Abstract
Purpose
This phase II trial evaluated the efficacy and safety of cixutumumab, a human anti–insulin-like growth factor receptor 1 (IGF-1R) monoclonal IgG1 antibody, and explored potential biomarkers in postmenopausal women with hormone receptor–positive breast cancer.
Experimental Design
Patients with hormone receptor–positive breast cancer that progressed on antiestrogen therapy received (2:1 randomization) cixutumumab 10 mg/kg and the same antiestrogen (arm A) or cixutumumab alone (arm B) every 2 weeks (q2w). Primary endpoint was progression-free survival (PFS); secondary endpoints included overall survival (OS) and safety. Correlative analyses of IGF-1R, total insulin receptor (IR), and IR isoforms A (IR-A) and B (IR-B) expression in tumor tissue were explored.
Results
Ninety-three patients were randomized (arm A, n = 62; arm B, n = 31). Median PFS was 2.0 and 3.1 months for arm A and arm B, respectively. Secondary efficacy measures were similar between the arms. Overall, cixutumumab was well tolerated. IGF-1R expression was not associated with clinical outcomes. Regardless of the treatment, lower IR-A, IR-B, and total IR mRNA expression in tumor tissue was significantly associated with longer PFS [IR-A: HR, 2.62 (P = 0.0062); IR-B: HR, 2.21 (P = 0.0202); and total IR: HR, 2.18 (P = 0.0230)] and OS [IR-A: HR, 2.94 (P = 0.0156); IR-B: HR, 2.69 (P = 0.0245); and total IR: HR, 2.72 (P = 0.0231)].
Conclusions
Cixutumumab (10 mg/kg) with or without antiestrogen q2w had an acceptable safety profile, but no significant clinical efficacy. Patients with low total IR, IR-A, and IR-B mRNA expression levels had significantly longer PFS and OS, independent of the treatment. The prognostic or predictive value of IR as a biomarker for IGF-1R–targeted therapies requires further validation.
Introduction
Approximately 70% to 75% of breast cancers are positive for expression of the estrogen receptor (ER; ref. 1). For patients with hormone receptor–positive tumors, the preferred first-line treatment comprises blockade of estradiol synthesis or hormone receptor activity using aromatase inhibitors or antiestrogen agents. Although endocrine therapies are useful and well tolerated, most patients with metastatic breast cancer respond to this form of treatment for approximately 12 to 18 months before developing refractory disease (2). Resistance to sequential hormone therapies can potentially lead to tumors with complete hormone independence (2, 3). New therapeutic modalities able to provide additional benefit to patients with hormone receptor–positive, antiestrogen refractory, advanced and metastatic breast cancer are required.
A growing body of evidence has implicated the overexpression of insulin-like growth factor receptor 1 (IGF-1R) in the development of resistance to antiestrogen therapy in hormone receptor–positive breast cancer (4, 5). Santen and colleagues investigated the mechanism by which breast cancer tumor cells develop resistance to antiestrogen therapy and posited that tumors adapt despite antiestrogen treatment, developing enhanced sensitivity to the growth-promoting effects of estradiol (2). Estradiol activates many signaling molecules, including IGF-1R, and it appears that estradiol hypersensitivity may be derived from interactions between estradiol and IGF-1R (3, 6). Results from a recent study suggest that targeting IGF-1R or downstream signaling components may resensitize breast cancer cells to antiestrogen therapies (7). The IGF-1R ligands IGF-I and IGF-II also mediate strong mitogenic and anti-apoptotic effects through IGF-1R in several cancer cell lines, including breast cancer (8). Additionally, the use of IGF-1R inhibitors has profound effects on the growth of tamoxifen-resistant breast cancer cell lines (9, 10). For example, the IGF-1R tyrosine kinase inhibitor AG1024 reduced the proliferation of tamoxifen-resistant cells in a dose-dependent manner as well as the phosphorylation of IGF-1R and known downstream signaling molecules (9). The monoclonal antibody αIR-3 inhibited the growth of tamoxifen-resistant cancer cells by 50% when compared with tamoxifen-sensitive cells (10). Taken together, the data suggest that blocking the binding of growth factors upstream of these pathways to their receptors might prevent this adaptive estradiol hypersensitivity and thus ameliorate antiestrogen resistance (2).
The insulin receptor (IR), which is closely related to IGF-1R, is also overexpressed in breast cancer cells. Studies with insulin analogues, blocking and stimulating anti-IR antibodies, small molecule inhibitors, and various strains of mice have demonstrated a role for IR in breast cancer development and progression (11–13). IR is found as two isoforms, IR-A and IR-B, as a result of alternative splicing (14, 15). Harrington and colleagues demonstrated that IR-A is more predominant than IR-B in breast cancer (16). Insulin binds to both isoforms with comparable affinity; however, IGF-II, which is expressed by breast cancer tumor stroma, binds with high affinity to IR-A but not IR-B (12, 15). Tissue-specific expression and differential ligand-binding specificity indicate a functional significance for these two isoforms (15). Although their individual roles have not been completely elucidated, IR-B is more associated with metabolic signaling, whereas IR-A is more associated with mitogenic signaling and anti-apoptotic effects (17). IGF-II activates IR-A to initiate proliferative, prosurvival, and metastatic signaling, which may limit the antitumor activity of antibodies that only block the interaction of IGF-II with IGF-1R (16–19). These data implicate IR-A as a possible predictive biomarker to select and monitor patients eligible for IGF-1R-targeted therapy (16). In breast cancer, IHC studies measuring total IR protein expression have suggested a correlation between elevated IR expression and positive outcomes in primary and node-negative cancers and poor outcomes in advanced cancers (20–22).
Cixutumumab (IMC-A12, NSC742460) is a fully human anti–IGF-1R monoclonal IgG1 antibody that inhibits IGF-I and IGF-II binding and downstream signaling mechanisms (8). Data from nonclinical studies and preliminary pharmacokinetic data from phase I trials CP13-0501 (clinicaltrials.gov identifier NCT00785538) and CP13-0502 (clinicaltrials.gov identifier NCT00785941) suggest that cixutumumab should induce therapeutic benefit when dosed at 10 mg/kg every 14 days (8, 23). In this phase II trial (ClinicalTrials.gov: NCT00728949), we assessed the efficacy and tolerability of cixutumumab as a single agent to test whether hormone receptor–positive breast cancer cells that developed resistance to antiestrogen therapy may benefit from IGF-1R blockade. To understand whether IGF-1R inhibition restores tumor sensitivity to antiestrogen therapy in these patients, this study also evaluated the antitumor effect of cixutumumab in combination with antiestrogens. Given that IGF-II can activate both IGF-1R and IR-A, we hypothesized that IGF-II-mediated IR signaling may facilitate de novo resistance to IGF-1R antibodies. As a result, patients with high IR expression in tumor tissue would be unlikely to benefit from IGF-1R blockade. Therefore, the relationship between IGF-1R, IR-A, and IR-B mRNA expression in tumor tissue and clinical efficacy of cixutumumab in patients with breast cancer was also explored.
Patients and Methods
Eligibility criteria
Postmenopausal women with hormone receptor–positive (either ERs, progesterone receptors, or both) breast cancer were enrolled. Key inclusion criteria were as follows: patients age ≥18 years with an intact uterus and amenorrhea for ≥12 months who had received prior antiestrogen therapy with at least one antiestrogen agent administered for ≥3 months in the adjuvant or metastatic setting and experienced disease progression while on or within 12 months after receiving the last dose of endocrine therapy. Histologically or cytologically confirmed invasive breast cancer, which at the time of study entry was either stage III (locally advanced) disease not amenable to curative therapy or stage IV disease, and either measurable or evaluable disease were required. Patients had a life expectancy of >3 months and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2. Patients must have completed any prior chemotherapy and/or radiotherapy prior to the administration of the first dose of study therapy.
Key exclusion criteria included the following: the presence of uncontrolled brain or leptomeningeal metastases, the presence of poorly controlled diabetes mellitus, and prior history of allergic reactions attributed to compounds of chemical or biologic composition similar to that of cixutumumab. Patients with a history of diabetes mellitus were allowed to participate, provided that their blood glucose was within normal range (fasting glucose at study entry <120 mg/dL or below upper limit of normal) and that they were on a stable dietary and/or therapeutic regimen for this condition.
The trial was conducted according to good clinical practice guidelines, Declaration of Helsinki, and all patients gave informed consent before undergoing any study procedures.
Study design and treatment
This was a multicenter, open-label, randomized, phase II trial that enrolled postmenopausal patients with advanced or metastatic breast adenocarcinoma that progressed on prior antiestrogen therapy. Patients were randomized 2:1 to arms A and B, respectively. Patients in arm A and arm B received cixutumumab administered at 10 mg/kg intravenously over 1 hour every 2 weeks; however, patients in arm A also received the same dose and schedule of antiestrogen therapy to which their disease became refractory. A treatment cycle was defined as 4 weeks. Patients continued to receive treatment until there was evidence of progressive disease, unacceptable toxicity, or consent was withdrawn.
Baseline and treatment assessments
Patients were evaluated for response according to the Response Evaluation Criteria in Solid Tumors guidelines v.1.0 (24), with radiologic evaluation every two cycles (i.e., every 8 weeks) as is typically done in early (exploratory) phase II studies. The same imaging-based assessments were used to characterize each identified and reported lesion at baseline and at reassessment during treatment. Imaging-based evaluations (included contrast-enhanced CT scan or MRI) were preferred to evaluation by clinical examination when both methods were used to assess the antitumor effect of the treatment. The baseline tumor burden (unidimensionally measurable and nonmeasurable disease) was assessed ≤28 days prior to randomization. The investigator prospectively identified the target lesions to be followed to evaluate the patient’s objective response to the therapy. Confirmatory scans were required no fewer than 4 weeks after initial documentation of objective response.
Adverse events (AEs) were summarized from the Medical Dictionary for Regulatory Activities System Organ Class and preferred term, classified from verbatim terms, and assessed at least every 2 weeks throughout the study. The incidence and percentage of patients with at least one occurrence of a preferred term were included, according to the most severe grade listed in the NCI-Common Terminology Criteria for AE (NCI-CTCAE) version 3.0 (25). Causality (relationship to study drug) was summarized separately. Laboratory results were classified according to the NCI-CTCAE, version 3.0. The incidence of laboratory abnormalities was summarized; laboratory results not corresponding to an NCI-CTCAE version 3.0 term were not graded.
qPCR assays for IR-A, IR-B, and IGF-1R
Pretreatment formalin-fixed paraffin-embedded tumor tissue was collected and analyzed using the qPCR assays designed, validated, and tested previously by Harrington and colleagues (16). A >50% tumor cellularity was required from the biopsied samples for qPCR analysis; no other pathology assessments were completed.
Statistical methods
The analyses of efficacy variables were performed on the intent-to-treat (ITT) population. All patients receiving at least one dose of study drug were included in the safety analysis. Each study arm was compared with historical standards obtained with fulvestrant (26). A median progression-free survival (PFS) of ≥4.4 months observed in either arm would warrant further investigation. Comparison between arms was performed by log-rank tests and HR determined by a Cox proportional hazards model; however, the study was not powered for the analysis of PFS.
Kaplan–Meier method was used to evaluate PFS and overall survival (OS; ref. 27). Median PFS and OS and 90% confidence interval (CI) of the medians were provided. Objective response rate [complete response (CR) +partial response (PR)] and disease control rate [DCR; CR + PR + stable disease (SD)] were presented along with the 95% CI.
In exploratory analyses, the Cox proportional hazard model was used to examine associations between each marker (dichotomized using the cutoff points at the 25th, median, and 75th percentile of the marker distribution) and PFS and OS. Because of the small sample size in arm B, Cox regressions (28) were performed for the pooled group (arms A and B combined) and for arm A separately. P values in the respective analyses were obtained using the log-rank test (29) stratified for the marker expression class. All tests of significance were conducted at a two-sided α-level of 0.05. No multiplicity adjustments across markers and endpoints were performed.
Results
Patients and treatment
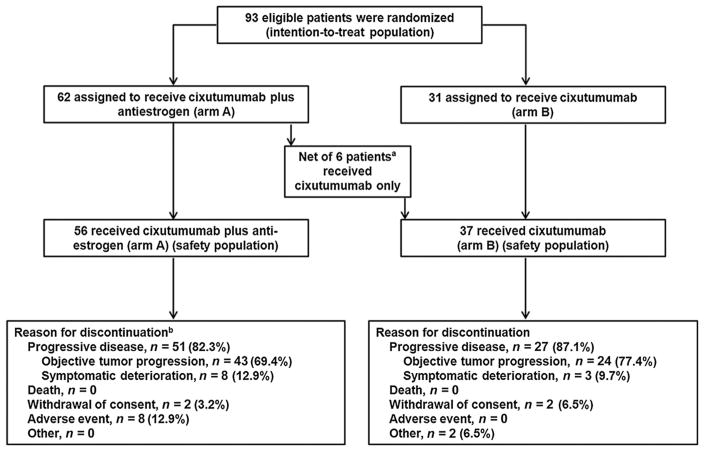
A total of 93 patients were randomized at 9 centers (Fig. 1). The efficacy analysis of the ITT population comprised 62 patients randomized to receive cixutumumab plus antiestrogen (arm A) and 31 patients randomized to receive cixutumumab monotherapy (arm B). Fourteen patients randomized to the combination arm only received cixutumumab monotherapy, and 8 patients randomized to the cixutumumab monotherapy arm received cixutumumab plus antiestrogen therapy. Thus, an exploratory analysis of efficacy was also done on the per-protocol population, comprised of 48 patients in arm A and 23 patients in arm B. Analysis of the safety population was based on actual treatment and included 56 patients who received cixutumumab plus antiestrogen (arm A) and 37 patients who received cixutumumab monotherapy (arm B). Baseline patient demographics and disease characteristics are outlined in Table 1. Covariates were generally balanced between treatment arms. Median age was slightly higher in arm A (61.0 years; range, 31.8–80.2) than in arm B (57.6 years; range, 38.5–83.8). Arm A also had more patients with an ECOG PS 2 and previous biologic therapy. Median duration of treatment with cixutumumab was 8.3 weeks (range, 2.0–160.3) in arm A, and 8.3 weeks (range, 2.0–68.9) in arm B. Median number of cycles was 2 (range, 1–40) in arm A and 2 (range, 1–17) in arm B.
Figure 1.
Patient disposition. aFourteen patients randomized to receive cixutumumab plus antiestrogen therapy received cixutumumab only, and 8 patients randomized to receive single-agent cixutumumab received cixutumumab plus antiestrogen therapy. bOne patient was still on treatment as of the study cutoff date of January 2, 2013.
Table 1.
Baseline patient demographics and disease characteristics (ITT population)
| Arm A (N = 62) | Arm B (N = 31) | Total (N = 93) | |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 62 (100.0) | 31 (100.0) | 93 (100.0) |
| Race, n (%) | |||
| White | 55 (88.7) | 26 (83.9) | 81 (87.1) |
| Black or African American | 5 (8.1) | 4 (12.9) | 9 (9.7) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 2 (3.2) | 1 (3.2) | 3 (3.2) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 1 (1.6) | 1 (3.2) | 2 (2.2) |
| Not Hispanic nor Latino | 61 (98.4) | 30 (96.8) | 91 (97.8) |
| Age, y | |||
| Mean (SD) | 59.4 (11.1) | 57.9 (11.2) | 58.9 (11.1) |
| Median | 61.0 | 57.6 | 59.7 |
| Min–max | 31.8–80.2 | 38.5–83.8 | 31.8–83.8 |
| ECOG PS, n (%) | |||
| 0 | 30 (48.4) | 16 (51.6) | 46 (49.5) |
| 1 | 26 (41.9) | 14 (45.2) | 40 (43.0) |
| 2 | 6 (9.7) | 1 (3.2) | 7 (7.5) |
| Prior therapy, n (%) | |||
| Chemotherapy | 47 (75.8) | 22 (71.0) | 69 (74.2) |
| Hormonala | 62 (100.0) | 31 (100.0) | 93 (100.0) |
| Fulvestrant | 20 (32.3) | 13 (41.9) | 33 (35.5) |
| Exemestane | 17 (27.4) | 8 (25.8) | 25 (26.9) |
| Letrozole | 9 (14.5) | 5 (16.1) | 14 (15.1) |
| Anastrozole | 8 (12.9) | 6 (19.4) | 14 (15.1) |
| Tamoxifen | 5 (8.1) | 1 (3.2) | 6 (6.5) |
| Other | 5 (8.1) | 1 (3.2) | 6 (6.5) |
| Radiotherapy | 42 (67.7) | 23 (74.2) | 65 (69.9) |
| Biologicb | 22 (35.5) | 6 (19.4) | 28 (30.1) |
| Other | 23 (37.1) | 13 (41.9) | 36 (38.7) |
| Hormone receptor, n (%) | |||
| ER+ | 60 (96.8) | 31 (100.0) | 91 (97.8) |
| ER− | 1 (1.6) | 0 (0.0) | 1 (1.1) |
| PgR+ | 45 (72.6) | 23 (74.2) | 68 (73.1) |
| PgR− | 15 (24.2) | 7 (22.6) | 22 (23.7) |
| Metastatic sites, n (%) | |||
| Breast | 6 (9.7) | 3 (9.7) | 9 (9.7) |
| Lung | 23 (37.1) | 13 (41.9) | 36 (38.7) |
| Liver | 24 (38.7) | 11 (35.5) | 35 (37.6) |
| Bone | 46 (74.2) | 25 (80.6) | 71 (76.3) |
| Brain | 1 (1.6) | 3 (9.7) | 4 (4.3) |
| Skin | 4 (6.5) | 1 (3.2) | 5 (5.4) |
| Lymph nodes | 27 (43.5) | 16 (51.6) | 43 (46.2) |
| Pleural | 11 (17.7) | 5 (16.1) | 16 (17.2) |
| Peritoneal | 3 (4.8) | 0 (0.0) | 3 (3.2) |
| Soft tissue | 7 (11.3) | 5 (16.1) | 12 (12.9) |
| Other | 8 (12.9) | 0 (0.0) | 8 (8.6) |
Abbreviations: Arm A, cixutumumab + antiestrogen; Arm B, cixutumumab; ECOG PS, Eastern Cooperative Oncology Group performance status; ER+/−, estrogen receptor positive/negative; Min, minimum; Max, maximum; N, number of patients for each treatment; n, number of patients per category for each treatment; PgR+/−, progesterone receptor positive/negative.
Two patients in arm A and 3 patients in arm B received more than one prior antiestrogen therapy.
Twenty-four patients had bevacizumab (19 in arm A, 5 in arm B), 3 patients had trastuzumab (2 in arm A, 1 in arm B), and 1 patient in arm A had tipifarnib.
Efficacy
The study did not meet the primary endpoint of PFS (Table 2, Fig. 2A). In the ITT population, the median PFS was 2.0 (90% CI, 1.9–3.4) and 3.1 (90% CI, 1.9–4.2) months for arm A and arm B, respectively. PFS rate at 6 months was 20.1% (arm A) and 15.2% (arm B). The censoring rate was 24.2% and 16.1% in arm A and arm B, respectively. Only 1 patient in arm A had a PR (1.6%), for an objective response rate of 1.6% for arm A and 0% for arm B (Table 2). Clinical benefit was assessed by DCR, which was 40.3% (95% CI, 28.1–53.6) for arm A and 51.6% (95% CI, 33.1–69.8) for arm B (Table 2).
Table 2.
Primary and secondary efficacy measures (ITT population)
| Efficacy outcomes | Arm A (N = 62) | Arm B (N = 31) |
|---|---|---|
| Median PFS (months) (90% CI)a | 2.0 (1.9, 3.4) | 3.1 (1.9, 4.2) |
| Q25, Q75a | (1.8, 5.7) | (1.8, 4.4) |
| 6-month PFS rate, % (90% CI)a | 20.1 (11.3, 30.6) | 15.2 (6.0, 28.2) |
| 1-year PFS rate, % (90% CI)a | 15.0 (7.5, 25.0) | 7.6 (1.9, 18.7) |
| 2-year PFS rate, % (90% CI)a | 3.0 (0.4, 10.8) | NR |
| Best overall response, n (%) | ||
| CR | 0 (0.0) | 0 (0.0) |
| PR | 1 (1.6) | 0 (0.0) |
| SD | 24 (38.7) | 16 (51.6) |
| Progressive disease | 32 (51.6) | 15 (48.4) |
| Not evaluable/available | 5 (8.1) | 0 (0.0) |
| Objective response rate (CR+PR), % (95% CI)b | 1.6 (0.0, 8.7) | 0.0 (0.0, 11.2) |
| Disease control (CR+PR+SD), % (95% CI)b | 40.3 (28.1, 53.6) | 51.6 (33.1, 69.8) |
| Median OS (months) (90% CI)a | 20.3 (11.5, NR) | NR (17.8, NR) |
| Q25, Q75a | (6.2, NR) | (17.8, NR) |
| 6-month OS rate, % (90% CI)a | 76.7 (66.3, 84.3) | 96.8 (84.4, 99.4) |
| 1-year OS rate, % (90% CI)a | 60.8 (49.4, 70.4) | 80.4 (65.2, 89.5) |
| 2-year OS rate, % (90% CI)a | 46.6 (29.4, 62.1) | 62.5 (38.9, 79.2) |
Abbreviations: Arm A, cixutumumab + antiestrogen; Arm B, cixutumumab; N, number of patients for each treatment; n, number of patients; NR, not reached; Q25, 25th quartile; Q75, 75th quartile.
Estimated by the Kaplan–Meier method.
Estimated using binomial distribution.
Figure 2.
Kaplan–Meier curves of PFS and OS from the ITT population. PFS (A) and OS (B) are shown for the ITT population in arm A (solid line) and arm B (dotted line). n, number of patients; NR, not reached.
There were 20/56 (35.7%) and 14/37 (37.8%) deaths observed for arms A and B, respectively. The median OS was 20.3 months for arm A, whereas the median OS was not reached for arm B. In arms A and B, respectively, OS rates at 12 months were 60.8% and 80.4% and at 24 months were 46.6% and 62.5% (Table 2 and Fig. 2B). In an exploratory analysis, efficacy results were not different in the per-protocol population (Supplementary Table S1).
Safety
AEs reported in >20% of patients in either arm were weight decreased (42.9% in arm A vs. 48.6% in arm B), fatigue (39.3% vs. 54.1%), nausea (32.1% vs. 35.1%), diarrhea (23.2% vs. 40.5%), vomiting (17.9% vs. 27.0%), anorexia (16.1% vs. 27.0%), and hyperglycemia (14.3% vs. 21.6%; Table 3). Patients with any-grade AEs, any-grade or grade ≥3 study drug-related AEs, and any-grade or grade ≥3 serious AEs were similar between the treatment arms (Table 3). Grade ≥3 AEs were experienced by 22/56 (39.3%) patients in arm A and 19/37 (51.4%) patients in arm B (Table 3). The frequency of drug-related serious AEs was 5/56 (8.9%) in arm A and 2/37 (5.4%) in arm B. AEs leading to discontinuation of any study drug were 6/56 (10.7%) in arm A and 2/37 (5.4%) in arm B (Table 3). There were 3/56 (5.4%) AEs with an outcome of death in arm A and 2/37 (5.4%) in arm B, none of which were study drug related.
Table 3.
Adverse events reported in ≥10% (any grade, either arm) patients (safety population)
| Preferred term | Number of patients
|
||||
|---|---|---|---|---|---|
| Arm A (N = 56) n (%)
|
Arm B (N 37) n (%)
|
||||
| Any grade | Grade ≥ | 3 | Any grade | Grade ≥3 | |
| Patients with any TEAE | 55 (98.2) | 22 (39.3) | 36 (97.3) | 19 (51.4) | |
| Study drug-related AE | 48 (85.7) | 10 (17.9) | 31 (83.8) | 7 (18.9) | |
| Serious AE | 16 (28.6) | 13 (23.2) | 11 (29.7) | 10 (27.0) | |
| AE leading to discontinuation of any study drug | 6 (10.7) | 4 (7.1) | 2 (5.4) | 2 (5.4) | |
| Weight decreased | 24 (42.9) | 0 | 18 (48.6) | 1 (2.7) | |
| Fatigue | 22 (39.3) | 2 (3.6) | 20 (54.1) | 3 (8.1) | |
| Nausea | 18 (32.1) | 2 (3.6) | 13 (35.1) | 3 (8.1) | |
| Diarrhea | 13 (23.2) | 0 | 15 (40.5) | 3 (8.1) | |
| Back pain | 11 (19.6) | 2 (3.6) | 6 (16.2) | 1 (2.7) | |
| Constipation | 10 (17.9) | 1 (1.8) | 5 (13.5) | 2 (5.4) | |
| Vomiting | 10 (17.9) | 1 (1.8) | 10 (27.0) | 2 (5.4) | |
| Abdominal pain | 9 (16.1) | 1 (1.8) | 6 (16.2) | 4 (10.8) | |
| Anorexia | 9 (16.1) | 0 | 10 (27.0) | 1 (2.7) | |
| Arthralgia | 9 (16.1) | 2 (3.6) | 3 (8.1) | 1 (2.7) | |
| Hyperglycemia | 8 (14.3) | 4 (7.1) | 8 (21.6) | 1 (2.7) | |
| Dizziness | 8 (14.3) | 0 | 3 (8.1) | 1 (2.7) | |
| Dyspnea | 8 (14.3) | 2 (3.6) | 6 (16.2) | 1 (2.7) | |
| Rash | 8 (14.3) | 0 | 4 (10.8) | 0 | |
| Muscle spasms | 7 (12.5) | 0 | 4 (10.8) | 0 | |
| Musculoskeletal pain | 4 (7.1) | 1 (1.8) | 5 (13.5) | 0 | |
| Cough | 4 (7.1) | 0 | 5 (13.5) | 0 | |
| Pruritus | 2 (3.6) | 0 | 4 (10.8) | 0 | |
| Decreased appetite | 2 (3.6) | 0 | 5 (13.5) | 0 | |
| Vision blurred | 0 | 0 | 4 (10.8) | 0 | |
| Dysgeusia | 0 | 0 | 5 (13.5) | 0 | |
NOTE: Patients reporting more than one adverse event within a preferred term or a system organ class were only counted once for that preferred term or system organ class.
Abbreviations: AE, adverse event; arm A, cixutumumab + antiestrogen; arm B, cixutumumab; N, number of patients for each treatment; n, number of patients per category for each treatment; TEAE, treatment-emergent adverse event.
Correlative analyses of IR-A, IR-B, and IGF-1R mRNA expression levels in tumor tissue
A majority of the tissue specimens were taken from primary tumor tissue (90%); the remaining samples were biopsied from metastatic lesions. The distributions of mRNA gene expression levels IGF-1R, IR-A, and IR-B are provided in Supplementary Table S2. The mean IR-A expression was higher than the mean IR-B and IGF-1R expression (mean copies/ng RNA: IR-A = 287.3, IR-B = 65.5, and IGF-1R = 12.2).
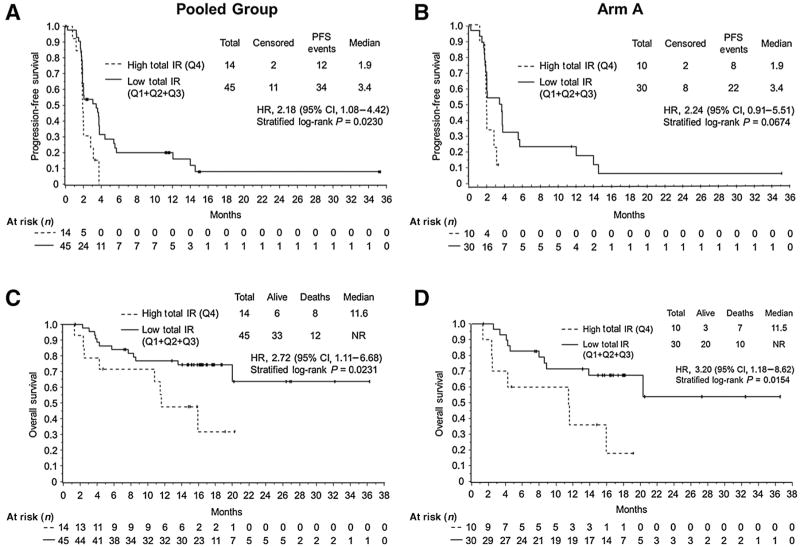
Cox regressions were performed to correlate each mRNA gene expression dichotomized at the 25th, median, and 75th percentile cutoff points with PFS and OS from the pooled group (arm A + arm B) and arm A alone, separately. No multiplicity adjustments across markers or endpoints were performed. Statistically significant associations were observed between the low expression of total IR, IR-A, and IR-B and PFS and OS for the pooled group using the 75th percentile cutoff point (Fig. 3, Supplementary Table S3, and Supplementary Figs. S1 and S2). In particular, all patients, regardless of treatment, who had high IR expression (n = 14) had a median PFS of 1.9 months, and patients with low IR expression (n = 45) had a median PFS of 3.4 months (HR, 2.18; 95% CI, 1.08–4.42; P = 0.0230; Fig. 3; Supplementary Table S3). Similar statistically significant results for PFS and OS were observed for IR-A (Supplementary Table S3; Supplementary Fig. S1) and IR-B for the pooled group using the 75th percentile cutoff point (Supplementary Table S3; Supplementary Fig. S2). Likewise, in arm A, patients with low expression of total IR, IR-A, and IR-B had longer PFS and OS than patients with high expression (Fig. 3; Supplementary Table S3; Supplementary Figs. S1 and S2; significant for OS: P = 0.0154, P = 0.0154, and P = 0.0031 for total IR, IR-A, and IR-B, respectively). The correlative analysis results for the 25th percentile and median cutoff points were similar to those for the 75th percentile cutoff point in terms of the direction of HR comparing high to low expression; however, these results were not statistically significant (Supplementary Table S4).
Figure 3.
Kaplan–Meier curves of PFS and OS from pooled group and arm A dichotomized by total insulin receptor (IR) expression (high vs. low) using the 75th percentile of marker distribution as cutoff point. PFS and OS curves are presented for the arms dichotomized for high (dotted line) or low (solid line) total IR expression on the pooled group (n = 59) and arm A population (n = 40). A, PFS: pooled group; B, PFS: arm A; C, OS: pooled group; D, OS: arm A. Arm A, cixutumumab + antiestrogen; n, number of patients; NR, not reached; Pooled group, cixutumumab + antiestrogen (arm A) + cixutumumab monotherapy (arm B); Q, quartile.
Discussion
The objective of this phase II trial was to analyze the antitumor efficacy of cixutumumab in combination with antiestrogen (arm A) or as cixutumumab monotherapy (arm B) in patients with hormone receptor–positive advanced or metastatic breast cancer that progressed on prior antiestrogen therapy. Furthermore, we also studied the biomarkers that may be associated with clinical efficacy of anti–IGF-1R antibody in hormone receptor–positive breast cancer.
In our trial, cixutumumab administered at 10 mg/kg every 2 weeks with or without antiestrogen therapy did not demonstrate significant clinical efficacy in the ITT population. The median PFS was 2.0 and 3.1 months for arm A and arm B, respectively, which indicates that this analysis is at the first or second disease assessment. Effective progression could have come earlier, but would have been asymptomatic; otherwise, the patient would have discontinued treatment for symptomatic disease progression. The 6-month PFS rates were 20.1% (arm A) and 15.2% (arm B). Although there was a shorter PFS in the combination arm in the ITT population, the data were very heterogeneous. These data are in alignment with the efficacy data demonstrated by another IGF-1R blocking therapy (30). In a phase II trial, Robertson and colleagues showed that ganitumab, a monoclonal IgG1 antibody that blocks IGF-1R, in combination with endocrine treatment in a similar patient population, did not improve the primary endpoint of PFS (median PFS, 3.9 months for ganitumab plus endocrine therapy and 5.7 months for placebo plus endocrine therapy; HR, 1.17; P = 0.44; ref. [30]). Limitations of our trial include the lack of an appropriate control arm and the small sample size in arm B, which did not permit relevant statistical analyses.
In the current trial, cixutumumab with or without antiestrogen had an acceptable safety profile. In the safety population, a lower number of patients in arm A (22/56 [39.3%]) experienced grade ≥3 AEs than in arm B (19/37 [51.4%]). Overall, the most frequently reported grade ≥3 AEs were fatigue, nausea, abdominal pain, and hyperglycemia (5/93 [5.4%] for each). The safety, tolerability, and AE profile of cixutumumab were consistent with the safety profile in previous publications, including fatigue, diarrhea, mild skin toxicities, and hyperglycemia (31–33). Overall, six patients from arm A, and two patients from arm B discontinued treatment due to AEs.
In addition to investigating clinical outcomes of modulating IGF pathways, this trial also analyzed biomarkers that might be predictive of clinical efficacy of cixutumumab in patients with breast cancer. Besides active IGF signaling, the overexpression of IR and IGF-1R in breast cancer tissue compared with normal tissue was involved in tumor growth and progression (12, 13, 18, 34, 35). A number of preclinical trials and clinical correlative analyses have found a prognostic value of IGF signaling in breast cancer (36, 37). In women with node-negative breast cancer, both undetectable and very high levels of IR expression were associated with reduced disease-free survival (38). Law and colleagues demonstrated that phosphorylated IGF-1R and IR are present in all breast cancer subtypes, and along with total levels of IR, are prognostic factors for poor survival (39). IR-A is the predominant IR isoform in breast cancer cells, suggesting that IGF signaling may be mediated by this IR isoform (40).
In this trial, we observed an association between efficacy and baseline tumor-specific IR expression, but not IGF-1R expression. Due to the small sample size of arm B, correlative analyses of IGF-1R, total IR, IR-A, and IR-B mRNA expression were performed on the pooled group from arms A and B (n = 59) and on arm A alone (n = 40). Notably, a significant association was observed between low mRNA expressions of IR-A, IR-B, and total IR and longer PFS and OS in the pooled group using the 75th percentile cutoff point. The correlation between total IR expression and OS should be interpreted with caution, as there was a high censoring of OS. Another limitation was that the study design did not allow us to discriminate between the prognostic and predictive value of IR. Additionally, samples were not taken in this study to determine the level of sustained pathway blockade. However, evidence of IGF-1R blockade has been seen with elevated serum levels of IGF-1 and IGFBP-3 in studies using similar doses of cixutumumab (41–44).
In conclusion, this trial did not meet the primary efficacy endpoint of PFS in the ITT population. Clinical outcomes of PFS for both arms A and B were similar or inferior to historical standards (2.0 and 3.1 months vs. 3 months, respectively), but were much lower than the desirable PFS of ≥4.4 months, which was worthy of further investigation (45). As the study did not meet the primary objective, no further investigation is warranted. The results from our correlative studies suggest an association between the expression of IR-A, IR-B, and total IR with both PFS and OS. Clinical validity of total IR and IR isoforms as potential biomarkers predictive of antitumor efficacy of IGF-1R-targeted therapies is yet to be established.
Supplementary Material
Translational Relevance.
This randomized, open-label, phase II trial evaluated the efficacy and safety of cixutumumab in patients with hormone receptor–positive, advanced or metastatic breast cancer refractory to antiestrogen therapy (NCT00728949). In addition, this study aimed to address the critical unmet need for the development of biomarkers for insulin-like growth factor receptor 1 (IGF-1R)–targeted therapies. In particular, mRNA expression of insulin receptor (IR)-A, IR-B, and total IR in tumor tissue was significantly associated with progression-free survival and overall survival in this study (lower marker levels corresponded with better outcomes). These results indicate that any potential practical use of IR as a predictive or prognostic biomarker would require clinical validation prior to use in guiding patient treatment.
Acknowledgments
The authors would thank all the patients and investigators who participated in this study. The authors also thank Richard Willey for statistical analysis support and Einav Yosef Leberknight for clinical operational support. Eli Lilly and Company contracted with inVentiv Health Clinical for writing support provided by Andrea Humphries, PhD, and Asifa Haider, PhD, and editorial support provided by Noelle Gasco and Rod Everhart, ELS.
Grant Support
This study was funded by Eli Lilly and Company.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
P. Haluska reports receiving speakers bureau honoraria from Boehringer Ingleheim, is a consultant/advisory board member for Boehringer Ingleheim, Bristol-Myers Squibb, and Medimmune, and reports receiving commercial research support from Bristol-Myers Squibb, ImClone, and Medimmune. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: H. Youssoufian, D. Grebennik
Development of methodology: H. Youssoufian, D. Grebennik
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): W.J. Gradishar, D.A. Yardley, R. Layman, J.A. Sparano, E. Chuang, D.W. Northfelt, G.N. Schwartz, H. Youssoufian, J. Cosaert, D. Grebennik, P. Haluska
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): W.J. Gradishar, D.A. Yardley, D.W. Northfelt, H. Youssoufian, S. Tang, R. Novosiadly, A. Forest, T.S. Nguyen, J. Cosaert, D. Grebennik, P. Haluska
Writing, review, and/or revision of the manuscript: W.J. Gradishar, D.A. Yardley, R. Layman, J.A. Sparano, E. Chuang, D.W. Northfelt, G.N. Schwartz, H. Youssoufian, S. Tang, R. Novosiadly, A. Forest, T.S. Nguyen, J. Cosaert, D. Grebennik, P. Haluska
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Forest
Study supervision: W.J. Gradishar, D.A. Yardley, J.A. Sparano, J. Cosaert, D. Grebennik, P. Haluska
References
- 1.Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40:862–71. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Santen RJ, Song RX, Zhang Z, Yue W, Kumar R. Adaptive hypersensitivity to estrogen: mechanism for sequential responses to hormonal therapy in breast cancer. Clin Cancer Res. 2004;10:337S–45S. doi: 10.1158/1078-0432.ccr-031207. [DOI] [PubMed] [Google Scholar]
- 3.Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–18. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 5.Chong K, Subramanian A, Sharma A, Mokbel K. Measuring IGF-1, ER-α and EGFR expression can predict tamoxifen-resistance in ER-positive breast cancer. Anticancer Res. 2011;31:23–32. [PubMed] [Google Scholar]
- 6.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura S, et al. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95:155–65. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Moerkens M, Ramaiahgari S, de Bont H, Price L, Meerman J, et al. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13:R52. doi: 10.1186/bcr2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–21. [PubMed] [Google Scholar]
- 9.Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 10.Parisot JP, Hu XF, DeLuise M, Zalcberg JR. Altered expression of the IGF-1 receptor in a tamoxifen-resistant human breast cancer cell line. Br J Cancer. 1999;79:693–700. doi: 10.1038/sj.bjc.6690112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–51. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belfiore A, Frasca F. IGF and insulin receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:381–406. doi: 10.1007/s10911-008-9099-z. [DOI] [PubMed] [Google Scholar]
- 13.Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:277–89. doi: 10.1007/s10911-013-9303-7. [DOI] [PubMed] [Google Scholar]
- 14.Milazzo G, Giorgino F, Damante G, Sung C, Stampfer MR, Vigneri R, et al. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 1992;52:3924–30. [PubMed] [Google Scholar]
- 15.Knudsen L, De Meyts P, Kiselyov VV. Insight into the molecular basis for the kinetic differences between the two insulin receptor isoforms. Biochem J. 2011;440:397–403. doi: 10.1042/BJ20110550. [DOI] [PubMed] [Google Scholar]
- 16.Harrington SC, Weroha SJ, Reynolds C, Suman VJ, Lingle WL, Haluska P. Quantifying insulin receptor isoform expression in FFPE breast tumors. Growth Horm IGF Res. 2012;22:108–15. doi: 10.1016/j.ghir.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 18.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–95. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, Chang YS, Jallal B, Viner J. Targeting the insulin-like growth factor axis for the development of novel therapeutics in oncology. Cancer Res. 2012;72:3–12. doi: 10.1158/0008-5472.CAN-11-0550. [DOI] [PubMed] [Google Scholar]
- 20.Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–73. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan AM, O’Malley FP, Ennis M, Fantus IG, Goodwin PJ. Insulin receptor is an independent predictor of a favorable outcome in early stage breast cancer. Breast Cancer Res Treat. 2007;106:39–47. doi: 10.1007/s10549-006-9471-x. [DOI] [PubMed] [Google Scholar]
- 22.Mathieu MC, Clark GM, Allred DC, Goldfine ID, Vigneri R. Insulin receptor expression and clinical outcome in node-negative breast cancer. Proc Assoc Am Physicians. 1997;109:565–71. [PubMed] [Google Scholar]
- 23.Wu JD, Odman A, Higgins LM, Haugk K, Vessella R, Ludwig DL, et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res. 2005;11:3065–74. doi: 10.1158/1078-0432.CCR-04-1586. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, v3.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 26.Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81. [Google Scholar]
- 28.Cox DR. Regression models and life-tables. J R Stat Soc Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 29.Bland JM, Altman DG. The logrank test. BMJ. 2004;328:1073. doi: 10.1136/bmj.328.7447.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson JF, Ferrero JM, Bourgeois H, Kennecke H, deBoer RH, Jacot W, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14:228–35. doi: 10.1016/S1470-2045(13)70026-3. [DOI] [PubMed] [Google Scholar]
- 31.Ma CX, Suman VJ, Goetz M, Haluska P, Moynihan T, Nanda R, et al. A phase I trial of the IGF-1R antibody Cixutumumab in combination with temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat. 2013;139:145–53. doi: 10.1007/s10549-013-2528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7:2575–88. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoffski P, Adkins D, Blay JY, Gil T, Elias AD, Rutkowski P, et al. An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur J Cancer. 2013;49:3219–28. doi: 10.1016/j.ejca.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Hartog H, Wesseling J, Boezen HM, van der Graaf WT. The insulin-like growth factor 1 receptor in cancer: old focus, new future. Eur J Cancer. 2007;43:1895–904. doi: 10.1016/j.ejca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L, et al. Elevated insulin receptor content in human breast cancer. J Clin Invest. 1990;86:1503–10. doi: 10.1172/JCI114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creighton CJ, Casa A, Lazard Z, Huang S, Tsimelzon A, Hilsenbeck SG, et al. Insulin-like growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J Clin Oncol. 2008;26:4078–85. doi: 10.1200/JCO.2007.13.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinaldi S, Peeters PH, Berrino F, Dossus L, Biessy C, Olsen A, et al. IGF-I, IGFBP-3 and breast cancer risk in women: the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2006;13:593–605. doi: 10.1677/erc.1.01150. [DOI] [PubMed] [Google Scholar]
- 38.Mathieu MC, Clark GM, Allred DC, Goldfine ID, Vigneri R. Insulin receptor expression and clinical outcome in node-negative breast cancer. Proc Assoc Am Physicians. 1997;109:565–71. [PubMed] [Google Scholar]
- 39.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–46. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 40.Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, et al. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–9. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 41.Weigel B, Malempati S, Reid JM, Voss SD, Cho SY, Chen HX, et al. Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2014;61:452–6. doi: 10.1002/pbc.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:256–62. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz GK, Tap WD, Qin LX, Livingston MB, Undevia SD, Chmielowski B, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2013;14:371–82. doi: 10.1016/S1470-2045(13)70049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajan A, Carter CA, Berman A, Cao L, Kelly RJ, Thomas A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15:191–200. doi: 10.1016/S1470-2045(13)70596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris AL, Dowsett M, Smith IE, Jeffcoate S. Aminoglutethimide induced hormone suppression and response to therapy in advanced postmenopausal breast cancer. Br J Cancer. 1983;48:585–94. doi: 10.1038/bjc.1983.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.