Abstract
The consumption of one meal of seafood containing domoic acid (DA) at levels high enough to induce seizures can cause gross histopathological lesions in hippocampal regions of the brain and permanent memory loss in humans and marine mammals. Seafood regulatory limits have been set at 20 mg DA/kg shellfish to protect human consumers from symptomatic acute exposure, but the effects of repetitive low-level asymptomatic exposure remain a critical knowledge gap. Recreational and Tribal-subsistence shellfish harvesters are known to regularly consume low levels of DA. The aim of this study was to determine if chronic low-level DA exposure, at doses below those that cause overt signs of neurotoxicity, has quantifiable impacts on cognitive function. To this end, female C57BL/6NJ mice were exposed to asymptomatic doses of DA (≈ 0.75 mg/kg) or vehicle once a week for several months. Spatial learning and memory were tested in a radial water maze paradigm at one, six and 25 weeks of exposure, after a nine-week recovery period following cessation of exposure, and at three old age time points (18, 24 and 28 months old). Mice from select time points were also tested for activity levels in a novel cage environment using a photobeam activity system. Chronic low-level DA exposure caused significant spatial learning impairment and hyperactivity after 25 weeks of exposure in the absence of visible histopathological lesions in hippocampal regions of the brain. These cognitive effects were reversible after a nine-week recovery period with no toxin exposure and recovery was sustained into old age. These findings identify a new potential health risk of chronic low-level exposure in a mammalian model. Unlike the permanent cognitive impacts of acute exposure, the chronic low-level effects observed in this study were reversible suggesting that these deficits could potentially be managed through cessation of exposure if they also occur in human seafood consumers.
1. Introduction
Domoic acid (DA) is a neurotoxic amino acid that is naturally produced by some species of diatoms of the genus Pseudo-nitzschia found in phytoplankton communities in oceans throughout the world (Bates, 2000; Lundholm and Moestrup, 2000; Marchetti et al., 2008; Martin et al., 1990). During harmful algal blooms (HABs) of toxigenic Pseudo-nitzschia, DA can accumulate in filter-feeding fish and shellfish making them unsafe for consumption by marine mammals and humans (Scholin et al., 2000; Todd, 1993). Current evidence suggests that HABs in general are increasing in magnitude and frequency globally as oceans continue to warm (Moore et al., 2008; Van Dolah, 2000). In 2015, an unprecedented coastwide toxic Pseudo- nitzschia bloom was linked to a warm water anomaly that spanned the west coast of North America resulting in large scale closures of razor clam (Siliqua patula), rock crab (Cancer anthonyi, C. antennarius, and C. productus) and Dungeness crab (C. magister) fisheries in multiple states (McCabe et al., 2016). Record-breaking levels of DA were detected in coastal food webs and seafood resources resulting in devastating economic losses and ecological damage (McCabe et al., 2016).
Domoic acid was first recognized as a seafood toxin in 1987 when over 100 people became ill after consuming DA-contaminated mussels (Mytilus edulis) harvested near Prince Edward Island in Canada (Perl et al., 1990). The condition was termed amnesic shellfish poisoning (ASP) and symptoms included gastrointestinal distress, confusion, disorientation, seizures, permanent short-term memory loss, and in the most severe cases death (Perl et al., 1990). In the aftermath of this event, a seafood safety regulatory limit was set at 20 mg DA/kg shellfish (Marien, 1996). The regulatory limit was based on the lowest level of DA reported to cause observable toxic effects from mussel consumption in the Canadian event (1 mg DA/kg body weight) and oral dose studies with primates where effects were not observed at 0.5 and 0.75 mg/kg body weight doses, but clinical effects were observed at 1 mg/kg body weight doses (Iverson et al., 1990; Marien, 1996; Wekell et al., 2004). The acute reference dose (ARfD) for acute DA exposure was determined as 0.075 mg/kg by taking 0.75 and applying a safety factor of 10. This limit was set to protect human health from acute toxicity in the context of one meal of 270 grams of clams in a 70 kg adult and includes a 10-fold safety factor to protect sensitive individuals such as young, old, and health compromised (Marien, 1996). It does not take into account consumption over multiple days, larger meal sizes, and/or potential effects of chronic long-term low-level (below the regulatory limit) toxin exposure. Although the risks of acute exposure to symptomatic doses of DA have been minimized by testing shellfish and regulating harvests based on toxin loads (≥20 mg DA/kg shellfish = harvest closure), there are no regulations in place for protection from long-term low-level repetitive DA exposure (< 20 mg DA/kg shellfish = harvest open). The effects of chronic low-level asymptomatic exposure have not been well studied and are of particular concern for coastal communities including recreational and Tribal harvesters who regularly consume shellfish such as razor clams that are known to retain low levels of DA in edible tissues for more than a year after HAB events (Lefebvre and Robertson, 2010; Wekell et al., 1994). In a study with long-term whole animal exposures to low asymptomatic doses of DA, zebrafish (Danio rerio) had altered gene transcriptomes and impaired mitochondrial function in whole brains after nine months of weekly exposures (Hiolski et al., 2014). Additionally, chronic low-level exposure did not confer resistance to DA, but instead increased toxin sensitivity making the neurologic effects of subsequent exposures more pronounced in chronically exposed animals (Hiolski et al., 2014).
In addition to human health risks, DA poisoning is a significant health risk for marine mammals that is likely increasing as HABs increase. The first DA poisoning event documented in marine mammals occurred in 1998 in Monterey Bay, CA when hundreds of California sea lions (Zalophus californianus) stranded on beaches exhibiting signs of neurotoxicity in the form of seizures, tremors and ataxia (Scholin et al., 2000). Over the last 18 years following the first documented DA poisoning event in marine mammals, dozens to hundreds of sea lions continue to be sickened each year with exceptionally high numbers in the most recent years (Bejarano et al., 2008; McCabe et al., 2016). From 2010–2013, The Marine Mammal Center in Sausalito, CA diagnosed an average of 64 ± 21 sea lions per year with DA poisoning, while numbers rose to over 200 in 2014 and 2015 (McCabe et al., 2016). Sea lions have served as a valuable sentinel for human health and a mammalian model for the elucidation of mechanisms of DA toxicity with natural environmental exposure. Acute toxicity as described above results from DA exposure high enough to elicit outward signs of neuroexcitotoxicity such as seizures within hours after exposure. A persistent toxicity syndrome has also been defined for neurologic effects that continue long after an initial acute exposure and is characterized by episodic seizures and permanent spatial memory loss (Cook et al., 2015; Goldstein et al., 2008; Muha and Ramsdell, 2011). As with humans, the effects of low-level repetitive exposure (levels below those that elicit visible neurotoxic symptoms) have not been studied in marine mammals and are likely to impact chronically exposed animals.
The objectives of this study were to determine if chronic asymptomatic DA exposure at doses below those that cause the overt signs of neurotoxicity described above leads to underlying health impacts in the mammalian system. To this end, female C57BL/6NJ mice were chronically exposed to low asymptomatic doses of DA via intraperitoneal injection (IP) once a week beginning at 3 months of age and examined at multiple time points for spatial memory and learning as well as general activity levels and gross morphology in hippocampal regions of the brain. Results of this study are applicable to both human and marine mammal health.
2. Methods
2.1. Test Animals
Eight-week-old male and female C57BL/6NJ mice were obtained from The Jackson Laboratory and acclimated for four weeks. All mice were housed at the animal research facility at the University of Washington and provided free access to a standard rodent diet (PicoLab® Rodent Diet 20, Lab Diet, USA) and water ad libitum in a controlled environment with a 12-hr light/dark cycle. Dose response assays were performed with male and female mice to determine if there were sex differences in toxin susceptibility using scratching as a toxicological endpoint (Tasker et al., 1991). Five IP doses were used to calculate 50% effective concentrations (EC50s) for male and female mice for comparison of DA sensitivity (n = 4 mice per sex per dose). Sex differences were not observed and female mice were chosen for subsequent long-term chronic exposure experiments because females were easier to handle during injections. Additional eight- week-old female C57BL/6NJ mice were purchased from The Jackson Laboratory and allowed to acclimate for four weeks before long-term asymptomatic exposure studies began. Animal handling and experimental procedures were performed in accordance with protocols approved by the Animal Care and Use Committee at the University of Washington.
2.2. Quantification of Asymptomatic Exposure Doses
The target asymptomatic exposure dose was chosen based on EC50 values and results from a pilot study where mice were injected weekly for six weeks with doses approximately 40% of the EC50 to identify a dose that would not elicit overt signs of neurotoxicity after repetitive exposure. All DA solutions were prepared from powdered DA (purchased from Tocris and from Sigma Aldrich) dissolved in nanopure water and quantified via high performance liquid Chromatography (HPLC) (Quilliam et al., 1989). Stock solutions were then diluted to target concentrations in phosphate buffered saline (PBS), analyzed by HPLC to confirm target doses and administered via IP injections of 200 μl in mice. Mice were weighed at all injections and doses were quantified for all animals at all time points.
2.3. Chronic Exposure Experiments
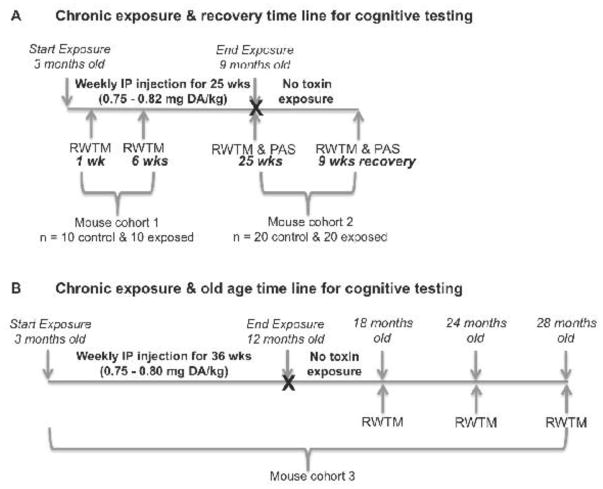
Female C57BL/6NJ mice were IP-injected once a week with either PBS (control) or approximately 0.75 to 0.82 mg DA/kg body weight from three months of age to nine months of age (25 weeks of exposure). One cohort of mice was sampled at one week and six weeks (n=10 exposed, n=10 control) of exposure and a second separate cohort of mice was sampled at 25 weeks (n=20 exposed, n=20 control) of exposure and all were tested for spatial learning and memory using a modified Barnes radial water tread maze (RWTM) (Pettan-Brewer et al., 2013). Mice from cohort two that were tested at 25 weeks of exposure (n=20 exposed, n=20 control) were also tested for activity level in a novel cage environment via an open field photobeam activity system (PAS), then given a nine-week recovery period of no toxin exposure and retested for spatial learning and memory via RWTM and novel cage activity via the open field PAS (Figure 1A).
Figure 1.
Schematic of chronic exposures and cognitive testing time lines for A) 25 week chronic exposure and nine week recovery experiments and B) 36 week chronic exposure and old age experiments. RWTM = Radial Water Tread Maze; PAS = Photobeam Activity System
To determine if chronic exposure to low-levels of DA over an early adult lifespan affects cognitive function in old age, a third cohort of mice were IP-injected weekly with PBS (control) or approximately 0.75 to 0.80 mg DA/kg body weight from three months of age to 12 months of age (36 weeks of exposure) and allowed to recover (no exposure) for six, 12, and 16 months followed by tests for spatial memory and learning via RWTM at three old-age time points (18 months of age (n=21 exposed, n=22 control), 24 months of age (n=18 exposed, n=18 control) and 28 months of age (n=9 exposed, n=7 control), respectively (Figure 1B).
2.4. Radial Water Tread Maze (RWTM)
A radial water tread maze (RWTM) was used to test spatial learning and memory in chronically exposed and control mice. The RWTM is a modification of the Morris water maze and the Barnes radial maze, both widely used to assess spatial learning and memory (Vorhees and Williams, 2006). The RWTM apparatus consists of a 30-inch circular galvanized enclosure with nine holes placed in the sides at regular intervals. Eight of the holes are decoys and one hole leads to a dark, escape “safe box” with a warm heating pad, food and gel. All holes extend the same distance before terminating or turning 90° to prevent direct visual determination of the escape route. The tub is filled with one inch of room temperature water and has a bright light positioned directly over the entire apparatus. The water and light provide the escape incentives and the enclosure contains unique visual images and objects along the sides that function as spatial cues for the animal.
Mice were tested individually and given four training days. During training days, mice were placed in the center of the enclosure and required to find the escape route within a three- minute interval. The escape route was the same for all mice for all training days. This was repeated three times a day for the four consecutive training days. If successful, the animal was allowed to stay in the warm safe box for one minute before entering the next training trial. If not successful within three minutes, mice were hand-guided to the correct exit and allowed to stay in the safe box for one minute before entering the next training trial. Mice were then tested for spatial learning and short-term memory on day five and long-term memory on day 12 by placing individual mice in the center of the enclosure three times and allowing three minutes to find the correct escape route. If not successful on test days, mice were not guided to the correct escape route. Failure or escape success was recorded as well as the time taken to successfully escape within three minutes.
Control (n = 10) and chronically exposed (n = 10) mice in cohort one were tested at one and six weeks of exposure (Figure 1A). The spatial cues and escape port were changed between the 1 and 6 week RWTM experiments to create a novel learning environment for these mice. A second cohort of control (n = 20) and chronically exposed (n = 20) mice were tested at 25 weeks of exposure and then again after a nine week recovery period with no DA exposure. The spatial cues were not changed between RWTM tests for these mice in order to determine if mice retained spatial memory after nine weeks. Finally, to determine if long-term early life low-level DA exposure impacts cognitive function with age, a third cohort of mice were chronically exposed once a week for approximately nine months from three months of age to one year of age (Figure 1B). The mice were then maintained without toxin exposure for six, 12, and 16 months before RWTM testing at three old age time points 18 months, 24 months, and 28 months of age, respectively (Figure 1B). The numbers of mice available for the old age time points were; n = 22 control and n = 21 exposed at 18 months, n = 18 control and n = 18 exposed at 24 months, and n = 7 control and n = 9 exposed at 28 months of age. The spatial cues were not changed between RWTM tests at 18 and 24 months of age time points to determine if spatial memory was maintained after 6 months. Spatial cues were changed between 24 and 28 months of age RWTM tests to create a novel learning challenge at the oldest age time point.
2.5. Open Field Photobeam Activity System (PAS)
Activity level in a novel environment was measured in chronically exposed and control mice using the open field Photobeam Activity System (PAS) (San Diego Instruments, San Diego, CA) in a standard sized mouse cage. This system records activity as a sum of photobeam breaks set in XY grids (a total of 32 photobeams with one inch spacing per grid). Individual mice were placed in the open field PAS and activity was measured as the sum of photobeam breaks in a lower mobility grid (horizontal movement) and an upper rearing grid (vertical movement) for a five-minute period on three consecutive days. All data were recorded by San Diego Instruments Software and exported to Microsoft Excel. Mean total activity levels (defined as the sum of all movements that interrupted a photobeam during a 5-minute interval on three consecutive days for each individual mouse) were compared using t tests and Prism software with p values set at < 0.05. Control (n = 20) and chronically exposed (n = 20) mice were tested via the open field PAS at 25 weeks of exposure and then again after a nine week recovery period with no toxin exposure (Figure 1A).
2.6. Brain Immunohistochemistry and Histology
Immunohistochemical examination was performed to determine if long-term asymptomatic DA exposure caused visible damage in hippocampal regions of the brain. At 22 weeks of chronic low-level exposure, mice (n = 6 control; n = 7 exposed) were sedated and trans-cardially perfused with 1x ice-cold PBS, followed by 4% PFA (in PBS) for fixation. Brains were extracted, post-fixed in 4% PFA for 3–4 hours, and transferred to 30% sucrose for cryoprotection. Coronal sections were generated at 40 μm thickness with a Leica CM3050 S cryostat (Leica Biosystems), and stored at 4°C in 1x TBS (0.05% azide, preservative). Two hippocampal sections (200 μm apart) per animal were processed free-floating and DAPI stained for 10 min (Invitrogen D21490/DAPI-Fluoro- Pure Grade, 300 nM working solution), and washed 3x with TBS. Tissues were mounted on superfrost/Plus slides (Fisherbrand), then coverslipped (Slip-Rite #1.5 coverglass, Thermo Scientific) using Fluoromount-G (Southern Biotech) and allowed to dry overnight before imaging. All sections were imaged on a Keyence Biorevo BZ-9000 digital widefield microscope using a 40x/0.95 objective lens. To generate images to measure hippocampal area, both hippocampal hemispheres were imaged at 20x; a series of coarsely z-spaced (2 μm) stacks were tiled together to generate a merged and full- focused ‘map’ of each entire hippocampal hemisphere. Imaris software was used to generate the total number of surfaces within each field of view to determine total cell count based on DAPI staining. Values for each of the four fields of view within an animal were summed to obtain a grand total for each outcome within each animal. Hippocampal ‘map’ images (20x tiled/merged images) were used to measure hippocampal areas (mm2) by hand-tracing in ImageJ (version 1.50g, NIH, USA). Total hippocampal area and subregion-specific areas (CA1, CA3, DG) were measured for both hemispheres within each section.
At the end of the old age RWTM experiments, heads including brain were collected from 28 month old mice at necropsy (n = 7 control; n = 9 exposed) and placed in 10% buffered formalin for 48 hours, transferred to 70% alcohol, and subsequently decalcified and processed in a standard manner into paraffin blocks for sectioning and hematoxylin and eosin staining. Histology slides were evaluated for toxicity and age-related lesions in an artifact-independent manner using a geropathology grading platform (Ladiges, 2016).
2.7. Statistical Analyses
Comparisons of mean percent successful escape levels and escape times on five and 12 day memory tests for all RWTM experiments were tested for statistical significance via t tests (p<0.05) using Prism software. F tests were conducted to test for equal variance and when applicable non-parametric t tests were used with the Holm-Sidak method for correction for multiple t tests. To determine if there were quantifiable differences in learning rates during the four training days of RWTM experiments, the linear regression slopes of mean percent successful escape levels over the four-day training period for control, 25 week-exposed and nine week-recovery mice were compared. Generalized linear models with a Poisson distribution were used to determine if the rate of change in percent success varied between each group (Zuur, 2009). The Poisson distribution is suitable for count or proportion data and accounted for heteroscedasticity between groups (Zuur, 2009). A linear relationship was fit to the percent success data because the limited number of data points would not adequately inform the more complex models often used in other learning curve analyses (Yelle, 1979). These learning rate analyses were conducted in the R programming environment (R Core Team, 2013). Comparisons of novel cage activity in the PAS at 25 weeks exposure and nine weeks recovery were also tested for statistical significance via t test (p < 0.05) using Prism software.
All brain immunohistochemistry data were analyzed with JMP software (version 12.0.1, SAS Institute, Inc.), and all data were confirmed to have equal variance and normal distribution before analyses. Differences in hippocampal cell counts between control and DA groups were assessed by t test (p < 0.05).
3. Results
3.1. Dose response and Determination of Asymptomatic Exposure Dose
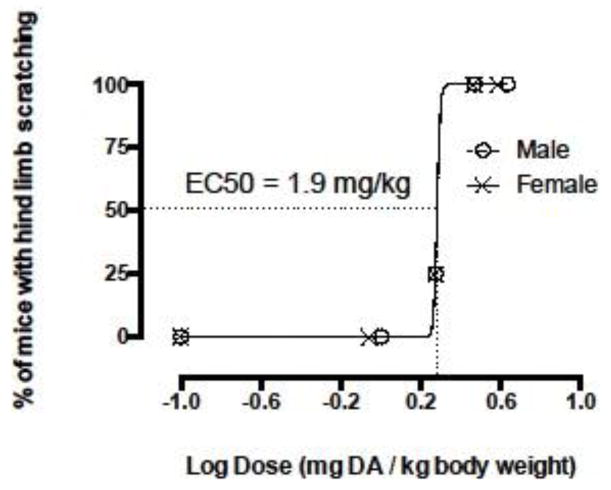
Dose-response assays were performed to determine if there were sex differences in toxin susceptibility in order to choose the most visibly sensitive sex for long-term exposure experiments. Four doses of 1.0 ± 0.05, 1.9 ± 0.12, 2.95 ± 0.07, and 4.3 ± 0.1 (mean ± sd) μg DA/g body weight for males and 0.9 ± 0.03, 1.9 ± 0.02, 2.9 ± 0.07, and 3.9 ± 0.08 (mean ± sd) μg DA/g body weight for females and a control were used to calculate EC50s (Figure 2). EC50s for male and female C57BL/6NJ mice were the same and showed no visible sex differences in susceptibility using hind limb scratching as a measure of affect (Figure 2). Consequently, female mice were chosen for use in the remaining long-term asymptomatic exposure experiments. The target asymptomatic exposure dose for long-term experiments was chosen as 0.75 mg DA/kg total body weight based on EC50 values and results from a pilot study where repetitive weekly doses of approximately 0.75 mg DA/kg did not elicit overt signs of DA toxicity (i.e. scratching, tremors and/or seizures) over six weeks.
Figure 2.
Dose-response relationship of intraperitoneal (IP) injection doses of domoic acid (DA) and the percent of animals affected at each dose for male and female C57BL/6NJ mice (n = 4 at each dose for each sex). Hind limb scratching was used to quantify excitotoxicity in mice and EC50s were the same for male and female mice. Effective concentration (EC50) = the dose at which 50% of the mice tested exhibited hind limb scratching.
3.2. Spatial Learning and Memory Outcomes
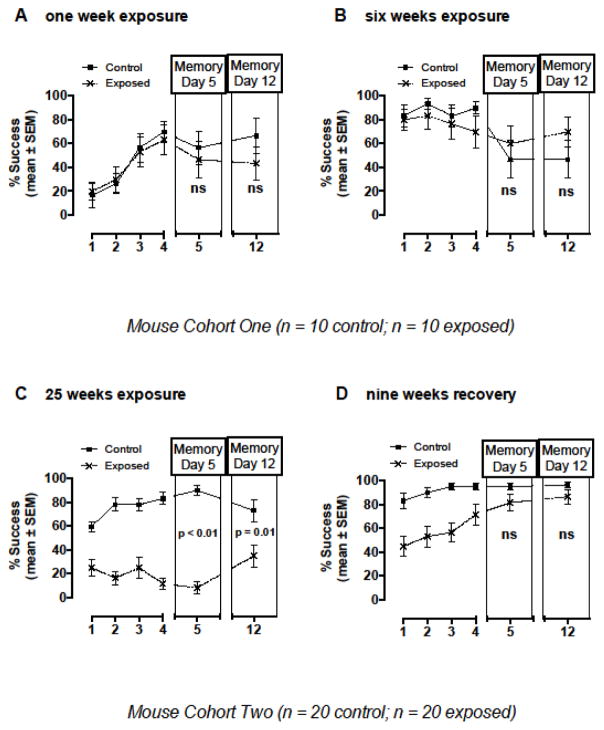
Exposure to 0.82 ± 0.06 mg DA/kg total body weight via a single IP injection (one week exposure) and via six weekly IP injections (6 weeks exposure) did not elicit significant differences in spatial learning (as measured via percent successful escape levels over the four training days) and short and long-term memory, as measured by mean percent successful escape levels on day five and 12 test days, respectively, in the RWTM (Figure 3A and B). Mice at one week of exposure (Figure 3A) had not experienced the RWTM before this experiment. The same mice were tested at six weeks of exposure and although the RWTM spatial cues were changed to create a new learning environment, the initial percent successful escape rate was higher (above 60%) and likely represents a familiarity with the RWTM spatial learning system. Mean percent of successful escape times were not significantly different at one and six weeks of exposure (Figure 3A and B).
Figure 3.
Spatial learning and memory outcomes tested using a radial water tread maze (RWTM) in mice that were exposed to low-level asymptomatic doses of domoic acid via intraperitoneal (IP) injection once a week for A) one, B) six, and C) 25 weeks, and at a recovery time point nine weeks after cessation of toxin exposure (D). Percent successful escape levels were not significantly different at the one and six week exposure time points, but were significantly lower in exposed mice at the 25-week exposure time point compared to controls. After a nine-week recovery period with no toxin exposure, percent successful escape levels on memory test days five and 12 were no longer significantly different between exposed and control mice (D). Statistically significant p values are shown; ns = not significant
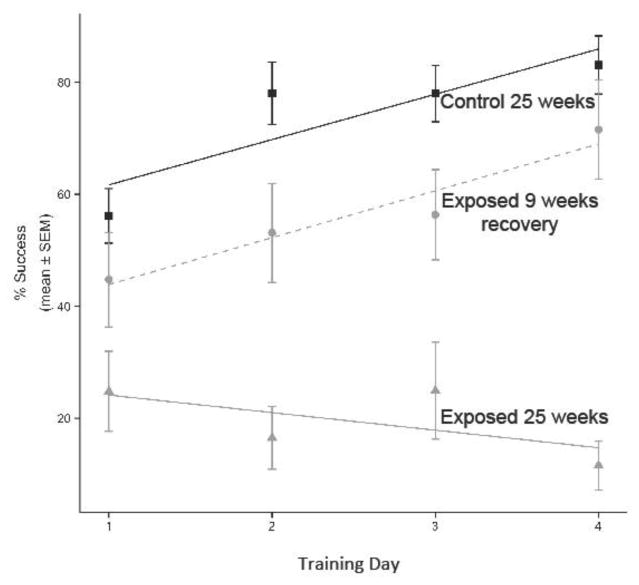
Exposure to 0.75 ± 0.5 mg DA/kg total body weight via IP injection for 25 weeks did elicit significant spatial learning and memory impairment as measured by percent successful escape levels during training days and percent successful escape levels on short term (day five) and long term (day 12) memory test days in the RWTM (p ≤ 0.01; Figure 3C). These mice from cohort two (Figure 1A) had not experienced the RWTM before and initial success rate of the first trial on the first training day was the same for both exposed and control treatments at less than 40% (data not shown). After these mice were allowed a recovery period of nine weeks without toxin exposure, spatial learning and memory were significantly improved as measured by the mean percent successful escape levels on memory test day five and 12 shown in Figure 3D (p > 0.05) as well as the mean percent successful escape rates recorded during training days (Figure 4). The best fit model describing variation in percent success over the training periods of the week 25 control group, week 25 exposed group, and nine-week recovery exposed group, included slope as a predictor of differences in learning rates. Specifically, learning rates were best explained by variables for group, day, and an interaction term between day and group (i.e. the slope) (ΔAIC > 2). A post hoc Tukey’s test determined the slopes of week 25 control and nine-week recovery exposed groups were not significantly different (p > 0.05), and that the slopes of those two groups were both significantly higher than the week 25 exposed group (p < 0.05). These results reveal significant spatial learning impairment after 25 weeks of exposure, but improvement to near control level learning rates after a nine-week recovery period of no toxin exposure (Figure 4).
Figure 4.
Generalized linear models of spatial learning rates over the four training days for the radial water tread maze (RWTM) experiments in control mice (solid black line), exposed mice at 25 weeks of chronic exposure (solid gray line) and recovery mice (the same exposed mice nine weeks after cessation of toxin exposure; dashed gray line). The slopes of control and 25 week- exposed mice were significantly different. The slopes of the control and recovery mice were not significantly different from each other, but both were significantly different from the 25 week exposed mice (p < 0.05). The data reveal significant spatial learning impairment at 25 weeks of exposure that is no longer present after a nine-week recovery period of no toxin exposure.
3.3. Activity Outcomes
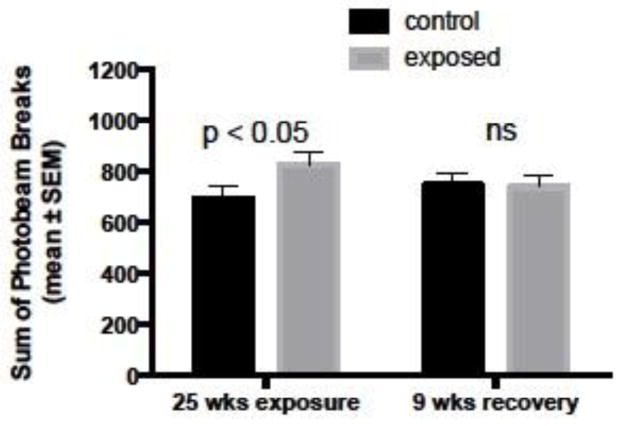
Chronic asymptomatic exposure to 0.75 ± 0.5 mg DA/kg total body weight via IP injection for 25 weeks caused a significant increase in novel cage activity level compared to controls (Figure 5). As with the RWTM results, activity levels in chronically exposed mice returned to control levels after a nine-week recovery period of no toxin exposure (Figure 5). Total novel cage activity was quantified by the sum of photobeam breaks in both horizontal and vertical planes occurring in one five-minute interval on three consecutive days. Other than exhibiting higher activity levels and cognitive deficits, chronically exposed mice appeared outwardly healthy and in good body condition as did controls. Test mice were weighed throughout the study and body weights were 23.3 ± 1.3 and 23.1 ± 1.4 (mean ± sd) g after twelve weeks of injections and 25.2 ± 1.8 and 26.8 ± 2.3 (mean ± sd) g after 25 weeks of injections for control and exposed mice, respectively.
Figure 5.
Novel cage activity levels quantified using an open field photobeam activity system (PAS) in control mice (n = 20) and mice that were exposed to asymptomatic doses of domoic acid via intraperitoneal (IP) injection once a week for 25 weeks (n = 20). Chronically exposed mice were significantly more active than control mice after 25 weeks of low-level domoic acid exposure, but returned to control levels after a nine-week recovery period with no toxin exposure. Statistically significant p values are shown; ns = not significant
3.4. Old Age Spatial Learning and Memory Outcomes
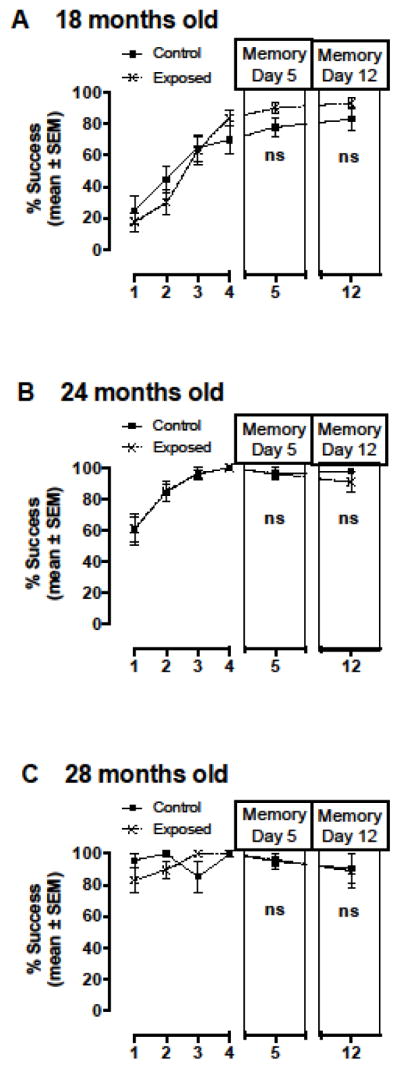
Chronic asymptomatic exposure to DA from three months of age to one year of age did not differentially affect cognitive function with age as measured via RWTM at 18, 24 or 28 months of age between control and exposed mice (Figures 6). These results suggest that the recovery observed in chronically exposed mice after a nine-week recovery period (Figure 3C and Figure 4) was permanent and sustained into old age. Percent successful escape levels were not significantly different between control and exposed mice at any of the old age time points (Figure 6). Spatial cues were not changed between the 18 and 24 month time points and the higher initial percent success at 24 months (approximately 60%; Figure 6B) compared to 18 months (approximately 20%; Figure 6A) suggest that these mice remembered the cues six months later. In an effort to create a novel learning challenge for the final time point, spatial cues were changed between the 24 and 28 month time points. Percent successful escape levels of ≥ 80% in initial trials (before training with the new spatial cues) revealed that previous training experience in the RWTM also leads to higher success in future RWTM experiments even if the spatial learning environment is changed (Figure 6C). Escape time was highly variable and was not significantly longer in exposed mice compared to controls (Data not shown).
Figure 6.
Spatial learning and memory outcomes tested using a radial water tread maze (RWTM) at three old age time points A) 18, B) 24 and C) 28 months of age in control mice and mice that were exposed to low-level asymptomatic doses of domoic acid via intraperitoneal (IP) injection once a week for 36 weeks from three months of age to one year of age (Figure 1B). Spatial cues were the same between 18 and 24 month time points, but were changed between the 24 and 28 month time points to create a novel learning challenge. Impairment was not observed as measured by percent successful escape levels suggesting that long term early life exposure did not significantly impact cognitive function with age and that recovery from the impairments observed immediately following 25 weeks of exposure as shown in Figure 3D was maintained into old age. ns = not significant
3.5. Brain Immunohistochemistry and Histology Outcomes
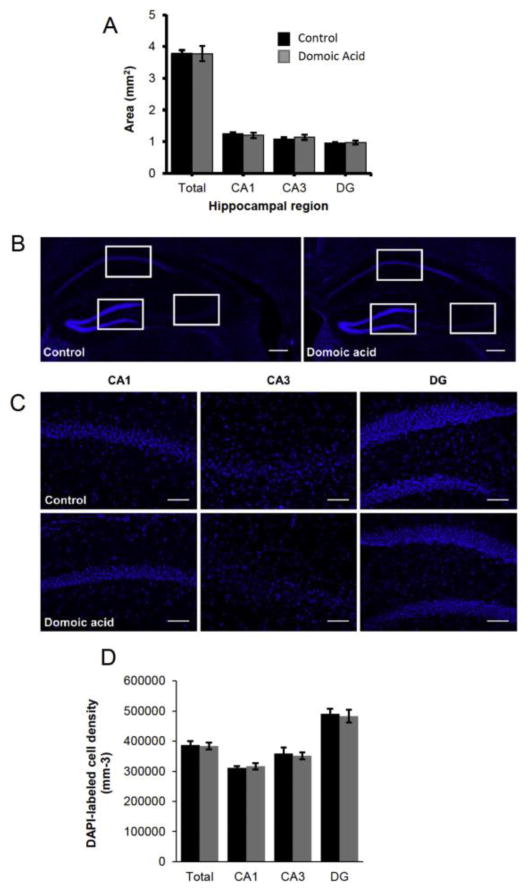
Chronic asymptomatic domoic acid exposure for 22 weeks did not differentially affect cell number or area in hippocampal regions of the brain between control and exposed mice, showing that hippocampal lesions characteristic of acute DA poisoning did not occur with chronic low-level exposure (Figure 7).
Figure 7.
Hippocampal damage characteristic of acute domoic acid poisoning in mammals was not observed in chronically exposed mice at 22 weeks of exposure to asymptomatic doses of domoic acid. A) Area measurements of CA1, CA3, and DG of the hippocampus, as well as all regions combined, are not significantly different between control and domoic acid-exposed mice (p>0.05). B) Example of DAPI labeling of cell nuclei in control (left) and domoic acid-exposed (right) hippocampus. C) Examples of 20X images of DAPI labeling in CA1 (left), CA3 (middle), and DG (right) regions of the hippocampus in control (top row) and domoic-acid exposed (bottom row) mice. D) Densities of DAPI-labeled nuclei are not significantly different between control and domoic acid-exposed mice in any hippocampal region (p>0.05).
Chronic asymptomatic exposure to DA from three months of age to one year of age did not appear to differentially affect the presence or progression of either age-related or background lesions in these mice at 28 months of age based on assessment of hematoxylin and eosin stained brain sections.
4. Discussion
The prevalence of domoic acid (DA) in seafood products is likely to increase as DA- producing algal blooms continue to become more frequent, more toxic, longer-lasting and cover larger geographic areas (Lefebvre et al., 2016; McCabe et al., 2016; Moore et al., 2008; Van Dolah, 2000). Current seafood safety regulations for the United States and the European Union target single high-level DA exposure and have been effective at protecting seafood consumers from acute high level DA exposure characterized by seizures, memory loss and brain lesions. Our findings of cognitive deficits as well as increased novel environment activity levels with repetitive asymptomatic DA exposures suggest that there is a critical need to consider chronic low-level exposure risks as recreational and subsistence harvesters from Tribal communities and the general public are known to consume low levels of DA on a regular basis (Ferriss et al., in press; Grattan et al., 2009; Lefebvre and Robertson, 2010). A recently published study assessed chronic DA exposure via razor clam consumption in 513 adult men and women from three Pacific Northwest Native American Tribes (Grattan et al., 2016). Razor clams in the Pacific Northwest are known to retain low levels of DA for over a year after HAB events (Wekell et al., 1994). The study found an association between long-term, low-level exposure to DA through heavy razor clam consumption and memory functioning (Grattan et al., 2016). These findings led to the first razor clam advisory issued by the Washington State Department of Health (WDOH) to incorporate long term exposure to seafood with toxin levels below the regulatory limit by advising harvesters not to consume more than 15 clams per month for twelve consecutive months (http://www.doh.wa.gov/Newsroom/2016NewsReleases/16116RazorClamsAdvisoryNewsRelease). The present study was designed with weekly exposures to low asymptomatic doses of DA for multiple months in order to model chronic exposure and determine if long-term low-level exposure causes quantifiable cognitive impacts in a mammalian model system. The findings presented here were considered by WDOH in the formation of this advisory.
To our knowledge, this is the first study to examine the impacts of weekly low-level exposures to DA for multiple months at doses below those to cause overt signs of excitotoxicity. Quantifiable changes in cognitive function (spatial memory and learning) and motor activity were observed (Figures 3, 4 and 5) and provide novel information regarding chronic low-level exposure risks. Previous laboratory studies in mammalian systems have investigated the effects of single high-level symptomatic exposures to DA via oral exposure (Burbacher and Petroff, 2016; Faustman et al., 2013; Truelove et al., 1997; Tryphonas et al., 1990c), intravenous (IV) injection (Tryphonas et al., 1990a) and intraperitoneal (IP) injection (Baron et al., 2013; Tryphonas et al., 1990b), providing a plethora of information on the toxicokinetics, behavioral impacts, and brain histology of acute toxicity in whole animals. Neurobehavioral impacts of acute DA exposure documented in rodent and non-human primate models include; sedation, hyperactivity, scratching, gagging, loss of balance control, tremors, seizures, spatial memory and learning impairment, and gross histologic lesions in the brain (Iverson et al., 1990; Nijjar and Madhyastha, 1997; Scallet et al., 2005; Schmued et al., 1995; Vieira et al., 2015a; Vieira et al., 2015b). A few laboratory studies using adult and neonatal whole animal rodent models have addressed lower dose exposures for periods of several days and have documented adverse effects such as kidney damage (Funk et al., 2014), lower seizure threshold (Gill et al., 2010), and persistent changes in behavioral and molecular indicators of stress response (Gill et al., 2012). One 64-day oral gavage study using 5 mg DA/kg body weight/day doses in rats reported no clinical abnormalities (Truelove et al., 1996). Oral doses reported to cause neurotoxic effects in rodent models (35 mg/kg in mice and 80 mg/kg in rats) are dramatically different than in primate models (1 mg/kg) and humans (1.9 mg/kg) (see table 3 in Lefebvre and Robertson, 2010), and are likely due to toxin uptake differences (Lefebvre and Robertson, 2010). For this reason, IP injection exposures of asymptomatic doses were used for our long-term chronic exposure model designed to identify subclinical health impacts.
Our findings of significant cognitive deficits in the absence of gross morphological lesions in hippocampal regions of the brain (Figure 7) indicate a new mode of action for chronic low-level exposure. Acute exposures that elicit seizures also cause permanent hippocampal brain lesions and permanent cognitive impairment, while the chronic low-level exposure impacts on cognitive function and hyperactivity reported here were reversible (with significant improvement after nine weeks recovery and full improvement after six months recovery) and were not associated with permanent visible brain lesions indicative of cell death (Figures 3C and D, 4, 5, and 7). Additionally, recovery from spatial learning and memory impairment was maintained into old age (Figure 6) suggesting full and sustained recovery from the initial cognitive impacts observed after chronic exposures at 25 weeks. A recently published study reported a loss of inhibitory neuron function with DA treatment in organotypic hippocampal brain slice cultures of mice, but not a loss in the total number of hippocampal neurons and may provide insight into a reversible mechanism (Hiolski et al., 2016). Additional whole animal chronic exposure studies and cognitive testing are currently underway to better define cognitive effects and to elucidate the physiological and cellular processes that are impacted during long-term low-level exposure leading to reversible cognitive deficits. Validation of laboratory model results are needed in naturally-exposed wildlife and humans, but the findings presented here have profound implications for human health and clinical treatment if similar patterns of cognitive deficits and recovery in the absence of permanent brain lesions also occur in chronically exposed human consumers.
5. Conclusions
We found that weekly exposure for six months to low levels of DA at doses below those that elicit the characteristic signs of acute DA poisoning caused significant spatial memory and learning impairment as well as hyperactivity in mice. The effects of chronic low-level exposure were reversible with a recovery period of no toxin exposure and recovery was maintained into old age. These results define a new paradigm for chronic low-level DA toxicity in that cognitive impairments were documented in the absence of seizures and visible brain lesions and were recoverable, unlike acute DA poisoning characterized by seizures, permanent brain lesions and permanent memory loss (Cook et al., 2015; Todd, 1993). These findings provide evidence that repetitive low-level exposure should be considered in seafood safety management practices as well as in wildlife health diagnostics.
Acknowledgments
This research was funded by the National Institute of Health (NIH) R01 ES021930 (to DJM and KAL), the National Science Foundation (NSF) OCE-1314088 (to DJM and KAL) and the National Institute of Health R24 AG047115 (to WCL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron AW, Rushton SP, Rens N, Morris CM, Blain PG, Judge SJ. Sex differences in effects of low level domoic acid exposure. Neurotoxicology. 2013;34:1–8. doi: 10.1016/j.neuro.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Bates SS. Domoic acid-producing diatoms: Another genus added! Journal of Phycology. 2000;36(6):978–983. [Google Scholar]
- Bejarano AC, Gulland FM, Goldstein T, St Leger J, Hunter M, Schwacke LH, VanDolah FM, Rowles TK. Demographics and spatio-temporal signature of the biotoxin domoic acid in California sea lion (Zalophus californianus) stranding records. Mar Mammal Sci. 2008;24(4):899–912. [Google Scholar]
- Burbacher TM, Petroff RL. Thesis (MS) University of Washington; Seattle: 2016. Toxicokinetics of domoic acid in a nonhuman primate model (Macaca fascicularis) [Google Scholar]
- Cook PF, Reichmuth C, Rouse AA, Libby LA, Dennison SE, Carmichael OT, Kruse-Elliott KT, Bloom J, Singh B, Fravel VA, Barbosa L, Stuppino JJ, Van Bonn WG, Gulland FM, Ranganath C. Algal toxin impairs sea lion memory and hippocampal connectivity, with implications for strandings. Science. 2015;350(6267):1545–1547. doi: 10.1126/science.aac5675. [DOI] [PubMed] [Google Scholar]
- Faustman EM, Burbacher T, Griffith W, Park J. In: Toxicokinetics of domoic acid (da) in pregnant and non-pregnant mice after repeated oral administrations. Faustman EM, Burbacher T, Griffith W, editors. ProQuest Dissertations Publishing; 2013. [Google Scholar]
- Ferriss BE, Lefebvre KA, Ayres D, Borchert J, Marcinek DJ. Acute and chronic dietary exposure to domoic acid in recreational harvesters: a survey of shellfish consumption behavior. Environment International. doi: 10.1016/j.envint.2017.01.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JA, Janech MG, Dillon JC, Bissler JJ, Siroky BJ, Bell PD. Characterization of renal toxicity in mice administered the marine biotoxin domoic Acid. Journal of the American Society of Nephrology: JASN. 2014;25(6):1187. doi: 10.1681/ASN.2013080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DA, Bastlund JF, Watson WP, Ryan CL, Reynolds DS, Tasker RA. Neonatal exposure to low-dose domoic acid lowers seizure threshold in adult rats. Neuroscience. 2010;169(4):1789–1799. doi: 10.1016/j.neuroscience.2010.06.045. [DOI] [PubMed] [Google Scholar]
- Gill DA, Perry MA, McGuire EP, Pérez-Gómez A, Tasker RA. Low-dose neonatal domoic acid causes persistent changes in behavioural and molecular indicators of stress response in rats. Behavioural Brain Research. 2012;230(2):409–417. doi: 10.1016/j.bbr.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Goldstein T, Mazet JA, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc Biol Sci. 2008;275(1632):267–276. doi: 10.1098/rspb.2007.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan LM, Boushey C, Tracy K, Trainer VL, Roberts SM, Schluterman N, Morris JG., Jr The association between razor clam consumption and memory in the CoASTAL cohort. Harmful Algae. 2016;57(Part B, Sp. Iss. SI):20–25. doi: 10.1016/j.hal.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan LM, Trainer V, Roberts S, Boushey C, Tracy K, Fialkowski M, Bubacher T, Morris JG. Human impacts of low level domoic acid exposure in the Pacific Northwest: An update of the CoASTAL cohort studies. Fifth Symposium on Harmful Algae in the U.S. Conference Proceedings; 2009. p. 46. [Google Scholar]
- Hiolski EM, Ito S, Beggs JM, Lefebvre KA, Litke AM, Smith DR. Domoic acid disrupts the activity and connectivity of neuronal networks in organotypic brain slice cultures. Neurotoxicology. 2016;56:215–224. doi: 10.1016/j.neuro.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiolski EM, Kendrick PS, Frame ER, Myers MS, Bammler TK, Beyer RP, Farin FM, Wilkerson H-w, Smith DR, Marcinek DJ, Lefebvre KA. Chronic low-level domoic acid exposure alters gene transcription and impairs mitochondrial function in the CNS. Aquatic Toxicology (Amsterdam) 2014;155:151–159. doi: 10.1016/j.aquatox.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson F, Truelove J, Tryphonas L, Nera EA. The toxicology of domoic acid administered systemically to rodents and primates. Can Dis Wkly Rep. 1990;16(Suppl 1E):15–19. [PubMed] [Google Scholar]
- Ladiges WC. Pathology assessment is necessary to validate translational endpoints in preclinical aging studies. Pathobiology of aging and age-related diseases. 2016:6. doi: 10.3402/pba.v6.31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Quakenbush L, Frame E, Huntington KB, Sheffield G, Stimmelmayr R, Bryan A, Kendrick P, Ziel H, Goldstein T, Snyder JA, Gelatt T, Gulland F, Dickerson B, Gill V. Prevalence of algal toxins in Alaskan marine mammals foraging in a changing arctic and subarctic environment. Harmful Algae. 2016;55:13–24. doi: 10.1016/j.hal.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Robertson A. Domoic acid and human exposure risks: a review. Toxicon. 2010;56(2):218–230. doi: 10.1016/j.toxicon.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Lundholm N, Moestrup O. Morphology of the marine diatom Nitzschia navis- varingica, sp, nov (Bacillariophyceae), another producer of the neurotoxin domoic acid. Journal of Phycology. 2000;36(6):1162–1174. [Google Scholar]
- Marchetti A, Lundholm N, Kotaki Y, Hubbard K, Harrison PJ, Armbrust EV. Identification and assessment of domoic acid production in oceanic Pseudo-nitzschia (Bacillariophyceae) from iron-limited waters in the northeast subarctic Pacific. Journal of Phycology. 2008;44(3):650–661. doi: 10.1111/j.1529-8817.2008.00526.x. [DOI] [PubMed] [Google Scholar]
- Marien K. Establishing tolerable dungeness crab (Cancer magister) and razor clam (Siliqua patula) domoic acid contaminant levels. Environmental Health Perspectives. 1996;104(11):1230–1236. doi: 10.1289/ehp.104-1469507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Haya K, Burridge LE, Wildish DJ. Nitzschia-pseudodelicatissima-A source of domoic acid in the Bay of Fundy, Eastern Canada. Marine Ecology-Progress Series. 1990;67(2):177–182. [Google Scholar]
- McCabe RM, Hickey BM, Kudela RM, Lefebvre KA, Adams NG, Bill BD, Gulland FDM, Thomson RE, Cochlan WP, Trainer VL. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophysical Research Letters. 2016:43. doi: 10.1002/2016GL070023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SK, Trainer VL, Mantua NJ, Parker MS, Laws EA, Backer LC, Fleming LE. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ Health. 2008;7(Suppl 2):S4. doi: 10.1186/1476-069X-7-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muha N, Ramsdell JS. Domoic acid induced seizures progress to a chronic state of epilepsy in rats. Toxicon: official journal of the International Society on Toxinology. 2011;57(1):168–171. doi: 10.1016/j.toxicon.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Nijjar MS, Madhyastha MS. Effect of pH on domoic acid toxicity in mice. Molecular and Cellular Biochemistry. 1997;167(1–2):179–185. doi: 10.1023/a:1006862311940. [DOI] [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990;322(25):1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- Pettan-Brewer C, Touch DV, Wiley JC, Hopkins HC, Rabinovitch PS, Ladiges WC. A novel radial water tread maze tracks age-related cognitive decline in mice. Pathobiology of aging and age-related diseases. 2013;3:20679. doi: 10.3402/pba.v3i0.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam MA, Sim PG, McCulloch AW, McInnes AG. High-Performance Liquid- Chromatography of Domoic Acid, a Marine Neurotoxin, with Application to Shellfish and Plankton. International Journal of Environmental Analytical Chemistry. 1989;36(3):139–154. [Google Scholar]
- R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org/ [Google Scholar]
- Scallet AC, Schmued LC, Johannessen JN. Neurohistochemical biomarkers of the marine neurotoxicant, domoic acid. Neurotoxicol Teratol. 2005;27(5):745–752. doi: 10.1016/j.ntt.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Scallet AC, Slikker W., Jr Domoic acid-induced neuronal degeneration in the primate forebrain revealed by degeneration specific histochemistry. Brain Res. 1995;695(1):64–70. doi: 10.1016/0006-8993(95)00799-v. [DOI] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R, 3rd, Miller PE, McLellan WA, Moeller PD, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403(6765):80–84. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- Tasker RA, Connell BJ, Strain SM. Pharmacology of systemically administered domoic acid in mice. Can J Physiol Pharmacol. 1991;69(3):378–382. doi: 10.1139/y91-057. [DOI] [PubMed] [Google Scholar]
- Todd ECD. Domoic acid and amnesic shellfish poisoning: A review. Journal of Food Protection. 1993;56(1):69–83. doi: 10.4315/0362-028X-56.1.69. [DOI] [PubMed] [Google Scholar]
- Truelove J, Mueller R, Pulido O, Iverson F. Subchronic toxicity study of domoic acid in the rat. Food Chem Toxicol. 1996;34(6):525–529. doi: 10.1016/0278-6915(96)81814-x. [DOI] [PubMed] [Google Scholar]
- Truelove J, Mueller R, Pulido O, Martin L, Fernie S, Iverson F. 30-day oral toxicity study of domoic acid in cynomolgus monkeys: lack of overt toxicity at doses approaching the acute toxic dose. Nat Toxins. 1997;5(3):111–114. doi: 10.1002/1522-7189(1997)5:3<111::AID-NT5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Iverson F. Acute parenteral neurotoxicity of domoic acid in cynomolgus monkeys (M. fascicularis) Toxicol Pathol. 1990a;18(2):297–303. doi: 10.1177/019262339001800208. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Nera E, Iverson F. Acute neurotoxicity of domoic acid in the rat. Toxicol Pathol. 1990b;18(1(1)):1–9. doi: 10.1177/019262339001800101. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Todd E, Nera E, Iverson F. Experimental oral toxicity of domoic acid in cynomolgus monkeys (Macaca fascicularis) and rats. Preliminary investigations. Food Chem Toxicol. 1990c;28(10):707–715. doi: 10.1016/0278-6915(90)90147-f. [DOI] [PubMed] [Google Scholar]
- Van Dolah FM. Marine algal toxins: Origins, health effects, and their increased occurrence. Environmental Health Perspectives. 2000;108:133–141. doi: 10.1289/ehp.00108s1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AC, Alemañ N, Cifuentes JM, Bermúdez R, Peña ML, Botana LM. Brain Pathology in Adult Rats Treated With Domoic Acid. Veterinary Pathology. 2015a;52(6):1077–1086. doi: 10.1177/0300985815584074. [DOI] [PubMed] [Google Scholar]
- Vieira AC, Martínez JMC, Pose RB, Queijo ÁA, Posadas NA, Botana LM. Dose–response and histopathological study, with special attention to the hypophysis, of the differential effects of domoic acid on rats and mice. Microscopy Research and Technique. 2015b;78(5):396–403. doi: 10.1002/jemt.22486. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekell JC, Gauglitz EJ, Jr, Barnett HJ, Hatfield CL, Simons D, Ayres D. Occurrence of domoic acid in Washington state razor clams (Siliqua patula) during 1991–1993. Nat Toxins. 1994;2(4):197–205. doi: 10.1002/nt.2620020408. [DOI] [PubMed] [Google Scholar]
- Wekell JC, Hurst J, Lefebvre KA. The origin of the regulatory limits for PSP and ASP toxins in shellfish. Journal of Shellfish Research. 2004;23(3):927–930. [Google Scholar]
- Yelle LE. The learning curve: Historical review and comprehensive survey. Decision Sciences. 1979;10(2):302–328. [Google Scholar]
- Zuur A. Mixed effects models and extensions in ecology with R. Springer; New York: 2009. [Google Scholar]