mammary carcinoma was allowed to grow s.c. for some time, which led to a systemic accumulation of MSCs in blood and spleen and an impairment of the alloreactive T lymphocyte response (data not shown). In this model, the tumor antigen induced a strong immune response that was therapeutic for mice bearing small tumors but that was no longer effective at late time points coincident with a diffused disease (Fig. 4B). This situation is common for many human clinical trials in which the patients enter the immunotherapy protocol with a metastatic disease.
Our results have an immediate relevance for cancer patients; in fact, the NO aspirin isomer NCX 4016 is actually in clinical pivotal phase II development for peripheral vascular disease and has already demonstrated a safer profile than aspirin in clinical trials (41) in which large numbers of volunteers/patients have been treated without major side effects.
Supplementary Material
Acknowledgments
We thank Lisa Smith for editing the manuscript and Pierantonio Gallo for assistance with graphics. This work was supported by Fondo per gli Investimenti della Ricerca di Base–Ministero dell'Instruzione, dell'Universitá, e della Ricerca Contract RBAU01935A; the Italian Association for Cancer Research; Cassa di Risparmio di Verona, Vicenza, Belluno, e Ancona, Bando 2001 “Ambiente e sviluppo sostenibile”; European Union Contract 005033-EICOSANOX (to the G. d'Annunzio University Foundation); and Italy–U.S. Cooperation Program for the Therapy of Cancer Grant T00.A17. I.M. and P.S. are supported by a fellowship from the Italian Foundation for Cancer Research.
Author contributions: P.Z. and V.B. designed research; C.D.S., P.S., I.M., L.D., C.M., and M.I. performed research; C.D.S., P.S., P.D.S., M.P.C., P.M., P.Z., and V.B. analyzed data; M.B., P.D.S., and C.G. contributed new reagents/analytic tools; and C.D.S., M.P.C., P.M., and V.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ARG, arginase; MSC, myeloid suppressor cell; TET, Ld H-2 tetramer; MLR, mixed leukocyte reaction; NOS, NO synthase; CTL, cytotoxic T lymphocyte; l-NMMA, Ng-monomethyl-l-arginine; l-norv, l-norvaline.
References
- 1.Rosenberg, S. A., Yang, J. C. & Restifo, N. P. (2004) Nat. Med. 10, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serafini, P., De Santo, C., Marigo, I., Cingarlini, S., Dolcetti, L., Gallina, G., Zanovello, P. & Bronte, V. (2003) Cancer Immunol. Immunother. 53, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronte, V., Serafini, P., Mazzoni, A., Segal, D. M. & Zanovello, P. (2003) Trends Immunol. 24, 301–305. [DOI] [PubMed] [Google Scholar]
- 4.Almand, B., Clark, J. I., Nikitina, E., van Beynen, J., English, N. R., Knight, S. C., Carbone, D. P. & Gabrilovich, D. I. (2001) J. Immunol. 166, 678–689. [DOI] [PubMed] [Google Scholar]
- 5.Melani, C., Chiodoni, C., Forni, G. & Colombo, M. P. (2003) Blood 102, 2138–2145. [DOI] [PubMed] [Google Scholar]
- 6.Baniyash, M. (2004) Nat. Rev. Immunol. 4, 675–687. [DOI] [PubMed] [Google Scholar]
- 7.Terabe, M., Matsui, S., Park, J. M., Mamura, M., Noben-Trauth, N., Donaldson, D. D., Chen, W., Wahl, S. M., Ledbetter, S., Pratt, B., et al. (2003) J. Exp. Med. 198, 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronte, V., Wang, M., Overwijk, W. W., Surman, D. R., Pericle, F., Rosenberg, S. A. & Restifo, N. P. (1998) J. Immunol. 161, 5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte, V., Chappel, D. B., Apolloni, E., Cabrelle, A., Wang, M., Hwu, P. & Restifo, N. P. (1999) J. Immunol. 162, 5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 10.Seung, L. P., Rowley, D. A., Dubey, P. & Schreiber, H. (1995) Proc. Natl. Acad. Sci. USA 92, 6254–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte, V., Serafini, P., De Santo, C., Marigo, I., Tosello, V., Mazzoni, A., Segal, D. M., Staib, C., Lowel, M., Sutter, G., et al. (2003) J. Immunol. 170, 270–278. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoni, A., Bronte, V., Visintin, A., Spitzer, J. H., Apolloni, E., Serafini, P., Zanovello, P. & Segal, D. M. (2002) J. Immunol. 168, 689–695. [DOI] [PubMed] [Google Scholar]
- 13.Apolloni, E., Bronte, V., Mazzoni, A., Serafini, P., Cabrelle, A., Segal, D. M., Young, H. A. & Zanovello, P. (2000) J. Immunol. 165, 6723–6730. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez, P. C., Zea, A. H., DeSalvo, J., Culotta, K. S., Zabaleta, J., Quiceno, D. G., Ochoa, J. B. & Ochoa, A. C. (2003) J. Immunol. 171, 1232–1239. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez, P. C., Quiceno, D. G., Zabaleta, J., Ortiz, B., Zea, A. H., Piazuelo, M. B., Delgado, A., Correa, P., Brayer, J., Sotomayor, E. M., et al. (2004) Cancer Res. 64, 5839–5849. [DOI] [PubMed] [Google Scholar]
- 16.Kusmartsev, S., Nefedova, Y., Yoder, D. & Gabrilovich, D. I. (2004) J. Immunol. 172, 989–999. [DOI] [PubMed] [Google Scholar]
- 17.Brito, C., Naviliat, M., Tiscornia, A. C., Vuillier, F., Gualco, G., Dighiero, G., Radi, R. & Cayota, A. M. (1999) J. Immunol. 162, 3356–3366. [PubMed] [Google Scholar]
- 18.Wallace, J. L., Ignarro, L. J. & Fiorucci, S. (2002) Nat. Rev. Drug Discovery 1, 375–382. [DOI] [PubMed] [Google Scholar]
- 19.Napoli, C., Ackah, E., De Nigris, F., Del Soldato, P., D'Armiento, F. P., Crimi, E., Condorelli, M. & Sessa, W. C. (2002) Proc. Natl. Acad. Sci. USA 99, 12467–12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorucci, S., Santucci, L., Cirino, G., Mencarelli, A., Familiari, L., Soldato, P. D. & Morelli, A. (2000) J. Immunol. 165, 5245–5254. [DOI] [PubMed] [Google Scholar]
- 21.Mariotto, S., Cuzzolin, L., Adami, A., Del Soldato, P., Suzuki, H. & Benoni, G. (1995) Br. J. Pharmacol. 115, 225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nath, N., Kashfi, K., Chen, J. & Rigas, B. (2003) Proc. Natl. Acad. Sci. USA 100, 12584–12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bronte, V., Cingarlini, S., Apolloni, E., Serafini, P., Marigo, I., De Santo, C., Macino, B., Marin, O. & Zanovello, P. (2003) J. Immunol. 171, 6396–6405. [DOI] [PubMed] [Google Scholar]
- 24.Sangaletti, S., Stoppacciaro, A., Guiducci, C., Torrisi, M. R. & Colombo, M. P. (2003) J. Exp. Med. 198, 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovero, S., Amici, A., Carlo, E. D., Bei, R., Nanni, P., Quaglino, E., Porcedda, P., Boggio, K., Smorlesi, A., Lollini, P. L., et al. (2000) J. Immunol. 165, 5133–5142. [DOI] [PubMed] [Google Scholar]
- 26.Mendiratta, K. S., Thai, G., Elashi, N. K., Thull, N. M., Matar, M., Bronte, V. & Pericle, F. (2001) Cancer Res. 61, 859–863. [PubMed] [Google Scholar]
- 27.Groves, J. T. (1999) Curr. Opin. Chem. Biol. 3, 226–235. [DOI] [PubMed] [Google Scholar]
- 28.Bronte, V., Apolloni, E., Cabrelle, A., Ronca, R., Serafini, A., Zamboni, P., Restifo, N. P. & Zanovello, P. (2000) Blood 96, 3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 29.Kusmartsev, S., Cheng, F., Yu, B., Nefedova, Y., Sotomayor, E., Lush, R. & Gabrilovich, D. (2003) Cancer Res. 63, 4441–4449. [PubMed] [Google Scholar]
- 30.Li, Q., Pan, P. Y., Gu, P., Xu, D. & Chen, S. H. (2004) Cancer Res. 64, 1130–1139. [DOI] [PubMed] [Google Scholar]
- 31.Perez, G. M., Melo, M., Keegan, A. D. & Zamorano, J. (2002) J. Immunol. 168, 1428–1434. [DOI] [PubMed] [Google Scholar]
- 32.Munder, M., Eichmann, K. & Modolell, M. (1998) J. Immunol. 160, 5347–5354. [PubMed] [Google Scholar]
- 33.Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. (2000) J. Immunol. 164, 6166–6173. [DOI] [PubMed] [Google Scholar]
- 34.Terabe, M., Matsui, S., Noben-Trauth, N., Chen, H., Watson, C., Donaldson, D. D., Carbone, D. P., Paul, W. E. & Berzofsky, J. A. (2000) Nat. Immunol. 1, 515–520. [DOI] [PubMed] [Google Scholar]
- 35.Xia, Y. & Zweier, J. L. (1997) Proc. Natl. Acad. Sci. USA 94, 6954–6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace, J. L., Vergnolle, N., Muscara, M. N., Asfaha, S., Chapman, K., McKnight, W., Del Soldato, P., Morelli, A. & Fiorucci, S. (1999) Gastroenterology 117, 557–566. [DOI] [PubMed] [Google Scholar]
- 37.Williams, J. L., Nath, N., Chen, J., Hundley, T. R., Gao, J., Kopelovich, L., Kashfi, K. & Rigas, B. (2003) Cancer Res. 63, 7613–7618. [PubMed] [Google Scholar]
- 38.Bak, A. W., McKnight, W., Li, P., Del Soldato, P., Calignano, A., Cirino, G. & Wallace, J. L. (1998) Life Sci. 62, 367–373. [DOI] [PubMed] [Google Scholar]
- 39.Williams, J. L., Kashfi, K., Ouyang, N., del Soldato, P., Kopelovich, L. & Rigas, B. (2004) Biochem. Biophys. Res. Commun. 313, 784–788. [DOI] [PubMed] [Google Scholar]
- 40.Napoli, C., Cirino, G., Del Soldato, P., Sorrentino, R., Sica, V., Condorelli, M., Pinto, A. & Ignarro, L. J. (2001) Proc. Natl. Acad. Sci. USA 98, 2860–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorucci, S., Santucci, L., Gresele, P., Faccino, R. M., Del Soldato, P. & Morelli, A. (2003) Gastroenterology 124, 600–607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.