Abstract
Circulating cell-free DNA (cfDNA) has been described as a prognostic marker for several diseases. Its prognostic value for short-term outcome in stroke patients treated with intravenous thrombolysis remains unexplored. cfDNA was measured on admission in 54 tissue plasminogen activator (tPA)-treated patients and 15 healthy controls using a real-time quantitative polymerase chain reaction assay. Neurological outcome was assessed at 48 h. Predictors of neurological improvement were evaluated by logistic regression analysis, and the additional predictive value of cfDNA over clinical variables was determined by integrated discrimination improvement (IDI). Stroke patients presented higher baseline cfDNA than healthy controls (408.5 (179–700.5) vs. 153.5 (66.9–700.5) kilogenome-equivalents/L, p = 0.123). A trend towards lower cfDNA levels was found in patients who neurologically improved at 48 h (269.5 (143.3–680) vs. 504 (345.9–792.3) kilogenome-equivalents/L, p = 0.130). In logistic regression analysis, recanalization at 1 h and cfDNA < 302.75 kilogenome-equivalents/L was independently associated with neurological improvement after adjustment by age, gender and baseline National Institutes of Health Stroke Scale score. The addition of cfDNA to the clinical predictive model improved its discrimination (IDI = 21.2% (9.2–33.3%), p = 0.009). These data suggest that cfDNA could be a surrogate marker for monitoring tPA efficacy by the prediction of short-term neurological outcome.
Keywords: Stroke, biomarkers, circulating DNA, thrombolysis, outcome, neurological improvement
Introduction
Stroke represents the fifth cause of death and one of the leading causes of disability worldwide.1 Nowadays, intravenous thrombolysis with tissue plasminogen activator (tPA) remains the only approved treatment for acute stroke.2 Objective tests predicting tPA response in acute stroke patients, and therefore, short-term neurological outcome, might be used to guide physicians in the indication of endovascular therapies in tPA non-responders or to optimize resources improving patient’s allocation.
Circulating cell-free DNA (cfDNA) is found in plasma under several conditions,3,4 cell death being the major factor accounting for cfDNA release.5 Several studies have assessed the predictive value of cfDNA as a prognostic marker for long-term outcome in stroke patients6,7 as well as its role in differentiating between ischaemic and haemorrhagic stroke.8 However, these studies enrolled few tPA-treated patients. Therefore, hypothesizing that high cfDNA level might reflect higher cell death, we aimed to explore the prognostic value of cfDNA in acute stroke patients treated with tPA regarding short-term neurological outcome.
Methods
Patients and clinical evaluation
Our target group consisted of acute ischemic stroke patients admitted to the stroke unit of our centre within the first 4.5 h after symptoms onset. Fifty-four patients with non-lacunar stroke involving the middle cerebral artery or the basilar artery territories and 15 age- and gender-matched healthy controls were evaluated. All patients received recombinant tPA in a standard dose, and recanalization was assessed at the end of tPA infusion by transcranial Doppler with the thrombolysis in brain ischaemia score.9 All patients underwent a complete workup on neurological assessment and ancillary tests as previously published.10 Neurological outcome was assessed at 48 h, and neurological improvement was defined as a decrease of four or more points in the National Institutes of Health Stroke Scale (NIHSS).11 Written informed consent was obtained from all patients or relatives, and the study protocol was approved by the local Ethics Committee.
Blood sampling and cfDNA measurement
Blood samples were taken on admission, before tPA administration (<4.5 h), centrifuged (8 min–2000 × g) and DNA from 400 μL of serum was extracted using MagNA Pure and Nucleic Acid Isolation Kit I (Roche Diagnostics, Basel, Switzerland). cfDNA was measured using quantitative polymerase chain reaction (PCR) analysis (LightCycler 480 Real-Time PCR; Roche Diagnostics) using LC480 Probes Master Kit (Roche Diagnostics). β-Globin hydrolysis probes system comprises primers beta-globin-354F and beta-globin-455 R and a dual-labelled fluorescent probe betaglobin-402 T. PCR conditions were 95°C for 5 min and 61°C for 15 min for 48 cycles. The final size of the amplicon was 102 bp. The experiment was repeated twice to evaluate the reproducibility of the results.
Statistical analysis
SPSS v15.0 [Chicago, SPSS Inc.] software was used for statistical analysis. Normality was assessed by Kolmogorov–Smirnov test. Normally distributed variables were analysed by Student t test, and non-normally distributed variables were analysed by Mann–Whitney U or Kruskal–Wallis tests. In the univariate analysis, intergroup differences were assessed by Pearson χ 2 test for categorical variables. Cut-off points were obtained from receiver operating characteristic curves. To build predictive models, all clinical variables associated with neurological improvement at p value <0.15 in univariate analysis (Oxfordshire Stroke Project Classification, Trial of Org 10172 in Acute Stroke Treatment classification, 1-h recanalization, coronary artery disease and previous stroke) were included in a forward stepwise logistic regression analysis. Afterwards, cfDNA was added by Enter method to build a second predictive model. For both models, odds ratios (ORs) were adjusted by age, gender and baseline NIHSS score. Comparisons between models were made with integrated discrimination improvement (IDI) index.12
Results
Demographic data are summarized in Table 1. cfDNA levels trended to be higher in stroke patients compared to controls (408.5 (179–700.5) vs. 153.5 (66.9–700.5) kilogenome-equivalents/L, p = 0.123; Figure 1(a)). There were no significant associations between cfDNA levels and baseline characteristics of stroke patients (Table 2), neither baseline stroke severity or aetiology, although patients with previous stroke and those with previous disability had non-significant higher cfDNA levels. Regarding neurological outcome, 33 patients (61.1%) improved at 48 h. No patient developed tPA-related complications such as symptomatic haemorrhagic transformation or malignant oedema. Patients who improved at 48 h trended to have lower cfDNA levels (286.5 (152–688) vs. 526 (382.8–927.5) kilogenome-equivalents/L, p = 0.123; Figure 1(b)).
Table 1.
Baseline characteristics and univariate predictors of neurological improvement at 48 h.
| Neurological improvement | |||||
|---|---|---|---|---|---|
| All (N = 54) | Yes (N = 33) | No (N = 21) | p Value | ||
| Age (years) | 77.0 (70–82) | 77.5 (71.5–82) | 77 (56.5–83) | 0.804 | |
| Gender (male) | 44.4% | 45.5% | 42.9% | 0.851 | |
| Hypertension | 68.5% | 66.7% | 71.4% | 0.713 | |
| Diabetes | 20.4% | 24.2% | 14.3% | 0.497 | |
| Dyslipidaemia | 35.8% | 42.4% | 25.0% | 0.200 | |
| Atrial fibrillation | 48.1% | 48.5% | 47.6% | 0.951 | |
| Tobacco use | 13.2% | 15.2% | 10.0% | 0.697 | |
| Coronary disease | 25.9% | 33.3% | 14.3% | 0.119 | |
| Previous stroke | 13.0% | 6.1% | 23.8% | 0.096 | |
| Previous disability | 3.8% | 3.2% | 4.8% | 0.999 | |
| Baseline NIHSS | 14.2 ± 7.1 | 13.5 ± 5 | 15.3 ± 9 | 0.429 | |
| SBP | 154 ± 27 | 156 ± 27 | 152 ± 29 | 0.591 | |
| DBP | 81 ± 14 | 82.9 ± 14 | 81.5 ± 15 | 0.0.928 | |
| Glycaemia | 117.5 (97–164) | 120 (100–169) | 112 (92—129) | 0.188 | |
| Proximal occlusion | 44.4% | 45.5% | 42.9% | 0.851 | |
| OCSP | TACI | 63.0% | 72.7% | 47.6% | 0.041* |
| PACI | 25.9% | 24.2% | 28.6% | ||
| POCI | 11.1% | 3.0% | 23.8% | ||
| LACI | 0% | – | – | ||
| TOAST | Atherothrombotic | 18.5% | 12.1% | 28.6% | 0.115 |
| Cardioembolic | 57.4% | 57.6% | 57.1% | ||
| Lacunar | 0% | – | – | ||
| Undetermined | 22.2% | 30.3% | 9.5% | ||
| Uncommon | 1.9% | 0% | 4.8% | ||
| Time to tPA (min) | 183 ± 74 | 176 ± 65 | 195 ± 87 | 0.383 | |
| 1-h recanalization | 39.2% | 54.8% | 15.0% | 0.004* | |
NIHSS: National Institutes of Health Stroke Scale; SBP: systolic blood pressure; DBP: diastolic blood pressure; OCSP: Oxfordshire Stroke Project Classification; TACI: total anterior circulation infarct; PACI: partial anterior circulation infarct; POCI: posterior arterial circulation infarct; LACI: lacunar infarct; TOAST: Trial of Org 10172 in Acute Stroke Treatment classification; tPA: tissue plasminogen activator.
*p < 0.05.
Figure 1.
Differences in baseline cfDNA levels between different groups. Box plots represent median (interquartile range) of cfDNA levels between the following comparisons: (a) stroke patients (N = 54) versus healthy controls (N = 15). (b) Patients with neurological improvement at 48 h (N = 33) versus patients without neurological improvement at 48 h (N = 21). cfDNA: circulating cell-free DNA.
Table 2.
Factors associated with cfDNA levels.
| Yes | No | P Value | ||
|---|---|---|---|---|
| Gender (male) | 383 (173–1435) | 346 (159–628) | 0.121 | |
| Hypertension | 383 (158–665) | 405 (173–999) | 0.999 | |
| Diabetes | 185 (127–688) | 383 (179–913) | 0.512 | |
| Dyslipidaemia | 383 (167–913) | 346 (164–657) | 0.978 | |
| Atrial fibrillation | 384 (172–672) | 309 (151–801) | 0.972 | |
| Tobacco use | 597 (237–1447) | 346 (158–673) | 0.373 | |
| Coronary disease | 321 (147–808) | 383 (172–650) | 0.961 | |
| Previous stroke | 639 (102–2045) | 383 (166–657) | 0.632 | |
| Previous disability | 617 (135–1099) | 383.5 (167–688) | 0.943 | |
| Proximal occlusion | 408 (206–665) | 309 (115–801) | 0.470 | |
| OCSP | TACI | 252 (143–578) | 0.041 | |
| PACI | 657 (180–999) | |||
| POCI | 1099 (432–1135) | |||
| LACI | – | |||
| TOAST | Atherothrombotic | 179 (100–371) | 0.433 | |
| Cardioembolic | 433 (173–808) | |||
| Lacunar | – | |||
| Undetermined | 297 (172–575) | |||
| Uncommon | – | |||
| 48-h improvement | 227 (135–688) | 457 (309–927) | 0.123 | |
| Correlation coefficient | p Value | |||
| Age | R = −0.075 | 0.589 | ||
| Baseline NIHSS | R = −0.096 | 0.492 | ||
| SBP | R = 0.072 | 0.612 | ||
| DBP | R = 0.095 | 0.503 | ||
| Glycaemia | R = −0.167 | 0.242 | ||
| Time to tPA | R = −0.065 | 0.646 | ||
NIHSS: National Institutes of Health Stroke Scale; SBP: systolic blood pressure; DBP: diastolic blood pressure; OCSP: Oxfordshire Stroke Project Classification; TACI: total anterior circulation infarct; PACI: partial anterior circulation infarct; POCI: posterior arterial circulation infarct; LACI: lacunar infarct; TOAST: Trial of Org 10172 in Acute Stroke Treatment classification; tPA: tissue plasminogen activator.
*p < 0.05.
A cut-off point of cfDNA <302.75 kilogenome-equivalents/L was found to have 81% sensitivity and 55% specificity for the prediction of 48 h neurological improvement. Patients with cfDNA levels below this cut-off point were significantly more prone to improve at 48 h than those with higher levels (81.8% vs. 46.7%, p = 0.010). In logistic regression analysis, after adjusting by age, gender and baseline NIHSS score, the presence of arterial recanalization 1 h after tPA (adjusted OR (adjOR) = 43.8(3.1–620.9), p = 0.005) and cfDNA levels <302.75 kilogenome-equivalents/L (adjOR = 27.1(2.63–279.2), p = 0.006) were the only independent predictors of 48 h improvement in our cohort. When models including or not the biomarker were compared, assessing cfDNA levels resulted in improved discrimination (IDI = 21.2% (9.2–33.3%), p = 0.0005; Figure 2, Table 3).
Figure 2.
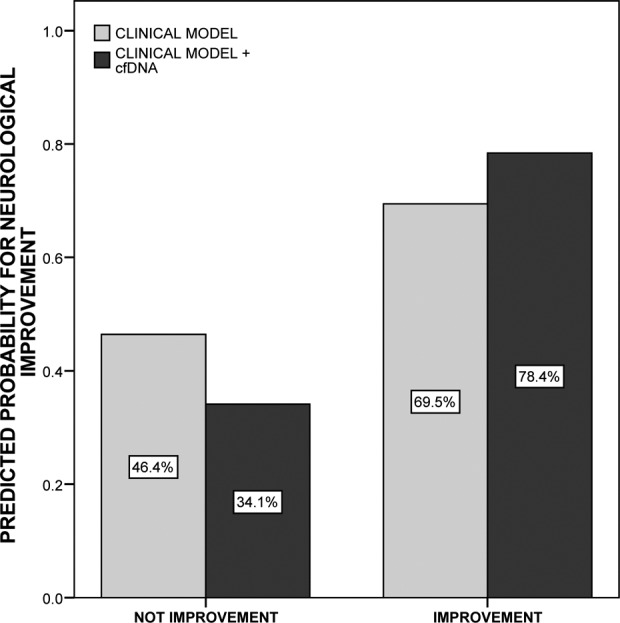
Comparison between predictive models for 48 h neurological improvement. Light bars represent the predictive ability of the clinical model, composed by 1-h recanalization adjusted by age, gender and baseline National Institutes of Health Stroke Scale score. Dark bars represent the predictive ability of the second model, constructed by adding cfDNA levels <302.75 kilogenome-equivalents/L over the only clinical model. The integrated discrimination improvement index was 21.2% (9.2–33.3%), p = 0.0005. cfDNA: circulating cell-free DNA.
Table 3.
Logistic regression analysis and additional predictive value of the model including cfDNA for 48-h improvement.a
| 48-h improvement | |||
|---|---|---|---|
| Clinical model | Clinical + cfDNA model | ||
| Logistic regression | 1 h recanalization | 9.8 (2.05–46.78); p = 0.004* | 43.84 (3.10–620.91); p = 0.005* |
| NIHSS admission | 0.92 (0.83–1.02); p = 0.095 | 0.93 (0.82–1.05); p = 0.221 | |
| Age | 1.02 (0.97–1.08); p = 0.471 | 1.01 (0.94–1.09); p = 0.735 | |
| Gender (male) | 2.23 (0.56–8.83); p = 0.254 | 7.22 (0.75–69.60); p = 0.087 | |
| cfDNA | – | 27.1 (2.63–279.2); p = 0.006* | |
| IDI statistics | IDI events | – | 8.9% |
| IDI non-events | – | 12.3% | |
| IDI | – | 21.2% (9.2–33.3) | |
| p Value | Reference | 0.0005* | |
cfDNA: circulating cell-free DNA; OCSP: Oxfordshire Stroke Project Classification; TOAST: Trial of Org 10172 in Acute Stroke Treatment classification; adjOR: adjusted odds ratio; NIHSS: National Institutes of Health Stroke Scale; IDI: integrated discrimination improvement.
aThe table represents the comparison between either predictive models, including or not the biomarker cfDNA. For the logistic regression analysis, variables entered on step 1 (p < 0.05) were OCSP, TOAST, 1 h recanalization, coronary disease and previous stroke. The table shows just the variables entered in the final step. adjORs and 95% confidence intervals are given for each of the variables.
*p < 0.005.
Discussion
Our study showed that baseline cfDNA was an independent predictor of neurological improvement at 48 h in stroke patients treated with tPA. Moreover, cfDNA <302.75 kilogenome-equivalents/L improved the prediction of neurological improvement regarding clinical variables and after adjustment by age, gender and baseline NIHSS score. Although higher cfDNA levels have been previously associated with long-term disability and mortality after stroke,6,7 a diagnostic test, which is able to identify early those patients with a higher chance of short-term neurological improvement, could be easily used by clinicians, to indicate early the rescue procedures (activation of interventional teams, inclusion in clinical trials) for patients with a low chance to improve after intravenous tPA treatment or, conversely, patients with a high chance to improve could be safely discharged from scarce resources such as stroke unit’s beds.
High levels of cfDNA in acute stroke patients have been associated also with infarct volume or baseline neurological impairment, which seems logical while cfDNA is an indicator of cell death.13,14 In accordance with these data, we found that patients with previous stroke and those with previous disability had higher cfDNA levels, which may indicate a sustained inflammatory response or a greater level of cell death in these patients, although these results did not reach statistical significance. The main finding of our study, however, was an association with short-term outcome. Since this association has been noted in tPA-treated patients, it might not indicate just a higher infarct size or neurological deficit, but a greater BBB damage as has been described,8 and therefore a higher chance of complications related to this breakdown in tPA-treated patients such as haemorrhagic transformation or oedema. However, high cfDNA levels have also been associated with other conditions related to cell death.3,4 Although in our study cfDNA levels were not related to baseline comorbidities or vascular risk factors, we cannot exclude that these differences were related to some conditions not assessed in the present study.
Our study has some limitations. First, our sample size was relatively small and this fact could be responsible for missing some associations, such as a less number of patients developing neurological worsening or none with tPA-related complications. Second, our data from short-term neurological outcome has been derived from a cohort of tPA-treated patients and extrapolation of the results to all stroke patients should be carefully interpreted. Third, final infarct volume was not measured, and so this parameter could not be used for further adjustments, although some other parameter reflecting similar information such as baseline NIHSS score was used to adjust the model.
Besides these limitations, cfDNA could represent a surrogate marker for monitoring tPA efficacy with the prediction of short-term neurological outcome, improving the prediction that may be done with clinical information. However, our results are still of preliminary nature, and future studies are needed to replicate these results, as well as the possible underlying association with symptomatic haemorrhagic transformation.
Compliance with ethical research standards
All research on human subjects presented in this article was conducted in accordance with the ethical research standards prescribed by the responsible national/institutional committee on human experimentation and with the WMA Declaration of Helsinki as of its seventh revision in 2013. Informed consent was obtained from all human subjects participating in the study.
Acknowledgement
Neurovascular Research Laboratory takes part in the Spanish stroke research network INVICTUS (RD12/0014/0005).
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been funded by Instituto de Salud Carlos III, grant FIS PI15/354, co-financed by the European Regional Development Fund (FEDER). AB is supported by a Rio Hortega contract CM13/00265 from the Instituto de Salud Carlos III.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. American heart association statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 3. Macher H, Egea-Guerrero JJ, Revuelto-Rey J. Role of early cell-free DNA levels decrease as a predictive marker of fatal outcome after severe traumatic brain injury. Clin Chim Acta 2012; 414: 12–17. [DOI] [PubMed] [Google Scholar]
- 4. Miranda ML, Macher HC, Muñoz-Hernández R. Role of circulating cell-free DNA levels in patients with severe preeclampsia and HELLP syndrome. Am J Hypertens 2013; 26: 1377–1380. [DOI] [PubMed] [Google Scholar]
- 5. Fournie GJ, Courtin JP, Laval F, et al. Plasma DNA as a marker of cancerous cell death. Investigations in patients suffering from lung cancer and in nude mice bearing human tumors. Cancer Lett 1995; 91: 221–227. [DOI] [PubMed] [Google Scholar]
- 6. Rainer TH, Wong LK, Lam W, et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem 2003; 49: 562–569. [DOI] [PubMed] [Google Scholar]
- 7. Geiger S, Holdenrieder S, Stieber P, et al. Nucleosomes as a new prognostic marker in early cerebral stroke. J Neurol 2007; 254: 617–623. [DOI] [PubMed] [Google Scholar]
- 8. Rainer TH, Wong KS, Lam W, et al. Comparison of plasma β-globin DNA and S-100 protein concentrations in acute stroke. Clin Chim Acta 2007; 376: 190–196. [DOI] [PubMed] [Google Scholar]
- 9. Burgin WS, Malkoff M, Felberg RA, et al. Transcranial Doppler ultrasound criteria for recanalization after thrombolysis for middle cerebral artery stroke. Stroke 2000; 31: 1128–1132. [DOI] [PubMed] [Google Scholar]
- 10. Mendioroz M, Fernandez-Cadenas I, Rosell A, et al. Osteopontin predicts long-term functional outcome among ischemic stroke patients. J Neurol 2011; 258: 486–493. [DOI] [PubMed] [Google Scholar]
- 11. Brott TG, Haley EC, Jr, Levy DE, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke 1992; 23: 632–640. [DOI] [PubMed] [Google Scholar]
- 12. Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2010; 48: 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geiger S, Holdenrieder S, Stieber P, et al. Nucleosomes in serum of patients with early cerebral stroke. Cerebrovasc Dis 2006; 21: 32–37. [DOI] [PubMed] [Google Scholar]
- 14. Tsai NW, Lin TK, Chen SD, et al. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta 2011; 412: 476–479. [DOI] [PubMed] [Google Scholar]



