Abstract
Since the initial recognition of acquired immunodeficiency syndrome (AIDS) in 1981, an increased burden of cervical cancer was identified among human immunodeficiency virus (HIV)-positive women. Introduction of antiretroviral therapy (ART) decreased risks of opportunistic infections and improved overall survival. HIV-infected women are living longer. Introduction of the human papillomavirus (HPV) vaccine, cervical cancer screening and early diagnosis provide opportunities to reduce cervical cancer associated mortality. In line with 2030 Sustainable Development Goals to reduce mortality from non-communicable diseases, increased efforts need to focus on high burden countries within sub-Saharan Africa (SSA). Despite limitations of resources in SSA, opportunities exist to improve cancer control. This article reviews advancements in cervical cancer control in HIV-positive women.
Keywords: Cervical cancer, Human immunodeficiency virus (HIV), Chemoradiation therapy, Limited resource setting, Cervical cancer screening
Highlights
-
•
The burden of cervical cancer and HIV-infection in women is concentrated in sub-Saharan Africa.
-
•
Cervical cancer control programs among HIV-positive women are highlighted in this review.
-
•
Gaps in knowledge exist in cervical cancer control in HIV-positive women.
1. HIV and cervical cancer: the early years (1980–2000)
The history of human immunodeficiency virus (HIV) and its link with cancer started with the first descriptions of acquired immune deficiency syndrome (AIDS). In August 1981, a report published a link of Kaposi's sarcoma (KS) in the setting of other opportunistic infections and this was soon recognized as a hallmark of AIDS (Durack, 1981). The Center for Disease Control (CDC) identified Non-Hodgkin lymphoma (NHL) as the second most common cancer and included it as part of AIDS defining conditions in 1985. Unlike KS and NHL, the increased incidence of invasive cervical cancer only became evident with time; ultimately, in 1993, cervical cancer became an AIDS defining illness among women with HIV (Centers for Disease Control, 1992).
Early in the HIV era, the story of cervical cancer and its impact on survival of HIV-positive women was overshadowed by the initial focus on men. In 1983, CDC reported AIDS among two women whose only risk factor was sexual exposure to males with diagnosis of AIDS (Centers for Disease Control, 1983). The initial reports on cervical disease in HIV-positive women noted an increased prevalence of human papillomavirus (HPV) disease and cervical dysplasia among HIV-positive women compared to HIV-negative women (Centers for Disease Control, 1990, Henry et al., 1989). In a study of 114 women evaluating cervical dysplasia and cancer, 37 women had cervical cancer under the age of 50 and 7/37 (19%) were HIV-positive and presented with more advanced stage at diagnosis (Maiman et al., 1990). In addition, among otherwise healthy women with HIV, the mean CD4 cell count was 362 cells/μl, which was above the cutoff for AIDS defining illnesses. In the same cohort, the findings supported a more advanced, multifocal and larger lesion of cervical intraepithelial neoplasia (CIN) among the HIV-positive compared to HIV-negative women. These three findings would later get corroborated in larger cohort studies. The longer latency period leading up to the development of cervical cancer, even in immunocompromised women, contributed to the early perception of lower incidence of cervical cancer among HIV-positive women.
Population-based match registry studies provide the best estimates of the increased risk of cervical cancer conferred as result of HIV-associated immune suppression. Studies reported increased cervical cancer among HIV-positive compared to the general population with standardized incidence ratios (SIRs) of 4.2 to 8.9 (Chaturvedi et al., 2009, Engels et al., 2006). It is important to note that countries with an existing national cervical cancer program reported significantly lower numbers of cervical cancer in HIV-positive women. In the North American prospective study on women with HIV, there was no reported significantly increased incidence of invasive cervical cancer in this highly-managed HIV population with a SIR of 1.32 (Massad et al., 2009). An earlier age of diagnosis of cervical cancer among HIV-positive compared to HIV-negative women is a consistent finding across studies. Early in 1984 the first case of AIDS in Africa was reported from Kinshasa in the Democratic Republic of Congo and soon it became apparent the rates of AIDS in Kinshasa were 15 to 30 times higher than those within USA (Mann et al., 1986). The burden of HIV disease in sub-Saharan Africa (SSA) would ultimately exceeded any other part of the world, accounting for nearly two thirds of all HIV cases. By 2001, 20 years after the first report of AIDS, the United Nations Program on HIV/AIDS (UNAIDS) reported an estimated 40 million adults and children were living with HIV (UNAIDS, 2002). In SSA alone there were 28.5 million people infected with HIV and of those 15 million were women ages 15–49. As heterosexual transmission is a key pathway for HIV spread in SSA, younger women were diagnosed with HIV, setting them at increased risk for cervical cancer, particularly as the number of years of immunosuppression lengthened.
The introduction of antiretroviral therapy (ART) led to drastic shift in the presentation of cancer along the progression of HIV disease. The three-drug combination compared to two or one drug therapy resulted in significant increase in CD4 + cell count and decrease in plasma HIV RNA (Collier et al., 1996). Additional studies supported the benefit of multidrug therapy (now known as highly active ART or HAART), but most important beyond improvement in short term benefit the three-drug combination in particular the addition of protease inhibitors, improved overall survival (Palella et al., 1998). By 2003, World Health Organization (WHO) supported the initiation of ART on a global scale for HIV + patients with CD4 + cell count at < 350 cells/mm3 and ultimately extended the recommendation for all HIV-positive patients irrespective of CD4 cell count level (World Health Organization, 2004).
After introduction of HAART, the incidence of KS and NHL declined dramatically, but the decrease in cervical cancer incidence was marginal. The international collaboration on HIV and cancer reported analysis of 23 prospective studies from North America, Europe and Australia on pre-and post- HAART (International Collaboration on HIV and Cancer, 2000). Based on 1992–1996 timeline as the pre-HAART compared to 1997–1999 when HAART use was more widespread, the incidence of KS decreased from 15.2 to 4.9 per 1000 person-years and the incidence rate of NHL decreased from 6.2 to 3.6, but there was no statistically significant change in incidence rates for cervical cancer. Beyond these AIDS-defining cancers, increased incidence of non-AIDS cancers such as anal cancer, liver cancer, lung cancer and Hodgkin lymphoma was recognized. It became apparent that HIV-positive individuals have increased risk of viral-associated cancers. In a meta-analysis of HIV/AIDS and immunosuppressed transplant patients, a similar pattern of cancer risk was detected. Among 444,175 HIV/AIDS patients and 31,977 transplant patients, HPV-associated cancers were increased with SIR for cervical cancer at 5.82 among HIV-positive women compared to 2.13 among transplant recipients (Grulich et al., 2007). In addition, SIR for vulva/vaginal and anal cancers were 6.45 and 28.75 among HIV-positive individuals compared to 22.7 and 4.85 in transplant recipients. Chronic immunosuppression was a shared risk factor increasing long term risk of virus-associated cancers.
2. HIV and cervical cancer control
2.1. Epidemiology of cervical cancer
The burden of HIV and cervical cancer is concentrated in SSA (Fig. 1). The most important risk factor for cervical cancer development is chronic persistent HPV infection. Women with HIV are more likely to have persistent HPV infection leading to cervical abnormalities and cancer. This is particularly important in SSA, which has been most heavily affected by the HIV pandemic. Cervical cancer is one of the most common cancers worldwide especially in low and middle-income countries (LMIC) with about 500,000 new cases around the world and 270,000 deaths (Torre et al., 2015). Cervical cancer is also one of the most common cause of cancer related morbidity and mortality in women around the world, with much of the burden falling on LMICs. Per Globocan 2012, incidence of cervical cancer in the developed world was 7 cases per 100,000 compared to 22 cases per 100,000 populations in the developing world and mortality of 3 cases per 100,000 in the developed world compared to 18 per 100,000 in the developing world. For example, in Botswana, a country in SSA, incidence of cervical cancer is 30 per 100,000 and mortality is 18 per 100,000 (Grover et al., 2015a, Torre et al., 2015). Despite aggressive public health measures to reduce new HIV infection, in 2015, Botswana estimates HIV infection prevalence among adults to be at 22% with 62% of adults on ART (GBD 2015 HIV Collaborators et al., 2016). HIV infected women on HAART can expect to live longer; in high income countries, a 25-year-old women with HIV in 2015 has an estimated median survival to age of 73.9 years and management of chronic conditions such as HPV infection and cervical cancer must be controlled to optimize overall survival (Lohse and Obel, 2016).
Fig. 1.
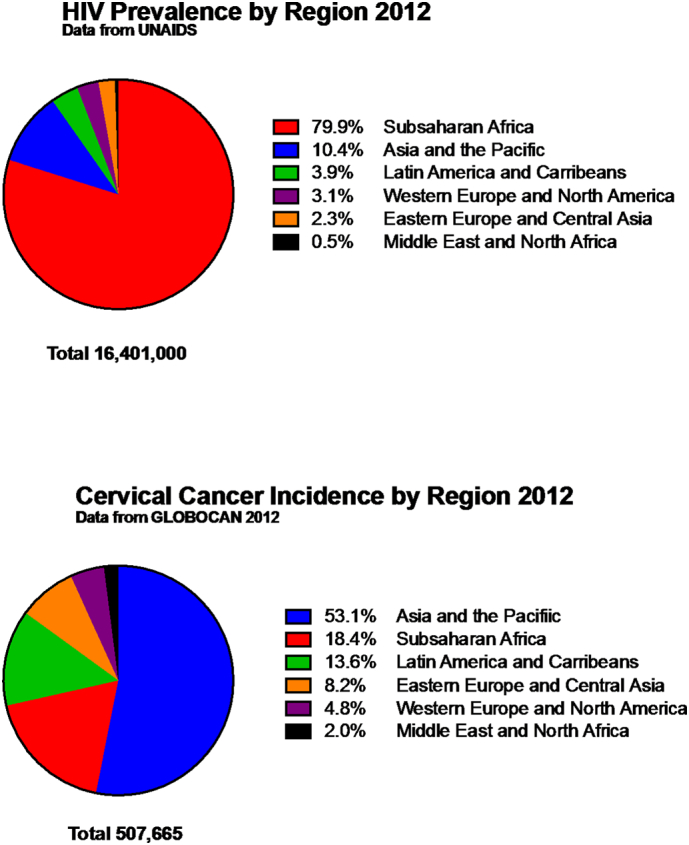
2012 Global burden of HIV and cancer.
3. HPV and HIV
3.1. HPV vaccine
There are currently three HPV vaccines approved by FDA: Gardasil™ (Merck & Co., Inc.), Cervarix™ (GlaxoSmithKline) and Gardasil 9™ (Merck & Co., Inc.) (FUTURE II Study Group, 2007, Joura et al., 2015, Paavonen et al., 2009). All three vaccines prevent infection against HPV16 and HPV 18, two high risk HPV sub-types that contribute up to 70% of cervical cancer (Clifford et al., 2003). In addition, the quadrivalent vaccine covers HPV 6 and HPV 11, which prevent genital warts. The newest addition, the 9-valent vaccine, also expands coverage to HPV 31,33,45,52 and 58. The guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents recommend the HPV vaccination for ages 13 to 26 (Masur et al., 2014). The maximal benefit from HPV vaccine is likely to be gained among HIV-infected but HPV naïve children and the HPV vaccine can be started as early as age 9. There are no HPV vaccine studies on efficacy specific to HIV population, although data from HIV-negative population report high efficacy for prevention of CIN for vaccine specific sub-types. Studies specific to HIV infected population address concerns regarding safety and immunogenicity of the HPV bivalent and quadrivalent vaccine. Among HIV-negative women, the quadrivalent HPV vaccine demonstrated 98% efficacy for preventing type specific CIN and 100% of genital warts, and similar results were also reported with 9-valent HPV vaccine (FUTURE II Study Group, 2007, Joura et al., 2015).
Appropriate antibody response to HPV vaccine is important to establishing long term immunity. The Adolescent Medicine Trials Network for HIV/AIDS Interventions conducted a study comparing antibody response to the quadrivalent HPV vaccine among HIV-positive women aged 16 to 23 years with HPV-negative for vaccine types covered, and found the vaccine was well tolerated (Kahn et al., 2013). Women who were on ART showed 100% seroconversion for HPV 6, 11, 16 and 18 with geometric mean titers (GMTs) equivalent to historical non-infected control; the GMTs were lower among HIV-positive participants not on ART with 7.6% not seroconverting for HPV 18 and 3.6% not seroconverting for HPV 16. The AIDS Clinical Trial Group (ACTG) in their study on the quadrivalent HPV vaccine among women aged 13 to 45 years reported the seroconversion rate was highest among women with CD4 count ≥ 200 cells/μl compared to those ≤ 200 cells/μl (Kojic et al., 2014). The overall seroconversion rate was 75% for all 4 HPV types, and > 90% for women with CD4 count ≥ 200 cells/μl. Among those with low CD4 count ≤ 200 cells/μl, a high HIV RNA viral load ≥ 10,000 copies/ml further reduced the seroconversion rate. The role of low to undetectable HIV viral load as predictor of higher antibody response to vaccinations in HIV infected children and adults has been documented with other vaccines (Overton et al., 2005). Studies have also been conducted on the safety and immunogenicity of quadrivalent vaccine for HIV infected boys and girls ages 7–12, documented safety and high seroconversion rates up to 90–100% (Levin et al., 2010, Weinberg et al., 2012). But there is a lack of data on vaccine uptake in this prenatally HIV infected group who potentially can gain the most from a primary cervical cancer prevention provided by the HPV vaccine.
4. Cervical cancer screening in HIV-infected women
HPV infections are more prevalent and persistent in HIV-infected women (Kojic et al., 2014). In a cohort study on the natural history of HIV, HPV infection with any of 37 high or low risk HPV types was highly prevalent within the cervix (83%) and anal canal (90%). Among HIV-infected women on ART in SSA (Rwanda), testing for 13 high risk types yielded three times higher prevalence rate among HIV-positive (32%) compared to HIV-negative women (8%) (Sinayobye et al., 2014). The majority of HPV infections acquired after initial sexual exposure in immunocompetent women is transient. In a study among HIV positive and negative young women ages 13 to 18, after an initial HPV infection, the mean time to loss of the initial infection was significantly shorter among HIV-negative (403 days) compared to HIV-positive women (689 days). The likelihood of clearance among HIV-positive women decreased with lower CD4 cell count and co-infection with ≥ 2 high-risk HPV types (Moscicki et al., 2004). Even among women with normal range of CD4 count, the HIV infection conferred a higher prevalence of persistent HPV infection. Co-infection of HPV and HIV predisposes women to persistent HPV infection, a known precursor of invasive cancer of lower genital tract. In women, HPV infection is associated with cancer of the cervix, vulva, vagina, anus and oropharynx. As there is no current approved therapeutic HPV vaccine, cervical cancer screening strategies for HIV is critical to long term management of pre-invasive disease.
Multiple cervical cancer screening strategies such cytology based method with Papanicolaou test (Pap test), HPV DNA testing, and visual inspection with acetic acid (VIA) or visual inspection with lugol's iodine (VILI), have been examined in HIV women. The success of any strategy is highly dependent on expanding access to all HIV-infected women, obtaining high follow-up rate from the point of screening through the course of treatment and positioning cervical cancer screening program within the context of comprehensive HIV care. Women's Interagency HIV Study (WIHS) studied the role of Pap test screening, every six months, on the incidence of abnormal cytology and invasive cervical cancer. During 5 year follow up, 73% of HIV-positive women had at least one abnormal Pap test compared to 42% in HIV-negative; the majority of the abnormalities were mild at Atypical Squamous cell of Undetermined Significance (ASCUS) although regression of abnormal findings was significantly lower among HIV-positive vs HIV-negative (Massad et al., 2001). With median follow-up at 10. 3 years, WIHS reported the incidence of invasive cervical cancer was not significantly elevated in HIV-positive women at (21.4 of 100,000 person-years) compared with HIV-negative women (0 of 100,000 person-years; P = 0.59), highlighting the benefit of cervical cancer screening (Massad et al., 2009). The screening schedule as adopted within the WIHS study, Pap test every 6 months is currently not the standard of care nor is this feasible outside of clinical trial. In adolescent women, whether HIV infection is acquired via sexual or perinatal exposure, the first cervical cancer screening is recommended to start within the first year of sexual exposure rather than delay to age 21 as is the guideline for normal risk women. Based on CDC guidelines, for women with newly diagnosed HIV-infection, the first cervical cancer screening is recommended to start at the time of HIV diagnosis with repeat in 6 or 12 months (Kaplan et al., 2009). The length of screening can be extended to every 3 years after three consecutive negative annual screening tests. Women with cytological abnormalities demonstrating ASCUS with positive reflex HPV DNA testing, ASC-H, LSIL or HSIL should undergo colposcopic evaluations. For women older than 30 years, either abnormal cytology or positive HPV testing will also require additional assessment with colposcopy and biopsies as indicated.
In limited resource settings with no established national cervical cancer control, a “see-and-treat” approach has been supported by World Health Organization, as this can triage women to immediate treatment with cryotherapy without additional procedures such as colposcopy and biopsy. Screen and immediate treat also reduce loss to follow-up rate. Two potential strategies, HPV testing and VIA, have been evaluated in the HIV-positive population. VIA studies conducted among HIV-positive women showed good performance with a range of sensitivity at 48–76% and specificity at 69–92% for CIN2 +, although test performance is dependent on quality control of the operator (Akinwuntan et al., 2008, Chibwesha et al., 2016, Firnhaber et al., 2013). Utilizing community engaged process with community health workers, the Zambian VIA program successfully screened > 102,000 women, 28% of them HIV-positive (Parham et al., 2015). A “see-and-treat” program was possible for 87% of participants, HIV-positive women were less likely to be eligible for cryotherapy treatment due to large cervical lesion or risk of invasive cancer. Management of HIV-positive women require special precautions when using a VIA based screening model. Alternatively, HPV testing has high sensitivity with lower specificity for the detection of cervical CIN2 +. Studies among HIV patients conducted in Zambia, South Africa and Tanzania provide a range of sensitivity (88–94%) with specificity (51–82%) (Chibwesha et al., 2016, Dartell et al., 2014, Kuhn et al., 2010). Beyond the test performance, HPV testing offers several advantages: limited training requirements for sample collection, reduced time for sample collection compared to VIA, possibility of self-sampling reducing burden on human and facility resources. Based on the existing health infrastructure, immediate triage to cryotherapy treatment or VIA/VILI followed by treatment is recommended. Certain challenges in HPV primary screening need to be evaluated including issues of cost and same day test and treatment. Implementation studies are needed using point of care HPV testing to increase uptake of screening in HIV-positive women.
5. Treatment of cervical cancer in HIV-infected patients
5.1. Medical management of immunosuppression
Cancer management of HIV-positive patients requires an awareness and management of potential medical complication. The time point of cervical cancer diagnosis may coincide with initial recognition of the HIV infection. Coordination of care can help optimize adherence to ART, monitoring of medication interaction during cancer therapy and psychosocial support of the women. (See Table 1) In cancer patients, the interaction of the tumor and active HIV infection results in significant T cell dysfunction at diagnosis. At the initiation of cancer therapy, baseline values of CD4 + cell count and HIV RNA viral are needed. HIV-positive patients can experience myelosuppression increasing risk of neutropenia related bacterial infection and potential reactivation of dormant viral infections. Chemotherapy drugs can induce immunodeficiency by depletion of CD4 T lymphocytes more so than CD8 T cells (Mackall et al., 1997), and to a lesser extent B cells and natural killer cells. Among patients with AIDS-related lymphoma on HAART, the CD4 T cells count dropped by 50% after 3 months of treatment but recovered within one month post treatment (Powles et al., 2002). In study conducted in South Africa, HIV-positive cervical cancer patients had median CD4 cell count at ≤ 354 cells/μl at the initiation of concurrent chemoradiation (Simonds et al., 2012). Similar values were reported in a study out of Botswana with 10% of patients having CD4 ≤ 200 cells/μl. Risk of opportunistic infections increase with CD4 + cell count ≤ 200 cells/μl (Dryden-Peterson et al., 2016). Guidelines exist regarding initiation of prophylactic medication for opportunistic infections, and include initiation of trimethoprim-sulfamethoxazole double strength for Pneumocystis pneumonia at any CD4 cell count with AIDS defining illness (Kaplan et al., 2009). In addition, HIV care clinics can provide guidance on current primary varicella vaccination, influenza vaccine, pneumococcal vaccine and patients current Hepatitis B immune status.
Table 1.
Antiretroviral drugs in use and common side effects.
| Categories | Drug name (trade name) | Abbreviation | FDA approved | Side effects (potential interaction with chemotherapeutic drugs) |
|---|---|---|---|---|
| Nucleoside/nucleotide reverse transcriptase inhibitors (NRTI/NtRT) | Zidovudine (Retrovir) | AZT | 1987 | Nausea, headache, myalgia, anemia, neutropenia (overlapping neutropenia toxicity with taxanes and antimetabolites, nausea and vomiting with cisplatin and cyclophosphamide) |
| Tenofovir (Viread) | TDF | 2001 | Nausea, diarrhea, headache, renal insufficiency (may need dose adjustment with cisplatin, worsening of nausea and vomiting with cisplatin and cyclophosphamide) | |
| Lamivudine (Epivir) | 3TC | 1995 | Minimal toxicity | |
| Emtricitabine (Emtriva) | FTC | 2003 | Minimal toxicity, hyperpigmentation | |
| Abacavir (Ziagen) | ABC | 1998 | Hypersensitivity reaction (hypersensitivity interaction with taxanes) | |
| Non-nucleoside reverse transcriptase inhibitors (NNRTI) | Efavirenz (Sustiva) | EFV | 1998 | Rash, CNS common (eg, insomnia, vivid dreams, confusion, visual hallucinations, depression), hepatotoxicity (monitoring for chemotherapy drugs requiring liver clearance ifosfamide, gemcitabine and etoposide) |
| Etravirine (Intelence) | ETR | 2008 | Rash, nausea (skin reaction with anthracyclines, antimetabolites) | |
| Nevirapine (Viramune) | NVP | 1996 | Rash, nausea & vomiting, hepatotoxicity (monitoring for chemotherapy drugs requiring liver clearance ifosfamide, gemcitabine and etoposide) | |
| Rilpivirine (Edurant) | RPV | 2011 | Depressive disorders, insomnia, headache, rash | |
| Protease inhibitors (PI) | Atazanavir (Reyataz) | ATV | 2003 | Indirect hyperbilirubinemia, prolonged QT interval, hyperglycemia, skin rash (20%), hyperlipidemia |
| Darunavir (Prezista) | DRV | 2006 | Rash, nausea, diarrhea, hyperlipidemia, hyperglycemia (diarrhea with irinotecan and antimetabolites) | |
| Lopinavir/ritonavir (Kaletra) | LTV | 2000 | Nausea, vomiting, diarrhea, asthenia, hyperlipidemia, hyperglycemia (nausea and vomiting with cisplatin) | |
| Ritonavir (Norvir) | RTV | 1996 | Nausea, vomiting, diarrhea, asthenia, hyperlipidemia, oral paresthesias, hyperglycemia, prolong QT interval (nausea and vomiting with cisplatin) | |
| Integrase inhibitors (II) | Raltegravir (Isentress) | RAL | 2007 | Nausea, diarrhea, headache, CK elevations, myopathy/rhabdomyolysis (rare) |
| Dolutegravir (Tivicay) | DTG | 2013 | Insomnia, Cholesterol and TG elevations, CK elevations, liver enzyme elevations, hyperglycemia | |
| Elvitegravir (Vitekta) | EVG | 2014 | Immune reconstitution syndrome | |
| Chemokine receptor antagonist (CCR5 antagonist) | Maraviroc (Selzentry) | MVC | 2007 | Constipation, dizziness, upper respiratory tract infection, rash, hepatotoxicity |
| Fusion inhibitor (FI) | Enfuvirtide (Fuzeon) | ENF | 2003 | Injection-site reactions (e.g. pain, erythema, induration, nodules), nausea, diarrhea |
5.2. Cervical cancer staging
The guidelines for cervical cancer staging continue to prioritize a clinical rather than imaging based staging, although controversies exist (Jolly et al., 2017). In LMIC where most cervical cancer is diagnosed, an appropriate trained physician can stage cervical cancer by combination of pelvic exam, chest x-ray to exclude lung metastasis and abdominal ultrasound to evaluate for hydronephrosis. Cervical cancer patients with HIV are on average 10 years younger than HIV-negative patients (Dryden-Peterson et al., 2016, Simonds et al., 2015). A high proportion of cervical cancer in LMIC is diagnosed at advanced stage; this reflects an overall lack of cervical cancer screening and early diagnosis. Evaluation utilizing computed tomography (CT) scans and [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography PET/CT scans are utilized with high frequency in staging of cervical cancer in high income countries. Compared to surgical staging, clinical staging is likely to under-stage an estimated 20–30% of early stage disease (Lagasse et al., 1980). In early stage when decisions regarding eligibility for surgical management are under consideration, accurate assessment of pelvic lymph nodes is critical. In a prospective study of PET/CT scan in early-stage disease, sensitivity for nodal involvement was 72% and specificity of 99.7% and these values increased to 100% and 99.6% for nodes > 0.5 cm (Sironi et al., 2006). Based on PET/CT scan finding of extra pelvic disease, potential modification of treatment planning is done utilizing newer modalities such as intensity-modulated radiation therapy (IMRT). Persistent generalized lymphadenopathy among HIV-positive patients can indicate a host of conditions including: reactive hyperplasia, extrapulmonary tuberculosis, opportunistic infections, lymphoma or metastatic cancer. Analysis of PET/CT scan among HIV-positive lymphoma patients have reported high false positive rate and decreased accuracy for patients with HIV plasma RNA viral load > 200 copies/ml (Dunleavy et al., 2010, Mhlanga et al., 2014). In addition, the use of PET/CT scanning as a tool to assess post treatment response may be limited due to lower positive predictive value in HIV infected cancer patients (Dunleavy et al., 2010). Studies on the predictive value of PET/CT scans in HIV-positive cervical cancer staging is lacking.
5.3. Surgical management
Surgery plays a role in the management of early stage cervical cancer and offers some advantages over chemoradiation therapy. A gynecologic oncologist or gynecology surgeon is trained to safely perform radical hysterectomy and lymphadenectomy for cervical cancer. Severe shortage of trained surgical oncologists in many LMIC limit access to life saving procedures for cervical cancer patients. Where there is a shortage of radiation therapy (RT) services, neoadjuvant chemotherapy followed by radical hysterectomy has been evaluated as an alternative approach for limited resource settings (Kim et al., 2013). HIV-positive women with newly diagnosed cervical cancer in early stages are eligible for surgical management and appropriate pre-surgical preparation can optimize the perioperative recovery. Co-infection with hepatitis C virus or hepatitis B virus is common among HIV patients and influences selection of specific ART (Nunez and Soriano, 2005). Baseline pre-surgical evaluation may yield below normal platelets or HIV-associated immune thrombocytopenic purpura (HIV-ITP) (Scaradavou, 2002). Surgical outcome study on 308 HIV-positive patients undergoing abdominoperineal resection for anal cancer reported risk of post-operative hemorrhage at 5.1% compared to 1.5% in HV-negative patients with no difference in rates of post-operative infection (Leeds et al., 2016). Utilizing national records in the US (1994–2007), HIV-infected compared to HIV-negative women undergoing gynecology procedures had increased infection complications and extended length of hospital stay (Penman-Aguilar et al., 2012). Surgical techniques including minimally invasive surgery in gynecologic procedures may decrease risk of post-operative infection.
6. Chemoradiation therapy
Most cervical cancer patients in LMICs present with locally advanced disease and most patients with cervical cancer in LMICs with HIV-infection tend to be younger making mortality in this group even more challenging to cope with (Simonds et al., 2015).
Treatment of locally advanced cervical cancer includes RT, which includes external beam RT and brachytherapy and concurrent cisplatin based-chemotherapy (Chuang et al., 2016, Eifel et al., 2004, Koh et al., 2015). Randomized studies in Western countries have recognized improved survival in patients treated with concurrent chemoradiation compared to RT alone and thus it is warranted to prescribe this treatment to patients in developing countries (Chuang et al., 2016, Eifel et al., 2004, Koh et al., 2015). However, access to RT is limited in LMICs where the burden of cervical cancer and HIV is highest (Grover et al., 2015b).For example, of the 54 countries in Africa, 28 have no radiation capacity altogether and only 20 countries have high-dose or low-dose rate brachytherapy (Grover et al., 2017). As a result, women in settings without access to any RT are treated with neoadjuvant chemotherapy followed by radical hysterectomy and external beam RT with concurrent chemotherapy followed by radical hysterectomy if no brachytherapy is available (Kim et al., 2013).
6.1. Palliative care
Palliative care for cervical cancer patients can be delivered at both low and high resource cancer center and provides the most humanistic care of the cancer patient and her family. Palliative care services facilitate optimal management of the cervical cancer patient integrating a multidisciplinary model including physical and psychosocial needs. Cervical cancer patients with progression of the disease may experience weight loss, fatigue, loss of mobility, malodorous vaginal discharge from tumor necrosis with bacterial overgrowth and vesico-vaginal fistula. Women suffer from pelvic and bone pain as the cancer spreads to the pelvic side wall invading the sciatic nerve or obstructing the ureter outlet leading to hydronephrosis. Based on WHO estimates, 80% of the population globally lack access to appropriate opioid analgesic, there is need to expand access to essential medicine such as morphine (Connor and Bermedo, 2014). The most basic of cancer pain management following the WHO pain ladder can be administered in limited resource setting (World Health Organization, and Reproductive Health, World Health Organization, 2014). Most patients (50–83%) at the end of life prefer to die at home (Gomes et al., 2012). Depending on a nation's health policy and health system resources, as many as 13–85% are dying in the hospital thus failing the wishes of the patient and their family (Pivodic et al., 2016). In SSA, significant strides were made in integration of HIV care within the community including palliative care services and these existing frameworks provide a basis upon which to expand home palliative services to cancer patients.
6.2. Survival outcome
Data regarding treatment outcomes of HIV-infected cervical cancer from Western Countries are limited since HIV-infected patients were excluded from large randomized studies evaluating outcomes of patients receiving RT vs. concurrent chemotherapy and RT. (Eifel et al., 2004, Rose et al., 1999, Rose et al., 2007) Previous studies in cervical cancer outcomes from SSA showed poor treatment completion rates in HIV-positive women, resulting in poorer treatment response compared to HIV-negative patients (Simonds et al., 2012). HIV-positive women had higher rates of grade 3 or 4 hematological toxicities with CRT (Simonds et al., 2015). However, in these studies, the majority of patients were started on ART at treatment initiation for cervical cancer, which may account for worse treatment outcome and tolerability. Other recent studies looking at outcomes of women with or without HIV in modern post-ART era have shown worse survival in HIV-infected women. However, they are limited in adequate treatment information or include women treated with palliative and radical intent (Dryden-Peterson et al., 2016, Ferreira et al., 2017). Perhaps worse outcome seen in these studies is associated with receipt of inadequate treatment due to factors associated with patient's HIV management or physician bias.
In the modern post-ART era, where cervical cancer patients are well managed in regards to their HIV infection, studies from SSA did not note any difference in treatment toxicities for HIV-positive and negative patients treated with CRT (Mdletshe et al., 2016). Similarly, cervical cancer patients who are able to initiate curative intent chemotherapy and RT are found to have no difference in outcome by HIV-status. Factors associated with survival in this group are similar to what has been noted for HIV-negative patients, i.e. total radiation dose, receipt of chemotherapy and baseline hemoglobin levels (Dryden-Peterson et al., 2016).
Further larger studies are needed to study outcomes of cervical cancer patients in post-ART era with robust treatment and toxicity information to truly dissect role of immune dysfunction vs. inability to receive adequate therapy and the factors associated with receipt of inadequate therapy (HIV, physician bias or lack of access) as the cause of poor outcomes of cervical cancer patients with HIV.
6.3. Gaps in knowledge
Large multicenter prospective treatment trials have been lacking in the HIV-positive group of patients. Cohort studies have provided the basis for evidence up until this point. A large International Atomic Energy Agency study recruited in African sites was prematurely halted and preliminary results have not been made available. The AIDS malignancy consortium has been set up to attempt to develop these much-needed collaborative studies and a small Phase I study has been completed looking at chemoradiation feasibility in 3 African sites. The initial results are awaited in 2017.
The current NCI clinical trials database reveals that there are no active treatment studies that either specifically study HIV positive patient outcomes in cervical cancer or allow HIV positivity as an inclusion criteria (National Institute of Health, n.d.). It is particularly concerning that no targeted agents have been, or will be, tested in the short term in this patient population. With the burden of disease carried in developing countries the delay in focusing on this group will have potentially detrimental outcomes for future patients (Table 2).
Table 2.
Clinical and research needs.
| Prevention HPV vaccine uptake among children with HIV at target age group Long-term efficacy of HPV vaccine in HIV-infected women Screening Implementation of HPV DNA based screening in HIV comprehensive care programs Point of care test using HPV DNA for “see and treat” strategies Cervical cancer screening strategies in older HIV-positive women Inclusion of HIV-positive women in studies of novel HPV disease biomarkers Treatment Surgical management of early stage cervical cancer in HIV patients and long term survival data Use of targeted cancer therapeutics and interactions with antiretroviral drugs Large multicenter treatment trials inclusive of HIV patients Adjuvant chemotherapy for high risk cervical cancer HIV-positive women post chemoradiation therapy Neoadjuvant chemotherapy in limited resource setting inclusive of HIV-positive women |
7. Conclusion
The world's attention is turning to management of non-communicable diseases (NCD). The 2030 Sustainable Development Goals on NCD's is to reduce by one third premature mortality from NCD's which extends to cervical cancer, the second most common cause of cancer associated mortality for women (Torre et al., 2015). Many of the initiatives including reducing tobacco exposure, increasing access to vaccinations and increasing access to essential medicines are cross cutting to many NCD's. HIV-infected women as a special population, are at increased risk for cervical cancer. Overall risk of co-morbid conditions will increase with aging of the HIV-positive population. Opportunities for cervical cancer control include implementation of effective HPV vaccination in children with HIV, expansion of cervical cancer screening program in LMIC and early diagnosis and treatment of high grade CIN and cervical cancer. Given the prolonged life expectancy with the advent of ART, opportunities to enhance cervical cancer control in partnership with a comprehensive care approach to HIV disease management ought to be thoroughly explored.
Acknowledgments
Funding
Rahel G. Ghebre is partially funded by Rwanda Human Resources for Health Program which is in part funded by Centers for Disease Control and the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Conflicts of interest
The authors report no conflicts of interest.
References
- Akinwuntan A.L., Adesina O.A., Okolo C.A., Oluwasola O.A., Oladokun A., Ifemeje A.A. Correlation of cervical cytology and visual inspection with acetic acid in HIV-positive women. J. Obstet. Gynaecol. 01 August 2008;28(6):638–641. doi: 10.1080/01443610802259977. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS) - New York. MMWR Morb. Mortal. Wkly Rep. 7 Jan 1983;31(52):697–698. [PubMed] [Google Scholar]
- Centers for Disease Control Risk for cervical disease in HIV-infected women-—New York City. MMWR Morb. Mortal. Wkly Rep. 30 Nov 1990;39(47):846–849. [PubMed] [Google Scholar]
- Centers for Disease Control 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm. Rep. 18 Dec 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- Chaturvedi A.K., Madeleine M.M., Biggar R.J., Engels E.A. Risk of human papillomavirus-associated cancers among persons with AIDS. J. Natl. Cancer Inst. 19 Aug 2009;101(16):1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibwesha C.J., Frett B., Katundu K., Bateman A.C., Shibemba A., Kapambwe S. Clinical performance validation of 4 point-of-care cervical cancer screening tests in HIV-infected women in Zambia. J Low Genit Tract Dis. 01 July 2016;20(3):218–223. doi: 10.1097/LGT.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang L.T., Temin S., Berek J.S. Management and care of women with Invasive Cervical Cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline summary. J. Oncol. Pract. Jul 2016;12(7):693–696. [Google Scholar]
- Clifford G.M., Smith J.S., Aguado T., Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br. J. Cancer. 07 July 2003;89(1):101–105. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A.C., Coombs R.W., Schoenfeld D.A., Bassett R.L., Timpone J., Baruch A. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N. Engl. J. Med. 18 April 1996;334(16):1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- Connor S.R., Bermedo MCS. 2014. Global Atlas of Palliative Care at the End of Life. [Google Scholar]
- Dartell M.A., Rasch V., Iftner T., Kahesa C., Mwaiselage J.D., Junge J. Performance of visual inspection with acetic acid and human papillomavirus testing for detection of high-grade cervical lesions in HIV positive and HIV negative Tanzanian women. Int. J. Cancer. 15 August 2014;135(4):896–904. doi: 10.1002/ijc.28712. [DOI] [PubMed] [Google Scholar]
- Dryden-Peterson S., Bvochora-Nsingo M., Suneja G., Efstathiou J.A., Grover S., Chiyapo S. HIV Infection and Survival Among Women With Cervical Cancer. J. Clin. Oncol. 29 August 2016;34(31):3749–3757. doi: 10.1200/JCO.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy K., Little R.F., Pittaluga S., Grant N., Wayne A.S., Carrasquillo J.A. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 15 April 2010;115(15):3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D.T. Opportunistic infections and Kaposi's sarcoma in homosexual men. N. Engl. J. Med. 10 Dec 1981;305(24):1465–1467. doi: 10.1056/NEJM198112103052408. [DOI] [PubMed] [Google Scholar]
- Eifel P.J., Winter K., Morris M., Levenback C., Grigsby P.W., Cooper J. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J. Clin. Oncol. 01 March 2004;22(5):872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- Engels E.A., Pfeiffer R.M., Goedert J.J., Virgo P., McNeel T.S., Scoppa S.M. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 1 Aug 2006;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- Ferreira M.P., Coghill A.E., Chaves C.B., Bergmann A., Thuler L.C., Soares E.A. Outcomes of cervical cancer among HIV-infected and HIV-uninfected women treated at the Brazilian National Institute of Cancer. AIDS. 20 February 2017;31(4):523–531. doi: 10.1097/QAD.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firnhaber C., Mayisela N., Mao L., Williams S., Swarts A., Faesen M. Validation of cervical cancer screening methods in HIV positive women from Johannesburg South Africa. PLoS One. 2013;8(1):e53494. doi: 10.1371/journal.pone.0053494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 10 May 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- GBD 2015 HIV Collaborators. Wang H., Wolock T.M., Carter A., Nguyen G., Kyu H.H. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 01 August 2016;3(8):361. doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes B., Higginson I.J., Calanzani N., Cohen J., Deliens L., Daveson B.A. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann. Oncol. 01 August 2012;23(8):2006–2015. doi: 10.1093/annonc/mdr602. [DOI] [PubMed] [Google Scholar]
- Grover S., Raesima M., Bvochora-Nsingo M., Chiyapo S.P., Balang D., Tapela N. Cervical cancer in Botswana: current state and future steps for screening and treatment programs. Front. Oncol. 03 November 2015;5:239. doi: 10.3389/fonc.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Xu M.J., Yeager A., Rosman L., Groen R.S., Chackungal S. A systematic review of radiotherapy capacity in low- and middle-income countries. Front. Oncol. 22 Jan 2015;4:380. doi: 10.3389/fonc.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Longo J., Einck J., Puri P., Brown D., Chino J. The unique issues with brachytherapy in low- and middle-income countries. Semin. Radiat. Oncol. 01 April 2017;27(2):136–142. doi: 10.1016/j.semradonc.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich A.E., van Leeuwen M.T., Falster M.O., Vajdic C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 7 Jul 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- Henry M.J., Stanley M.W., Cruikshank S., Carson L. Association of human immunodeficiency virus-induced immunosuppression with human papillomavirus infection and cervical intraepithelial neoplasia. Am. J. Obstet. Gynecol. Feb 1989;160(2):352–353. doi: 10.1016/0002-9378(89)90441-9. [DOI] [PubMed] [Google Scholar]
- International Collaboration on HIV and Cancer Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J. Natl. Cancer Inst. 15 Nov 2000;92(22):1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- Jolly S., Uppal S., Bhatla N., Johnston C., Maturen K. Improving global outcomes in cervical cancer: the time has come for international federation of gynecology and obstetrics staging to formally incorporate advanced imaging. J. Global Oncol. 2017 doi: 10.1200/JGO.2016.007534. (JGO. 2017.007534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joura E.A., Giuliano A.R., Iversen O.E., Bouchard C., Mao C., Mehlsen J. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 19 February 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- Kahn J.A., Xu J., Kapogiannis B.G., Rudy B., Gonin R., Liu N. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin. Infect. Dis. 01 September 2013;57(5):735–744. doi: 10.1093/cid/cit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.E., Benson C., Holmes K.K., Brooks J.T., Pau A., Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR Recomm. Rep. 2009;58(RR-4):1–207. [PubMed] [Google Scholar]
- Kim H.S., Sardi J.E., Katsumata N., Ryu H.S., Nam J.H., Chung H.H. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur. J. Surg. Oncol. 01 February 2013;39(2):115–124. doi: 10.1016/j.ejso.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Koh W.J., Greer B.E., Abu-Rustum N.R., Apte S.M., Campos S.M., Cho K.R. Cervical cancer, version 2.2015. J. Natl. Compr. Cancer Netw. 2015;13(4):404. doi: 10.6004/jnccn.2015.0055. (Apr quiz 404) [DOI] [PubMed] [Google Scholar]
- Kojic E.M., Kang M., Cespedes M.S., Umbleja T., Godfrey C., Allen R.T. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin. Infect. Dis. 01 July 2014;59(1):127–135. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn L., Wang C., Tsai W.Y., Wright T.C., Denny L. Efficacy of human papillomavirus-based screen-and-treat for cervical cancer prevention among HIV-infected women. AIDS. 23 October 2010;24(16):2553–2561. doi: 10.1097/QAD.0b013e32833e163e. [DOI] [PubMed] [Google Scholar]
- Lagasse L.D., Creasman W.T., Shingleton H.M., Ford J.H., Blessing J.A. Results and complications of operative staging in cervical cancer: experience of the gynecologic oncology group. Gynecol. Oncol. 01 February 1980;9(1):90–98. doi: 10.1016/0090-8258(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Leeds I.L., Alturki H., Canner J.K., Schneider E.B., Efron J.E., Wick E.C. Outcomes of abdominoperineal resection for management of anal cancer in HIV-positive patients: a national case review. World J. Surg. Oncol. 2016;14(1) doi: 10.1186/s12957-016-0970-x. (x August 05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M.J., Moscicki A.B., Song L.Y., Fenton T., Meyer W.A., Read J.S. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J. Acquir. Immune Defic. Syndr. 01 October 2010;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse N., Obel N. Update of survival for persons with HIV infection in Denmark. Ann. Intern. Med. 15 November 2016;165(10):749–750. doi: 10.7326/L16-0091. [DOI] [PubMed] [Google Scholar]
- Mackall C.L., Fleisher T.A., Brown M.R., Andrich M.P., Chen C.C., Feuerstein I.M. Distinctions between CD8 + and CD4 + T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 15 May 1997;89(10):3700–3707. [PubMed] [Google Scholar]
- Maiman M., Fruchter R.G., Serur E., Remy J.C., Feuer G., Boyce J. Human immunodeficiency virus infection and cervical neoplasia. Gynecol. Oncol. Sep 1990;38(3):377–382. doi: 10.1016/0090-8258(90)90077-x. [DOI] [PubMed] [Google Scholar]
- Mann J.M., Francis H., Quinn T., Asila P.K., Bosenge N., Nzilambi N. Surveillance for AIDS in a central African city. Kinshasa, Zaire. JAMA. 20 Jun 1986;255(23):3255–3259. [PubMed] [Google Scholar]
- Massad L.S., Ahdieh L., Benning L., Minkoff H., Greenblatt R.M., Watts H. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women's interagency HIV study. J. Acquir. Immune Defic. Syndr. 15 August 2001;27(5):432–442. doi: 10.1097/00126334-200108150-00003. [DOI] [PubMed] [Google Scholar]
- Massad L.S., Seaberg E.C., Watts D.H., Minkoff H., Levine A.M., Henry D. Long-term incidence of cervical cancer in women with human immunodeficiency virus. Cancer. 1 Feb 2009;115(3):524–530. doi: 10.1002/cncr.24067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H., Brooks J.T., Benson C.A., Holmes K.K., Pau A.K., Kaplan J.E. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 01 May 2014;58(9):1308–1311. doi: 10.1093/cid/ciu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdletshe S., Munkupa H., Lishimpi K. Acute toxicity in cervical cancer HIV-positive vs. HIV-negative patients treated by radical chemo-radiation in Zambia. Southern African Journal of Gynaecological Oncology. 2016;8(2):37–41. [Google Scholar]
- Mhlanga J.C., Durand D., Tsai H.L., Durand C.M., Leal J.P., Wang H. Differentiation of HIV-associated lymphoma from HIV-associated reactive adenopathy using quantitative FDG PET and symmetry. Eur. J. Nucl. Med. Mol. Imaging. 01 April 2014;41(4):596–604. doi: 10.1007/s00259-013-2671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki A.B., Ellenberg J.H., Farhat S., Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 01 July 2004;190(1):37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- National Institute of Health Clinical trials search results. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/results?protocolsearchid=8693745&vers=1 (Accessed 3 May 2017)
- Nunez M., Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect. Dis. 01 June 2005;5(6):374–382. doi: 10.1016/S1473-3099(05)70141-9. [DOI] [PubMed] [Google Scholar]
- Overton E.T., Sungkanuparph S., Powderly W.G., Seyfried W., Groger R.K., Aberg J.A. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin. Infect. Dis. 01 October 2005;41(7):1045–1048. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]
- Paavonen J., Naud P., Salmeron J., Wheeler C.M., Chow S.N., Apter D. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 25 July 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- Palella F.J., Delaney K.M., Moorman A.C., Loveless M.O., Fuhrer J., Satten G.A. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 26 March 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Parham G.P., Mwanahamuntu M.H., Kapambwe S., Muwonge R., Bateman A.C., Blevins M. Population-level scale-up of cervical cancer prevention services in a low-resource setting: development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS One. 17 April 2015;10(4):e0122169. doi: 10.1371/journal.pone.0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman-Aguilar A., Whiteman M.K., Cox S., Posner S.F., Meikle S.F., Kourtis A.P. Complications of common gynecologic surgeries among HIV-infected women in the United States. Infect. Dis. Obstet. Gynecol. 2012;2012:610876. doi: 10.1155/2012/610876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivodic L., Pardon K., Morin L., Addington-Hall J., Miccinesi G., Cardenas-Turanzas M. Place of death in the population dying from diseases indicative of palliative care need: a cross-national population-level study in 14 countries. J. Epidemiol. Community Health. 01 January 2016;70(1):17–24. doi: 10.1136/jech-2014-205365. [DOI] [PubMed] [Google Scholar]
- Powles T., Imami N., Nelson M., Gazzard B.G., Bower M. Effects of combination chemotherapy and highly active antiretroviral therapy on immune parameters in HIV-1 associated lymphoma. AIDS. 08 March 2002;16(4):531–536. doi: 10.1097/00002030-200203080-00003. [DOI] [PubMed] [Google Scholar]
- Rose P.G., Bundy B.N., Watkins E.B., Thigpen J.T., Deppe G., Maiman M.A. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 15 April 1999;340(15):1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- Rose P.G., Ali S., Watkins E., Thigpen J.T., Deppe G., Clarke-Pearson D.L. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a gynecologic oncology group study. J. Clin. Oncol. 01 July 2007;25(19):2804–2810. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- Scaradavou A. HIV-related thrombocytopenia. Blood Rev. 01 March 2002;16(1):73–76. doi: 10.1054/blre.2001.0188. [DOI] [PubMed] [Google Scholar]
- Simonds H.M., Wright J.D., du Toit N., Neugut A.I., Jacobson J.S. Completion of and early response to chemoradiation among human immunodeficiency virus (HIV)-positive and HIV-negative patients with locally advanced cervical carcinoma in South Africa. Cancer. 01 June 2012;118(11):2971–2979. doi: 10.1002/cncr.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds H.M., Neugut A.I., Jacobson J.S. HIV status and acute hematologic toxicity among patients with cervix cancer undergoing radical Chemoradiation. Int. J. Gynecol. Cancer. 01 June 2015;25(5):884–890. doi: 10.1097/IGC.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinayobye J., Sklar M., Hoover D.R., Shi Q., Dusingize J.C., Cohen M. Prevalence and risk factors for high-risk human papillomavirus (hrHPV) infection among HIV-infected and uninfected Rwandan women: implications for hrHPV-based screening in Rwanda. Infect Agent Cancer. 2014;9:40. doi: 10.1186/1750-9378-9-40. (December 08 eCollection 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi S., Buda A., Picchio M., Perego P., Moreni R., Pellegrino A. Lymph node metastasis in patients with clinical early-stage cervical cancer: detection with integrated FDG PET/CT. Radiology. 01 January 2006;238(1):272–279. doi: 10.1148/radiol.2381041799. [DOI] [PubMed] [Google Scholar]
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. Mar 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- UNAIDS . World Health Organization; 2002. Report on the Global HIV/AIDS Epidemic.http://data.unaids.org/pub/report/2002/brglobal_aids_report_en_pdf_red_en.pdf (Accessed 3 May 2017) [Google Scholar]
- Weinberg A., Song L.Y., Saah A., Brown M., Moscicki A.B., Meyer W.A. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis. 01 October 2012;206(8):1309–1318. doi: 10.1093/infdis/jis489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. IAPAC Mon. 2004 [PubMed] [Google Scholar]
- World Health Organization. Reproductive Health, World Health Organization . Comprehensive Cervical Cancer Control: A Guide to Essential Practice. 2nd ed. World Health Organization; 2014. Chronic diseases, health promotion. [Google Scholar]


