Abstract
The role of pattern of circulating endothelial cell-derived microparticles, platelet-derived microparticles (PMPs), and monocyte-derived microparticles (MMPs) in metabolic syndrome (MetS) patients with chronic heart failure (CHF) is not still understood. The aim of the study was to investigate a pattern of circulating microparticles (MPs) in MetS patients with CHF in relation to neurohumoral and inflammatory activation. The study retrospectively involved 101 patients with MetS and 35 healthy volunteers. Biomarkers were measured at baseline of the study. The results of the study have shown that numerous circulating PMPs- and MMPs in subjects with MetS (with or without CHF) insufficiently distinguished from level obtained in healthy volunteers. We found elevated level of CD31+/annexin V+ MPs in association with lower level of CD62E+ MPs. Therefore, we found that biomarkers of biomechanical stress serum N-terminal brain natriuretic peptide and inflammation (high-sensitive C-reactive protein ,osteoprotegerin) remain statistically significant predictors for decreased CD62E+ to CD31+/annexin V+ ratio in MetS patients with CHF. In conclusion, decreased CD62E+ to CD31+/annexin V+ ratio reflected that impaired immune phenotype of MPs may be discussed as a surrogate marker of CHF development in MetS population.
Keywords: Chronic heart failure, metabolic syndrome, circulating microparticles, cardiovascular risk factors inflammation, neurohumoral activation
Introduction
The traditionally recognized metabolic syndrome (MetS) is defined as risk-factor clustering related to the development of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD).1 MetS includes abdominal obesity, insulin resistance, dyslipidemia, and elevated blood pressure and associates with other comorbidities including the prothrombotic and pro-inflammatory states.2 Accumulating evidence has shown that MetS is a powerful risk factor for CVD event as well as all cause and CVD mortality in total population.3–5 The underlying pathophysiological mechanisms resulting in the MetS, that is, insulin resistance (IR), associate with activation of neurohumoral mechanisms, immunity, cytokine production, systemic pro-inflammatory response, and oxidative stress.6–8 All these factors may have effect on the development of CVD through inducing endothelial dysfunction9,10 and microvascular inflammation.11
Recent studies have shown a controversial role of MetS in patients at high risk of chronic heart failure (CHF) and in subjects with documented CHF. Although MetS associates with cardiovascular risk factors and CVD outcomes,12–15 prognostic impact of MetS on CHF progression is not fully confirmed and widely discussed.16,17 Therefore, it is still unclear whether MetS may induce development and progression of cardiac failure through imbalance between endothelial injuries and repair.18,19 Probably, microparticles (MPs) corresponding cell-to-cell cooperation, immunity, tissue reparation, and vascular function are key factors that coordinate microvascular integrity and function.20
Extracellular MPs are microvesicles with sizes ranging between 50 nm and 1000 nm, released from plasma membrane of wide variety of cells, including endothelial cells, mononuclear cells, platelets, and by specific (cytokine stimulation, apoptotic agents, mononuclear cooperation, coagulation, and so on) and nonspecific (shear stress) stimuli.21 Circulating endothelial-derived microparticles (EMPs) depending on their origin (apoptotic-derived or activated-endothelial cell production) are capable of transferring biological information (regulating peptides and hormones) or even genetic material, as well as proteins and lipid components, from one cell to another without direct cell-to-cell contact to maintain cell homeostasis.22,23 EMPs derived from activated endothelial cells may have pro-angiogenic and cardio-protective properties.24 In opposite, apoptotic-derived EMPs originated from damaged endothelial cells are discussed as a marker of endothelial cell injury and vascular aging.25
Platelet-derived microparticles (PMPs) are heterogeneous population of microvesicles that are secreted from chemokine and cytokine activated platelets. PMPs mediate multiple cellular responses that predominantly affected protein and lipid metabolism, coagulation, and inflammation.26 Elevated PMPs show a relation to clinical outcomes and mortality in several patient populations.27
Numerous studies have shown that monocyte-derived microparticles (MMPs) are realized from activated and/or apoptotic monocytes in response to various stimuli, that is, antigen stimulation, growth factors, inflammatory interleukins, chemokines and cytokines, and so on.28–30 Elevated level of circulating MMPs is documented in almost all thrombotic diseases, infective, rheumatic and autoimmune diseases, stroke, myocardial infarction, atrial fibrillation as well as in metabolic, ischemia/hypoxia states, and critical conditions.31–33 However, the significance of MPs in MetS patients as an inductor of development and progression of CHF remains controversial. An example of this controversy is that it is still unknown if circulating MPs found in peripheral blood cause injury of endothelium and worsening of CHF whether they are the result of disease progression in response to endothelial dysfunction and vascular dysintegrity.34,35 The aim of the study was to investigate the pattern of circulating EMPs, PMPs, and MMPs in MetS patients with CHF in relation to neurohumoral and inflammatory activation.
Methods
The study retrospectively involved 101 patients with MetS (54 subjects with CHF and 47 patients without CHF) without documented coronary artery stenosis >50% at least of one artery and 35 healthy volunteers who were examined between February 2013 and November 2013. The study was approved by the local ethics committee of State Medical University, Zaporozhye, Ukraine. The study was performed in conformity with the Declaration of Helsinki. All the patients have given their informed written consent for participation in the study.
MetS was diagnosed based on the National Cholesterol Education Program Adult Treatment Panel III criteria.36 Patients were enrolled in the MetS cohort when at least three of the following components were defined: waist circumference ≥90 cm or ≥80 cm in men and women, respectively; high-density lipoprotein (HDL) cholesterol <1.03 mmol/L or <1.3 mmol/L in men and women, respectively; triglycerides (TG) ≥1.7 mmol/L; blood pressure ≥130/85 mmHg or current exposure of antihypertensive drugs; fasting plasma glucose ≥5.6 mmol/L. Subjects with defined T2DM or treatment with oral antidiabetic agents or insulin were not enrolled in the study. Current smoking was defined as consumption of one cigarette daily for 3 months. Anthropometric measurements were made using standard procedures.
Methods for visualization of coronary arteries
Contrast-enhanced multispiral computed tomography angiography was performed for all the patients with dysmetabolic disorder prior to their inclusion in the study on Optima CT660 scanner (GE Healthcare, Milwaukee, USA) using non-ionic contrast Omnipaque (Amersham Health, Ireland).37 Subjects with atherosclerotic lesions >50% of diameter at least of one coronary artery were excluded for further enrollment in the study.
Transthoracic echocardiography
Transthoracic echocardiography was performed according to a conventional procedure on ultrasound scanner ACUSON (SIEMENS, Germany) in B-mode and Tissue Doppler imaging with phased probe of 2.5–5 MHz. Left ventricular (LV) end-diastolic and end-systolic volumes and LV ejection fraction (LVEF) were measured by modified Simpson’s method.38
Calculation of glomerular filtration rate
Glomerular filtration rate (GFR) was calculated using Chronic Kidney Disease Epidemiology Collaboration formula.39
Measurement of circulating biomarkers
To determine circulating biomarkers, blood samples were collected at baseline in the morning (at 7–8 a.m.) into cooled silicone test tubes wherein 2 mL of 5% Trilon B solution were added. Then they were centrifuged upon permanent cooling at 6000 r/min for 3 min. Plasma was collected and refrigerated immediately to be stored at a temperature of −70°C. Serum N-terminal brain natriuretic peptide (NT-proBNP), adiponectin, serum receptor activator of NF-κB ligand (RANKL), and osteoprotegerin (OPG) were measured by high-sensitive enzyme-linked immunosorbent assays using commercial kits (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany) according to the manufacturers’ recommendations. The inter-assay coefficients of variation were as follows: NT-proBNP: 4.5%, adiponectin: 5%, RANKL: 7.0%; and OPG: 8.2%.
High-sensitive C-reactive protein (hs-CRP) was measured by commercially available standard kit (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). The intra-assay and inter-assay coefficients of variation were <5%.
Fasting insulin level was measured by a double-antibody sandwich immunoassay (Elecsys 1010 analyzer; F. Hoffmann-La Roche Diagnostics, Mannheim, Germany). The intra-assay and inter-assay coefficients of variation were <5%. The lower detection limit of insulin level was 1.39 pmol/L.
IR was assessed by the homeostasis model assessment for IR (HOMA-IR)40 using the following formula:
Concentrations of total cholesterol , cholesterol of low-density lipoproteins (LDLs-C), and cholesterol of HDLs-C were measured by enzymatic method.
Assay of circulating EMPs
Circulating MPs were isolated from 5 mL of venous citrated blood drawn from the fistula-free arm. No hemolysis in the samples was found. All samples were not frozen before analysis. To prevent the contamination of samples platelet-free plasma (PFP) was separated from whole blood. PFP was centrifuged at 70,476 × g for 70 min. MP pellets were washed with DMEM (Sigma-Aldrich Chemie Gmbh Munich, Germany) (supplemented with 10 μg/mL polymyxin B, 100 UI of streptomycin, and 100 U/mL penicillin) and centrifuged again (70,476 × g for 90 min).41 The obtained supernatant was extracted, and MP pellets were re-suspended into the remaining 200 μL of supernatant. PFP, MPs, and supernatant were diluted 5-, 10-, and 5-fold in phosphate-buffered saline, respectively. Only 100 μL of supernatant was prepared for further analysis through incubation with different fluorochrome-labeled antibodies or their respective isotypic immunoglobulins (Beckman Coulter, Pasadena, California, USA).
MPs were labeled and characterized by flow cytometry by phycoerythrin-conjugated monoclonal antibody against CD31 (platelet endothelial cell adhesion molecule-1), CD41a, CD64, CD105, CD144 (vascular endothelial-cadherin), CD62E (E-selectin), and Annexin V (BD Biosciences, San Jose, CA, USA) followed by incubation with fluorescein isothiocyanate-conjugated Annexin V (BD Biosciences) per high-definition fluorescence activated cell sorter (HD-FACS) methodology independently after supernatant diluted without freeze.42
The samples were incubated in the dark for 15 min at room temperature according to the manufacturer’s instructions. The analysis of area, height, and width forward scatter (FSC) and side scatter (SSC) parameters as well as side scatter width (SSC-W) was performed. Particle sizing by dynamic light scattering revealed a characteristic size of the MPs (Sigma, St Louis, Missouri, USA). A MPs’ gate was established on the FACS Aria instrument (BD Biosciences, San Jose, CA, USA) by preliminary standardization experiments using a blend of size-calibrated fluorescent beads, with sizes ranging from 0.1 µm to 1.0 µm. Two size gates were defined based on forward angle light scattering from polystyrene microsphere (0.5–0.9 µm) accordingly to the standard protocol. The upper and the outer limit of the MP gate was established just above the size distribution of the 0.9-µm beads in a FSC-A and SSC-A setting (log scale) using the ‘auto-gate’ function. Accordingly, MPs’ gate was defined less than a 0.4 µm polystyrene microsphere extending down to the noise threshold level, that is, equivalent to cell-derived MPs <1 µm diameter. The lower limit was the noise threshold of the instrument, and an absolute minimum threshold of 200 was set at the SSC-A parameter (instead of FSC-A) to avoid exclusion of the smallest events. In order to separate true events from background noise, we defined MPs as particles that were less than 1.0 µm in diameter and expressed cell specific markers.
For each sample, 500,000 events have been analyzed. Compensation tubes were used with similar reagents as were used in the sample tubes. Data were constructed as numerous MPs depending on marker presentation (positive or negative) and determination of MP populations (Figure 1).
Figure 1.
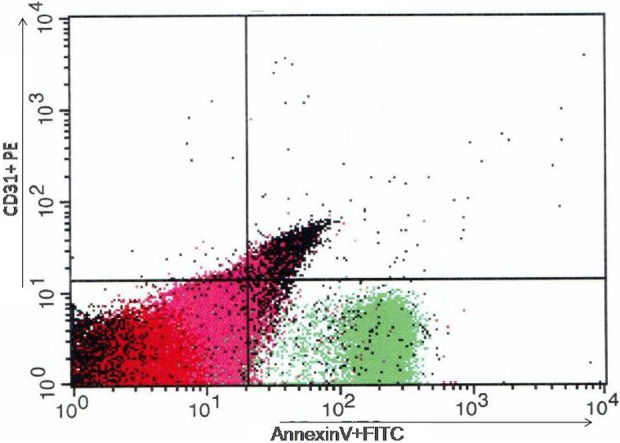
Distribution of MPs according presentation of Annexin V FITS and CD31 PE. The results of flow cytometry analysis. MPs: microparticles
Calculation of the number of MPs per liter plasma was based upon the particle count per unit time, the flow rate of the flow cytometer, and the net dilution during sample preparation of the analyzed MP suspension. MP-exposed antigen concentrations were calculated in each sample by multiplying the total concentration of positive MPs by the mean fluorescence intensity of the antigen exposure of the total positive MP population. The reproducibility of EPCs using standard protocol was 4.5%
Determination of MP populations
CD41a+ was used as a more specific marker of platelets, and CD64+ was considered as a more specific marker of monocytes. CD31 antigen was determined as an essential marker for endothelial cells, platelets, and leukocytes. CD144+ was used to identify a pure population of endothelial cells. CD31+/annexin V+ and CD144+/CD31+/annexin V+ MPs were defined as apoptotic EMPs, MPs labeled for CD105+ or CD62E+ were determined as MPs produced due to activation of endothelial cells.43
Statistical analysis
Statistical analysis of the results obtained was performed in SPSS system for Windows, version 22 (SPSS Inc, Chicago, Illinois, USA). The data were presented as mean (M) and standard deviation (±SD) as well as median (Me) and 25–75% interquartile range (IQR). To compare the main parameters of patient cohorts, two-tailed Student t test or Mann-Whitney U test were used. To compare categorical variables between groups, χ2test and Fisher exact test were used. Univariable and multivariable regression analysis was used for determining the predictors of decreased CD62E+ to CD31+/annexin V+ ratio. All sufficient predictors with p <0.2 obtained by univariable regression analysis were included in the multivariate regression model. A two-tailed probability value of <0.05 was considered as significant.
Results
general characteristic of patients participating in the study was reported in Table 1. There was a significant difference between healthy volunteers and entire patient cohort in body index mass (BMI), waist circumference, cardiovascular risk factors (hypertension, dyslipidemia, and adherence to smoking), CHF class, blood pressure levels, heart rate, LVEF, HOMA-IR, lipid abnormalities, and Framingham risk score. However, MetS patients without CHF have demonstrated lower incidence of dyslipidemia, lower concentrations of LDL-C, hs-CRP, sRANKL, OPG, NT-proBNP compared with MetS subjects with CHF. Therefore, higher LVEF, TG, HDL-C, and HOMA-IR were found in MetS patients without CHF in comparison to MetS patients with CHF.
Table 1.
general characteristic of patients participating in the studya.
| Healthy volunteers (n = 35) | Entire cohort of enrolled MetS patients (n = 101) | MetS patients without CHF (n = 47) | MetS patients with CHF (n = 54) | |
|---|---|---|---|---|
| Age (years) | 46.12 ± 4.22 | 48.34 ± 7.80 | 48.30 ± 3.94 | 48.42 ± 6.10 |
| males (n (%)) | 23 (65.7%) | 64 (63.3%) | 30 (63.8%) | 34 (63.0%) |
| BMI (kg/m2) | 21.5 (16.1–23.5) | 28.4 (16.5–32.4)b | 28.2 (16.7–31.0) | 28.5 (16.8–32.1) |
| Waist circumference (sm) | 78 (63–89) | 93 (76–103)b | 92 (77–105) | 95 (90–104) |
| Hypertension (n (%)) | – | 68 (67.3%)b | 32 (68.0%) | 36 (66.7%) |
| I NYHA class CHF | – | 17 (16.8%)b | – | 17 (31.5%)c |
| II NYHA class CHF | – | 22 (21.9%)b | – | 22 (40.7%)c |
| III NYHA class CHF | – | 15 (14.9%)b | – | 15 (27.8%)c |
| Dyslipidemia (n (%)) | – | 59 (58.4%)b | 26 (55.3%) | 33 (61.1%)c |
| Adherence to smoking (n (%)) | 6 (17.1%) | 31 (30.7%)b | 16 (34.0%) | 15 (27.7%) |
| Framingham risk score (%) | 2.55 ± 1.05 | 8.12 ± 2.88b | 8.09 ± 2.12 | 9.28 ± 2.32 |
| Systolic BP (mm Hg) | 122 ± 5 | 138 ± 6b | 137 ± 4 | 139 ± 5 |
| Diastolic BP (mm Hg) | 72 ± 4 | 87 ± 6b | 87 ± 5 | 88 ± 4 |
| Heart rate, beats per 1 min | 66 ± 6 | 75 ± 7b | 71 ± 6 | 78 ± 5 |
| LVEF (%) | 66.8 (61.2–73.5) | 50.6 (42.5–55.3)b | 52.4 (48.3–57.5) | 44.2 (40.3–48.1)c |
| GFR (mL/min/1.73 m2) | 102.1 (91.4–113.2) | 93.1 (79.5–109.7) | 92.5 (83.1–107.4) | 93.8 (80.4–106.8) |
| HbA1c (%) | 4.75 (4.36–5.12) | 6.7 (5.3–8.2)b | 6.82 (5.61–8.37) | 6.64 (5.53–8.31) |
| fasting blood glucose (mmol/L) | 4.52 (4.43–4.76) | 6.50 (5.8–7.0)b | 6.46 (5.73-6.86) | 6.54 (5.69–6.98) |
| Insulin (µU/mL) | 4.98 (1.5–14.1) | 15.45 (13.69–16.62)b | 15.2 (12.5–15.7) | 15.6 (12.9–16.8) |
| HOMA-IR (mmol/L × µU/mL) | 1.01 (0.91–1.07) | 4.46 (4.17–5.20)b | 4.36 (4.12–5.18) | 4.53 (4.11–5.12) |
| creatinine (μmol/L) | 62.1 (55.7–82.4) | 71.2 (59.9–87.2) | 70.5 (59.6–88.3) | 72.3 (56.1–86.9) |
| Total cholesterol (mmol/L) | 4.76 (4.21–5.05) | 5.3 (4.6–6.0)b | 5.3 (4.5–5.9) | 5.4 (4.8–5.8) |
| LDL-C (mmol/L) | 3.10 (2.78–3.21) | 3.60 (3.20–4.18)b | 3.48 (3.30–4.07) | 3.80 (3.20–4.20)c |
| HDL-C (mmol/L) | 1.13 (1.05–1.17) | 0.94 (0.92–1.06)b | 1.01 (0.90–1.13) | 0.94 (0.88–1.04) |
| TG (mmol/L) | 1.18 (1.07–1.30) | 1.68 (1.44–1.98)b | 1.77 (1.62–1.95) | 1.45 (1.42–1.51)c |
| hs-CRP (mg/L) | 4.11 (0.97–5.03) | 7.96 (4.72–9.34)b | 7.80 (4.92–9.43) | 8.13 (5.90–10.85)c |
| sRANKL (pg/mL) | 16.10 (2.1–30.1) | 29.10 (15.2–56.7)b | 24.10 (14.7–36.9) | 34.20 (20.1–55.2)c |
| OPG, (pg/mL) | 88.3 (37.5–136.6) | 804.5 (579.9–1055.3)b | 718.5 (572.1–846.2) | 882.5 (697.1–1046.2)c |
| Adiponectin (mg/L) | 6.17 (3.44–10.15) | 13.65 (10.12–24.93)b | 13.61 (9.74–22.35) | 14.12 (10.12–23.10) |
| NT-proBNP (pg/mL) | 96.1 (64.5–125.8) | 687.5 (84.7–1244.5)b | 92.2 (55.8–133.2) | 1475.3 (584.7–2293.5)c |
SE: standard error; IQR: inter quartile range; BMI: body mass index: TG: triglycerides, BP: blood pressure; BMI: body mass index: CHF: chronic heart failure; LVEF: left ventricular ejection fraction; GFR: glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; hs-CRP: high-sensitive C reactive protein; sRANKL: serum receptor activator of NF-κB ligand; MetS: metabolic syndrome; OPG: osteoprotegerin.
aData are presented as mean and ± SE; median and 25–75% IQR. Categorical variables are expressed as numerous (n) and percentages (%). P-value is a comparison of mean or median variables (ANOVA test).
bsignificant difference between healthy subjects and entire cohort of enrolled MetS patients.
cSignificant difference between MetS subjects with and without CHF.
Table 2 reports the numbers of circulating MPs in patients participating in the study. Numerous PMPs and MMPs with immune phenotypes labeled as CD41a+ and CD64+ were similar in healthy volunteers and entire patient cohort. Controversially, there is lower circulating level of activated EMPs with phenotype CD62E+ and CD105E+ in MetS patients compared with healthy volunteers (p < 0.001 for all cases). There were no significant differences between numbers of circulating MPs labeled as CD144+ and CD144+/CD31+ originated from endothelial cells obtained from healthy volunteers and MetS patients. Although circulating levels of Annexin V+, CD144+/annexin V+, and CD144+/CD31+/annexin V+ MPs derived from apoptotic cells including endothelial cells were similar in both cohorts, CD31+/annexin V+ MPs were significantly elevated in MetS patient (p < 0.001). CD62E+ to CD31+/annexin V+ ratio was significantly elevated in healthy persons when compared with MetS patients (p < 0.001), while CD105E+ to CD31+/annexin V+ ratio was not. Interestingly, similarities of circulating levels of MPs different origin were determined in both MetS patient cohorts apart from CD31+/annexin V+ MPs. Therefore, CD62E+ to CD31+/annexin V+ ratio was found to be higher in the MetS patients without CHF compared with MetS patients with CHF.
Table 2.
Numbers of microparticles in participators of the studya.
| Immune phenotype of MPs | Healthy volunteers (n = 35) | Entire cohort of enrolled MetS patients (n = 101) | MetS patients without CHF (n = 47) | MetS patients with CHF (n = 54) |
|---|---|---|---|---|
| CD41a+ MPs (n/µL) | 23 (19–28) | 25 (16–33) | 23 (15–31) | 27 (19–36) |
| CD64+ MPs (n/µL) | 3.9 (3.5–4.6) | 4.2 (3.2–5.1) | 4.0 (3.4–4.8) | 4.3 (3.6–5.2) |
| CD62E+ MPs (n/µL) | 1.35 (0.95–1.68) | 1.03 (0.86–1.13)b | 1.05 (0.88–1.18) | 0.98 (0.89–1.12) |
| CD105E+ MPs (n/µL) | 2.32 (1.92–2.56) | 2.24 (1.85–2.41)b | 2.37 (1.92–2.68) | 2.09 (1.58–2.50) |
| CD144+ MPs (n/µL) | 0.29 (0.22–0.36) | 0.33 (0.24–0.39) | 0.30 (0.22–0.37) | 0.35 (0.21–0.40) |
| CD144+/CD31+ MPs (n/µL) | 0.87 (0.27–1.25) | 0.92 (0.36–1.32) | 0.89 (0.32–1.29) | 0.93 (0.41–1.33) |
| Annexin V+ MPs (n/µL) | 4655 (3724–6237) | 5495 (3988–6957) | 5114 (3695–6547) | 5844 (4213–7167) |
| CD144+/annexin V+ MPs (n/µL) | 0.95 (0.11–1.78) | 1.15 (0.13–2.41) | 1.08 (0.13–2.39) | 1.17 (0.15–2.55) |
| CD144+/CD31+/annexin V+ MPs (n/µL) | 0.82 (0.27–1.55) | 1.01 (0.39–1.70) | 0.94 (0.38–1.52) | 1.12 (0.40–1.67) |
| CD31+/annexin V+ MPs (n/µL) | 0.154 (0.03–0.21) | 0.316 (0.261–0.374)b | 0.285 (0.253–0.318) | 0.355 (0.294–0.382)c |
| CD62E+ to CD31+/annexin V+ ratio, unit | 8.77 (7.95–9.18) | 3.26 (3.23–3.30)b | 3.68 (3.47–3.81) | 2.76 (2.42–3.04)c |
| CD105E+ to CD31+/annexin V+ ratio, unit | 15.1 (8.59–23.4) | 7.07 (4.85–10.90) | 8.31 (6.02–10.65) | 5.89 (4.11–7.67) |
IQR: inter quartile range; MPS: microparticles; MetS: metabolic syndrome; CHF: chronic heart failure;
aData are presented as median and 25–75% IQR. P-value is a comparison of mean or median variables between both cohorts (ANOVA test).
bSignificant difference between healthy subjects and entire cohort of enrolled patients.
cSignificant difference between MetS subjects with and without CHF.
There was weak correlation between numerous CD31+/annexin V+ MPs and BMI (r = 0.27, p = 0.001), NT-proBNP (r = 0.26, p = 0.001), OPG (r = 0.26, p = 0.001), hs-CRP (r = 0.25, p = 0.001), and Framingham risk score (r = 0.21, p = 0.001). Numerous CD62E+ MPs correlated positively with BMI (r = 0.22, p = 0.001), waist circumference (r = 0.22, p < 0.001), and negatively with OPG (r = −0.23, p = 0.001), hs-CRP (r = −0.21, p = 0.001), smoking (r = −0.20, p = 0.001).
There was correlation between CD62E+ to CD31+/annexin V+ ratio, cardiovascular risk factors, hemodynamic performances, and other biomarkers. We found that CD62E+ to CD31+/annexin V+ ratio was directly related with NT-proBNP (r = −0.512, p = 0.001), BMI (r = 0.46, p = 0.001), OPG (r = −0.412, p = 0.001), hs-CRP (r = −0.445, p = 0.001), HOMA-IR (r = −0.414, p = 0.001), eGFR (r = 0.312, p = 0.001), TG (r = −0.304, p = 0.001), dyslipidemia (r = −0.248, p = 0.001), creatinine (r = −0.242, p = 0.001), Framingham risk score (r = −0.23, p = 0.001), waist circumference (r = 0.23, p < 0.001), gender (r = 0.228, p < 0.001 for male), age (r = −0.225, p = 0.001), and smoking (r = −0.212, p = 0.001). Therefore, CD62E+ to CD31+/annexin V+ ratio is associated positively with numerous of MetS components (r = 0.42, p = 0.003).
No significant association CD62E+ to CD31+/annexin V+ ratio with fasting plasma glucose, HbA1c, means of systolic and diastolic BP was found. We did not find possible age- and gender-related correlation between metabolic status and the presence of EMPs.
By multivariate regression analyses, NT-proBNP (β coefficient = −0.42, p = 0.012), OPG (β coefficient = −0.32, p = 0.026), hs-CRP (β coefficient = −0.21, p = 0.044), and BMI (β coefficient = 0.142, p = 0.036) were found as independent factors to decrease of CD62E+ to CD31+/annexin V+ ratio (Table 3).
Table 3.
Univariable and multivariable associations with decrease of CD62E+ to CD31+/annexin V+ ratio.a
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| β coefficient | p value | β coefficient | p value | |
| Framingham risk score (%) | −0.014 | 0.34 | – | – |
| eGFR | 0.012 | 0.22 | – | – |
| HOMA-IR | 0.018 | 0.26 | – | – |
| Waist circumference | 0.052 | 0.38 | – | – |
| BMI | 0.16 | 0.046 | 0.142 | 0.036 |
| NT-proBNP | −0.46 | 0.001 | −0.42 | 0.012 |
| OPG | −0.36 | 0.001 | −0.32 | 0.026 |
| hs-CRP | −0.28 | 0.001 | −0.21 | 0.044 |
| Adiponectin | −0.015 | 0.22 | – | – |
| TG | −0.032 | 0.42 | – | – |
| Creatinine | −0.025 | 0.36 | – | – |
BMI: body mass index; eGFR: estimated glomerular filtration rate; HOMA-IR: homeostasis model assessment for insulin resistance; NT: proBNP: N-terminal pro-brain natriuretic peptide; hs-CRP: high-sensitive C-reactive protein; TG: triglycerides; OPG: osteoprotegerin
aThe multivariate regression model included all variables with p < 0.2.
Discussion
The results of the study have shown that circulating numerous of PMPs and MMPs in subjects with MetS (with or without CHF) insufficiently distinguished from level obtained in healthy volunteers. We found elevated level of apoptotic EMPs labeled CD31+/annexin V+ MPs in association with lower level of activated endothelial cell-derived MPs phenotyped as CD62E+ MPs. All these lead to decreased CD62E+ to CD31+/annexin V+ ratio among patients with MetS in comparison with healthy volunteers as well as in MetS patients with CHF compared with those who did not demonstrate CHF. Thus, development of CHF in MetS patients was closely related to altered balance between activated EMPs and apoptotic endothelial cell-derived MPs. This phenomenon was described as impaired phenotype of circulating MPs that might probably preexist CHF and appeared to be clinically significant endothelial dysfunction.20 Whether impaired phenotype of EMPs is the result of early stages of endothelial injury due to neurohumoral and inflammatory activation associated with dysmetabolic states or CHF development or circulating MPs that are able to directly induce endothelial dysintegrity is still not fully clear.35,44
Indeed, the ability of endothelium to release activated EMPs with pro-angiogenic capacity may have a causality role in improving the clinical outcomes in CHF subjects with known MetS in comparison to none MetS subjects.44 Interestingly, circulating numbers of MPs that are phenotypically nearly identical to CD31+/annexin V+ MPs were closely associated with cardiovascular risk factors, while they were not elevated in dysmetabolic disorders without known atherosclerosis or/and cardiovascular diseases.43–47 Probably, subpopulations of MPs labeled as annexin V+ are not sensitive marker of early endothelial injury and this requires performing measurements of double- and triple-labeled annexin V+ MPs, such as CD31+/annexin V+ MPs. The results of the study report that numerous CD31+/annexin V+ MPs are not only elevated in MetS patients but also they increase sufficiently in CHF development in MetS population. Therefore, NT-proBNP, OPG, hs-CRP, and BMI independently predicted decrease of CD62E+ to CD31+/annexin V+ ratio reflected impaired immune phenotype in MetS with and without CHF.
We suggested that decreased CD62E+ to CD31+/annexin V+ ratio and probably elevated apoptotic EMPs level may discuss surrogate markers of vascular dysfunction at early stages in MetS patients with high risk of CHF development. In fact, apoptotic EMPs play a pivotal role in the development of vascular complications in MetS and diabetes through promoting various processes, that is, coagulation, thrombosis, and angiogenesis.47,48 In contrast, activated EMPs may avoid inducing tissue injury and worsening vasomotion via genome involved mechanisms, and they are able to protect the endothelium from damage. Therefore, PMPs and leukocyte-derived MPs have probably not sufficient effect on vascular integrity and vascular complications among MetS.49 These findings support our hypothesis that imbalance between activated and apoptotic EMPs may predict CV diseases and events in general population and patients with known T2DM and MetS.50,51
Surprisingly, in our study, independent associations of CD62E+ to CD31+/annexin V+ ratio with cardiovascular risk factors were not found, while association of TG and lipid abnormality with CD62E+ to CD31+/annexin V+ ratio was shown. A recent study has shown that dyslipidemia and especially increased TG level in MetS patient populations may have a negative effect on ability of endothelium to produce activated macrovesicles with angiogenic capacities and secreted apoptotic-derived MPs.52,53 Therefore, it is discussed the question regarding dyslipidemia-induced apoptotic-related EMPs production.54 In fact, infiltration of subintima by LDL may induce production of free radicals, oxidation of cytockeleton, and membrane vesiculation of endothelial cells.55 The oxidative-driven vesiculation of endothelial cells may relate to low intensity inflammation in vasculature, which associates with over production of cytokines, that is, hs-CRP, adiponectin, and OPG.56 Moreover, membrane vesiculation may enhance inflammatory cytokines in convey of biomechanical stress.57 As well-known hs-CRP and OPG appear to be sufficiently increased in MetS and they may be compensatory up-regulated in the atherosclerosis and microvascular inflammation.58 Therefore, there was NT-proBNP-dependent regulation of microvesiculation in endocardial endothelium.59 The clinical significance of this phenomenon is still not clear and planned/ongoing clinical studies with large sample population are absent.60
Although initially there was skepticism regarding origin of imbalance of activated and apoptotic EMP in patients with impaired glucose metabolism and dyslipidemia, we suppose that inflammatory cytokine over production and probably lipid abnormalities may consider a possible cause of predominantly immune phenotype of MPs not directly related with glucose impairment and other parameters of MetS. Obviously, patients with different types of dysmetabolic disorders might have different patterns of MPs,61 which contribute the development of CHF.62,63 Thus, pattern of MPs correlates with parameters usually used in the characterization of HF, including BNPs, and authors have believed that it presents any advantages over the currently used biomarkers to stratify the patients at the risk of negative clinical outcomes. Importantly, decreased CD62E+ to CD31+/annexin V+ ratio reflected impaired immune phenotype of MPs beyond MetS parameters and other traditional CV risk factors. Finally, determination of impaired phenotype of EMPs appears to be as novel biological marker of CHF development in MetS population.
Study limitations
This study has some limitations. The first limitation is the lack of standardization of MP measurements, while commercial flow cytometers are existed. This study is specifically assessing MPs between 50 and 1000 nm that might also include exosomes (50–>100 nm), small apoptotic bodies (<1000 nm) and other microvesicles originated from various cells. At the same time, exosomes are not able to express Annexin antigen, while they are defined as CD 63+ CD9+ microvesicles. Because exosomes and MPs are often released concomitantly, differentiation of these two microvesicular species might be difficult and this is a study limitation. It is necessary to note that a large pool of MPs might be produced after blood sampling due to destruction of platelets and blood cells. In this study, we used platelet free plasma to prevent the contamination of samples with MPs originated from platelets. Therefore, preparation of MP isolates from samples is the most sophisticated step for further examination. The next limitation might relate to complicated assay and suffers from resolution of MP detection technique that is worth considering. Indeed, there were several technical-related difficulties in the measurement of MPs affected centrifugation of samples, labeling of MPs, using HD-FACS methodology and final assay of results obtained. Although HD-FACS methodology is widely used, theoretically overlap between two or more fluorochromes might reflect some obstacles for further interpretation of obtained results, especially including size gating in MP determination. Therefore, rotor type and centrifugation time theoretically may influence on purity of extracellular vesicles. Overall, the definition of a blood MP using flow cytometry is still an area of great debate. However, flow cytometry is commonly used procedure, while it is not standardized and is difficult for use.
Additionally, we cannot discuss whether CD62E+ to CD31+/annexin V+ ratio is better than traditional measures (BNP, and so on.) at predicting patient outcomes, because the design of clinical study does not correspond with clinical events’ evaluation. The advantage of CD62E+ to CD31+/annexin V+ ratio over the currently used biomarkers requires to be reassayed in the large clinical trial in the future. Another limitation of this study is that a specific role of MPs is also possible and has not been characterized in depth in MetS patients. However, the authors suppose that these optionally technically restrictions might have no significant impact on the study data interpretation. Additionally, retrospective, relatively small sample size may limit the significance of this study.
In conclusion, decreased CD62E+ to CD31+/annexin V+ ratio reflected impaired immune phenotype of MPs might discuss a surrogate marker of CHF development in MetS population. Biomarkers of biomechanical stress (NT-proBNP) and inflammation (hs-CRP, OPG) were found as significant predictors for decreased CD62E+ to CD31+/annexin V+ ratio in MetS patients especially with CHF.
Acknowledgments
We thank all patients for their participation in the investigation, staff of the Regional Zaporozhye Hospital (Ukraine), and the doctors, nurses, and administrative staff in Regional Center of cardiovascular diseases (Zaporozhye, Ukraine) and City Hospital # 6 (Zaporozhye, Ukraine), general practices, and site-managed organizations that assisted with the study.
Footnotes
Authors’ Contributions: Alexander E Berezin initiated the hypothesis and designed the study protocol, contributed to collect, analyze, and interpret the data, performed statistical analysis, and wrote the manuscript. Alexander A Kremzer contributed to enroll the patients, collected and analyzed the data, reviewed the source documents, and drafted the article. Tatyana A Berezina contributed to the study protocol design, enrolled the patients in the study, collected the data, analyzed and interpreted the data obtained, and drafted the article. Yulia V Martovitskaya performed biomarker measurements, including determination of MPs, and analyzed and interpreted the data received by flow cytometry. All authors revised the manuscript critically, had consolidated agreement to be accountable for all aspects of the work, and finally approved the version to be published.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ogbera AO. Prevalence and gender distribution of the metabolic syndrome. Diabetol Metab Syndr 2010; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120(16): 1640–1645. [DOI] [PubMed] [Google Scholar]
- 3. Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev 2008; 29(7): 777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vidigal Fde C, Ribeiro AQ, Babio N, et al. Prevalence of metabolic syndrome and pre-metabolic syndrome in health professionals: LATINMETS Brazil study. Diabetol Metab Syndr 2015; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005; 112(20): 3066–3072. [DOI] [PubMed] [Google Scholar]
- 6. McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care 2005; 28(2): 385–390. [DOI] [PubMed] [Google Scholar]
- 7. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004; 27: 813–823. [DOI] [PubMed] [Google Scholar]
- 8. Brasil AR, Norton RC, Rossetti MB, et al. C-reactive protein as an indicator of low intensity inflammation in children and adolescents with and without obesity. J Pediatr 2007; 83(5): 477–480. [DOI] [PubMed] [Google Scholar]
- 9. Petersson H, Daryani A, Risérus U. Sagittal abdominal diameter as a marker of inflammation and insulin resistance among immigrant women from the Middle East and native Swedish women: a cross-sectional study. Cardiovasc Diabetol 2007; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jia G, Sowers JR. Endothelial dysfunction potentially interacts with impaired glucose metabolism to increase cardiovascular risk. Hypertension 2014; 64(6): 1192–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zaghloul A, Al-Bukhari TA, Al-Pakistani HA, et al. Soluble endothelial protein C receptor and high sensitivity C reactive protein levels as markers of endothelial dysfunction in patients with type 1 and type 2 diabetes mellitus: their role in the prediction of vascular complications. Diabetes Res Clin Pract 2014; 106(3): 597–604. [DOI] [PubMed] [Google Scholar]
- 12. Rezende FAC, Rosado LEFPL, Ribeiro RCL, et al. Body mass index and waist circumference: association with cardiovascular risk factors. Arq Bras Cardiol 2006; 87(6): 728–734. [DOI] [PubMed] [Google Scholar]
- 13. Rankinen T, Sarzynski MA, Ghosh S, et al. Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors? Circ Res 2015; 116(5): 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carnethon MR, Loria CM, Hill JO, et al. Risk factors for the metabolic syndrome: the Coronary Artery Risk Development in Young Adults (CARDIA) study 1985–2001. Diabetes Care 2004; 27(11): 2707–2715. [DOI] [PubMed] [Google Scholar]
- 15. Ahmadi A, Leipsic J, Feuchtner G, et al. Is metabolic syndrome predictive of prevalence, extent, and risk of coronary artery disease beyond its components? Results from the multinational coronary CT angiography evaluation for Clinical Outcome: An International Multicenter Registry (CONFIRM). PLoS One 2015; 10(3): e0118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perrone-Filardi P, Savarese G, Scarano M, et al. Prognostic impact of metabolic syndrome in patients with chronic heart failure: data from GISSI-HF trial. Int J Cardiol 2015; 178: 85–90. [DOI] [PubMed] [Google Scholar]
- 17. Santulli G. β-Blockers in diabetic patients with heart failure. JAMA Intern Med 2015; 175(4): 657. [DOI] [PubMed] [Google Scholar]
- 18. Barteneva NS, Fasler-Kan E, Bernimoulin M, et al. Circulating microparticles: square the circle. BMC Cell Biol 2013; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markiewicz M, Richard E, Marks N, et al. Impact of endothelial microparticles on coagulation, inflammation, and angiogenesis in age-related vascular diseases. J Aging Res 2013; 2013: 734509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berezin AE. Impaired pattern of endothelial derived microparticles in heart failure patients. J Mol Genet Med 2015; 9: 1. [Google Scholar]
- 21. Wu ZH, Ji CL, Li H, et al. Membrane microparticles and diseases. Eur Rev Med Pharmacol Sci 2013; 17(18): 2420–2427. [PubMed] [Google Scholar]
- 22. Tetta C, Bruno S, Fonsato V, et al. The role of microvesicles in tissue repair. Organogenesis 2011; 7(2): 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez MC, Andriantsitohaina R. Microparticles in angiogenesis: therapeutic potential. Circ Res 2011; 109: 110–119. [DOI] [PubMed] [Google Scholar]
- 24. Rautou PE, Vion AC, Amabile N, et al. Microparticles, vascular function, and atherothrombosis. Circ Res 2011; 109(5): 593–606. [DOI] [PubMed] [Google Scholar]
- 25. Kurtzman N, Zhang L, French B, et al. Personalized cytomic assessment of vascular health: evaluation of the vascular health profile in diabetes mellitus. Cytometry B Clin Cytom 2013; 84(4): 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milioli M, Ibáñez-Vea M, Sidoli S, et al. Quantitative proteomics analysis of platelet-derived microparticles reveals distinct protein signatures when stimulated by different physiological agonists. J Proteomics 2015; 121: 56–66. [DOI] [PubMed] [Google Scholar]
- 27. Ohuchi M, Fujino K, Kishimoto T, et al. Association of the plasma platelet-derived microparticles to platelet count ratio with hospital mortality and disseminated intravascular coagulopathy in critically ill patients. J Atheroscler Thromb 2015; 22: 773–782. [DOI] [PubMed] [Google Scholar]
- 28. Nomura S, Shimizu M. Clinical significance of procoagulant microparticles. J Intensive Care 2015; 3(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ Res 2012; 110: 356–369. [DOI] [PubMed] [Google Scholar]
- 30. Burnier L, Fontana P, Kwak BR, et al. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost 2009; 101: 439–451. [PubMed] [Google Scholar]
- 31. Hjuler Nielsen M, Irvine H, Vedel S, et al. Elevated atherosclerosis-related gene expression, monocyte activation and microparticle-release are related to increased lipoprotein-associated oxidative stress in familial hypercholesterolemia. PLoS One 2015; 10(4): e0121516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ederhy S, Di Angelantonio E, Mallat Z, et al. Levels of circulating procoagulant microparticles in nonvalvular atrial fibrillation. Am J Cardiol 2007; 100: 989–994. [DOI] [PubMed] [Google Scholar]
- 33. Suades R, Padró T, Alonso R, et al. Lipid-lowering therapy with statins reduces microparticles shedding from endothelium, platelets and inflammatory cells. Thromb Haemost 2013; 110: 366–377. [DOI] [PubMed] [Google Scholar]
- 34. Boulanger CM, Scoazec A, Ebrahimian T, et al. , Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 2001; 104: 2649–2652. [DOI] [PubMed] [Google Scholar]
- 35. Berezin AE, Kremzer AA, Martovitskaya YV, et al. The predictive role of circulating microparticles in patients with chronic heart failure. BBA Clin 2015; 3: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106(25): 3143–3421. [PubMed] [Google Scholar]
- 37. Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation 2008; 118: 586–606. [DOI] [PubMed] [Google Scholar]
- 38. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358–367. [DOI] [PubMed] [Google Scholar]
- 39. Levey AS, Stevens LA, Schmid CH, et al. for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 41. Cvjetkovic A, Lotvall J, Lasser C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles 2014; 3: 23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A 2010; 77(6): 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lacroix R, Judicone C, Mooberry M, et al. The ISTH SSC Workshop. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. Epub ahead of print 2 April 2013. DOI: 10.1111/jth.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leroyer AS, Tedgui A, Boulanger CM. Microparticles and type 2 diabetes. Diabetes Metab 2008; 34(1): S27–S32. [DOI] [PubMed] [Google Scholar]
- 45. Amabile N, Cheng S, Renard JM, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J 2014; 35(42): 2972–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nomura S. Dynamic role of microparticles in type 2 diabetes mellitus. Curr Diabetes Rev 2009; 5(4): 245–251. [DOI] [PubMed] [Google Scholar]
- 47. Puddu P, Puddu GM, Cravero E, et al. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can J Cardiol 2010; 26(4): 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jimenez JJ, Jy W, Mauro LM, et al. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res 2003; 109(4): 175–180. [DOI] [PubMed] [Google Scholar]
- 49. Arteaga RB, Chirinos JA, Soriano AO, et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol 2006; 98(1): 70–74. [DOI] [PubMed] [Google Scholar]
- 50. Berezin AE. Circulating endothelial-derived apoptotic microparticles as novel perspective biomarker for diabetes. Diabetes Res Treat 2014; 1(4): 117–120. [Google Scholar]
- 51. Berezin AE, Kremzer AA, Samura TA, et al. Inflammatory phenotype of circulating endothelial-derived microparticles in chronic heart failure patients with metabolic syndrome. J Mol Pathophysiol 2015; 4(2): 51–58. [Google Scholar]
- 52. Chironi GN, Boulanger CM, Simon A, et al. Endothelial microparticles in diseases. Cell Tissue Res 2009; 335(1): 143–151. [DOI] [PubMed] [Google Scholar]
- 53. Shantsila E. Endothelial microparticles: a universal marker of vascular health? J Hum Hypertens 2009; 23: 359–361. [DOI] [PubMed] [Google Scholar]
- 54. Müller G, Schneider M, Biemer-Daub G, et al. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal 2011; 23(7): 1207–1223. [DOI] [PubMed] [Google Scholar]
- 55. Camussi G, Deregibus MC, Bruno S, et al. Exosome / microvesiclemediated epigenetic reprogramming of cells. Am J Canc Res 2011; 1: 98–110. [PMC free article] [PubMed] [Google Scholar]
- 56. Poulsen MK, Nybo M, Dahl J, et al. Plasma osteoprotegerin is related to carotid and peripheral arterial disease, but not to myocardial ischemia in type 2 diabetes mellitus. Cardiovas Diabetol 2011; 10: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Chen LM, Liu ML. Microvesicles and diabetic complications - novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacol Sin 2014; 35(4): 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 2010; 107(9): 1047–1057. [DOI] [PubMed] [Google Scholar]
- 59. Montoro-García S, Shantsila E, Marín F, et al. Circulating microparticles: new insights into the biochemical basis of microparticle release and activity. Basic Res Cardiol 2011; 106(6): 911–923. [DOI] [PubMed] [Google Scholar]
- 60. Berezin A, Zulli A, Kerrigan S, et al. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin Biochem 2015; 48: 562–568. [DOI] [PubMed] [Google Scholar]
- 61. Agouni A, Andriantsitohaina R, Martinez MC. Microparticles as biomarkers of vascular dysfunction in metabolic syndrome and its individual components. Curr Vasc Pharmacol 2014; 12(3): 483–492. [DOI] [PubMed] [Google Scholar]
- 62. Berezin AE, Kremzer AA, Samura TA, et al. Immune phenotypes of endothelial-derived microparticles in dysmetabolic patients. J Proteomics Bioinformatics 2015; 8: 060–066. [Google Scholar]
- 63. Berezin AE, Kremzer AA, Samura TA, et al. Apoptotic microparticles to progenitor mononuclear cells ratio in heart failure: relevance of clinical status and outcomes. JCvD 2014; 2(2): 50–57. [Google Scholar]


