Abstract
Background and aims
Predicting the clinical course of Crohn’s disease (CD) is relevant for treatment selection. Currently, such diagnostic tools are lacking. In a previous pilot study, morphometric tissue image analysis showed promise in predicting the clinical phenotype and need for surgery. In this study, we aimed to validate our previous results on a larger cohort.
Methods
Colonic biopsies from CD patients with colonic or ileocolonic disease and at least five years of post-biopsy clinical follow-up were analyzed. The results were used to predict post-biopsy clinical phenotypes and outcomes. Data analysis was performed using multivariate regression models, discriminant score (DS) computations and Neural Network (NNET).
Results
Multivariate analysis of morphometric variables differentiated between B1 and B2 phenotypes (sensitivity 81%, specificity 74%, accuracy on cross-validation 75%; area under the curve (AUC) of 0.74 (CI 0.6–0.84; NNET model sensitivity 87%, specificity 67% on the testing population)). Differentiation between B1 and B3 phenotypes was also possible (sensitivity 69%, specificity 76%, accuracy 70.5% on cross-validation; AUC 0.78 (CI 0.68–0.89); NNET model sensitivity 78%, specificity 77% on the testing population)). Differentiating between B2 and B3 phenotypes was not possible using morphometric variables. Multivariate analysis predicted surgery (sensitivity 67%, specificity 72.5%, accuracy 69%; AUC 0.72 (CI 0.61–0.82); NNET model sensitivity 80%, specificity 91% on the testing population)).
Conclusions
This study validates previous results and suggests that morphometric image analysis of early biopsies from Crohn’s colitis patients may contribute to the prediction of future outcomes such as clinical phenotype and surgery. Prospective validation on larger cohorts is still needed.
Keywords: Inflammatory bowel disease, histology, morphometry, image analysis, phenotype prediction, Crohn’s disease
Introduction
Crohn’s disease (CD) is characterized by chronic inflammation of the gastrointestinal tract. The inflammatory process is thought to result from an interaction between genetic background, environmental factors, the host immune system and the gut microflora.1,2 The clinical phenotype of CD can be classified into three main categories as previously described in detail.3 B1 phenotype implies an inflammatory type disease without stricturing or penetrating complications. B2 phenotype is dominated by luminal stricture formation and B3 phenotype by penetrating complication such as fistulas and abscesses. Disease course and phenotype may change over the years and up to 80% of patients may develop disease complications.4,5 Histopathological findings are important tools in the diagnosis of CD. No findings are pathognomonic; however, some are considered highly suggestive when coupled with a likely clinical scenario, endoscopic findings and imaging results.6
Current management of CD may include the use of early aggressive therapy with immunosuppressive, biological agents or a combination of both. This strategy may offer advantages by disease course modification,7–10 but entails significant short- and long-term complications such as increased risk of infections and malignancy.11–13 Balancing efficacy and risks is a major therapeutic challenge, which can be significantly assisted by the ability to predict disease course. Previously, multiple clinical, serological and genetic characteristics have been shown to correlate with disease phenotype and complications.14–22 However, despite these observations, to date, prediction of disease course and complications is suboptimal and presents a major medical need.
Morphometry is a quantitative field that investigates changes in shape, size, architectural complexity and orientation of objects. Several methods for extraction of morphological parameters from an object exist. These include length, angles, perimeter shape and distribution in the space. Architectural complexities are typically analyzed by fractal and lacunar geometry algorithms.23 Morphometry has been used to predict lymph node metastasis, tumor progression and patient survival in squamous cell carcinoma of the vulva and renal cell carcinoma, and in the assessment of dysplasia and progression to adenocarcinoma in Barrett’s esophagus.23–26
In a previous pilot study, we were able to show that morphometric analysis may contribute to the prediction of the clinical phenotype and surgery in patients with Crohn’s colitis.27 In the present study, we sought to further evaluate and validate the histomorphometric features of early colonic biopsies from patients with Crohn’s colitis and their relationship to evolving clinical phenotypes.
Methods
Patients
CD patients with colonic involvement were selected from our database according to clinical phenotypes as outlined in the Montreal classification.3 Patients had either isolated colonic disease or a combination of small-bowel and colonic disease. We analyzed biopsies obtained at the first documented colonoscopy for each patient, which was around the time of diagnosis in the majority of cases. All patients had at least five years of post-biopsy follow-up. Clinical data were extracted from patient files and included the clinical phenotype, medical and surgical history. The clinical data were coded and subsequently analyzed and correlated with the histological findings. The observer who performed the morphometric analysis was completely blinded to the clinical variables and patient identity. The study was approved by the institutional review board.
Morphometric analysis
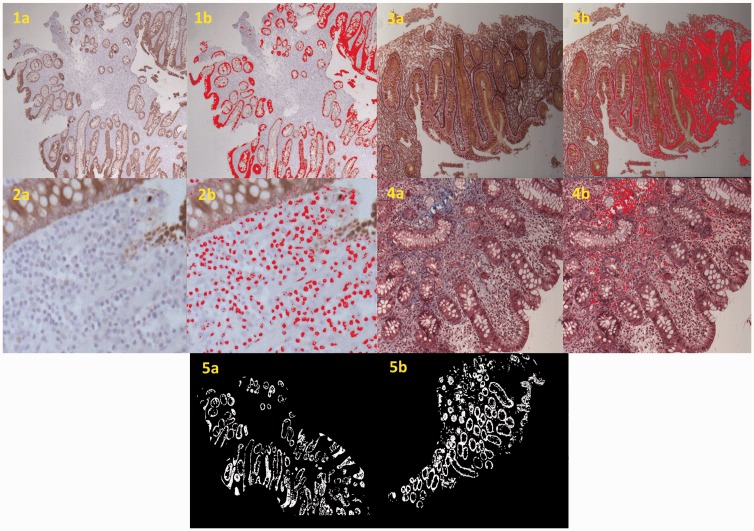
New slides were prepared from the original paraffin blocks. The histological stains included: hematoxylin and eosin (H&E) for diagnosis, confirmation and evaluation of the inflammatory cells type and numbers; pancytokeratin (CK) to highlight and quantify the mucosal crypts; and Mason-Tri-chrome (TC) and reticulin (RET) for the presence of mature and young collagen, respectively. Staining was performed according to standard procedures. Slides were scanned using the 2.1 Dotslide virtual microscopy system (Olympus, Germany and Japan). From each slide, three to four representative images were collected. The number of scanned fields and the magnification used were identical for all slides with each histological stain. The ImagePro plus 7.0 program (Media Cybernetics, USA) was used to analyze and quantify the collagen fibers, the inflammatory cells and the crypt architecture. The MATLAB program (MathWorks, USA) was used to analyze the fractal and lacunar dimensions and the orientation of the crypts in order to assess architectural complexity and distortions of the mucosal crypts. Figure 1 shows the morphometric analysis of different quantitative features in the colonic mucosa.
Figure 1.
Examples of morphometric measurements. The left image in each pair represents the clean stained histological specimen whereas the right image displays the quantified variable during image analysis (1a + 1b: CK stain highlighting the crypts; 2a + 2b: CK stain negative highlights the inflammatory cells; 3a + 3b: reticulin stain highlighting young collagen; 4a + 4b: Mason TC stain highlighting mature collagen; 5a + 5b: image masking highlights the mucosal architecture). CK: pancytokeratin; TC: Tri-chrome.
Statistical analysis
Univariate analysis was performed and morphometric variables associated with specific clinical characteristics and evolving clinical phenotype were identified. Variables identified as significant on univariate analysis were then used in a multivariate model (discriminant analysis) to adjust for confounding variables and to single out independent and significant predictors of disease phenotype. Validation of the results was obtained by using the “leave one out method.” Using the independent variables and their coefficients, discriminant scores (DS) were computed and receiver operating characteristic (ROC) analysis of the DS was calculated to obtain an area under the curve (AUC). In order to better handle the complexity and the large number of variables, a neural network (NNET) model with a feed forward architecture was trained using the morphometric variables data in order to develop a mathematical classifier that best predicts the clinical phenotypes. Our model used for function fitting a two-layer feed forward network with a sigmoid transfer function both in the hidden and the output layers. The output data consisted of the patients’ clinical phenotype. We used 50%–70% of the cases as the training population for our model, and 30%–50% of the cases as the testing population. Statistical analysis was performed with SPSS version 23 (SPSS Inc, Chicago, IL, USA).
Results
Patients
Patient characteristics are summarized in Table 1. The patient groups did not differ in average age at diagnosis, the average post-biopsy clinical follow-up or the gender. Differences were seen between groups in clinical data such as surgery, smoking and extraintestinal manifestations. Four patients in our cohort received 5 aminosalicylate (5ASA) or steroid treatment prior to biopsy (within one to two years). In the rest of the cases, biopsy was performed before any CD medications were started. The time lag between the diagnosis and the first documented biopsy was available only as an ordinal (not interval) variable measured in years (not months). As a result, the calculated median disease duration at time of biopsy was 0 years (less than one year, interquartile range (IQR) 0–3).
Table 1.
Patient characteristics.
| Phenotypea |
||||
|---|---|---|---|---|
| B1 | B2 | B3 | p value | |
| Age at diagnosisb | 26.7 ± 13.9 | 27 ± 12.5 | 25.4 ± 12.6 | NS |
| Age groupa | ||||
| <17 | 12 (25%) | 4 (21%) | 9 (23%) | NS |
| 17-40 | 24 (51%) | 11 (58%) | 18 (46%) | NS |
| >40 | 11 (24%) | 4 (21%) | 12 (31%) | NS |
| Mean post-biopsy follow-up (years) | 11 | 14 | 13 | NS |
| Smoking | 25 (52%) | 4 (22%) | 12 (39%) | NS |
| CD extenta | ||||
| Colon | 18 (37%) | 6 (32%) | 10 (33%) | NS |
| Ileo-colon | 30 (62%) | 13 (68%) | 20 (66%) | NS |
| Peri-anal disease | 8 (17%) | 6 (21%) | 15 (52%) | 0.007 |
| Surgery | 10 (21%) | 10 (29%) | 19 (68%) | <0.001 |
| Extra-intestinal manifestations | 23 (48%) | 3 (19%) | 9 (32%) | NS |
According to Montreal classification. bMean ± standard deviation. CD: Crohn’s disease.
Morphometry and its correlation to clinical variables and disease phenotypes
Univariate analysis showed multiple morphometric variables associated with specific clinical characteristics (Table 2): Smoking was associated with smaller, more complex-shaped crypts. Smoking was also associated with a lower degree of mature collagen optical density; peri-anal disease was associated with more inflammatory cells and lower degree of mature collagen optical density; the older age group (17–40) according to the Montreal classification had a smaller crypt area. Univariate analysis demonstrated an association between the number of inflammatory cells and the optical density of mature collagen and disease phenotypes (Table 3).
Table 2.
Association between morphometric variables and specific clinical characteristics of the cohort.
| Morphometric variable | Mean | STD | p value | |
|---|---|---|---|---|
| Smoking status | ||||
| Relative area occupied by crypts | No | 0.178 | 0.08 | 0.023 |
| Yes | 0.140 | 0.07 | ||
| Mean complexity of crypts | No | 0.386 | 0.07 | 0.049 |
| Yes | 0.36 | 0.05 | ||
| Minimal optical density (Tri-chrome) | No | 104.2 | 23.5 | 0.038 |
| Yes | 94.8 | 19.3 | ||
| Peri-anal disease | ||||
| Inflammatory cells per mm2 | No | 4994 | 2947 | 0.046 |
| Yes | 6542 | 4323 | ||
| Optical density (Tri-chrome) | No | 12.4 | 5.7 | 0.041 |
| Yes | 9.8 | 5.2 | ||
| Age group | ||||
| Overall crypt area | <17 years | 964455 | 1181175 | 0.04 |
| 17–40 years | 481326 | 467595 | ||
STD: standard deviation.
Table 3.
Univariate analysis of the correlation between morphometric variables and the evolving clinical phenotypes.
| Morphometric variable | B1 phenotype | B2 phenotype | B3 phenotype | p value (ANOVA) | p value (Bonferroni) |
|---|---|---|---|---|---|
| Minimum number of inflammatory cell per mm2 | 3342 ± 1782 95% CI (2824–3859) | 3709 ± 2330 95% CI (2586–4832) | 4653 ± 2960 95% CI (3567–5739) | 0.005 | 0.047 (B1 VS B3) |
| Mean overall optical density, Tri-chrome | 100 ± 25 95% CI (92–107) | 108 ± 24 95% CI (96–120) | 115 ± 24 95% CI (106–124) | 0.02 | 0.02 (B1 VS B3) |
| Mean optical density (Red), Tri-chrome | 129 ± 26 95% CI (121–136) | 132 ± 29 95% CI (118–145) | 144 ± 27 95% CI (135–153) | 0.04 | 0.04 (B1 VS B3) |
| Minimal overall optical density, Tri-chrome | 85 ± 23 95% CI (78–88) | 92 ± 22 95% CI (81–102) | 99 ± 22 95% CI (91–107) | 0.03 | 0.03 (B1 VS B3) |
Values are mean ± standard deviation. ANOVA: analysis of variance; CI: confidence interval.
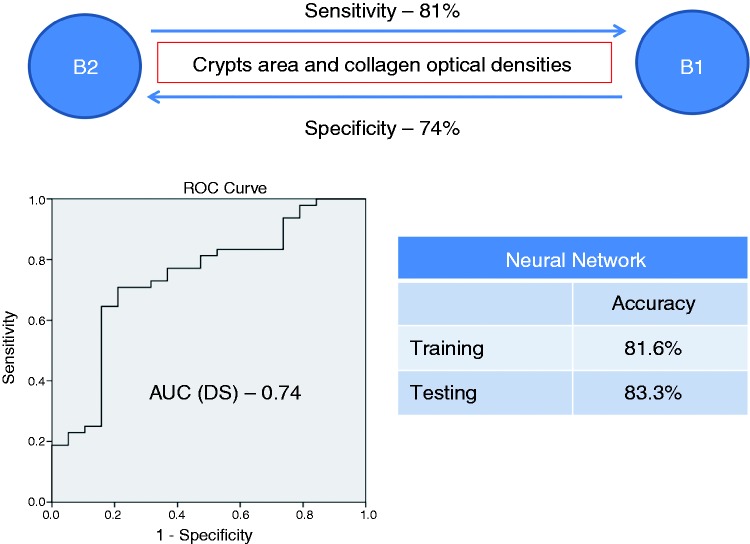
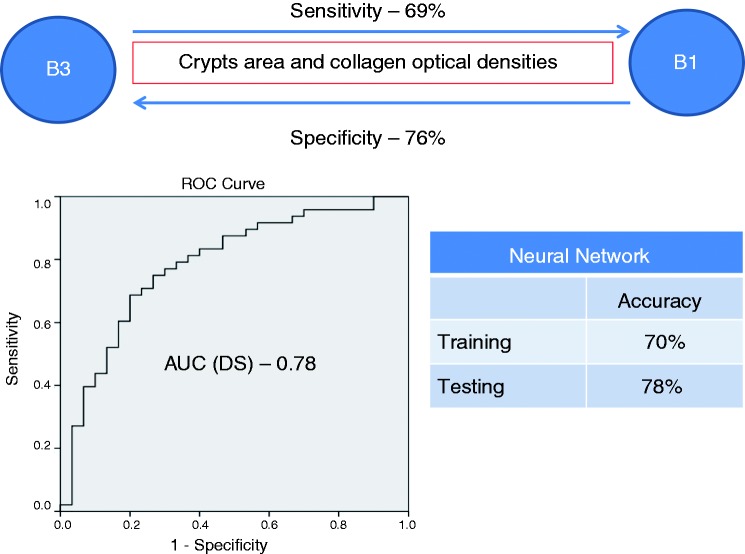
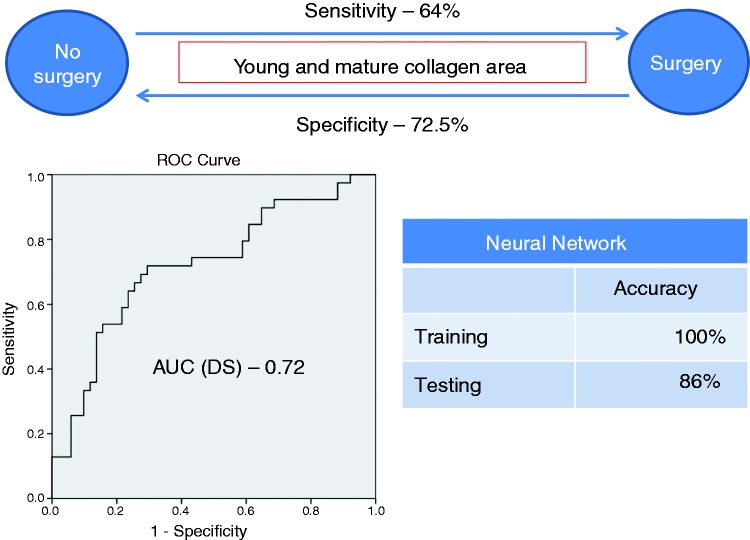
On multivariate analysis (Table 4) crypts and collagen optical densities were independent predictors for differentiating between B1 and B2 phenotypes (sensitivity 81%, specificity 74%, accuracy on cross-validation 75%). A ROC analysis of the discriminant score (DS) yielded an AUC of 0.74 (95% confidence interval (CI) 0.6–0.84) for this comparison. A NNET model was also able to differentiate between B1 and B2 phenotypes (sensitivity 87%, specificity 67% on the testing population). Morphometric measurements of crypts and collagen area and optical densities were found to be independent predictors for differentiating between B1 and B3 phenotypes (sensitivity 69%, specificity 76%, accuracy 70.5% on cross-validation). ROC analysis of the DS revealed an AUC of 0.78 (95% CI 0.68–0.89). A NNET model applied to this comparison yielded a sensitivity of 78%, and a specificity of 77% on the testing population. Differentiating between B2 and B3 phenotypes was not possible using morphometric variables. Multivariate analysis also predicted surgery (sensitivity 67%, specificity 72.5%, accuracy on cross-validation 69%). Morphometric measurements of collagen area were independent predictors in this analysis. ROC analysis of the DS for surgery revealed an AUC of 0.72 (95% CI 0.61–0.82). A NNET model predicted surgery with a sensitivity of 80% and a specificity of 91% on the testing population. Figures 2–4 show a schematic representation of the multivariate analysis between the clinical phenotypes.
Table 4.
Independent predictors found in multivariate analysis for distinguishing between disease phenotypes and the need for surgery.
| Table 4 | Variable | p value |
|---|---|---|
| B1 versus B2 | Mean percentage crypt area | 0.009 |
| Optical density reticulin | 0.002 | |
| Mason TC optical density | <0.001 | |
| B1 versus B3 | Mean percentage crypt area | 0.002 |
| Mason TC optical density | 0.001 | |
| Surgery | Crypt area | 0.003 |
| Maximal area of TC | 0.021 |
TC: Mason Tri-chrome.
Figure 2.
A schematic presentation of multivariate analysis and NNET model for predicting between B1 and B2 phenotypes. NNET: neural network; AUC: area under the curve; DS: discriminant score.
Figure 3.
A schematic presentation of multivariate analysis and NNET model for predicting between B1 and B3 phenotypes. NNET: neural network; AUC: area under the curve; DS: discriminant score.
Figure 4.
A schematic presentation of multivariate analysis and NNET model for predicting the need for surgery. NNET: neural network; AUC: area under the curve; DS: discriminant score.
Discussion
The present study describes the use of morphometric analysis for quantitative measurements of various elements in endoscopic colonic biopsies in order to evaluate their associations and potential role in predicting disease course and complications.
We found an association between smoking and crypt size and complexity and level of collagen optical density. This observation implies that smoking may have an effect on tissue inflammation and fibrosis early in the disease course. An association was also seen between the presence of peri-anal disease and the amount of inflammatory cells and levels of collagen optical density. This is very interesting as both smoking and peri-anal disease have been shown to be associated with more severe disease and inflammation.21,22 Further research into this area and the potential molecular mechanisms behind these phenomena is needed.
Currently, prediction of disease phenotype is suboptimal. Genetic factors such as NOD2 mutations are associated with a fibro-stenosing phenotype and an increased need for surgery.14 However, genetic variables, naturally available upon diagnosis, are useful in only approximately one-third of patients, and are even less relevant in certain geographical areas. A prominent example of this limitation is the low prevalence of NOD2/CARD15 mutations in the eastern Asian CD patient population as compared to Western populations.28 Serology may be an additional useful tool. Antibodies to anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-CBIRL (anti-flagelin) are associated with small-bowel and penetrating disease, respectively.15–17 Antibodies to perinuclear anti-neutrophil cytoplasmatic antibodies (pANCA) correlate with an ulcerative colitis (UC)-like disease.18 Furthermore, multiple positive serologies are associated with propensity for disease complications.19,20 However, although serological findings may be present at least to some extent at diagnosis, only limited data are available regarding the association of serology at early disease stages and late outcomes.29,30 Clinical characteristics such as cigarette smoking, early use of steroid and the presence of peri-anal disease have also been shown to be suggestive of a complicated disease course.21,22 However, despite these observations, to date, prediction of disease course and complications is suboptimal and presents a major medical need. In this study, several morphometric variables emerged as significant for prediction of disease phenotype and the need for surgery in the multivariate analysis and NNET model (crypts and collagen optical densities for comparison between B1 and B2 phenotypes; crypts and collagen area and optical densities for comparison between B1 and B3 phenotypes; collagen area for prediction of surgery). Using two different mathematical models provided an adequate validation of the potential predictive ability of morphometry in this cohort. These observations, in combination with known predictors, may enhance our ability to predict disease course and complications.
We recognize this study’s limitations: Firstly, this was a retrospective study of a small group of patients. A small number of patients in our cohort (four) received 5ASA or steroid therapy prior to biopsy, which theoretically could have influenced the histological findings. However, since this is a minority, we believe it did not significantly influence the average result of the different variable for each phenotype group. Validation of our results using additional, larger blinded cohorts and eventually in a bona fide prospective manner is needed. Secondly, we examined biopsies from patient with colonic CD involvement and did not include small-bowel biopsies. In addition, some patients had isolated colonic disease whereas others within our cohort had a combination of colonic and small-bowel disease, which may already confer a different clinical phenotype. We did not have detailed information regarding patients’ past complications (i.e. stricture location and extent, or nature of penetrating complications). However, since complicated disease can occur both in the small bowel and the colon, we felt these patients could be grouped into one cohort. Patients with isolated colonic disease represent only 25%–50% of CD patients, and further studies are needed in order to characterize and quantify the histological findings in other gastrointestinal segments in patients with combined small-bowel and colonic disease and in patients with isolated small-bowel disease.
In conclusion, this study validates our preliminary results that quantitative morphometric data may enable differentiation and prediction of clinical phenotypes and outcomes such as surgery. Because histological sampling is almost universally used in the workup of CD patients, this novel approach may be combined with other biologic variables and clinical predictors to significantly increase the ability to classify and predict the clinical course of CD colitis patients, thus improving their management. Prospective validation on larger cohorts is still needed.
Declaration of conflicting interests
Yehuda Chowers is a member of the Rambam Committee of Interns and Residents (CIR) and has served as a consultant and received lecture fees from Abbott, Schering Plough, Janssen and Takeda.
Funding
This work was supported by the departments of Gastroenterology and Pathology at the Rambam Health Care Campus.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347: 417–429. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV., Jr Inflammatory bowel disease extending its reach. Gastroenterology 2005; 129: 1117–1120. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- 4.Cosnes J, Gowerrousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140: 1785–1794. [DOI] [PubMed] [Google Scholar]
- 5.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001; 49: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Berre N, Heresbach D, Kerbaol M, et al. Histological discrimination of idiopathic inflammatory bowel disease from other types of colitis. J Clin Pathol 1995; 48: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: Increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis 2013; 7: 213–221. [DOI] [PubMed] [Google Scholar]
- 8.Rubin DT, Uluscu O, Sederman R. Response to biologic therapy in Crohn’s disease is improved with early treatment: An analysis of health claims data. Inflamm Bowel Dis 2012; 18: 2225–2231. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology 2005; 128: 862–869. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: Results from the CHARM study. Gastroenterology 2008; 135: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 11.Van Assche G, Lewis JD, Lichtenstein GR, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organisation: Safety. Am J Gastroenterol 2011; 106: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 12.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 13.Siegel CA, Finlayson SRG, Sands BE, et al. Adverse events do not outweigh benefits of combination therapy for Crohn’s disease in a decision analytic model. Clin Gastroenterol Hepatol 2012; 10: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abreu MT, Taylor KD, Lin YC, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn’s disease. Gastroenterology 2002; 123: 679–688. [DOI] [PubMed] [Google Scholar]
- 15.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology 2004; 126: 414–424. [DOI] [PubMed] [Google Scholar]
- 16.Papp M, Altorjay I, Dotan N, et al. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am J Gastroenterol 2008; 103: 665–681. [DOI] [PubMed] [Google Scholar]
- 17.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology 2005; 128: 2020–2028. [DOI] [PubMed] [Google Scholar]
- 18.Vasiliauskas EA, Plevy SE, Landers CJ, et al. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn’s disease define a clinical subgroup. Gastroenterology 1996; 110: 1810–1819. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante M, Henckaerts L, Joossens M, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut 2007; 56: 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieder F, Schleder S, Wolf A, et al. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated Crohn’s disease behavior. Inflamm Bowel Dis 2010; 16: 263–274. [DOI] [PubMed] [Google Scholar]
- 21.Lakatos PL, Czegledi Z, Szamosi T, et al. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behavior change in patients with Crohn’s disease. World J Gastroenterol 2009; 15: 3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology 2006; 130: 650–656. [DOI] [PubMed] [Google Scholar]
- 23.Sabo E, Beck AH, Montgomery EA, et al. Computerized morphometry as an aid in determining the grade of dysplasia and progression to adenocarcinoma in Barrett’s esophagus. Lab Invest 2006; 86: 1261–1271. [DOI] [PubMed] [Google Scholar]
- 24.Lavie O, Maini I, Pilip A, et al. Computerized nuclear morphometry for the prediction of inguinal lymph nodes metastases in squamous cell carcinoma of the vulva. Int J Gynecol Cancer 2006; 16: 556–561. [DOI] [PubMed] [Google Scholar]
- 25.Nativ O, Sabo E, Raviv G, et al. Value of nuclear morphometry for differentiating localized from metastatic renal cell carcinoma. Eur Urol 1998; 33: 186–189. [DOI] [PubMed] [Google Scholar]
- 26.Sabo E, Boltenko A, Sova Y, et al. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin Cancer Res 2001; 7: 533–537. [PubMed] [Google Scholar]
- 27.Klein A, Eliakim R, Karban A, et al. Early histological findings quantified by histomorphometry allow prediction of clinical phenotypes in Crohn’s colitis patients. Anal Quant Cytol Histol 2013; 35: 95–104. [PubMed] [Google Scholar]
- 28.Thia KT, Loftus EV, Jr, Sandborn WJ, et al. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol 2008; 103: 3167–3182. [DOI] [PubMed] [Google Scholar]
- 29.Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis 2004; 10: 55–60. [DOI] [PubMed] [Google Scholar]
- 30.Israeli E, Grotto I, Gilburd B, et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 2005; 54: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]